Abstract
The potential role of socioeconomic status (SES) in the survival of patients with myelodysplastic syndromes (MDS) has not been evaluated. We conducted the first study to assess the prognostic role of neighborhood SES among a cohort of 2,118 patients (age ≥ 66 years) who were diagnosed with incident MDS in the United States during 2001–2002. Principal component analysis was used to develop a summary SES score by combining multiple measures of neighborhood SES. The score was then used to classify the census tract each patient resided in into a category of high, medium, or low SES. Hazard ratios (HRs) were estimated using multivariate Cox proportional hazard models. After adjusting for age, gender, comorbidities, and histological subtypes, compared with MDS patients lived in high-SES census tracts, those resided in medium (HR = 1.14, 95% CI: 1.01–1.30) and low (HR = 1.17, 95% CI: 1.02–1.34) SES census tracts had significantly increased the risks of death. The impact of SES on survival was more apparent for patients with refractory anemia with ringed sideroblasts—patients residing in medium (HR = 1.85, 95% CI: 1.17–2.91) and low (HR = 2.06, 95% CI: 1.27–3.37) census tracts had a nearly two-fold increased the risk of mortality, compared with those living in high-SES census tracts. In conclusion, this population-based study suggests that neighborhood SES status is a significant and independent determinant of survival among elderly patients with MDS in the United States.
Keywords: Myelodysplastic syndromes, Survival, Socioeconomic status
Introduction
Previous studies about solid tumors (e.g. breast, colon/rectum, and bladder cancer) have observed poor survival among patients with low socioeconomic status (SES) [1, 2]. In the US, people living in disadvantaged neighborhoods have poorer access to health care [3–5]. There has been evidence that different treatments were given to patients with cancer in different socioeconomic groups [1]. For example, patients with breast, colon, and prostate cancer patients residing in low-SES areas tend to receive less aggressive treatments [6].
More recently, investigators have also looked at the role of SES in the prognosis of hematological malignancies with mixed results. In England and Wales, patients with non-Hodgkin lymphoma (NHL) and adult leukemia in affluent groups had higher survival rate, 5- and 10-year respectively[7, 8]. In Denmark, NHL patients with higher SES had better survival, but no significant association was observed between SES and the survival of patients with leukemia or Hodgkin lymphoma [9]. Two other NHL studies conducted in the US and Scotland also reported better survival among patients of higher SES [10, 11]. The survival of acute myeloid leukemia, however, did not appear to be linked to the SES of patients in a recently published US study [12].
To date, no studies have assessed the possible role of SES in the survival of patients with myelodysplastic syndromes (MDS), a group of hematological malignancies marked by poor prognosis and frequent (~30%) transformation to acute myeloid leukemia, although a few prognostic factors, such as age, gender, blast percentage, number of cytopenias, transfusion dependence, and cytogenetics, have been identified [13, 14]. It is particularly timely to evaluate SES in survival of patients with MDS, given that new expensive therapies are being adopted for MDS. Utilizing the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database [15], we conducted a large, population-based study to evaluate the impact of neighborhood SES on the survival of elderly MDS patients in the US.
Materials and methods
Data sources
The linkage of the SEER data with the Medicare records is the collaborative effort of the National Cancer Institute, the SEER registries, and the Center for Medicare and Medicaid Services (CMS). The SEER program consists of population-based tumor registries in 17 geographic areas, which cover ~26.2% of the US population and include the states of California, Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah, the metropolitan areas of Atlanta, Detroit, and Seattle (Puget Sound), as well as rural Georgia and American Indians/Alaska Natives residing in the state of Alaska.
The MDS histology types recorded in SEER are based on the International Classification of Diseases for Oncology (3rd Edition, ICO-O-3) codes [16], including (1) 9980: refractory anemia (RA); (2) 9982: RA with ringed sideroblasts (RARS); (3) 9983: RA with excess blasts (RAEB, including RAEB under the FAB classification and both RAEB-1 and RAEB-2 under the WHO recommendation); (4) 9984: RAEB in transformation (RAEB-t); (5) 9985: refractory cytopenia with multilineage dysplasia (RCMD); (6) 9986: MDS associated with 5q deletion; (7) 9987: therapy-related MDS; and (8) 9989: MDS, not otherwise specified. Since the morphologic feature of RAEB-t is considered more in line with that of AML, we conducted our analyses with and without the inclusion of patients with RAEB-t.
