Abstract
High soy (HS) diets are neuroprotective and promote vascular dilatation in the periphery. We hypothesized that a HS diet would promote vascular dilatation in the cerebrovasculature by mimicking estradiol's actions on the endothelial nitric oxide synthase (eNOS) system including increasing eNOS expression and decreasing caveolin-1 expression to increase nitric oxide (NO) production. Ovariectomized rats were fed HS or a soy-free diet (SF) ± low physiological estradiol (E2) for 4 weeks. Neither E2 nor HS altered middle cerebral artery (MCA) structure or vascular responses to acetylcholine, serotonin, or phenylephrine. Estradiol enhanced bradykinin-induced relaxation in an eNOS-dependent manner. Although E2 and HS increased eNOS mRNA expression in the brain and cerebrovasculature, they had no effect on eNOS protein expression or phosphorylation in the MCA. However, E2 decreased caveolin-1 protein in the MCA. In MCAs neither E2 nor HS altered estrogen receptor (ER) alpha expression, but E2 did reduce ER beta levels. These data suggest that HS diets have no effect on vascular NO production, and that E2 may modulate basal NO production by reducing the expression of caveolin-1, an allosteric inhibitor of NOS activity. However, the effects of E2 and HS on the cerebrovasculature are small and may not underlie their protective actions in pathological states.
Keywords: estrogen, estradiol, middle cerebral artery, vasoreactivity, vasodilatation
1. Introduction
Controversy surrounding the use of hormone therapy (HT) for cardiovascular and neuronal health has contributed to the decline in its post-menopausal use (Kelly et al 2005). Several prospective studies have concluded that post-menopausal hormone replacement is ineffective or even deleterious for cardiovascular health (Brass 2004). Such results are in contrast to epidemiological and laboratory animal studies that show beneficial effects of estrogens on the vasculature and brain (Amantea et al 2005; Gibson et al 2006; Duckles and Krause 2007). Many women are using alternative therapies for post-menopausal health including dietary soy and isoflavone supplements instead of, or in addition to, traditional HT (Newton et al 2002; Kurzer 2003). Soy isoflavones, genistein and daidzein, and their metabolites, are phytoestrogens, natural compounds that can bind to estrogen receptors (ERs) and mimic some of estrogen's actions.
Recent data from our laboratory (Schreihofer et al 2005; Lovekamp-Swan et al 2007; Lovekamp-Swan et al 2007) and others (Setchell 2001; Lephart et al 2004; Burguete et al 2006) show a beneficial effect of a HS diet on the rodent brain. In particular, HS diets protect the brain from experimental ischemia and reduce apoptosis in the ischemic cortex (Schreihofer et al 2005; Burguete et al 2006; Lovekamp-Swan et al 2007). This protective effect coincides with increases in antiapoptotic genes such as Bcl-XL and growth factors including brain derived neurotrophic factor (BDNF) (Pan et al 1999; File et al 2003; Lovekamp-Swan et al 2007; Lovekamp-Swan et al 2007). However, the precise target and pathways underlying the beneficial effects of soy in the brain remain to be fully elucidated.
HS diets enhance endothelial function and eNOS expression in the peripheral vasculature in rats (Catania et al 2002; Mahn et al 2005). The soy phytoestrogens daidzein and genistein enhance NOS and NO-mediated dilation in male rat carotid and basilar arteries and reduce the expression of the endogenous inhibitor of eNOS, caveolin-1 (Sobey et al 2004). Genistein also increases eNOS expression, decreases caveolin-1, and increases NO bioavailability in ovariectomized female rat myocardium (Tang et al 2005). In contrast, the reports on the effects of soy or soy isoflavones on endothelium dependent vasodilation in humans are decidedly mixed with some showing benefits (Cuevas et al 2003; Steinberg et al 2003; Lissin et al 2004; Colacurci et al 2005), and others failing to do so (Kreijkamp-Kaspers et al 2005; Evans et al 2007; Katz et al 2007; Matthan et al 2007).
