Abstract
Estradiol and progesterone bind to their respective receptors in the hypothalamus and hippocampus to influence a variety of behavioral and physiological functions, including reproduction and cognition. Work from our lab and others has shown that the nuclear receptor coactivators, steroid receptor coactivator-1 (SRC-1) and SRC-2, are essential for efficient estrogen receptor (ER) and progestin receptor (PR) transcriptional activity in brain and for hormone-dependent behaviors. While the expression of SRC-1 in brain has been studied extensively, little is known about the expression of SRC-2 in brain. In the present studies, we found that SRC-2 was highly expressed throughout the hippocampus, amygdala and hypothalamus, including the medial preoptic area (MPOA), ventral medial nucleus (VMN), arcuate nucleus (ARC), bed nucleus of the stria terminalis, supraoptic nucleus and suprachiasmatic nucleus. In order for coactivators to function with steroid receptors, they must be expressed in the same cells. Indeed, SRC-2 and ERα were coexpressed in many cells in the MPOA, VMN and ARC, all brain regions known to be involved in female reproductive behavior and physiology. While in vitro studies indicate that SRC-2 physically associates with ER and PR, very little is known about receptor-coactivator interactions in brain. Therefore, we used pull-down assays to test the hypotheses that SRC-2 from hypothalamic and hippocampal tissue physically associate with ER and PR subtypes in a ligand-dependent manner. SRC-2 from both brain regions interacted with ERα bound to agonist, but not in the absence of ligand or in the presence of the selective ER modulator, tamoxifen. Analysis by mass spectrometry confirmed these ligand-dependent interactions between ERα and SRC-2 from brain. In dramatic contrast, SRC-2 from brain showed little to no interaction with ERβ. Interestingly, SRC-2 from both brain regions interacted with PR-B, but not PR-A, in a ligand-dependent manner. Taken together, these findings reveal that SRC-2 is expressed in brain regions known to mediate a variety of steroid-dependent functions. Furthermore, SRC-2 is expressed in many ERα containing cells in the hypothalamus. Finally, SRC-2 from brain interacts with ER and PR in a subtype-specific manner, which may contribute to the functional differences of these steroid receptor subtypes in brain.
Keywords: steroid receptor coactivator-2 (SRC-2), estrogen receptor, progestin receptor, hypothalamus, hippocampus, reproductive behavior
INTRODUCTION
The ovarian steroid hormones, estradiol and progesterone, act in the brain to profoundly influence a variety of physiological and behavioral events, including development, cognition, and reproduction (Blaustein and Mani, 2006; Pfaff et al., 2009). Estradiol and progesterone elicit many of these biological effects by binding to receptors for estrogens (ER) and progestins (PR), respectively, in specific brain regions. ER and PR are members of the steroid/nuclear receptor superfamily of transcriptional activators (Mangelsdorf et al., 1995). In brain, these receptors act in a classic, genomic mechanism by interacting directly with DNA to regulate gene transcription. In addition, steroid receptors in brain can function independent of ligand on either DNA or at the membrane to rapidly activate cytoplasmic signaling pathways (Olesen et al., 2005; Kelly and Ronnekleiv, 2008; Micevych and Mermelstein, 2008; Vasudevan and Pfaff, 2008; Mani et al., 2009; Tetel and Lange, 2009).
Intracellular ER exist as two subtypes, α and β, which are transcribed from different genes (Jensen et al., 1968; Shyamala and Gorski, 1969; Kuiper et al., 1996). These two ER subtypes differ in their abilities to bind ligands (Kuiper et al., 1997; Hall and McDonnell, 1999; Jones et al., 1999; Damdimopoulos et al., 2008), distribution in brain (Shughrue et al., 1997; Osterlund et al., 1998; Greco et al., 2001; Mitra et al., 2003), and function in brain and behavior (Ogawa et al., 1998; Ogawa et al., 1999; Bodo and Rissman, 2006; Musatov et al., 2006). Furthermore, cell culture experiments indicate that ERα is a stronger transcriptional activator than ERβ due to differences in the AF-1 region (Delaunay et al., 2000). In primates and rodents PR are expressed in two forms; the full-length PR-B and the N-terminal truncated PR-A, which are encoded by the same gene but are regulated by different promoters (Kastner et al., 1990). Under certain cell and promoter contexts, PR-B is a stronger transcriptional activator than PR-A (Giangrande et al., 1997; Tung et al., 2006) due to an additional activation function in the up-stream sequences unique to PR-B (Sartorius et al., 1994; Wen et al., 1994). Studies using isoform specific knockout mice reveal that PR-A and PR-B have distinct functions in reproductive behavior and physiology (Mulac-Jericevic and Conneely, 2004; Mani et al., 2006).
Efficient steroid receptor transcription requires a class of proteins known as nuclear receptor coregulators, which consist of coactivators and corepressors. These coregulators play an important role in a variety of human diseases, including cancer and some neurological disorders (Lonard et al., 2007). Nuclear receptor coactivators are rate-limiting in steroid receptor-mediated gene transcription (Oñate et al., 1995; O’Malley, 2006; Rosenfeld et al., 2006). The importance of coactivators is further evident in their ability to reverse the squelching of the transcriptional activity of one steroid receptor by another (Oñate et al., 1995). In addition to functioning as a bridge between receptors and the general transcriptional machinery, nuclear receptor coactivators influence receptor transcription through a variety of mechanisms, including phosphorylation, acetylation, methylation, RNA splicing and chromatin remodeling (Lonard and O’Malley, 2006; Rosenfeld et al., 2006).
The p160 steroid receptor coactivator (SRC) family includes SRC-1/NcoA-1(Oñate et al., 1995); SRC-2/TIF2/GRIP1/NcoA2 (Voegel et al., 1996; Hong et al., 1997); and SRC-3/p/CIP/ACTR/AIB1/TRAM-1/RAC3 (Anzick et al., 1997; Suen et al., 1998). These coactivators dramatically enhance the transcriptional activity of a variety of steroid receptors, including ER and PR (Oñate et al., 1995; O’Malley, 2006; Rosenfeld et al., 2006). This p160 SRC family of coactivators, along with other coactivators, is thought to increase transcriptional activity through a variety of mechanisms, including acetylation of histones, methylation, phosphorylation and chromatin remodeling (O’Malley, 2006; Rosenfeld et al., 2006)]. In vitro studies indicate that under most conditions, ER and PR interact with the SRCs in the presence of an agonist, but not in the absence of ligand or in the presence of an antagonist or a selective receptor modulator (Oñate et al., 1995; McInerney et al., 1996; Shiau et al., 1998; Tanenbaum et al., 1998) but c.f. with (Oñate et al., 1998; Webb et al., 1998; Dutertre and Smith, 2003). Selective ER modulators (SERMs, e.g. tamoxifen) and selective PR modulators (SPRMs, e.g. RU486) regulate ER and PR activity, respectively, in a tissue-specific manner (Lewis-Wambi and Jordan, 2005; Wardell and Edwards, 2005; Han et al., 2007). Whether these receptor modulators block or activate receptor action appears be dependent on the cellular environment, including the ratio of coactivators and corepressors (Smith et al., 1997).
While SRC-2 shares some sequence homology with the other two members of the p160 coactivator family, distinct physiological functions of SRC-2 have been identified. SRC-2 knock-out mice reveal that this coactivator is important in fertility and ductal branching in mammary gland (Gehin et al., 2002; Fernandez-Valdivia et al., 2007; Mukherjee et al., 2007). Generation of mice in which SRC-2 is ablated specifically in cell types that express PR (PRCre/+SRC-2flox/flox) has revealed that SRC-2 functions in progestin-dependent embryo implantation (Fernandez-Valdivia et al., 2007). Microarray analysis of uteri from SRC-2 null mice reveal that this coactivator is critical for the ability of progesterone to repress specific genes involved in a variety of functions, including cell cycle and immunity (Jeong et al., 2007). SRC-2 also functions to regulate glucose production (Chopra et al., 2008) and bone mass (Modder et al., 2009). Finally, SRC-2 appears to be involved in ERα regulated cell proliferation of breast cancer cells (Karmakar et al., 2009; Xu et al., 2009).