Study population
All individuals with a diagnosis of MDS made between 2001 and 2002 (n = 3,638) were identified from the most recently linked database; their Medicare claims through the end of 2004 were also obtained. We then excluded 98 patients who did not have an exact month of diagnosis recorded, 71 patients who were identified from death certificates only, and 378 patients who were 65 years or younger. The rationale to limit the age of diagnosis to 66 years or older is to ensure a minimum of 12 months of Medicare claims prior to MDS diagnosis [17–19]. In addition, we also excluded 762 patients who did not have continuous Medicare Part A and Part B coverage or were enrolled in health maintenance organizations (HMOs) during the period of interest, which began 12 months before diagnosis and ended at the time of death or in December 2004, whichever was earlier. This exclusion was necessary because the claims of these 762 patients would not have been all reported to the CMS [20]. After exclusions described earlier, 2,329 patients were retained, of whom 211 (9.06%) did not have their census tract information on file. Patients without census tract information (n = 211) did not differ significantly from patients with known census tract (n = 2,118) with regard to age at diagnosis, gender, race/ethnicity, vital status, year of diagnosis, or whether the area they resided in was rural/urban. We conducted all analyses with two different groups of patients: (1) 2,118 patients with known census tracts; and (2) 2,118 patients with known census tracts plus 211 patients with randomly allocated census tracts within the same counties. Since analyses with the two groups yielded extremely similar results, we decided to only report results from analyses involving the 2,118 patients with known census tracts.
Study variables
Categorization of SES
The linked database provides geocoded residential information for each patient at the time of diagnosis, and the smallest geographic unit included in the database is census tract, an area containing an average of 4,000 residents. As all patients included in our analysis were diagnosed during 2001–2002, we focused on census tract level SES measures from the 2000 US census. A total of 16,682 census tracts were identified in the SEER regions. After excluding census tracts with a median household income of zero and census tracts without any residents above the age of 25 years (usually non-residential areas such as university campuses and a prison), we used seven SES indicators of the remaining 16,569 census tracts to develop a SES index score. Specifically, we utilized principle component analysis and followed a similar procedure reported by Yost et al. [21]. The SES indicators variables included education index, median household income, percent living 200% below poverty level, percent white-collar workers, percent age >16 years in workforce without job, median rent, and median house value, which reflect information on education, income, and occupation, three major determinants of SES [22], as well cost of living. The education index weighted the proportion of people attained a given level of education by the correspondent number of needed school years [23]. Only one principal component was extracted based on Kaiser Criteria and Scree test, and the component explained 68.5% of the variance. The output from the principle component analysis is a table of factor scores or weights for each variable. Variables with positive factor scores, i.e., education index, median household income, percent white-collar workers, median rent, and median house value, are associated with higher SES, and conversely variables with negative factor scores, i.e., percent living 200% below poverty level and percent age >16 years in workforce without job, are associated with lower SES. Using this index, we assigned a standardized score to each of the 16,569 tracts in the SEER regions in 2000 and then categorized these scores into tertiles: low, medium, and high SES. Each MDS patient included in our analysis was then assigned a corresponding neighborhood SES level based on their census tract at the time of diagnosis.
Other study variables
The vital status and date of death are included in the SEER–Medicare linked database. Survival time in this analysis was defined as the duration between the date of MDS diagnosis and the date of death due to any cause or December 31, 2004, whichever was earlier. Other variables of interested included age at diagnosis (categorized into five groups, 65–69, 70–74, 75–79, 80–84, and ≥85 years), gender, race/ethnicity (white and non-white), histological subtypes (as reflected by ICD-O-3 codes), and comorbidities. To measure comorbidities, we first examined inpatient records, outpatient and carrier files to ascertain all Medicare claims that were made within 1 year prior to the diagnosis of MDS and 1 month after the diagnosis; then, we used the health claim data to calculate a commonly used comorbidity index known as the Charlson index (with Romano adaptation) [24, 25], which has been widely used as a summary measure for comorbid conditions. The methods have been described in a more detailed way by Wang et al. [26]. In this study population, the most common comorbidities observed were diabetes (21.0%), congestive heart failure (20.6%), and chronic obstructive pulmonary disease (18.6%) (comorbid conditions not mutually exclusive).