In contrast to chronic in vivo effects of soy isoflavones in the periphery, little is known about the effects of soy on the cerebral vasculature. Because of differences in the anatomy and physiology of the cerebral and peripheral vasculature, one cannot extrapolate the findings in the periphery to the brain. Studies have shown that daidzein treatment enhances NO production in the basilar artery of male rats, but has no apparent effect on NOS expression (Sobey et al 2004). However the daidzein metabolite, equol, is similarly vasodilatory without NO dependence (Jackman et al 2007). Because the use of soy isoflavones and soy for health in humans is generally chronic and through oral consumption, we undertook the present study to determine whether dietary soy promoted relaxation of cerebral vessels in the ovariectomized female rat. We hypothesized that dietary soy would improve endothelial function through its estrogenic actions resulting in enhanced eNOS expression and activity.
2. Material and Methods
2.1. Animals and Treatments
The Medical College of Georgia Institutional Animal Care and Use Committee approved all animal protocols. Female Sprague-Dawley rats (200-225 g) (Harlan, Indianapolis, IN) were housed in a temperature-controlled room (22-25 °C) with 12hr dark-light cycles. After acclimating for one week, animals were randomly assigned to one of three groups: SF (soy-free diet + placebo), E2 (soy free diet + estradiol), or HS (high soy diet + placebo). The soy-free diet (Ziegler Brothers Inc. Gardners, PA) was designed to match macro- and micro-nutrient content of the HS diet, Teklad 8604 (Harlan Teklad, Madison, WI). The Teklad diet contains 600 μg/g soy isoflavones and results in an average of 6 μM of circulating total isoflavones (Lund et al 2001; Lovekamp-Swan et al 2007), an amount equal to or greater than a typical Asian diet which is high in soy (Setchell 2001). One week after initiation of diets, animals were bilaterally ovariectomized and subcutaneously implanted with a placebo pellet or a 0.18 mg, 60-day release 17β-estradiol pellet (Innovative Research of America, Sarasota, FL). Animals were kept on assigned diet for four weeks after ovariectomy. Prior to sacrifice, an iSTAT monitor (Abbot, Princeton, NJ) was used to assess blood values of glucose, hematocrit, pH, Na+, and K+ in 4 animals per group.
2.2. Tissue isolation
Rats were anesthetized with urethane (1.7 g/kg i.p.) and decapitated. RNA was isolated from 2 mm coronal sections. Tissue samples were microdissected and frozen on dry ice for subsequent processing (see below). Brain microcapillary cells were isolated from pooled brain samples (2/pool). Brains were homogenized on ice with a glass dounce in 0.5 mg/ml dispase solution. Homogenates were incubated for 45 min at 37° C, washed two times in 4 volumes of Hank's buffered saline (HBS), and centrifuged at 1000 × g for 10 min. The resulting pellet was resuspended in 20% BSA in HBS and centrifuged at 1200 × g for 20 min. Resulting pellets containing microvessels were frozen at -80 C for subsequent extraction. Major cerebral vessels, including the MCA, circle of Willis, and proximal portions of the anterior and posterior cerebral arteries, were carefully removed from the brain and flash frozen.
2.3. RNA collection and real time RT-PCR
Total RNA was isolated from tissue punches using a commercial kit with a DNAse treatment (RNEasy, Qiagen, Valencia, CA). RNA concentration was determined in triplicate using RiboGreen RNA-binding dye (Molecular Probes, Invitrogen, Carlsbad, CA) and stored at −80° C until used. RNA (500 ng) was reverse transcribed with oligo-dT (OmniScript, Qiagen, Valencia, CA), diluted, and stored at -80° C. Real-time RT-PCR was performed on 25 ng equivalents in triplicate on an Applied Biosystems (AB) 7500 Sequence Detection System using AB TaqMan Gene Expression Assays for eNOS (Rn02132634_s1), estrogen receptor-α (Rn01430446_m1), and estrogen receptor-β (Rn00688792_m1). Gene products were amplified in multiplex reactions with primer-limited GAPDH as an endogenous control using AB Universal Taq Master Mix according to manufacturers instructions. No significant differences in GAPDH expression were detected between groups. Threshold amplification cycle number (CT) data from multiple plates was combined using Applied Biosystems Relative Quantitation software (SDS1.2) and the ΔΔCt method. All data are expressed as mean fold change ± SE.