Studies from our lab and others reveal that nuclear receptor coactivators function in hormone action in the central and peripheral nervous systems (Tetel, 2009; Tetel et al., 2009). For example, SRC-1 is expressed in cells containing estradiol-induced PR (Tetel et al., 2007) and is important for hormone-dependent sexual differentiation of the brain (Auger et al., 2000), gene expression in brain (Apostolakis et al., 2002; Molenda et al., 2002; Charlier et al., 2005; Charlier et al., 2006) and sexual behavior (Apostolakis et al., 2002; Molenda et al., 2002; Charlier et al., 2005; Charlier et al., 2006; Molenda-Figueira et al., 2006). While the function of SRC-1 in brain has been well-studied, less is known about the role of SRC-2 in brain. While no detailed neuroanatomical studies have been reported, in situ hybridization and Western blot analyses reveal SRC-2 is expressed at high levels in the hippocampus, cerebellum and hypothalamus (Apostolakis et al., 2002; Nishihara et al., 2003; McGinnis et al., 2007). Moreover, in females SRC-2 is important in hormone-dependent lordosis and estradiol-induction of PR in the hypothalamus (Apostolakis et al., 2002). Finally, SRC-2, as well as SRC-1, function in glucocorticoid receptor mediated gene expression in astrocytes (Grenier et al., 2006; van der Laan et al., 2008).
A variety of cell culture studies indicate that receptor-coactivator interactions occur in a ligand-dependent manner (Oñate et al., 1995; McInerney et al., 1996; Shiau et al., 1998; Tanenbaum et al., 1998). Recent work from our lab reveals that SRC-1 from brain physically interacts with ER and PR in a receptor subtype-specific and ligand-dependent manner (Molenda-Figueira et al., 2008). However, it is not known if SRC-2 from brain physically associates with steroid receptors. In the present studies, we investigated the expression pattern of SRC-2 in the female rat hypothalamus and asked if SRC-2 and ERα were coexpressed in cells from hypothalamic regions known to regulate female reproduction. Furthermore, using pull-down assays, we tested the hypotheses that SRC-2 from brain regions rich in steroid receptors physically associate with ER and PR subtypes in a ligand-dependent manner.
EXPERIMENTAL PROCEDURES
Experimental animals
Adult female (175–200g) Sprague-Dawley rats from Charles River Laboratories. Inc. (Wilmington, MA) were housed four animals to a cage in a 14:10 light/dark cycle with lights off at 11 a.m. Animals were given food and water ad libitum. Female rats were anesthetized with Ketamine/Xylazine cocktail (100 mg Ketamine and 18 mg xylazine/0.75 ml/kg in saline) and ovariectomized. A one-week recovery period followed to allow clearing of endogenous hormones. All animal procedures were approved by the Institutional Animal Care and Use Committees of Skidmore College and Wellesley College.
Immunohistochemical analysis of SRC-2 expression in brain
For immunohistochemical studies, animals (n=6) were overdosed with sodium pentobarbitol (89 mg/kg) and chloral hydrate (425 mg/kg) and perfused with 4% paraformaldehyde. Five thousand units of sodium heparin dissolved in 1 ml of saline were injected into the left ventricle. Saline (0.15 M, 25 ml) preceded the flow of 250 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH=7.2) at a flow rate of 25 ml/min for 10 minutes. Brains were removed from the cranium, blocked and stored in 0.1 M sodium phosphate buffer (pH=7.2) containing 20% sucrose at 4oC overnight. Forty μm sections were cut through the hypothalamus on a freezing rotary microtome and stored in cryoprotectant at −20°C until immunohistochemistry.
Single-label Immnohistochemistry for SRC-2
Sections were initially rinsed in 0.05M Tris-buffered saline (TBS). Tissue was then rinsed in TBS and incubated in a solution of 1% H202, 20% normal goat serum and 1% bovine serum albumin in TBS for 20 minutes to decrease nonspecific staining and reduce endogenous peroxidase activity. Sections were incubated for 48 hours with a mouse monoclonal antibody generated against amino acids 959–1067 of human SRC-2 (TIF 2, 1.9 μg/ml, BD Trans Lab) in TBS containing 0.02% sodium azide (NaN3), 1% normal goat serum, 0.1% gelatin and Triton X-100 (pH 7.6 at 4°C). After rinsing, the tissue was incubated for 90 minutes in biotinylated goat-anti-mouse secondary antibody (3 ug/ml, Jackson Laboratory, West Grove, PA) containing NaN3 and Triton X-100 and 1.5% normal goat serum. Tissue was rinsed in TBS containing NaN3, gelatin and Triton X-100 followed by rinsing in TBS. Sections were then incubated for 90 minutes in TBS containing 1% avidin DH: biotinylated horseradish peroxidase H complex (Vectastain ABC Elite Kit, Vector, Burlingame, CA) followed by rinsing in TBS. Finally, sections were exposed to 0.05% diaminobenzine (DAB) with 3% hydrogen peroxide with TBS for approximately 10 minutes. The sections were rinsed in TBS and then mounted on microscope slides and coverslipped using DePeX mounting medium (Electron Microscopy Sciences, PA). One matched section for each brain area from the hypothalamus, hippocampus and amygdala (Paxinos and Watson, 1998) were investigated. SRC-2 immunoreactive cells were visualized under 100x magnification using an Olympus BX60 microscope.
Dual-label Immunofluorescence for ERα and SRC-2
Brain sections were mounted onto subbed slides, dried and then washed three times in PBS for 5 min. Tissue was permeabilized with 1% SDS (ACROS), 8% betamercaptoethanol (Sigma) and 4% normal goat serum (PelFreeze, Rogers, AR) in PBS. To detect ERα and SRC-2, sections were incubated in a cocktail containing a rabbit polyclonal antibody generated against the last 15 amino acids of rat ERα (1:15,000, C1355, Upstate) and the SRC-2 monoclonal, TIF2 (1.9 ug/ml), in TBS at 4°C overnight. Sections were washed with PBS and then incubated in 1% BSA and 4% normal goat serum in PBS for 30 min. Sections were incubated for one hour in a cocktail of fluorescently-labeled secondary antisera containing CY3-labeled goat anti-rabbit serum (1ug/ml, Jackson ImmunoResearch) for visualization of ERα and FITC-labeled goat anti-mouse serum (4 ug/ml, Jackson ImmunoResearch) for detection of SRC-2. Sections were washed in PBS, dried and slides were cover-slipped with Vectashield mounting medium (Vector Laboratories) diluted 1:1 with 0.3M Tris (pH 8.8). Images of immunofluorescence from the left side of one matched section per brain region for each rat (Paxinos and Watson, 1998) were captured at 200X using an Olympus Fluoview FV300 confocal system equipped with Argon and He-Ne lasers. Images of one optical section at a thickness of 1 μm were taken at the top of each brain section within a consistent region of interest for all animals per brain region and converted to tif files for analysis. Controls for the immunohistochemistry included the omission of the primary or secondary antibodies.
