Abstract
Objective: There exists a strong belief among physicians and the lay public that pregnancy adversely affects survival in patients with melanoma. The authors asked if there was any evidence to support this in patients with clinically localized disease. Methods: The authors reviewed the published literature on MEDLINE. Results: The authors found no compelling evidence in the literature that pregnancy has a negative impact on survival in patients with clinically localized cutaneous melanoma. Two recent population-based studies reported no negative impact of pregnancy on survival when pregnant melanoma patients were compared to nonpregnant gender-matched controls. A small increased risk of cause-specific death was noted in a recent population-based study, though this effect was small (HR, 1.52, p=0.47) and pregnant patients were more likely to have axial primary sites, which are associated with a poorer outcome. Conclusion: There is no compelling evidence that pregnancy adversely affects outcome in melanoma patients who have clinically localized disease. Continuing to recommend a delay in childbearing for these patients is not supported by the published medical literature.
There is a controversy in the literature about the influence of childbearing on survival of patients with cutaneous melanoma. This is an important clinical issue, as melanoma is the most commonly diagnosed cancer in pregnant women.1 It has been traditionally held that patients who are pregnant at the time of diagnosis of melanoma have a poor prognosis2 and that a subsequent pregnancy following treatment for melanoma increases risk of recurrence.3 Many young women who have been previously treated for melanoma are advised to delay or avoid pregnancy for fear that it increases risk for recurrence.
Part of the problem with the question of the effect of pregnancy on outcome in melanoma patients is a “numerator” problem. Dramatic reports of pregnant women who develop widespread metastatic melanoma, having been diagnosed years before, make headlines and engender fear among both patients and physicians.4 In addition, in any busy oncology practice it is not uncommon to remember the occasional patient who presented with recurrent melanoma concomitant with pregnancy.5 On the other hand, patients who have melanoma treated during pregnancy or become pregnant shortly before or after a diagnosis of melanoma, without sequelae, are far less memorable. These patients constitute the “denominator.”
Three important questions will be examined in this review. First, is the immunology of pregnancy potentially detrimental to melanoma patients? Second, are there any clinical data to support a decrease in survival due to pregnancy in melanoma patients with clinically localized disease or those at higher risk for recurrence? Finally, is there any evidence that pregnancy subsequent to treatment for clinically localized melanoma increases a patient's risk of recurrence?
Background
Concern about the implications of pregnancy in patients with melanoma was initially raised in 1951 when Pack et al2 reported poor outcomes in 32 patients with melanoma diagnosed during pregnancy.2 As a consequence, they recommended women avoid pregnancy for 3 to 5 years post-treatment. Byrd et al6 took this one step further, and in 1954 published a report stating that female melanoma patients should be surgically sterilized in order to prevent metastasis.6 These early reports laid the groundwork for a prevailing concern about the prognostic implications of pregnancy on prognosis in patients with cutaneous melanoma.
Recently published National Cancer Institute (NCI) Surveillance Epidemiology and End Results (SEER) data demonstrate an increasing trend in melanoma incidence in young women (aged 15–39).7 A significant proportion of patients who are treated for cutaneous melanoma are women between the ages of 15 and 45 years and, therefore, of childbearing age.8 In a review from the John Wayne Cancer Institute by Wong et al,9 the percentage of patients who were pregnant at the time of melanoma diagnosis was 8.7 percent. This is much higher than would be expected and most likely reflects the fact that the reporting institution is a tertiary cancer referral center. However, they noted that 48 percent of female patients referred to their center were between the ages of 15 and 40 years, so approximately half of the female patients were of childbearing age.9 Jacobs et al10 reported a much lower incidence of concurrent pregnancy and melanoma, but found that it was second only to breast cancer in women who presented at the University of Chicago with cancer occurring during pregnancy. Perhaps the most accurate estimate of the scope of the problem can be obtained from a population-based study from Sweden where Lambe et al8 examined a cohort of Swedish women born between 1925 and 1972 and found that 5.6 percent of cancers diagnosed in women of childbearing age occurred concurrently with pregnancy or lactation. The only cancer with an observed/expected ratio of 1 (all others were less common) was melanoma.8 In a recent population-based study from Norway, melanoma was the most common cancer diagnosed during pregnancy.1
Immunological Changes During Pregnancy
The immune system of the pregnant patient is modified so that the fetus, expressing paternal alloantigens, will not be rejected by the mother. The mother's immune system recognizes paternal fetal antigens as foreign and yet it is uncommon for an immune response to be mounted against these antigens. It has been demonstrated that 30 percent of women have immunoglobulin G (IgG) as well as cytotoxic T-cells, which recognize paternal human leukocyte antigens (HLA).11 There is abundant evidence in murine models to support maternal T-cell awareness of fetal alloantigens without evidence of a detrimental fetal effect.12–14 The precise mechanisms by which tolerance to alloantigens is established and maintained is unclear, but several alterations occur that may be important.