2.4. Protein collection and immunoblotting
For protein collection, a separate group of animals was sacrificed as described above. Coronal sections corresponding to AP +2 to -3 mm relative to bregma were placed on ice-cold glass slides. The cortex was carefully peeled away from the underlying tissue and frozen at -80°C. Protein was extracted using T-PER reagent (Pierce, Rockford, IL) supplemented with HALT Protease Inhibitor Cocktail (Pierce, Rockford, IL). Concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL), and samples were stored as aliquots to avoid multiple freeze-thaw cycles. Samples containing 15-50 μg of protein were separated on precast 4-20% polyacrylamide gels (Pierce Precise) under reducing conditions. Samples were transferred to nitrocellulose and blocked for one hour at room temperature using Odyssey Blocking Buffer (LiCor, Lincoln, NE). Blots were subjected to immunoblotting in primary antibody overnight at 4°C. Antibodies used were eNOS (BD Signal Transduction Laboratories #610296, 1:2500), phospho(Ser1177)-eNOS (Cell Signaling Technologies #95715, 1:1000), β-actin (Sigma #A5441, 1:10,000), and caveolin-1 (Abcam #18199, 1:1000). Secondary detection was accomplished with corresponding IRDye Conjugated antibodies (LiCor, Lincoln, NE) at 1:10,000. Blots were visualized using a LiCor Odyssey Infrared Scanner and quantified using Odyssey software. Results are expressed as a ratio of the protein of interest to β actin.
2.5. Measurements of vessel morphology
The first branch-free segment of the MCA most proximal to the Circle of Willis was mounted on two glass micropipettes (outer diameter, 125–150 μm) and secured with silk thread in a pressure myograph (Living Systems Instrumentation, Burlington, VT). The distal pipette was closed creating a zero-flow conditions and vessels demonstrating leaks were discarded. Vessels were allowed to equilibrate for 1 h at 75 mmHg and 37 °C in PSS. An active pressure response curve was then constructed with the lumen diameter being measured after 7 minutes at each intralumenal pressure (3-180mmHg). The vessels were then placed in calcium free PSS and the pressure response curve was repeated and lumen diameters and wall thickness were measured. Myogenic tone was assessed using the following formula: 1-(active diameter/passive diameter)*100 (Rigsby et al 2007).
2.6. MCA Isometric Force Measurement
The left and right segments of the MCA most proximal to the Circle of Willis were dissected, cleaned and cut into 2mm sections before mounting them on two tungsten wires (40μm diameter) in wire myograph (Model 610M, Danish Myo Technology, Denmark) under cold conditions in PSS. Vessels were allowed to warm to 37°C before applying a passive tension of 8mN to the vessels. 5-HT (10-6 mol/L) was used to test vessel viability. Cumulative dose response curves to 5-HT and phenylephrine (PE) (10-9-10-5 mol/L) were constructed, and the results were expressed as a percentage of the maximum contraction. The force on the vessels was returned to baseline by washing with PSS and the vessels were preconstricted with an EC:70 concentration of 5-HT before generating dose response curves to Ach or bradykinin (BK) (10-9-10-5 mol/L). The vessels were then washed back to baseline and after a ten minute re-equilibration period L-NAME (10-6 mol/L) was added to each bath. The vessels were incubated with L-NAME for 10 minutes before recording the force generated and repeating the Ach and BK dose response curves. For the dilator studies the results were expressed as a percentage of the 5-HT induced preconstriction.
2.7. Plasma NOx measurements
Plasma was collected from a separate group of rats. The concentration of nitrite and nitrate, stable metabolites of NO, were measured in plasma using a sensitive HPLC system (ENO-20; EiCom, Kyoto, Japan), according to the manufacturer's protocol as previously described (Foster et al 2009). The sensitivity limit of this assay is 0.1 pmol nitrite and sample quantification was achieved using a nitrite standard curve. Data are expressed as total NOx.
2.7. Statistical analysis
Differences in plasma NOx, mRNA, and protein expression levels were evaluated with one way analysis of variance (ANOVA) followed by Newman-Kuels post hoc tests for individual group differences. Each area of the brain was analyzed independently, and significance was determined at p<0.05. All dose response, pressure response, and myogenic tone data was analyzed using a repeated measured ANOVA with a Bonferroni correction.
3. Results
3.1. Chronic estrogen treatment, but not soy, enhances middle cerebral artery dilation
The HS diet did not mimic the effect of E2 in inhibiting weight gain and stimulating uterine growth (Table 1), and there were no differences among groups in blood glucose, hematocrit, pH, Na+, or K+ (Table 2). Using the pressure myograph we found no differences in the passive structure of the MCAs from the treatment groups over the range of intralumenal pressures (Figure 1A and 1B). The ability of the vessel to generate myogenic tone was unchanged over the entire range of intralumenal pressures studied and not altered by diet (at 100mmHg in percent tone, control: 28±6%, soy treated: 32±5%, E2 treated: 29±3%).