Statistical analysis
Frequencies and percentages were used to describe various characteristics of the study population. Kaplan–Meier product limit was used to describe the probability of survival at various times. The log-rank test was used to compare survival curves across SES categories. Unadjusted and adjusted Cox proportional hazard regression models were utilized to assess the impact of various characteristics on survival. Tests for trend were conducted by using the original value of each variable on a continuous scale, which means standardized census tract level SES score for neighborhood SES, age in years, and the actual Charlson index in its continuous format (as opposed to categories such as 1–2, ≥3). All analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, NC). All significance tests were two-sided with α = 0.05.
Results
More than 90% of the patients with MDS were white people, 54% were male, and 59% lived in big metropolitan areas (Table 1). The majority of patients had at least one comorbid condition. Those residing in low-SES areas were more likely to be female, non-white people, have comorbidities, and from Kentucky or Louisiana. The most frequently reported cause of death was neoplasm (56.5%), mainly leukemia (43.8% of neoplasm deaths), and MDS (38.4% of neoplasm deaths). Other major causes of death were diseases of the circulatory system (20.9%), the respiratory system (5.9%), and the digestive system (3.1%).
Table 1.
Selected demographic and clinical characteristics of 2,118 patients with MDS diagnosed during 2001–2002 and included in SEER–medicare
| Characteristic | Total n (%) | Socioeconomic status |
||
|---|---|---|---|---|
| Low n (%) | Medium n (%) | High n (%) | ||
| Gender | ||||
| Female | 973(45.9) | 282(48.0) | 381(48.0) | 310(42.1) |
| Male | 1,145(54.1) | 306(52.0) | 413(52.0) | 426(57.9) |
| Race | ||||
| White | 1,908(90.1) | 481(81.8) | 735(92.6) | 692(94.0) |
| Black | 131(6.2) | 84(14.3) | 36(4.5) | 11(1.5) |
| Other | 79(3.7) | 23(3.9) | 23(2.9) | 33(4.5) |
| Age (years) | ||||
| 66–69 | 208(9.8) | 63(10.7) | 80(10.1) | 65(8.8) |
| 70–74 | 425(20.1) | 129(22.0) | 135(17.0) | 161(21.9) |
| 75–79 | 504(23.8) | 136(23.1) | 209(26.3) | 159(21.6) |
| 80–84 | 547(25.8) | 147(25.0) | 200(25.2) | 200(27.2) |
| 85+ | 434(20.5) | 113(19.2) | 170(21.4) | 151(20.5) |
| Charlson index | ||||
| 0 | 1,031(48.7) | 261(44.4) | 387(48.7) | 383(52.0) |
| 1–2 | 778(36.7) | 220(37.4) | 287(36.2) | 271(36.8) |
| 3+ | 309(14.6) | 107(18.2) | 120(15.1) | 82(11.2) |
| Subtypea (ICD-O-3 Code) | ||||
| RA (9980) | 384(18.1) | 101(17.2) | 152(19.1) | 131(17.8) |
| RARS (9982) | 253(12.0) | 60(10.2) | 95(12.0) | 98(13.3) |
| RAEB (9983) | 276(13.0) | 79(13.5) | 108(13.6) | 89(12.1) |
| RAEB-t (9984) | 34(1.6) | 13(2.2) | 9(1.1) | 12(1.6) |
| RCMD (9985) | 64(3.0) | 13(2.2) | 26(3.3) | 25(3.4) |
| MDS associated with 5q deletion (9986) | 41(1.9) | 9(1.5) | 14(1.8) | 18(2.5) |
| Therapy-related MDS (9987) | 34(1.6) | 9(1.5) | 17(2.1) | 8(1.1) |
| MDS, not otherwise specified (9989) | 1,032(48.7) | 304(51.7) | 373(47.0) | 355(48.2) |
| Urban/rural | ||||
| Big metro | 1,245(58.8) | 223(37.9) | 441(55.5) | 581(78.9) |
| Metro | 532(25.1) | 162(27.6) | 228(28.7) | 142(19.3) |
| Urban | 110(5.2) | 38(6.5) | 62(7.8) | 10(1.4) |