The total number of immune effector cells is altered during pregnancy. Granulocytes increase, monocytes remain unchanged, and there is a significant decrease in lymphocytes compared with the quantity in nonpregnant patients. These alterations are consistent with the observation that adaptive immunity is preserved and innate immunity is somewhat downregulated in the pregnant patient––conditions that favor allograft tolerance. T-lymphocytes demonstrate impaired interleukin (IL)-2 and interferon gamma production after stimulation. Clinical evidence of this phenomenon includes the frequently observed remission of autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis, in the pregnant patient.15
Figures 1A and 1B.
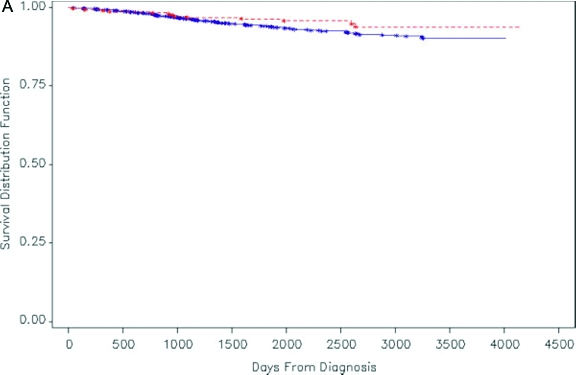
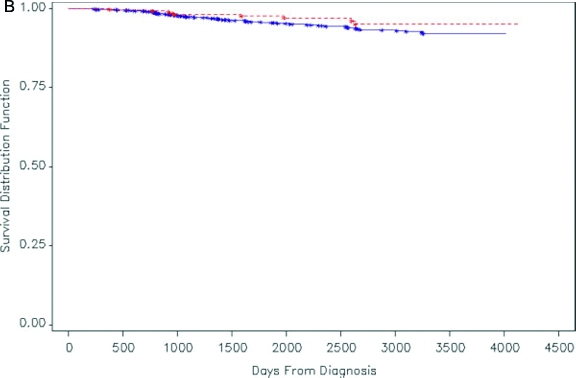
Kaplan-Meier survival distribution for women (both pregnant women and age-matched, nonpregnant women) in California who were diagnosed with melanoma during 1991–1999. Solid line: nonpregnant women with melanoma; dashed line: pregnant women with melanoma. (A) Patients with all stages of disease (although 82% have clinically localized disease). (B) Patients with clinically localized disease. A log rank test showed no significant difference in survival distributions between pregnant women and nonpregnant women with melanoma (p=0.13 for A, p=0.16 for B).
Reprinted with permission from O'Meara AT, Cress R, Xing G, Danielsen B, Smith LH. Malignant melanoma in pregnancy. A population-based evaluation. Cancer. 2005;103(6):1217–1226.