Table 1.
Body and uterus weight in soy free, estradiol treated, and soy fed rats (n=6/group, Mean±SE). Asterisks represent significant difference (P<0.05) from soy free group.
| Body Weight pre OVX (g) | Body Weight at sacrifice (g) | Uterus wet weight (g) | |
|---|---|---|---|
| Soy Free | 238±7 | 295±8 | 0.19±0.01 |
| Estradiol | 239±9 | 230±6* | 0.66±.06* |
| High Soy | 230±5 | 282±8 | 0.19±.02 |
Table 2.
Blood values in soy free, estradiol treated, and soy fed rats (n=6/group, Mean±SE).
| Glucose (mg/dL) | pH | Hct | Na mmol/L | K mmol/L | NOx μM | |
|---|---|---|---|---|---|---|
| Soy Free | 387±24 | 7.3±0.0 | 38±2 | 133±1 | 4.2±0.2 | 9.2±1.5 |
| Estradiol | 386±128 | 7.3±0.1 | 37±0 | 136±2 | 3.9±0.2 | 8.5±1.3 |
| High Soy | 401±18 | 7.3±0.1 | 36±2.8 | 135±2 | 4.3±0.3 | 10.4±1.5 |
Figure 1.
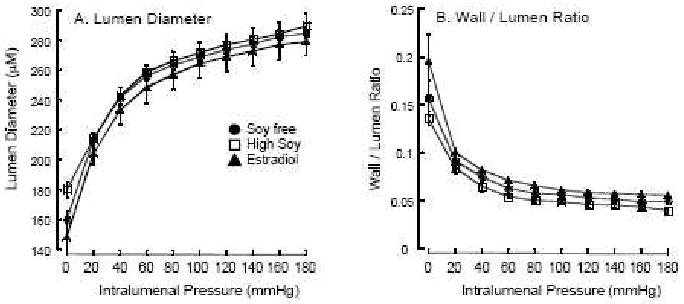
Passive MCA structure. Lumen diameter (A) and wall / lumen ratio (B) were measured by pressure myography over a range of pressures. The closed circles are soy-free controls (SF, n=4), closed triangles are E2-treated (E2, n=4), and the open squares are soy fed (HS, n=4).
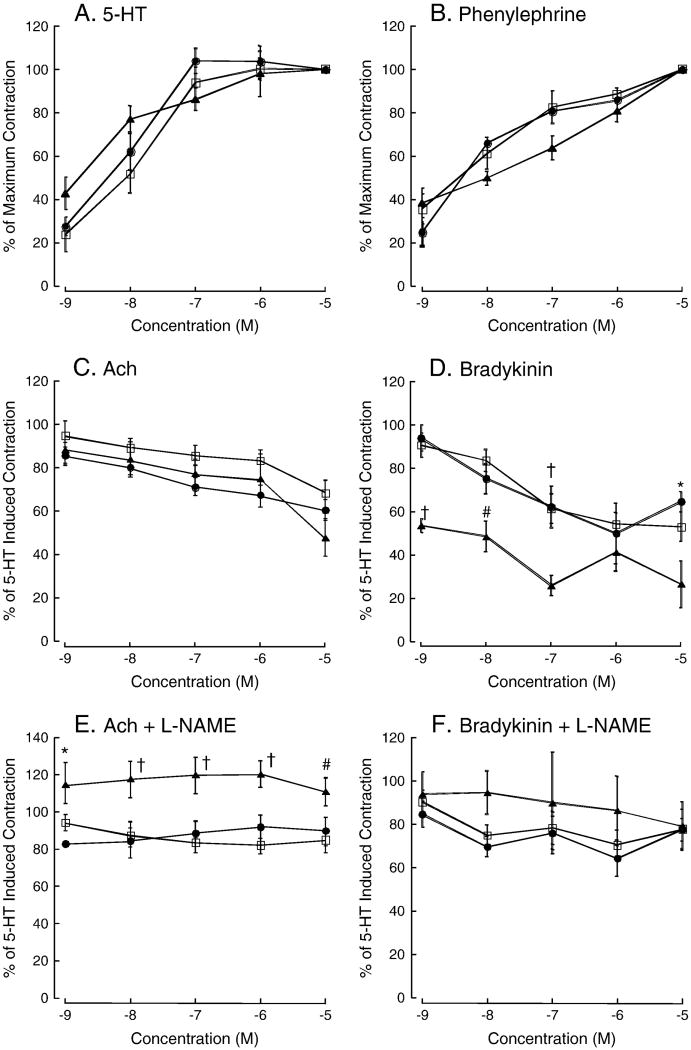
Vascular function was then assessed using wire myography. We found that neither E2 nor HS significantly altered constrictor responses or the maximum contraction to 5-HT or PE relative to control (Figure 2A and 2B). Similarly, we observed no significant effect of E2 or HS on Ach-induced relaxation (Figure 2C). Additional experiments assessed the NOS contribution to Ach-induced dilation using pre-incubation with the non-selective inhibitor L-NAME. L-NAME abolished Ach-induced dilation in all groups, however, only in the E2-treated rats did L-NAME pre-incubation result in a significant increase in vascular tone (2E) Vasodilatory responses to bradykinin (BK) were enhanced in MCA from E2-treated animals (Figure 2D), and L-NAME blocked this effect (Figure 2F). HS treatment did not alter the vascular response to any of the constrictors or dilators. The addition of L-NAME to the myograph chamber caused a small amount of constriction in