Recombinant flag and GST-tagged steroid receptors
Recombinant ER and PR fusion proteins were expressed in Spodoptera frugiperda (Sf9) insect cells by the Baculovirus/Monoclonal Antibody Facility of the Baylor College of Medicine as described previously (Tetel et al., 1999; Melvin et al., 2004). Full-length human ERα or ERβ were fused to a flag tag (viruses kindly provided by Lee Kraus, Cornell Univ.) (Kraus and Kadonaga, 1998; Melvin et al., 2004). Sf9 cell cultures for ER-flag were incubated with saturating doses of 200 nM estradiol, 200 nM 4-OH-tamoxifen, or no ligand. Full-length human PR-A or PR-B was fused to a glutathione S-transferase (GST) tag. Insect cell cultures for PR-GST (viruses kindly provided by David Bain, Univ. Colorado HSC) were incubated with saturating doses of 200 nM of the PR agonist R5020, 200 nM of the SPRM RU486, or in the absence of PR ligand. Sf9 cell pellets were homogenized in homogenization buffer (10 mM Tris, 10% glycerol, 400 mM NaCl, 1 mM DTT, 1mM EDTA, pH = 7.4) with protease inhibitors (1:10 dilution, P2714, Sigma, Saint Louis, MO). Samples were incubated on ice for 30 min, and then centrifuged for 30 min at 4°C at 40,000 rpm and supernatants were stored at −80°C.
Tissue preparation
Ovariectomized rats were overdosed with sodium pentobarbitol (89 mg/kg) and chloral hydrate (425 mg/kg) and then decapitated. Hypothalamic and hippocampal (containing a small portion of the cortex dorsal to the hippocampus) tissues were dissected out, flash frozen on dry ice and stored at −80°C. Brain tissue from female rats (n=54) was pooled in groups of three for each sample and homogenized in buffer (10 mM Tris, 10% glycerol, 400 mM NaCl, 1 mM DTT, 1mM EDTA, pH = 7.4) with protease inhibitors (1:10 dilution, P2714, Sigma). Samples were incubated on ice for 30 min., and then centrifuged for 30 min. at 4°C at 12,000 rpm and supernatants were aliquotted and frozen at −80°C.
ER flag-tagged pull-down
ER flag-tagged pull down assays were conducted at 4 C as described previously (Molenda-Figueira et al., 2008). Briefly, twenty-five microliters of packed Anti-flag M2 affinity gel resin (Sigma) were added to each siliconized centrifuge tube and pre-washed 3 times with TBS and 2 times with 100 mM glycine HCl (pH = 3.5). Resins were next washed 3 times with Wash Buffer + NaCl (50mM Tris-HCl, 100mM NaCl, 1% glycerol, 50mM Na Fluoride, pH = 7.4) + TX-100 (0.1% Triton X-100). Equal amounts of recombinant flag-tagged ER were added to the resin column and rotated on an end-over-end rotator for 1 hour. The resins, with immobilized ER, were washed 3 times with Wash Buffer + NaCl. Equal amounts of pooled hypothalamic or hippocampal whole cell extracts were added to the immobilized ER-flag and incubated on a rotator for 1 hour. The resins were washed 3 times with Wash Buffer + NaCl to eliminate non-specific binding, and then samples were eluted with 2% SDS sample buffer by boiling samples for 5 minutes and stored at −80°C until use.
Samples were analyzed by Western blot as described previously (Molenda-Figueira et al., 2006) for detection of SRC-2 interactions with ER. Blots were probed for SRC-2 from brain by incubating overnight with the mouse monoclonal antibody described above (TIF 2, 1.0 μg/ml, BD Trans Lab). Membranes were washed in TBS-T and incubated in a sheep anti-mouse antibody (1:6000, Amersham Biosciences, Uppsala, Sweden). Following washes in TBS-T, immunoreactive bands were detected with an enhanced chemiluminescence kit (ECL; New England Biolabs, Ipswich, MA), and the membranes were scanned using a PhosphorImager (STORM Scanner 860, Molecular Dynamics) and exposed to film (Blue Sensitive X-ray film, Laboratory Products Sales, Rochester, NY). Images from the PhosphorImager were imported into the ImageQuant analysis program (V.5.2, Molecular Dynamics) and analyzed for integrated density (area of band × mean optical density) of immunoreactive bands. Membranes were stripped for 2 hours at 70°C in stripping buffer (2% sodium laurel sulfate, 62.5 mM Tris HCl, 100 mM 2-mercaptoethanol, H2O, pH = 6.7) and re-probed for flag-tagged ER using a mouse monoclonal antibody generated against the flag-tag (0.5 μg/ml, anti-Flag M2, Sigma) and a sheep anti-mouse secondary antibody (1:80,000 dilution, Amersham Biosciences). Immunoreactive bands were analyzed as described above.
PR-GST pull-down
PR-GST pull down assays were done as described previously (Molenda-Figueira et al., 2008). Fifty microliters of Glutathione Sepharose 4B packed resins (0.05 μg/μl, Amersham Biosciences) were added to siliconized centrifuge tubes and pre-treated with ovalbumin (1 mg/ml, Fisher Scientific, Hampton, NH) for 15 minutes on an end-over-end rotator and rinsed three times with TG buffer (20 mM Tris-HCl, 10% glycerol; pH 8.0) containing 100 mM NaCl (TG + NaCl). Equal amounts of recombinant human PR-GST suspended in TG buffer were added to resins and incubated on a rotator for 1 hour. Following the incubation, the resins with immobilized PR-GST were washed four times with TG + NaCl. Equal amounts of pooled hypothalamic or hippocampal whole cell extracts were added to immobilized PR-GST and incubated on a rotator for 1 hour. The resins were washed four times with TG + NaCl.
Samples were eluted in 2% SDS sample buffer as described above and stored at −80°C until analysis. Samples were analyzed by Western blot, as described above, to detect SRC-2 interacting with PR. SRC-2 immunoreactive bands were detected using a PhosphorImager and analyzed as described above (STORM Scanner 860, Molecular Dynamics). Membranes were stripped and probed for PR-A and PR-B, using a mouse monoclonal antibody that recognizes the N-terminal amino acids 165–534 of both PR-A and PR-B (PR 1294, 0.1 μg/ml, kindly provided by Dean Edwards, Baylor College of Medicine), followed by a sheep anti-mouse secondary antibody (1:10,000, Amersham). PR-immunoreactive bands were analyzed as described above.
Mass Spectrometry
Rat hypothalamic extracts (approximately 40 mg of tissue per condition) were exposed to immobilized ERα in the presence of 200 nM estradiol or no ligand. Eluted samples were resolved in adjacent lanes by SDS-PAGE and the region of the gel corresponding to SRC-2 was excised, digested with trypsin and desalted as described previously (Zhao et al., 2003; Tilton et al., 2007). The peptide mixture was injected onto a C18 trap and then separated on a reversed phase nano-HPLC column (PicoFritTM, 75 μm × 10 cm; tip ID 15 μm) with a linear gradient of 0–50 % mobile phase B (0.1 % formic acid-90 % acetonitrile) in mobile phase A (0.1 % formic acid) over 120 min at 200 nl/min. LC-MS/MS experiments were performed with a LTQ linear ion trap mass spectrometer (ThermoFinnigan, San Jose, CA) equipped with a nanospray source; the mass spectrometer was coupled on-line to a ProteomX® nano-HPLC system (ThermoFinnigan, San Jose, CA). The mass spectrometer was operated in the data-dependent mode using XCalibur software. The most intense seven ions in each MS survey scan were automatically selected for MS/MS. This approach allows the detection of individual proteins in the nanogram range and has been used to identify SRC-1 in multiprotein complexes using immunoaffinity purification as well as low abundance transcription factors such as RelA/p65 NFkB (Zhao et al., 2003; Tilton et al., 2007). The acquired MS/MS spectra were searched with SEQUEST algorithm from the SWISSPROT Protein Database on the Bioworks 3.2 platform (ThermoFinnigan, San Jose, CA).
Statistical analysis
The amount of SRC-2 in each pull-down sample was normalized to the amount of SRC-2 in the input whole cell extract by creating a ratio of the integrated density of the SRC-2 immunoreactive band to the integrated density of input of SRC-2 for the experiment. Unless stated otherwise, the integrated density of immunoreactive bands was analyzed using a two-way ANOVA (SPSS) to determine differences between receptor subtypes and ligand conditions. A Tukey’s HSD test was used for post-hoc comparisons. Differences were considered significant at a probability of less than 0.05.