B-lymphocyte function appears to be normal, and antibody production is unchanged during pregnancy.15 Therefore, the immune system in pregnancy is more likely to respond with antibody and less likely with activated T-cells, a state that has been described as “Th2 bias.” This shift in Th1/Th2 (Th = T helper cells) balance during pregnancy is similar to that described in cancer patients, where Th1 responses are relatively downregulated and Th2 responses appear relatively more prominent, a potentially detrimental scenario for the melanoma patient.16
Another potential mechanism by which fetal allograft tolerance is established and maintained is the expression of the costimulatory molecule B7-H1, an immunomodulatory cell surface molecule expressed by trophoblast cells at the maternal/fetal interface that induces apoptosis in activated T cells. Expression is upregulated during pregnancy. Tumors may utilize a similar mechanism to escape immune surveillance, for B7-H1 has also been reported to be expressed in melanoma and renal cell carcinoma. B7-H1 expression at the maternal fetal interface appears to favor suppression of activated maternal leukocytes, making it a potentially useful mechanism for maintaining tolerance to fetal alloantigens.17
Another immunomodulatory molecule expressed by tumors as well as at the maternal/fetal interface is HLA-G, which is a nonclassical major histocompatibility complex (MHC) class I molecule with restricted tissue distribution. It is widely expressed on trophoblast and chorionic blood vessels, and, interestingly, on melanoma cell lines and fresh tissue biopsies from melanoma patients. Expression of HLA-G facilitates survival of HLA I loss tumors by inhibiting NK-mediated lysis. IL10, a Th2 cytokine, upregulates HLA-G expression on tumor cells. HLA-G exists in both membrane-bound and soluble forms and may be a mechanism for induction of systemic tolerance to tumor antigen in the pregnant patient.18
Indoleamine 2,3-dioxygenase (IDO) is an enzyme that has recently been described and found to play a role in fetal allograft tolerance. IDO degrades tryptophan, an essential amino acid necessary for T-cell activation. This promotes T-cell suppression and T-cell tolerance. Maternal T-regulatory cells may induce IDO expression on trophoblast and uterine dendritic cells and promote tolerance to paternal antigens by this mechanism.19
These mechanisms represent potential means of T-cell suppression in the pregnant patient, clearly designed to prevent rejection of the fetal allograft. These same mechanisms are, theoretically, detrimental to the patient with cancer, as T-cell recognition of tumor antigen is considered a central mechanism of effective antitumor response. With this in mind, is there any clinical evidence that pregnancy negatively impacts the survival of melanoma patients?
Melanoma and Pregnancy in Patients with Clinically Localized Disease
Level I/II evidence (randomized or nonrandomized clinical trials, respectively) to address the implications of pregnancy in the cancer patient does not exist. The highest level of evidence is level III, consisting of the most reliable level III data (population-based studies, or level IIIA), followed by consecutive case reports (level IIIB) and nonconsecutive case reports (level IIIC). Population-based studies avoid much of the bias inherent in consecutive case reports and nonconsecutive case series.
Figure 2.
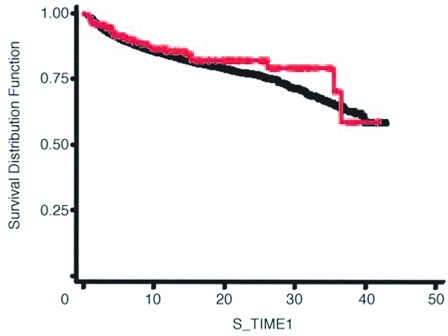
Kaplan-Meier curves of the probability of survival for women with pregnancy-associated melanoma compared with women with nonpregnancy-associated melanoma. Red line, women with melanoma diagnosed while pregnant; black line, women with melanoma diagnosed while nonpregnant; S_TIME1, survival time in years. Reprinted with permission from Lens MB et al. J Clin Oncol. 2004;22(21):4369–4375.
In 1981, Houghton et al20 used the Connecticut Tumor Registry to compare 12 pregnant patients with melanoma to 175 nonpregnant controls. At five-year follow up, there was no significant difference in survival between pregnant (55%) and nonpregnant (58%) patients. Pregnant patients, however, tended to have prognostically poorer primary sites, as well as more advanced stage melanoma.20 There are two other relatively recent population-based studies that address the question of whether pregnancy adversely affects survival in melanoma patients. Lens et al21 reported on 185 patients from the Swedish Cancer Registry who were pregnant at the time of diagnosis of clinically localized melanoma. The patients were diagnosed between the years 1958 and 1999. Researchers compared the outcome of these patients with 5,348 nonpregnant age- and gender-matched controls. They noted that pregnant melanoma patients had somewhat thicker melanomas, with a mean Breslow thickness of 1.28mm versus 1.07mm in controls. The anatomic location of the melanoma was quite similar, however, with approximately 35 percent of patients in each group with melanoma of the trunk and approximately 50 percent with melanoma of the extremity. No evidence of a decrease in survival was found in the pregnant patients compared to the nonpregnant controls (hazard ratio [HR] for pregnant patients, 1.08; 95 percent confidence interval [CI], 0.60–1.93).21