| Less urban | 189(8.9) | 136(23.1) | –b | –b |
| Rural | 42(2.0) | 29(4.9) | –b | –b |
RA refractory anemia, RARS RA with ringed sideroblasts, RAEB RA with excess blasts, RAEB-t RAEB in transformation, RCMD refractory cytopenia with multilineage dysplasia
SEER–Medicare confidentiality rules stipulate that cells size <5 should not be presented
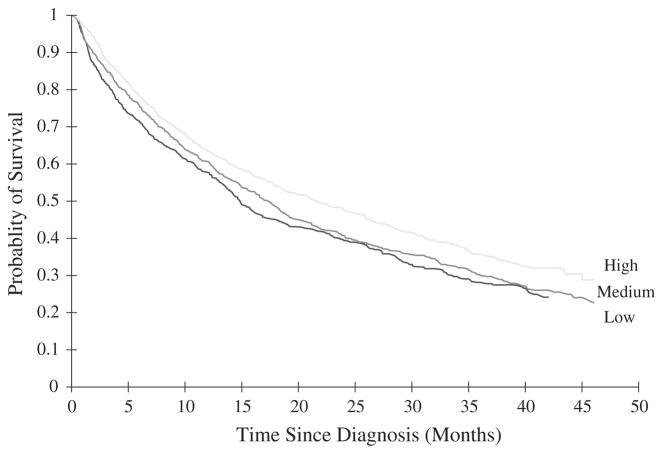
The median survival time for the overall study population was 17.8 [95% confidence interval (CI) 16.3–19.0] months. Survival of MDS patients was significantly associated with neighborhood SES (p value from log-rank test < 0.01) (Fig. 1). The median survival time for patients residing in low, medium, and high-SES neighborhoods were 14.9 (95% CI: 13.6–16.9) months, 17.4 (95% CI: 15.4–18.9) months, and 21.6 (95% CI: 18.6–25.8) months, respectively.
Fig. 1.
Socioeconomic status and survival of 2,118 MDS patients diagnosed during 2001–2002 and included in SEER-medicare
Analyses with unadjusted Cox proportional hazard models suggested that residing in low-SES neighborhoods, older age, male gender, and the presence of comorbid conditions were associated with a greater risk of death (i.e., shorter survival), whereas race did not appear to affect survival (Table 2). Residential area (urban/rural) was also not associated with survival, either (detailed data not shown due to small numbers of patients in certain cells). Different MDS subtypes showed varying prognosis—patients diagonised with RARS appeared to have longer survival than patients diagnosed with RA, RAEB, or RAEB-t.
Table 2.
Selected characteristics and survival of 2,118 MDS patients diagnosed during 2001–2002 and included in SEER-medicare (unadjusted analyses)
| Characteristic | Hazard ratio | 95% CI | p for trend |
|---|---|---|---|
| Socioeconomic status | |||
| High | 1.00 | ||
| Medium | 1.18 | 1.04–1.33 | |
| Low | 1.26 | 1.10–1.43 | <0.01 |
| Gender | |||
| Female | 1.00 | ||
| Male | 1.15 | 1.04–1.28 | |
| Race | |||
| White | 1.00 | ||
| Black | 0.89 | 0.71–1.11 | |
| Other | 1.03 | 0.78–1.36 | |
| Age (years) | |||
| 66–69 | 1.00 | ||
| 70–74 | 1.11 | 0.90–1.38 | |
| 75–79 | 1.22 | 0.99–1.50 | |
| 80–84 | 1.38 | 1.13–1.70 | |
| 85+ | 1.93 | 1.57–2.38 | <0.01 |
| Charlson index | |||
| 0 | 1.00 | ||
| 1–2 | 1.19 | 1.06–1.33 | |
| 3+ | 1.76 | 1.52–2.03 | <0.01 |
| Subtypea (ICD-O-3 Code) | |||
| RA (9980) | 1.00 | ||
| RARS (9982) | 0.71 | 0.57–0.88 | |
| RAEB (9983) | 2.22 | 1.86–2.66 | |
| RAEB-t (9984) | 2.79 | 1.93–4.04 | |
| RCMD (9985) | 0.92 | 0.64–1.32 | |
| MDS with 5q deletion (9986) | 1.06 | 0.70–1.61 | |
| Therapy-related MDS (9987) | 1.32 | 0.87–2.00 | |
| MDS, not otherwise specified (9989) | 1.25 | 1.08–1.45 | |
RA refractory anemia, RARS RA with ringed sideroblasts, RAEB RA with excess blasts, RAEB-t RAEB in transformation, RCMD refractory cytopenia with multilineage dysplasia