RESULTS
SRC-2 and ERα are coexpressed in individual cells in brain
SRC-2 immunoreactivity (SRC2-IR) was observed at high levels in the female rat ventral medial nucleus (VMN), arcuate nucleus (ARC), posterodorsal medial amygdala (MePD), medial preoptic area (MPOA) and supraoptic nucleus (Figure 1), as well as the bed nucleus of the stria terminalis (BNST), suprachiasmatic nucleus and hippocampus. Moderate to lower levels of SRC2-IR were detected in the habenular and paraventricular nuclei. These findings are consistent with, and extend, previous work revealing SRC-2 expression in the hypothalamus and hippocampus of rats (Apostolakis et al., 2002; McGinnis et al., 2007) and mice (Nishihara et al., 2003).
Figure 1.
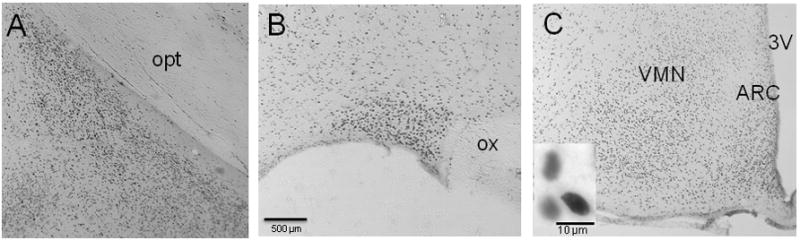
SRC-2 immunoreactive cells in the A) posterodorsal portion of the medial amygdala (MePD), B) supraoptic nucleus (SON), magnification bar = 500 μm, and C) ventromedial nucleus of the hypothalamus (VMN) and arcuate nucleus (ARC) of the female rat. Inset shows nuclear immunostaining of cells from the VMN, magnification bar = 10 μm. opt = optic tract; ox = optic chiasm; 3V = third ventricle.
Consistent with previous studies, we found ERα-IR cells in the same regions of the preoptic area, hypothalamus and amygdala as described previously in rats using [3H]autoradiography, in situ hybridization and immunohistochemistry (Stumpf and Sar, 1971; Pfaff and Keiner, 1973; Cintra et al., 1986; Lauber et al., 1990; Simerly et al., 1990; Blaustein, 1992; Tetel et al., 1994). Moreover, many ERα-IR cells also expressed SRC-2 in the VMN and ARC (Figure 2), as well as the MPOA and BNST. In addition, there were ERα-IR cells that did not express SRC-2 and SRC2-IR cells that lacked ERα. Omission of the primary monoclonal antibody for SRC-2 from the immunohistochemical procedure resulted in no detectable SRC-2 immunoreactive cells in any brain region. Omission of the primary antibody for ER from the immunohistochemical procedure resulted in no ER-IR cells. In further confirmation of the specificity of the double label immunofluorescent technique, as stated above, intensely labeled ER-IR cells devoid of SRC-2 were observed, as well as SRC2-IR cells that lacked ER-IR.
Figure 2.
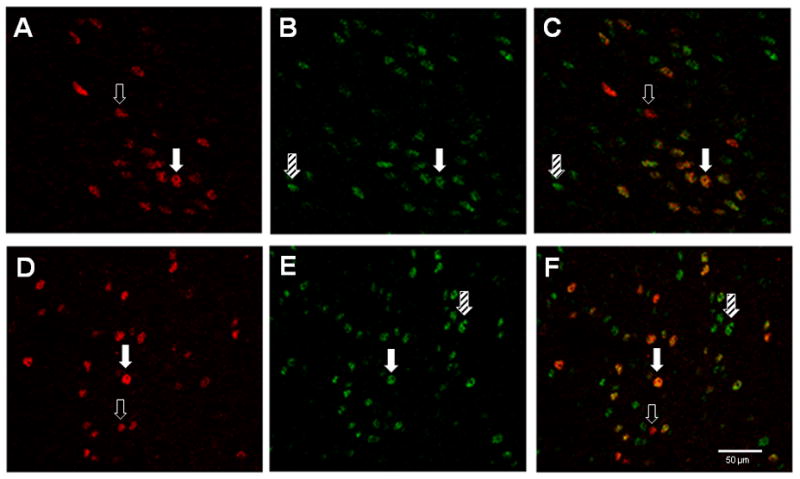
Coexpression of SRC-2 and estrogen receptors (ER) in cells in the ventromedial nucleus of the hypothalamus (A–C) and arcuate nucleus (D–F). Sections were simultaneously immunostained for ERα (A and D) and SRC-2 (B and E). Overlaid images from the VMN (C) and ARC (F) show cells expressing both ERα and SRC-2. Open arrows point to cells containing ERα only (red), hatched arrows point to cells containing SRC-2 only (green) and solid arrows point to cells expressing both ERα and SRC-2 (orange/yellow). Magnification bar = 50 μm
SRC-2 from brain associates more with agonist-bound ERα than with ERβ
ER-flag tag pull-down assays were performed to determine whether SRC-2 interacts with ERα or ERβ and whether these associations were ligand-dependent. Western blot analysis of pull-down assays revealed that SRC-2 from female rat hypothalamus interacted with ERα in the presence of the agonist, estradiol, while little interaction was detected when ERα was not bound to ligand or bound to the SERM, tamoxifen (Figure 3B). Quantification of these ligand-dependent interactions between SRC-2 from the hypothalamus and ERα are shown in Figure 3D. As in the hypothalamus, SRC-2 from the hippocampus physically interacted with ERα in the presence of estradiol, but not when unbound or bound to tamoxifen (Figures 3A & C; F(2, 24) = 37.06, P < 0.0001). These findings indicate that SRC-2 from hypothalamus or hippocampus interacts with ERα in a ligand-dependent manner.
Figure 3.
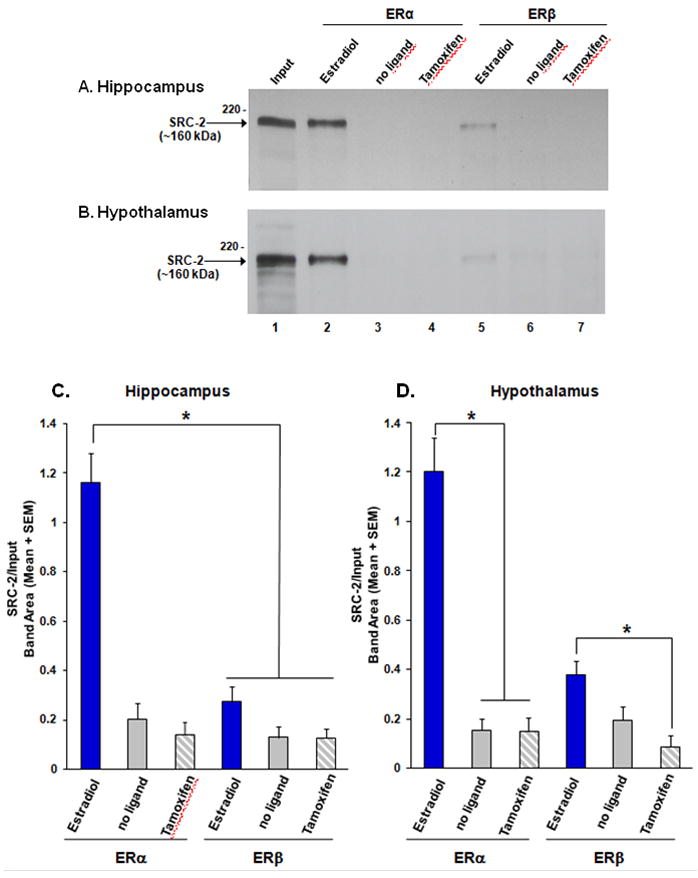
SRC-2 from rat hypothalamic whole cell extracts associates with ERα, but not ERβ, in a ligand-dependent manner. SRC-2 from A) hippocampus or B) hypothalamus interacts with ERα in the presence of estradiol (lane 2), but not in the absence of ligand (lane 3) or in the presence of the SERM, tamoxifen (lane 4). SRC-2 interacts weakly with ERβ bound to estradiol (lane 5) with little to no interaction in the absence of ligand or tamoxifen (lanes 6 & 7). Inputs (2% of total) of SRC-2 from hippocampal or hypothalamic extracts are shown in lane 1.