Another recent population-based study (level IIIA) was reported by O'Meara et al.22 This study included 412 pregnant and postpartum patients in California who were diagnosed with melanoma between 1991 and 1999. Postpartum patients were defined as those patients who were diagnosed with melanoma within the first year after delivery. The control group was an age-matched group of patients who were not pregnant at the time of diagnosis. The vast majority of patients had clinically localized melanoma (82%). The median Breslow thickness was 0.77mm in pregnant patients, 0.9mm in postpartum patients, and 0.81mm in the controls. The control group was well matched for clinical stage, anatomic site, and Breslow thickness. Researchers found no evidence of a decrease in survival of patients who were pregnant (HR=0.79; P=0.570) or postpartum (HR=0.58; P=0.162) compared to controls. Not surprisingly, Breslow thickness was the most important prognostic factor.22
In the most recent population-based (level IIIA) study, Stensheim et al1 used the Cancer and Medical Birth Registry of Norway to assess 42,511 women (516 pregnant, 531 lactating), aged 16 to 49, with various cancers. The most commonly diagnosed cancer during pregnancy was melanoma. When compared to age- and gender-matched nonpregnant controls, there was a slightly increased risk of cause-specific death if melanoma was diagnosed during pregnancy (HR=1.52; P=0.047). Interestingly, they noted that pregnant patients were more likely to have a primary tumor on the head and neck or trunk, compared with nonpregnant controls, where the extremity was the most common site. No difference in Breslow thickness was found between the pregnant versus nonpregnant patients; however, Breslow thickness was collected for only 55 percent of the pregnant patients.1
Level IIIB clinical evidence consists of consecutive case series. These studies report no evidence for a negative impact of pregnancy on survival of melanoma patients with clinically localized disease. Slingluff et al23 reported no dimunition in survival in 100 pregnant melanoma patients compared to age- and gender-matched nonpregnant controls. Seventy-five percent of the pregnant patients were alive at five years compared to 77 percent of nonpregnant controls. They observed that pregnant patients tended to have thicker lesions (median Breslow thickness 2.2 vs. 1.5mm) but reported no difference in survival.23 A retrospective study at Massachusetts General Hospital found a significantly greater tumor thickness in pregnant versus nonpregnant patients (2.28 vs. 1.22mm, respectively; P<0.007) and a slightly increased survival was noted for pregnancy-associated melanoma.24 In pregnancy- versus nonpregnancy-associated melanoma, patients with no evidence of metastasis had survival rates of 88.4 percent and 82.5 percent, respectively.24 In addition, in a large study by Wong et al,9 survival was no worse for 66 pregnant melanoma patients compared to 619 age- and gender-matched controls.9 In a small series from Memorial Sloan-Kettering Cancer Center (MSKCC), pregnant melanoma patients with clinically localized disease had an 80-percent survival at five years compared to 83 percent for patients who were nulliparous at diagnosis.3
Daryanani et al25 performed a retrospective, case-control study comparing the outcome of 46 patients with clinically localized melanoma arising in pregnancy to 368 nonpregnant controls seen at University Medical Center Groningen (1965–2001).25 There was no significant difference between the two groups in 10-year disease-free survival (DFS) or overall survival (OS). Pregnant patients with stage 126 melanoma had a DFS and OS of 88 and 94 percent, respectively, compared to nonpregnant controls (86% and 90%, respectively). In addition, there was no significant difference in survival between pregnant patients compared to controls with stage 2 melanoma. Researchers did observe that the median Breslow thickness was greater (2.0mm) in pregnant patients compared with controls (1.7mm), although this was also not statistically significant. Unfortunately, the investigators do not describe how the cases were identified, suggesting that the difference in thickness observed may be secondary to retrieval bias.25 A similar, recent, retrospective study by Silipo et al27 compared 10 pregnant patients to 30 well-matched controls. At five-year follow up, they found no significant difference in survival between the two groups.27
MacKie et al28 also evaluated the impact of timing of pregnancy on survival in female melanoma patients. These data were collected and analyzed within the auspices of the World Health Organization (WHO) Melanoma Program and published in Lancet in 1991. Although the patient groups were relatively small, researchers found no evidence of a negative impact of childbearing on survival in any subgroup of patients. In this study, the control group consisted of patients who had completed childbearing prior to the diagnosis of melanoma. Researchers did observe, as has been previously reported, that melanomas tended to be thicker in pregnant patients. The median Breslow thickness was 2.38mm in the pregnant patients compared to 1.49mm in the 86 control patients. In a multivariate analysis of factors affecting outcome, pregnancy was not significantly associated with a decrease in survival. Again, not surprisingly, known prognostic features (thickness and truncal site) were important factors for survival.28
Table 1 chronologically outlines past experience with melanoma, and the impact pregnancy has had on the survival of patients with clinically localized disease. Table 2 summarizes the Breslow thickness in pregnant versus nonpregnant patients with melanoma; more recent students have found no significant difference.21,22,25
Table 1.