all the vessels consistent with blocking basal NOS-mediated tone. There was a trend toward enhanced constriction in the HS and E2 treated rats but this did not reach statistical significance (change in force from baseline, SF: 0.18±0.03 mN, E2: 0.28±0.07 mN, HS: 0.22±0.04, ANOVA p = 0.08). Enhanced constriction in response to L-NAME is indicative of greater basal NOS activity, however, no significant changes were observed in plasma NOx levels (Table 2).
Figure 2.
Vascular reactivity. Dose response curves for 5-HT (A) and PE (B) in the MCA, the results are expressed as a percentage of the maximum vasoconstriction. Dose response curves for Ach (C) and BK (D), results are expressed as a percentage of the 5-HT induced preconstriction. Dose response curves to acetylcholine (E) and bradykinin (F) in the presences of L-NAME. The closed circles are soy-free controls (SF, n=12), closed triangles are E2-treated (E2, n=8), and the open squares are soy fed (HS, n=14). † indicates significantly different from both HS and soy free control, * indicates significantly different from soy free control, # indicates significantly different from HS. Results are presented as mean ± SE, and significance was set at P<0.05.
3.2. Effects of soy on eNOS, caveolin-1, and estrogen receptor expression in brain and cerebral vessels
Four weeks of HS diet significantly increased eNOS mRNA expression in the cortex and hippocampus (Figure 3A). E2 also significantly increased eNOS mRNA expression in the hippocampus (Figure 3A). In isolated cerebral microvascular cells (MVC) and major cerebral blood vessels (CV) HS increased eNOS mRNA expression, as did E2 in MVC (Figure 3A). In agreement with previous results in male rat basilar artery (Sobey et al 2004), no significant changes in eNOS protein levels were observed in the MCA by Western blotting (Figure 3B). Similarly, eNOS phosphorylation on the estrogen sensitive site, serine 1177, in the MCA was unchanged by the diets (Figure 3C). Caveolin-1, an endogenous inhibitor of NOS activity was decreased in E2-treated, but not HS rats (Figure 3D).
Figure 3.