C) SRC-2 from hippocampus physically associates with ERα bound to estradiol, but not in the absence of ligand or in the presence of tamoxifen. In contrast, SRC-2 interacts weakly with ERβ. D) Hypothalamic SRC-2 interacts with ERα, and to a much lesser extent with ERβ, in a ligand dependent manner. *p < 0.05, n = 4–5 per treatment group.
In dramatic contrast to the high levels of association between SRC-2 and estradiol-bound ERα, little interaction was detected between agonist-bound ERβ and SRC-2 from brain (Figure 3). SRC-2 from the hypothalamus interacted with ERβ in a ligand-dependent manner to some extent (Figure 4B; F(2, 9) = 6.004, P < 0.03), while there was a trend towards association of SRC-2 from hippocampus with ERβ bound to estradiol (Figure 3C & D). Moreover, SRC-2 from either brain region interacted more with estradiol-bound ERα than ERβ (hippocampus: F(1, 24) = 27.47, P < 0.0001; hypothalamus: (F(1, 18) = 13.58, P < 0.003). These findings suggest that SRC-2 physically associates with ER in a receptor sub-type specific manner.
Figure 4.
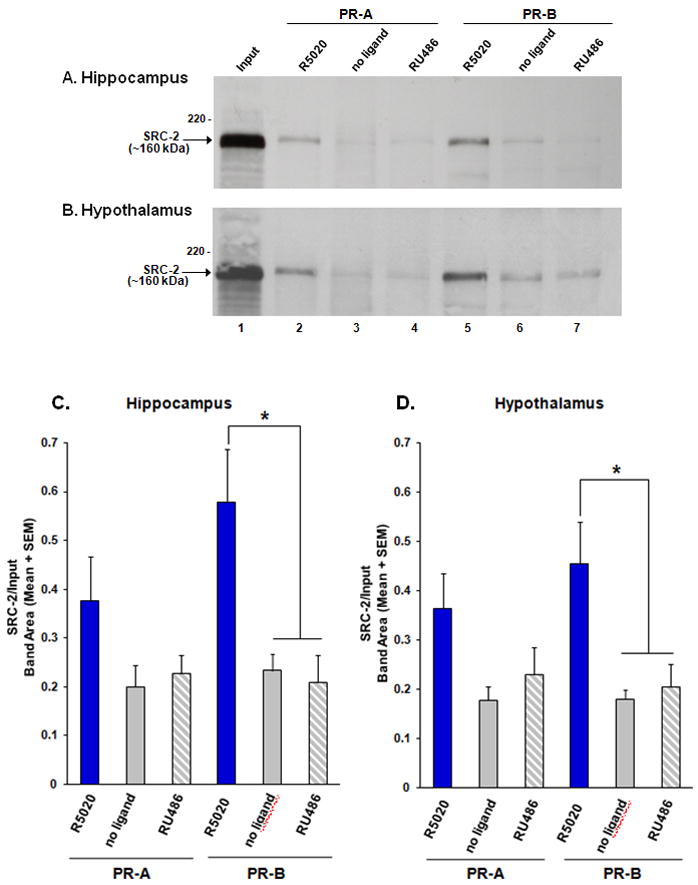
Progestin receptor (PR) interactions with SRC-2 from brain. SRC-2 from A) hippocampal or B) hypothalamic whole cell extracts interacts with PR-B, and to a lesser extent PR-A, in the presence of the agonist R5020, but not in the absence of ligand or in the presence of the SPRM, RU486. Inputs (2% of total) of SRC-2 from hippocampal or hypothalamic extracts are shown in lane 1.
SRC-2 from the C) hippocampus and D) hypothalamus interacts with PR-B in the presence of the agonist R5020, but not in the absence of ligand or in the presence of the SPRM, RU486. SRC-2 from each brain region interacts with PR-A to a lesser extent. *p < 0.05, n = 8 per treatment group.
Mass spectrometry confirms the ligand-dependent interactions of hypothalamic SRC-2 with ERα
In order to independently confirm the western blot data for estradiol-dependent binding of ERα to SRC-2 from rat brain, we employed an unbiased mass spectrometry approach. Rat hypothalamic extracts were exposed to immobilized ERα in the presence of estradiol or no ligand and eluted samples were resolved by SDS-PAGE. Gel slices corresponding to the putative SRC-2 region of the 2 lanes were digested with trypsin, and peptides analyzed by LC-MS/MS. Table 1 shows that database searching identified three highly significant, doubly charged peptides corresponding uniquely to rat SRC-2 (UniProt Accession # Q9WUI9) in the gel slice from the estradiol treated sample. All three peptides had XCorr values 3.5 and Cn 0.40 with excellent identification of fragments ions. These peptides corresponded to amino acids 100–112, (SDVSSTGQGVIDK), 693–705 (LLQDSSSPVDLAK), and 714–731 (ELNQESSGTAPGSEVTVK). In confirmation of the western blot analyses, no SRC-2 peptides were identified in the sample lane from unliganded ERα.
Table 1.
Mass Spectrometry Identification of SRC-2 Bound to Estradiol-Treated ERα
| Scan# | Peptide Sequence | MH+z | chargeXcorr | ΔCn | Sp | RSp | Ions | |
|---|---|---|---|---|---|---|---|---|
| 2740 | SDVSSTGQGVIDK | 1292.63285 | 2 | 3.47 | 0.40 | 1624.0 | 1 | 19/24 |
| 3248 | ELNQESSGTAPGSEVTVK | 1832.88723 | 2 | 3.97 | 0.60 | 530.3 | 1 | 18/34 |
| 4126 | LLQDSSSPVDLAK | 1372.73184 | 2 | 3.40 | 0.40 | 1830.2 | 1 | 19/24 |
PR interacts with neural SRC-2 in a receptor subtype-specific manner
PR-GST pull-down assays were done to determine if SRC-2 from rat brain physically interacts with PR-A or PR-B, and if these interactions occur in a ligand-dependent manner. Western blots from GST-tag pull-down assays indicated that SRC-2 from hippocampus interacted with PR-B in the presence of the agonist R5020, but little to no interactions were detected with PR-B in the absence of ligand or in the presence of the SPRM, RU486 (Figure 4A & C; F(2, 40) = 9.19, P < 0.002). Similar findings were made with SRC-2 from the hypothalamus, suggesting that SRC-2 from these brain regions interact with PR-B in a ligand-dependent manner (Figure 4B & D; F(2, 39) = 9.20, P < 0.002). While the ligand-dependent interactions of SRC-2 from brain with PR-A did not reach significance, there was a trend towards these interactions occurring in a ligand-dependent manner (Figure 4C & D).
DISCUSSION
In vitro studies indicate that SRC-2 interacts with ER and PR, and dramatically enhances the transcriptional activity of these steroid receptors (Voegel et al., 1996; Hong et al., 1997). While the function of SRC-1 in hormone action in brain and behavior has been studied in some depth (Tetel, 2009; Tetel et al., 2009), the role of SRC-2 in brain function has not been well-defined. Thus, the present study investigated if SRC-2 was expressed in steroid-sensitive brain regions. In order for SRC-2 to function with steroid receptors, SRC-2 must be expressed in receptor-containing cells. Therefore, we asked if individual cells coexpressed SRC-2 and ERα. Finally, we tested the hypothesis that SRC-2 from brain regions rich in steroid receptors physically associates with ER and PR subtypes in a ligand-dependent manner.