Impact of pregnancy on survival in patients with melanoma
| AUTHOR | PATIENTS (n) | SURVIVALa | STAGE |
|---|---|---|---|
| Stensheimd 2009 | 160 pregnant, 126 lactating, 4,460 age, gender-matched controls | (11.9 years) Pregnant HR=1.52, P=0.047 Lactating HR=1.10, P=NS Controls HR=1.00 |
All Stages |
| Silipob 2006 | 10 pregnant 30 age, anatomic site, stage controls |
(5 years) P=NS | pTis, pT1a, pT2a, pT3a, pT4ac |
| O'Mearad 2005 | 149 pregnant, 263 postpartum, 2,451 age-matched controls | Pregnant HR=0.79 Postpartum HR=0.58 Controls HR=1.00; P=NS |
All Stages |
| Lensd 2004 | 185 pregnant, 5,348 age-matched controls | (12.9 years) 85% pregnant; 82% nonpregnant P=NS |
Clinically localized |
| Daryananib 2003 | 46 pregnant, 368 age-, gender-matched controls | (10 years) Stage 1: P=NS; 88% pregnant; 86% nonpregnant Stage 2: P=NS; 67% pregnant; 73% nonpregnant |
1, 2c |
| Traversb 1995 | 45 pregnancy associated,e 420 age-matched controls | (5.4 years) 88.4% pregnant; 82.5% nonpregnant P value not given |
Clinically localized |
| MacKieb 1991 | 92 pregnant, 85 never pregnant, 143 completed all pregnancies, 68 between pregnancies | P=NS | Clinically localized |
| Slingluffb 1990 | 100 pregnant, 86 age-matched controls | (mean 6.8 years) 75% pregnant; 77% control P=NS |
All stages |
| Wongb 1989 | 66 pregnant, 619 controls matched for thickness, Clark's level, anatomic site, and histopathology | (5 years) 86% pregnant; 87% control P=NS |
Clinically localized |
| Reingtenb 1985 | 58 pregnant, 585 controls matched for age, primary site, disease stage, Clark's level, tumor thickness, ulceration, histologic type | (5 years) P=NS |
Clinically localized |
| Houghtond 1981 | 12 pregnant, 175 controls matched for age, anatomic site, stage at diagnosis | (5 years) 55% pregnant 58% nonpregnant |
All stages |
| Shiub 1976 | Stage 1: 20 pregnant 36 age-matched nulliparous controls Stage 2: 14 pregnant 11 age-matched nulliparous controls | (5 years) Stage 1: P=NS; 80% pregnant 83% nulliparous Stage 2: P<0.05*; 29% pregnant 55% nulliparous |
1f, 2g |
HR=hazard ratio (Cox proportional hazards model); NS=not significant
Overall survival
consecutive case series
American Joint Committee on Cancer (AJCC) staging (Balch, 2001)
small population-based studies
pregnant or within a year of delivery
melanoma localized to the primary site
metastases confined to regional lymph nodes
statistically significant difference between the pregnant and nulliparous groups
Table 2.