In multivariate analyses with adjusted Cox regression models, MDS patients residing in lower SES neighborhoods remained a negative prognostic factor (Table 3). After adjusting for age, gender, and comorbidities (Model 1), MDS patients residing in medium- and low-SES neighborhoods had a 16–22% increased risk of death when compared with patients living in high-SES areas. There was a significant trend between standardized SES score and survival—a higher SES score was linked to longer survival. As with the unadjusted analyses, older age, male gender, and the presence of comorbid conditions were significantly predicative of shorter survival. A significant inverse trend between age at diagnosis and survival was identified. Male patients with MDS had significantly shorter survival than female patients (HR = 1.22, 95% CI: 1.09–1.35). Compared with patients without comorbid conditions (i.e., had a Charlson index of 0), those with a Charlson index of 1–2 or ≥3 had hazard ratios of 1.18 (95% CI: 1.05–1.32) and 1.73 (95% CI: 1.50–2.00), respectively. Additionally, adjusting for MDS subtype (Model 2) did not have much impact on the results. Compared with RA patients, RARS patients had lower risk of death (HR = 0.73, 95% CI: 0.59–0.91), while RAEB (HR = 2.43, 95% CI: 2.03–2.92) and RAEB-t (HR = 3.40, 95% CI: 2.34–4.93) patients had higher risk of death (Table 3).
Table 3.
Selected demographic and clinical characteristics and survival of 2,118 MDS patients diagnosed during 2001–2002 and included in SEER-medicare (adjusted analyses)
| Characteristic | Model 1b |
Model 2b |
||
|---|---|---|---|---|
| RR(95% CI) | p for trend | RR(95% CI) | p for trend | |
| Socioeconomic status | ||||
| High | 1.00 | 1.00 | ||
| Medium | 1.16(1.03–1.31) | 1.14(1.01–1.30) | ||
| Low | 1.22(1.07–1.39) | <0.01 | 1.17(1.02–1.34) | <0.01 |
| Gender | ||||
| Female | 1.00 | 1.00 | ||
| Male | 1.22(1.09–1.35) | 1.14(1.03–1.27) | ||
| Age (years) | ||||
| 66–69 | 1.00 | 1.00 | ||
| 70–74 | 1.09(0.88–1.35) | 1.06(0.86–1.32) | ||
| 75–79 | 1.18(0.96–1.46) | 1.27(1.03–1.56) | ||
| 80–84 | 1.37(1.12–1.68) | 1.43(1.16–1.76) | ||
| 85+ | 1.94(1.58–2.39) | <0.01 | 2.11(1.71–2.60) | <0.01 |
| Charlson index | ||||
| 0 | 1.00 | 1.00 | ||
| 1–2 | 1.18(1.05–1.32) | 1.23(1.10–1.38) | ||
| 3+ | 1.73(1.50–2.00) | <0.01 | 1.83(1.58–2.12) | <0.01 |
| Subtypea (ICD-O-3 Code) | ||||
| RA (9980) | 1.00 | |||
| RARS (9982) | 0.73(0.59–0.91) | |||
| RAEB (9983) | 2.43(2.03–2.92) | |||
| RAEB-t (9984) | 3.40(2.34–4.93) | |||
| RCMD (9985) | 1.02(0.71–1.47) | |||
| MDS with 5q deletion (9986) | 1.23(0.82–1.86) | |||
| Therapy-related MDS (9987) | 1.47(0.96–2.24) | |||
| MDS, not otherwise specified (9989) | 1.27(1.09–1.47) | |||
RA refractory anemia, RARS RA with ringed sideroblasts, RAEB RA with excess blasts, RAEB-t RAEB in transformation, RCMD refractory cytopenia with multilineage dysplasia
In Model 1, socioeconomic status (low, medium, and high), gender, age (in 5-year age groups) and Charlson index (0, 1–2, and 3+) were mutually adjusted. Compared with Model 1, Model 2 additionally adjusted for histological subtypes
The analyses described earlier were also conducted separately for patients with one of the three major MDS subtypes (i.e., RA, RARS, and RAEB). The impact of SES on survival was most obvious among patients with RARS. Compared with RARS patients living in high-SES areas, the hazard ratios for RARS patients residing in medium and low-SES neighborhoods were 1.85 (95% CI: 1.17–2.91) and 2.06 (95% CI: 1.27–3.37), respectively; a significant trend was also observed. For patients with RA and RAEB, the hazard ratios were closer to 1 and did not reach statistical significance (details not shown).