Previous analyses by Western blots have shown that SRC-2 is expressed in the rat hypothalamus (Apostolakis et al., 2002; McGinnis et al., 2007). The present studies used immunohistochemistry to extend these findings to reveal that SRC-2 is expressed at high levels in the female rat MPOA, VMN, ARC, posterodorsal medial amygdala, bed nucleus of the stria terminalis, supraoptic nucleus, suprachiasmatic nucleus and hippocampus. In addition, moderate to lower levels of SRC-2 were detected in the habenular and paraventricular nucleus. Moreover, many cells in the MPOA, VMN and ARC coexpressed ERα and SRC-2. These results extend our previous findings that other nuclear receptor coactivators, SRC-1 and CBP, are expressed in ERα-containing cells in the female hypothalamus (Tetel et al., 2007). In addition, many ERα containing cells in these brain regions also express PR (Blaustein and Turcotte, 1989; Warembourg et al., 1989), suggesting that a subpopulation of the coexpressing cells identified in the present study also express PR. While not all ERα-containing cells expressed SRC-2, it may be that the immunohistochemical technique was not sensitive enough to detect low levels of SRC-2. Alternatively, it is possible that this sub-population of ERα-expressing cells is regulated by other coactivators. In addition, there were SRC2-IR cells that did not express ERα, suggesting that SRC-2 may function with other receptors, including those for androgens (AR) and glucocorticoids (GR), in these cells (Voegel et al., 1996; Hong et al., 1997). In future studies, it will be important to determine if SRC-2 is coexpressed with other steroid receptors, including ERβ, PR, AR and GR. The present findings provide neuroanatomical evidence suggesting that SRC-2 functions in ERα action in brain regions discussed above that are known to be involved in a variety of functions, including reproduction (Pfaff et al., 2009), stress (Krishnan and Nestler, 2008), circadian rhythms (Silver et al., 1996; Hastings et al., 2008), metabolism (King, 2006; Musatov et al., 2007) and cognition (Fugger et al., 2000; Bodo and Rissman, 2006).
We also tested the hypothesis that SRC-2 from female rat hippocampus and hypothalamus, regions rich in ER and PR (Pfaff and Keiner, 1973; MacLusky and McEwen, 1978; Blaustein et al., 1988)(Shughrue et al., 1997; Osterlund et al., 1998; Greco et al., 2001; Mitra et al., 2003), physically associates with ER and PR subtypes in a ligand-dependent manner. Using pull-down assays with brain tissue, we found that SRC-2 from hypothalamic and hippocampal extracts interacted with flag-tagged ERα when bound to the agonist estradiol. In contrast, little to no interactions were observed between SRC-2 and ERα in the absence of ligand or in the presence of tamoxifen, indicating that this SERM is acting as an antagonist in our assays. Analysis by mass spectrometry confirmed that SRC-2 from rat brain interacted with ERα in a ligand-dependent manner. These results are consistent with in vitro studies that show SRC-2 interacts with ERα in the presence of agonist (Voegel et al., 1996; Hong et al., 1997; Webb et al., 1998; Privalsky et al., 2009). In addition, these findings support previous studies that SRC-2 acts in the hypothalamus to modulate estrogen action (Apostolakis et al., 2002) and suggest that SRC-2 functions with ERα in cognition in the hippocampus (Fugger et al., 2000; Bodo and Rissman, 2006).
In contrast to ERα, ERβ did not interact with SRC-2 from hippocampus under any ligand condition, including when bound to estradiol. In addition, ERβ bound to estradiol interacted very weakly with SRC-2 from hypothalamus. This weak interaction between agonist-bound ERβ and SRC-2 from brain is in contrast to cell culture studies indicating over-expressed SRC-2 does interact with ERβ (Kraichely et al., 2000; Routledge et al., 2000; Zhao et al., 2005; Cvoro et al., 2008). One explanation for the differences between the present results using SRC-2 from rat brain and other studies using recombinant SRC-2 (Kraichely et al., 2000) may be that over-expression of coactivators leads to altered interactions with receptors. In addition, the presence of other factors in brain that may mediate appropriate receptor-coactivator associations point to the significance of using biologically-relevant tissue in studying these important interactions. Finally, cell culture studies suggest that both ERα and ERβ can recruit SRC-2 and other coactivators in the absence of ligand under certain phosphorylation conditions (Webb et al., 1998; Yi et al., 2002; Bai and Giguere, 2003). While we detected little to no interactions between ER and SRC-2 from brain in the absence of ligand, it will be important to investigate whether physiologically-relevant events that modulate ligand-independent activation can influence receptor-coactivator interactions in brain.
Both ERα and ERβ are expressed in the hypothalamus (Shughrue et al., 1997; Kuiper et al., 1998; Osterlund et al., 1998; Greco et al., 2001; Mitra et al., 2003). Hypothalamic ERα are necessary for the full expression of female sexual behavior (Rissman et al., 1997; Ogawa et al., 1998; Ogawa et al., 1999; Kudwa and Rissman, 2003; Musatov et al., 2006), while expression of ERβ in the hypothalamus seems to be more important in anxiety and the stress response (Isgor et al., 2003; Imwalle et al., 2005; Lund et al., 2005; Bodo and Rissman, 2006). These dramatic differential functions of the ER subtypes in brain and behavior may be explained in part by the differential interactions of these receptor subtypes with coactivators reported here. In addition, in some cell lines, ERα is a stronger transcriptional activator than ERβ (Delaunay et al., 2000). Thus, the present findings that SRC-2 from brain interacts strongly with ERα, but not ERβ, provide a possible mechanism for the functional differences of these ER subtypes.
Our results also show that SRC-2 from rat hippocampus or hypothalamus physically interact with PR-B in a ligand-dependent manner. SRC-2 from both of these brain regions associated more with PR-B in the presence of the agonist, R5020, than in the absence of ligand or in the presence of the SPRM, RU486. In contrast, while there was a trend towards ligand-dependent interactions of SRC-2 from the hippocampus or hypothalamus with PR-A, statistical significance was not reached, suggesting PR isoform specific interactions with SRC-2. These differential interactions between SRC-2 and the PR isoforms are supported by some cell culture studies (Giangrande et al., 2000), while other studies indicate that PR-A can interact with SRC-2 under certain promoter contexts (Heneghan et al., 2007). Cell culture studies suggest that under certain circumstances, PR-B is a stronger transcriptional activator than PR-A (Giangrande et al., 1997; Giangrande et al., 2000; Tung et al., 2006), likely due to the additional activation function (AF-3) of PR-B (Sartorius et al., 1994; Wen et al., 1994). Moreover, studies using PR-A and PR-B specific knock-outs reveal that both receptors are important for the full display of progesterone-facilitated lordosis (Mani et al., 2006). Interestingly, PR-A has a greater role than PR-B in ligand-independent lordosis facilitated by the cyclic AMP analogue, 8-bromo-cAMP (Mani et al., 2006). In future studies, it will be important to investigate the function of SRC-2 and other coactivators in ligand-independent activation of PR.
As discussed in our previous work (Molenda-Figueira et al., 2008), it should be noted that human ER and PR proteins were used to detect interactions with rat SRC-2 from brain. Therefore, we cannot exclude the possibility that SRC-2 from rat brain may interact differently with rat ER or PR compared with human. However, the ligand binding domains (LBDs) of human ER (α and β) and PR, the most important receptor region for mediating SRC-2 interactions (Heery et al., 1997; Feng et al., 1998), have highly homologous protein sequences (89–92% identical) with the LBDs of rat ER and PR, respectively (Kastner et al., 1990; Kato et al., 1993; Harris et al., 2002). Given that protein-protein interactions are sensitive to protein structure, in future studies it will be important to study the interactions of rat SRC-2 with rat ER and PR using other approaches such as coimmunoprecipitation assays. Even so, the high degree of homology between rat and human LBDs of ER and PR, the ligand dependency of the interactions detected in the present studies, and the confirmation by mass spectrometry, indicate that these results offer important information about the interactions between ER and PR with SRC-2 from brain.