Mean Breslow thicknessa in pregnant versus nonpregnant patients with melanoma
| AUTHOR | PATIENTS (n) | PREGNANT | NONPREGNANT | P |
|---|---|---|---|---|
| O'Meara 2005 | 149 pregnant 2,451 controls |
0.77b | 0.81 | NS |
| Lens 2004 | 185 pregnant 5,348 controls |
1.28 | 1.07 | NS |
| Daryanani 2003 | 46 pregnant 368 controls |
2.0b | 1.7 | NS |
| Travers 1995 | 45 pregnancy associatedc 420 controls |
2.28 | 1.22 | <0.007 |
| MacKie 1991 | 92 pregnant 85 never pregnant 143 completed pregnancy 68 between pregnancy |
2.38 | 1.49 never pregnant 1.96 completed pregnancy 1.48 between pregnancies |
0.002 |
| Slingluff 1990 | Stage 1 88 pregnant 79 controls |
1.87 | 1.45 | 0.052d |
| Wong 1989 | 66 pregnant 619 controls |
1.24 | 1.28 | NS |
NS=not significant
thickness given in mm
median thickness
pregnant or within a year of delivery
tumor thickness was slightly greater for pregnant patients by unpaired two-tailed t test
Melanoma and Pregnancy in High-Risk Melanoma Patients
There are little data to address the question of whether pregnancy has a negative impact on survival in patients with more advanced disease. A significant proportion of patients may decide to terminate pregnancy in the setting of recurrent or advanced melanoma; therefore, an appropriate evaluation of the outcome in this group is extremely difficult to measure. In a study reported by Shui et al3 from MSKCC, patients with regional melanoma (American Joint Committee on Cancer [AJCC] stage 3)26 were found to have a significant decrease in survival compared with nulliparous patients. The study was quite small, with only 14 pregnant patients and 11 controls. The five-year survival was 29 percent for the pregnant patients and 55 percent for the nonpregnant patients, not statistically significant but involving very small numbers of patients.3 There are no additional data that address this important question and it is unlikely that it can ever truly be addressed. Recommendations must be based on individual patients and their wishes and concerns.
Is there evidence of increased risk of recurrence or death in melanoma patients with clinically localized disease who subsequently become pregnant. A more common clinical scenario occurs when a patient has been treated for melanoma and wishes to become pregnant. Is there any evidence that this will increase the risk of recurrence? It is not uncommon for practitioners to recommend that women delay childbearing for 2 to 3 years following treatment for melanoma, although the data on which these recommendations are based are difficult to identify. In addition, delaying childbearing may significantly decrease the chance of successful childbearing in a large proportion of patients.29 In a consecutive case series study reported by Reintgen et al,30 there was no evidence that patients who became pregnant within five years of diagnosis had a decrease in survival when compared to control patients.30 The WHO study, by MacKie et al,28 found no significant difference in survival between women who had completed all pregnancies compared to those diagnosed with melanoma before or those diagnosed between pregnancies.28 Stensheim et al,1 as well as Lens et al, also found that pregnancy after diagnosis did not increase risk of death.1,21 Indeed, in the recent population-based report from Norway, childbearing subsequent to a diagnosis of melanoma was associated with a more favorable outcome compared with women who did not give birth after treatment for melanoma.1
Conclusion
There is no evidence that pregnancy adversely affects outcome in melanoma patients who have clinically localized disease. Continuing to recommend a delay in childbearing for these patients is not supported by available clinical evidence. It is unclear, however, whether pregnancy imparts a negative impact on survival in patients with more advanced disease. Recommendations in these patients must be individualized. In patients with advanced disease, immunological mechanisms that favor fetal allograft survival may have a negative impact on the pregnant melanoma patient's survival. In addition, there may be a small subset of pregnant melanoma patients in whom shared fetal/melanoma antigens in the setting of fetal allograft tolerance results in recurrence or rapid progression of disease. This anecdotal clinical observation does not, however, warrant interruption of pregnancy or modification of family planning in young women with clinically localized disease.