We repeated all analyses described earlier for a total of 1,690 patients with MDS and without previous history of cancer (i.e., de novo MDS) and observed extremely similar results. In addition, these analyses were also carried out after excluding patients with RAEB-t, and the results were basically the same.
Discussion
To the best of our knowledge, this was the first study to address the role of neighborhood SES in the prognosis of MDS. Our findings suggest that neighborhood SES is significantly associated with MDS survival, even after adjusting for comorbidities and other patient characteristics. Comorbidities may influence disease progression and the ability to tolerate treatments, which in turn could affect survival. In this study, we did observe an increased risk of death with a higher Charlson index. However, the impact of SES persisted after comorbidities and other patient characteristics were taken into account, suggesting that residing in lower SES neighborhoods independently predicts shorter survival after the diagnosis of MDS.
Health care factors, such as access to medical services, available medical expertise, treatments prescribed, and adherence to treatments, may play a role in the differences of outcomes with regard to SES. For the elderly population, the availability of help with transportation could be an important factor. In the US, people living in disadvantaged neighborhoods have poorer access to health care [3–5]. It has also been reported that patients with breast, colon, and prostate cancer patients residing in low-SES areas received less aggressive treatments [6]. It is conceivable that MDS patients with high SES may have been identified and/or treated earlier than MDS patients with low SES. Although we did not observe a significant association between age at diagnosis and neighborhood SES, we found that among patients of age 66–75 years of age, those who resided in high-SES neighborhoods were more likely to be diagnosed with RA and RARS, MDS subtypes that are known to have longer survival. One could also speculate that MDS patients living in high-SES neighborhoods may have access to medical care more easily or to “better” medical care leading to prolonged survival. Between May 2004 and May 2006, three drugs were approved by the Food and Drug Administration for the treatment of MDS, including azacitidine, lenalidomide, and decitabine. During the study period (2001–2004), however, treatment options for elderly patients with MDS mainly aimed at symptom management [27, 28] and included supportive care and palliative measures, such as hematopoietic growth factors, red blood cell or platelet transfusions, and antimicrobial agents [29]. In this study, we found that growth factors were more frequently received by patients with MDS who resided in high-SES neighborhoods (26.6%) than patients residing in medium (19.9%) or low (17.9%) level SES neighborhoods, whereas the history of receiving blood transfusions was similar across patients with different levels of neighborhood SES. After adjusting for the administration of growth factors and blood transfusions, neighborhood SES was still significantly associated with the survival of patients with MDS (RR for low vs. high neighborhood SES: 1.14, 95% CI: 1.00–1.31; p for trend = 0.01). We also found that the impact of SES on survival was more obvious for RARS, a subtype known to have better prognosis than other common MDS subtypes [30]. For subtypes known to be more aggressive (e.g. RAEB), it is possible that the biological characteristics of the disease play a more dominant role than external factors such as SES. Since the natural history of the more aggressive subtypes is shorter, there is also a limited time window for SES to exert any impact.
This study has several strengths. A large number of newly diagnosed, population-based MDS cases were included in this analysis. Given the rarity of MDS, the sample size was remarkable. Previous studies of MDS prognosis were mostly based on case series from one or few clinical institutions, so the representativeness of the patients might have been questionable. To the best of our knowledge, this was the first study aimed at systematically evaluating the potential role of SES in the survival of patients with MDS. The SEER-Medicare linked database not only provided an opportunity to examine SES at the census tract level, but also made it possible to identify comorbid conditions of all patients. It was important to adjust/control for comorbidities in the analysis, as they are common in the elderly patients and have been linked to the prognosis of MDS in recent studies [26, 31–33].