In summary, these findings indicate that SRC-2 is expressed in steroid sensitive regions in the hypothalamus. Moreover, a subpopulation of hypothalamic cells coexpress SRC-2 and ER, extending our previous work showing the coexpression of other coactivators (SRC-1 and CBP) with steroid receptors in brain (Tetel et al., 2007). In addition, using pull-down assays, we found that SRC-2 from hypothalamus and hippocampus interact differently with the ER and PR subtypes. These results support previous work showing that SRC-2 is important for ER-mediated induction of PR in brain and the expression of hormone-dependent female sexual behavior (Apostolakis et al., 2002). Presumably, these interactions between SRC-2 and receptors are important for reproductive development in males and females (Gehin et al., 2002; Fernandez-Valdivia et al., 2007; Mukherjee et al., 2007), pregnancy (Gehin et al., 2002), hormone-dependent gene transcription in brain (Apostolakis et al., 2002) and female sexual behavior (Apostolakis et al., 2002). In addition, the present findings reveal the importance of using biologically-relevant brain tissue when studying these interactions. Finally, estrogens and progestins are involved in a variety of human diseases. For example, estrogen therapy may reduce the risk of Alzheimer’s disease (Pike et al., 2009). Interestingly, expression in brain of an ERα mutant, that may prevent coactivator binding, has been correlated with Alzheimer’s disease (Ishunina and Swaab, 2009). In future studies, it will be critical to determine the functional significance of the differential interactions of SRC-2 with the receptor subtypes on gene expression in brain and the potential role in disease.
Acknowledgments
This research was supported in part by Endocrine Society Summer Research Fellowships (DIM and LKW) and National Institutes of Health RO1 DK61935 (MJT). We thank Cheryl Jenks for technical assistance on some of these studies.
Abbreviations
- AR
androgen receptor
- ARC
arcuate nucleus
- BNST
bed nucleus of the stria terminalis
- CBP
CREB binding protein
- ER
estrogen receptor
- GR
glucocorticoid receptor
- GST
glutathione-S-transferase
- IR
immunoreactivity
- LBD
ligand binding domain
- MePD
posterodorsal medial amygdala
- MPOA
medial preoptic area
- PR
progestin receptor
- SERM
selective estrogen receptor modulator
- SPRM
selective progestin receptor modulator
- SRC-1
steroid receptor coactivator-1
- SRC-2
steroid receptor coactivator-2
- VMN
ventral medial nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O’Malley B. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Molecular Endocrinology. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 mediates the development of sex specific brain morphology and behavior. Proceedings of the National Academy of Sciences USA. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Giguere V. Isoform-Selective interactions between estrogen receptors and steroid receptor coactivators promoted by estradiol and ErbB-2 signaling in living cells. Molecular Endocrinology. 2003;17:589–599. doi: 10.1210/me.2002-0351. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: Immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte JC. Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Mani SK. Feminine sexual behavior from neuroendocrine and molecular neurobiological perspectives. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Neurobiology. New York: Springer; 2006. pp. 95–150. [Google Scholar]
- Blaustein JD, King JC, Toft DO, Turcotte J. Immunocytochemical localization of estrogen-induced progestin receptors in guinea pig brain. Brain Res. 1988;474:1–15. doi: 10.1016/0006-8993(88)90664-6. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. Journal of Neuroscience. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Harada N, Ball GF, Balthazart J. Targeting steroid receptor coactivator-1 expression with locked nucleic acids antisense reveals different thresholds for the hormonal regulation of male sexual behavior in relation to aromatase activity and protein expression. Behav Brain Res. 2006;172:333–343. doi: 10.1016/j.bbr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O’Malley BW. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra A, Fuxe K, Harfstrand A, Agnati LF, Miller LS, Greene JL, Gustafsson JA. On the cellular localization and distribution of estrogen receptors in the rat tel- and diencephalon using monoclonal antibodies to human estrogen receptor. NeurochemistryInternational. 1986;8:587–595. doi: 10.1016/0197-0186(86)90196-8. [DOI] [PubMed] [Google Scholar]
- Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol. 2008;180:630–636. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- Damdimopoulos AE, Spyrou G, Gustafsson JA. Ligands Differentially Modify the Nuclear Mobility of Estrogen Receptors {alpha} and. Endocrinology. 2008;149:339–345. doi: 10.1210/en.2007-0198. [DOI] [PubMed] [Google Scholar]
- Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58:584–590. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-Binding Protein (CBP) with estrogen receptor- à: Regulation by phosphorylation sites in the A/B region depends on other receptor domains. Molecular Endocrinology. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- Feng W, Ribeiro RCJ, Wagner RL, Nguyen H, Apriletti JW, Fletterick RL, Baxter JD, Kushner PJ, West BL. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science. 1998;280:1747–1750. doi: 10.1126/science.280.5370.1747. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Amato P, Allred DC, Nguyen J, DeMayo FJ, Lydon JP. Progesterone-action in the murine uterus and mammary gland requires steroid receptor coactivator 2: relevance to the human. Front Biosci. 2007;12:3640–3647. doi: 10.2741/2340. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Molecular and Cellular Biology. 2002;22:5923–5937. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande PH, Pollio G, McDonnell DP. Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. Journal of Biological Chemistry. 1997;272:32889–32900. doi: 10.1074/jbc.272.52.32889. [DOI] [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel A, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. MolCell Biol. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: Effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. MolEndocrinol. 2006;20:254–267. doi: 10.1210/me.2005-0061. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Han SJ, Tsai SY, Tsai MJ, O’Malley BW. Distinct Temporal and Spatial Activities of RU486 on PR Function in Reproductive Organs of Ovariectomized Mice. Endocrinology. 2007 doi: 10.1210/en.2006-1561. [DOI] [PubMed] [Google Scholar]
- Harris HA, Bapat AR, Gonder DS, Frail DE. The ligand binding profiles of estrogen receptors alpha and beta are species dependent. Steroids. 2002;67:379–384. doi: 10.1016/s0039-128x(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Reddy AB. Two decades of circadian time. Journal of Neuroendocrinology. 2008;20:812–819. doi: 10.1111/j.1365-2826.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- Heneghan AF, Connaghan-Jones KD, Miura MT, Bain DL. Coactivator assembly at the promoter: efficient recruitment of SRC2 is coupled to cooperative DNA binding by the progesterone receptor. Biochemistry. 2007;46:11023–11032. doi: 10.1021/bi700850v. [DOI] [PubMed] [Google Scholar]
- Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Molecular and Cellular Biology. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Hippocampal estrogen receptor-alpha splice variant TADDI in the human brain in aging and Alzheimer’s disease. Neuroendocrinology. 2009;89:187–199. doi: 10.1159/000158573. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Suzuki T, Kawasima T, Stumpf WE, Jungblut PW, de Sombre ER. A two-step mechanism for the interaction of estradiol with rat uterus. Proceedings of the National Academy of Sciences USA. 1968;59:632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O’Malley BW, Demayo FJ. The p160 steroid receptor coactivator-2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148:4238–4250. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- Jones PS, Parrott E, White IN. Activation of transcription by estrogen receptor alpha and beta is cell type- and promoter-dependent. The Journal of biological chemistry. 1999;274:32008–32014. doi: 10.1074/jbc.274.45.32008. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Foster EA, Smith CL. Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-αlpha transcriptional activity. Endocrinology. 2009;150:1588–1596. doi: 10.1210/en.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO Journal. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Mouri N. The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid BiochemMolBiol. 1993;47:173–182. doi: 10.1016/0960-0760(93)90072-5. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Molecular and cellular endocrinology. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS. Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-alpha and estrogen receptor-beta: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology. 2000;141:3534–3545. doi: 10.1210/endo.141.10.7698. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Kadonaga JT. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 1998;12:331–342. doi: 10.1101/gad.12.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Mobbs CV, Pfaff DW. Estradiol Regulation of Estrogen Receptor Messenger Ribonucleic Acid in Rat Mediobasal Hypothalamus - An In Situ Hybridization Study. JNeuroendocrinol. 1990;2:605–611. doi: 10.1111/j.1365-2826.1990.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Lewis-Wambi JS, Jordan VC. Treatment of Postmenopausal Breast Cancer with Selective Estrogen Receptor Modulators (SERMs) Breast Dis. 2005;24:93–105. doi: 10.3233/bd-2006-24108. [DOI] [PubMed] [Google Scholar]
- Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O’Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–587. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Portillo W, Reyna A. Steroid hormone action in the brain: cross-talk between signalling pathways. J Neuroendocrinol. 2009;21:243–247. doi: 10.1111/j.1365-2826.2009.01844.x. [DOI] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Chen JZ, Mulac-Jericevic B, Conneely OM. Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Molecular Endocrinology. 2006;20:1322–1332. doi: 10.1210/me.2005-0466. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figuiera HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiology and Behavior. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney EM, Tsai MJ, O’Malley BW, Katzenellenbogen BS. Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proceedings of the National Academy of Sciences USA. 1996;93:10069–10073. doi: 10.1073/pnas.93.19.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin VS, Harrell C, Adelman JS, Kraus WL, Churchill M, Edwards DP. The role of the C-terminal extension (CTE) of the estrogen receptor à and B DNA binding domain in DNA binding and interaction with HMGB. The Journal of biological chemistry. 2004;279:14763–14771. doi: 10.1074/jbc.M313335200. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: An emerging mechanism of estrogen action in brain. Molecular Neurobiology. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Modder UI, Monroe DG, Fraser DG, Spelsberg TC, Rosen CJ, Gehin M, Chambon P, O’Malley BW, Khosla S. Skeletal consequences of deletion of steroid receptor coactivator-2/transcription intermediary factor-2. The Journal of biological chemistry. 2009;284:18767–18777. doi: 10.1074/jbc.M109.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Hormones and Behavior. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenda-Figueira HA, Murphy SD, Shea KL, Siegal NK, Zhao Y, Chadwick JG, Denner LA, Tetel MJ. Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes. Endocrinology. 2008;149:5272–5279. doi: 10.1210/en.2008-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Amato P, Allred DC, DeMayo FJ, Lydon JP. Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human. Nuclear receptor signaling. 2007;5:e011. doi: 10.1621/nrs.05011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. ProcNatlAcadSciUSA. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor-alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. ProcNatlAcadSciUSA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara E, Yoshida-Kimoya H, Chan C, Liao L, Davis RL, O’Malley BW, Xu J. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. The Journal of Neuroscience. 2003;23:213–222. doi: 10.1523/JNEUROSCI.23-01-00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW. Molecular biology. Little molecules with big goals. Science. 2006;313:1749–1750. doi: 10.1126/science.1132509. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene- deficient (betaERKO) male and female mice. ProcNatlAcadSciUSA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Oñate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, Edwards DP, O’Malley BW. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. Journal of Biological Chemistry. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Pfaff DW, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. JCompNeurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Tetel MJ, Schober JM. Neuroendocrinology: Mechanisms by which hormones affect behaviors. In: Bernston GG, Cacioppo JT, editors. Handbook of Neuroscience for the Behavioral Sciences. Wiley; 2009. [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky ML, Lee S, Hahm JB, Young BM, Fong RN, Chan IH. The p160 coactivator PAS-B motif stabilizes nuclear receptor binding and contributes to isoform-specific regulation by thyroid hormone receptors. The Journal of biological chemistry. 2009;284:19554–19563. doi: 10.1074/jbc.M109.007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Routledge EJ, White R, Parker MG, Sumpter JP. Differential Effects of Xenoestrogens on Coactivator Recruitment by Estrogen Receptor (ER) alpha and ERbeta. Journal of Biological Chemistry. 2000;275:35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Molecular Endocrinology. 1994;8:1347–1360. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Gorski J. Estrogen receptors in the rat uterus. Studies on the interaction of cytosol and nuclear binding sites. The Journal of biological chemistry. 1969;244:1097–1103. [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusable coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomoter rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of Androgen and Estrogen Receptor Messenger RNA- Containing Cells in the Rat Brain - An In Situ Hybridization Study. Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Molecular Endocrinology. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M. Estradiol concentrating neurons in the amygdala. PSEBM. 1971;136:102–106. doi: 10.3181/00379727-136-35204. [DOI] [PubMed] [Google Scholar]
- Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. Journal of Biological Chemistry. 1998;273:27645–27653. doi: 10.1074/jbc.273.42.27645. [DOI] [PubMed] [Google Scholar]
- Tanenbaum DM, Wang Y, Williams SP, Sigler PB. Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc Natl Acad Sci U S A. 1998;95:5998–6003. doi: 10.1073/pnas.95.11.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ. Nuclear receptor coactivators: Essential players in steroid hormone action in brain and behavior. Journal of Neuroendocrinology. 2009;21:229–237. doi: 10.1111/j.1365-2826.2009.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, Lange CA. Molecular genomics of progestin actions. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2009. pp. 1439–1465. [Google Scholar]
- Tetel MJ, Celentano DC, Blaustein JD. Intraneuronal convergence of tactile and hormonal stimuli associated with female reproduction in rats. Journal of Neuroendocrinology. 1994;6:211–216. doi: 10.1111/j.1365-2826.1994.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Siegal NK, Murphy SD. Cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and ovarian steroid receptors. J Neuroendocrinol. 2007;19:262–271. doi: 10.1111/j.1365-2826.2007.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, Auger AP, Charlier TD. Who’s in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30:328–342. doi: 10.1016/j.yfrne.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Molecular Endocrinology. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- Tilton RG, Haidacher SJ, Lejeune WS, Zhang X, Zhao Y, Kurosky A, Brasier AR, Denner L. Diabetes-induced changes in the renal cortical proteome assessed with two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2007;7:1729–1742. doi: 10.1002/pmic.200700017. [DOI] [PubMed] [Google Scholar]
- Tung L, Abdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Molecular endocrinology (Baltimore, Md. 2006;20:2656–2670. doi: 10.1210/me.2006-0105. [DOI] [PubMed] [Google Scholar]
- van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149:725–732. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Frontiers in Neuroendocrinology. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJS, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO Journal. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- Wardell SE, Edwards DP. Mechanisms controlling agonist and antagonist potential of selective progesterone receptor modulators (SPRMs) SeminReprodMed. 2005;23:9–21. doi: 10.1055/s-2005-864030. [DOI] [PubMed] [Google Scholar]
- Warembourg M, Jolivet A, Milgrom E. Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res. 1989;480:1–15. doi: 10.1016/0006-8993(89)91561-8. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Molecular Endocrinology. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Molecular and Cellular Biology. 1994;14:8356–8364. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P, Driscoll MD, Huang J, Bhagat S, Hilf R, Bambara RA, Muyan M. The effects of estrogen-responsive element- and ligand-induced structural changes on the recruitment of cofactors and transcriptional responses by ER alpha and ER beta. Molecular Endocrinology. 2002;16:674–693. doi: 10.1210/mend.16.4.0810. [DOI] [PubMed] [Google Scholar]
- Zhao C, Toresson G, Xu L, Koehler KF, Gustafsson JA, Dahlman-Wright K. Mouse estrogen receptor beta isoforms exhibit differences in ligand selectivity and coactivator recruitment. Biochemistry. 2005;44:7936–7944. doi: 10.1021/bi047691m. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang W, White MA, Zhao Y. Capillary high-performance liquid chromatography/mass spectrometric analysis of proteins from affinity-purified plasma membrane. Anal Chem. 2003;75:3751–3757. doi: 10.1021/ac034184m. [DOI] [PubMed] [Google Scholar]