References
- 1.Stensheim H, Moller B, van Dijk T, Fossa SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45–51. doi: 10.1200/JCO.2008.17.4110. [DOI] [PubMed] [Google Scholar]
- 2.Pack GT, Scharnagel IM. The prognosis for malignant melanoma in the pregnant woman. Cancer. 1951;4:324–334. doi: 10.1002/1097-0142(195103)4:2<324::aid-cncr2820040218>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Shiu MH, Schottenfeld D, Maclean B, Fortner JG. Adverse effect of pregnancy on melanoma: a reappraisal. Cancer. 1976;37(1):181–187. doi: 10.1002/1097-0142(197601)37:1<181::aid-cncr2820370126>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.McCrummen S. Weary Father Left to Count the Days. Doctors Hope Technology Can Sustain Fetus. Washington Post. 2005 Jun 27;:B01. [PubMed] [Google Scholar]
- 5.Saenz-Badillos J, Brady MS. Pregnancy associated melanoma occurring in two generations. J Surg Oncol. 2000;73(4):231–233. doi: 10.1002/(sici)1096-9098(200004)73:4<231::aid-jso9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Byrd BF, McGanity WJ. The effect of pregnancy on the clinical course of malignant melanoma. Southern Medical Journal. 1954;47(3):196–199. doi: 10.1097/00007611-195403000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambe M, Ekbom A. Cancers coinciding with childbearing: delayed diagnosis during pregnancy? BMJ. 1995;311(7020):1607–1608. doi: 10.1136/bmj.311.7020.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong JH, Sterns EE, Kopald KH, Nizze JA, Morton DL. Prognostic significance of pregnancy in stage I melanoma. Arch Surg. 1989;124(10):1227–1230. doi: 10.1001/archsurg.1989.01410100133023. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs IA, Chang CK, Salti GI. Coexistence of pregnancy and cancer. Am Surg. 2004;70(11):1025–1029. [PubMed] [Google Scholar]
- 11.van Kampen CA, Versteeg-vd Voort Maarschalk MF, Langerak-Langerak J, Roelen DL, Claas FH. Kinetics of the pregnancy-induced humoral and cellular immune response against the paternal HLA class I antigens of the child. Hum Immunol. 2002;63(6):452–458. doi: 10.1016/s0198-8859(02)00396-8. [DOI] [PubMed] [Google Scholar]
- 12.Tafuri A, Alferink J, MÖller P, Hämmerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270(5236):630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 13.Zhou M, Mellor AL. Expanded cohorts of maternal CD8+ T-cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol. 1998;40(1):47–62. doi: 10.1016/s0165-0378(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft.”. J Immunol. 1998;160(7):3086–3090. [PubMed] [Google Scholar]
- 15.Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21(24):3352–3357. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- 16.Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21(3):339–359. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- 17.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68(5):1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 18.Rouas-Freiss N, Paul P, Dausset J, Carosella ED. HLA-G promotes immune tolerance. J Biol Regul Homeost Agents. 2000;14(2):93–98. [PubMed] [Google Scholar]
- 19.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 20.Houghton AN, Flannery J, Viola MV. Malignant melanoma of the skin occurring during pregnancy. Cancer. 1981;48(2):407–410. doi: 10.1002/1097-0142(19810715)48:2<407::aid-cncr2820480231>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22(21):4369–4375. doi: 10.1200/JCO.2004.02.096. [DOI] [PubMed] [Google Scholar]
- 22.O'Meara AT, Cress R, Xing G, Danielsen B, Smith LH. Malignant melanoma in pregnancy. A population-based evaluation. Cancer. 2005;103(6):1217–1226. doi: 10.1002/cncr.20925. [DOI] [PubMed] [Google Scholar]
- 23.Slingluff CL, Jr., Reintgen DS, Vollmer RT, Seigler HF. Malignant melanoma arising during pregnancy. A study of 100 patients. Ann Surg. 1990;211(5):552–557. doi: 10.1097/00000658-199005000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travers RL, Sober AJ, Berwick M, et al. Increased thickness of pregnancy-associated melanoma. Br J Dermatol. 1995;132(6):876–883. doi: 10.1111/j.1365-2133.1995.tb16942.x. [DOI] [PubMed] [Google Scholar]
- 25.Daryanani D, Plukker JT, De Hullu JA, et al. Pregnancy and early-stage melanoma. Cancer. 2003 May 1;97(9):2248–2253. doi: 10.1002/cncr.11321. [DOI] [PubMed] [Google Scholar]
- 26.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 27.Silipo V, De Simone P, Mariani G, et al. Malignant melanoma and pregnancy. Melanoma Res. 2006;16(6):497–500. doi: 10.1097/01.cmr.0000232295.91536.09. [DOI] [PubMed] [Google Scholar]
- 28.MacKie RM, Bufalino R, Morabito A, Sutherland C, Cascinelli N. Lack of effect of pregnancy on outcome of melanoma. For The World Health Organisation Melanoma Programme. Lancet. 1991;337(8742):653–655. doi: 10.1016/0140-6736(91)92462-b. [DOI] [PubMed] [Google Scholar]
- 29.Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;23:1389. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 30.Reintgen DS, McCarty KS, Jr., Vollmer R, Cox E, Seigler HF. Malignant melanoma and pregnancy. Cancer. 1985;55(6):1340–1344. doi: 10.1002/1097-0142(19850315)55:6<1340::aid-cncr2820550630>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]