The design of the study also resulted in some limitations. The study subjects were restricted to elderly patients enrolled in Medicare (age ≥ 66 years), so the findings may not be applicable to MDS patients diagnosed at a younger age. However, as approximately 80% of MDS patients were 65 years or older at the time of diagnosis [30], our findings should be relevant with the vast majority of MDS patients. Medicare beneficiaries with enrollment in health maintenance organizations or without continuous coverage for Part A and Part B benefits were excluded from the analysis, as their health claims were unavailable or incomplete. We found that these patients were younger and lived in more affluent areas than Fee-for-Service participants, especially those enrolled in health maintenance organizations. This finding was consistent with what was reported in a previous study [34]. When we conducted analysis including participants without complete health claims and categorized their comorbidity index as unknown, we still observed an association between neighborhood SES and survival of patients with MDS (low vs. high SES: RR = 1.24, 95% CI: 1.10–1.39; medium vs. high SES: RR = 1.17, 95% CI: 1.05–1.30; p for trend <0.01). In addition, the assessment of SES might have been undermined by the fact that a census tract typically includes 4,000 residents—the census tract level SES may not accurately reflect an individual’s SES. Nevertheless, a large body of literature supports the strong influence of neighborhood characteristics on an individual’s health [3–5, 35, 36]. In this analysis, any deviation of individual level SES from neighborhood SES should not have been differential with respect to the outcome of interest (i.e., death after MDS diagnosis), as data on neighborhood SES were available before any deaths occurred. In this study, we did not have individual level data on SES or lifestyle factors, such as education, family income, smoking, and obesity.
In conclusion, lower neighborhood SES was associated with a significantly shorter survival among elderly MDS patients in the US, even though the mechanisms through which SES may operate remain unclear. This finding, if confirmed by future studies, may inspire other investigators to explore the underlying mechanisms. From a clinical perspective, diagnostic and therapeutic protocols need to be designed to serve both high and low SES patients with MDS.
Acknowledgments
This work was supported by a grant from the National Cancer Institute (K07 CA119108). Dr Gross’s efforts were supported by Beeson Career Development Award (1 K08 AG24842). The linked SEER–Medicare database was used for this study. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER–Medicare database.
Contributor Information
Rong Wang, Department of Epidemiology and Public Health, Yale University School of Medicine, 60 College St, Box 208034, New Haven, CT 06520-8034, USA.
Cary P. Gross, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA
Stephanie Halene, Section of Hematology, Yale University School of Medicine, New Haven, CT, USA.
Xiaomei Ma, Email: xiaomei.ma@yale.edu, Department of Epidemiology and Public Health, Yale University School of Medicine, 60 College St, Box 208034, New Haven, CT 06520-8034, USA.
References
- 1.Woods LM, Rachet B, Coleman MP. Origins of socioeconomic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 2.Kogevinas M, Pearce N, Susser M, et al., editors. Social inequalities and cancer, IARC Sci Publ No 138. International Agency for Research on Cancer; Lyon: 1997. pp. 1–15. [Google Scholar]
- 3.Auchincloss AH, Van Nostrand JF, Ronsaville D. Access to health care for older persons in the United States: personal, structural, and neighborhood characteristics. J Aging Health. 2001;13:329–354. doi: 10.1177/089826430101300302. [DOI] [PubMed] [Google Scholar]
- 4.Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005;46:15–31. doi: 10.1177/002214650504600103. [DOI] [PubMed] [Google Scholar]
- 5.Prentice JC. Neighborhood effects on primary care access in Los Angeles. Soc Sci Med. 2006;62:1291–1303. doi: 10.1016/j.socscimed.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113:582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 7.Rachet B, Mitry E, Shah A, Cooper N, Coleman MP. Survival from non-Hodgkin lymphoma in England and Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S104–S106. doi: 10.1038/sj.bjc.6604605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rachet B, Mitry E, Shah A, Cooper N, Coleman MP. Survival from adult leukaemia in England and Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S116–S118. doi: 10.1038/sj.bjc.6604609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roswall N, Olsen A, Christensen J, Rugbjerg K, Mellemkjaer L. Social inequality and incidence of and survival from Hodgkin lymphoma, non-Hodgkin lymphoma and leukaemia in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44:2058–2073. doi: 10.1016/j.ejca.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Burau KD, Fang S, Wang H, Du XL. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer. 2008;113:3231–3241. doi: 10.1002/cncr.23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray C, Morrison DS, McKay P. Socio-economic deprivation and survival of non-Hodgkin lymphoma in Scotland. Leuk Lymphoma. 2008;49:917–923. doi: 10.1080/10428190801933377. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez CP, Baz R, Jawde RA, et al. Impact of socioeconomic status and distance from treatment center on survival in patients receiving remission induction therapy for newly diagnosed acute myeloid leukemia. Leuk Res. 2008;32:413–420. doi: 10.1016/j.leukres.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Bennett J. The myelodysplastic syndromes: pathobiology and clinical management. In: Cheson B, editor. Basic and Clinical Oncology. Vol. 27. Marcel Dekker; New York: 2002. [Google Scholar]
- 14.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.Fritz A, editor. International classification of diseases for oncology. 3. World Health Organization; Geneva: 2000. [Google Scholar]
- 17.Piccirillo JF, Spitznagel EL, Jr, Vermani N, Costas I, Schnitzler M. Comparison of comorbidity indices for patients with head and neck cancer. Med Care. 2004;42:482–486. doi: 10.1097/01.mlr.0000124254.88292.a1. [DOI] [PubMed] [Google Scholar]
- 18.Sharma G, Freeman J, Zhang D, Goodwin JS. Trends in end-of-life ICU use among older adults with advanced lung cancer. Chest. 2008;133:72–78. doi: 10.1378/chest.07-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong YN, Mitra N, Hudes G, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. Jama. 2006;296:2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 20.Keating NL, Landrum MB, Meara E, Ganz PA, Guadagnoli E. Do increases in the market share of managed care influence quality of cancer care in the fee-for-service sector? J Natl Cancer Inst. 2005;97:257–264. doi: 10.1093/jnci/dji044. [DOI] [PubMed] [Google Scholar]
- 21.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/A:1011240019516. [DOI] [PubMed] [Google Scholar]
- 22.Liberatos P, Link BG, Kelsey JL. The measurement of social class in epidemiology. Epidemiol Rev. 1988;10:87–121. doi: 10.1093/oxfordjournals.epirev.a036030. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Deapen D, Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States) Cancer Causes Control. 1998;9:369–380. doi: 10.1023/A:1008811432436. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. (discussion 1081–1090) [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Gross CP, Halene S, Ma X. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res. 2009 doi: 10.1016/j.leukres.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shadduck RK, Latsko JM, Rossetti JM, Haq B, Abdulhaq H. Recent advances in myelodysplastic syndromes. Exp Hematol. 2007;35:137–143. doi: 10.1016/j.exphem.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Kasner MT, Luger SM. Update on the therapy for myelodysplastic syndrome. Am J Hematol. 2009;84:177–186. doi: 10.1002/ajh.21352. [DOI] [PubMed] [Google Scholar]
- 29.Steensma DP, Tefferi A. Risk-based management of myelodysplastic syndrome. Oncology (Williston Park) 2007;21:43–54. (discussion 57–48, 62) [PubMed] [Google Scholar]
- 30.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 31.Boehm A, Sperr WR, Leitner G, et al. Comorbidity predicts survival in myelodysplastic syndromes or secondary acute myeloid leukaemia after allogeneic stem cell transplantation. Eur J Clin Invest. 2008;38:945–952. doi: 10.1111/j.1365-2362.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 32.Della Porta MG, Kuendgen A, Malcovati L, et al. Myelodysplastic Syndrome (MDS)-Specific comorbidity index for predicting the impact of extra-hematological comorbidities on survival of patients with MDS. ASH Annual Meeting Abstracts. 2008;112:2677. [Google Scholar]
- 33.Pelz D, Nachtkamp K, Kündgen A, Strupp C, Haas R, Germing U. C015 Influence of comorbidity factors on the prognosis of patients with myelodysplastic syndromes (MDS) Leuk Res. 2007;31:S30–S31. doi: 10.1016/S0145-2126(07)70053-X. [DOI] [Google Scholar]
- 34.Blustein J, Hoy EC. Who is enrolled in for-profit vs. nonprofit Medicare HMOs. Health Aff (Millwood) 2000;19:210–220. doi: 10.1377/hlthaff.19.1.210. [DOI] [PubMed] [Google Scholar]
- 35.Do DP, Finch BK. The link between neighborhood poverty and health: context or composition? Am J Epidemiol. 2008:kwn182. doi: 10.1093/aje/kwn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55:125–139. doi: 10.1016/S0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]