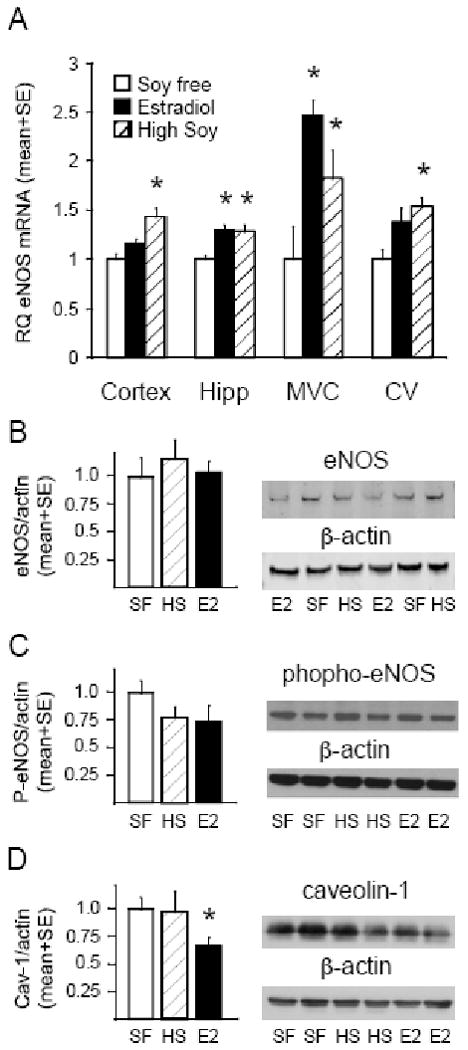
Expression of eNOS and caveolin-1 in brain and cerebral vessels. Relative eNOS mRNA levels in cerebral cortex (Cortex), hippocampus (Hipp), brain microvascular cells (MVC), and large cerebral vessels (CV) in soy free control (SF, open bars), E2 treated (filled bars), and HS (hatched bars) (panel A). Values represent relative mRNA quantity (RQ) corrected for GAPDH and normalized to the soy free control (mean ± SE; n=6). Normalized mean±SE and representative Western blot for eNOS (B), phospho serine 1177 e-NOS (C), and caveolin-1 (D) normalized to β-actin in large cerebral blood vessels from soy free control (SF, open bars), soy fed (HS, hatched bars), and E2 treated (E2, filled bars) rats (n=6). * indicates significant difference from soy free control, P<0.05.
Effects of HS and E2 on the expression of ERα and ERβ in the vasculature may play a role in the vasodilatory responses we observed. HS, but not E2, selectively increased ERα mRNA expression in the cerebral cortex. HS and E2 both increased ERα expression in the hippocampus and brain microvascular cells (Figure 4). However, neither HS nor E2 significantly altered ERα expression in major cerebral vessels (Figure 4). E2 decreased ERβ expression in the major cerebral vessels, but there were no other significant effects in any other region examined (Figure 4).
Figure 4.
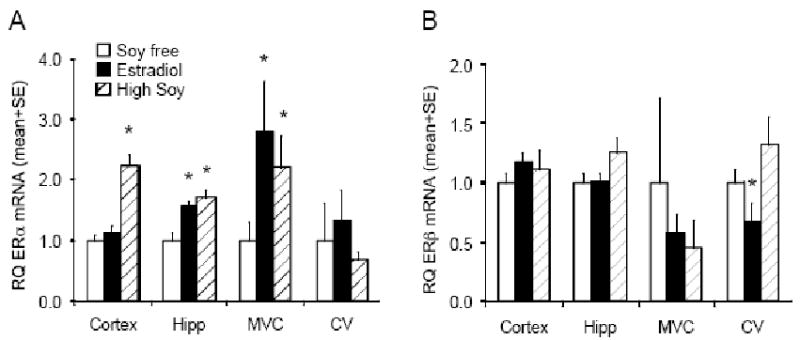
Expression of ER mRNA and protein in brain and cerebral vessels. Relative ERα (A) and ERβ (B) mRNA levels in cerebral cortex (Cortex), hippocampus (Hipp), brain microvascular cells (MVC), and large cerebral vessels (CV) in soy free control (SF, open bars), estrogen treated (E2, filled bars), and soy fed (HS, hatched bars). Values represent relative mRNA quantity (RQ) corrected for GAPDH and normalized to the soy free control (mean ± SE; n=6). * indicates significant difference from soy free control, P<0.05.
4. Discussion
Soy isoflavones, have been proposed to act as vasoactive alternatives to E2 in the peripheral vasculature. We have previously shown that consumption of a HS diet reduces the damage caused by cerebral ischemia in female rats (Schreihofer et al 2005; Lovekamp-Swan et al 2007). In the current study we assessed the effects of dietary soy supplementation on cerebral vasculature reactivity and eNOS. Contrary to our hypothesis we did not observe enhanced dilation in the MCA of ovariectomized female rats fed a HS diet for 4 weeks. However, in agreement with previous studies (Duckles and Krause 2007), chronic E2 enhanced NO dependent dilation in response to BK. This was associated with reduced expression of caveolin-1, an allosteric inhibitor of eNOS. Others have suggested that isoflavone treatment enhances the vasodilatory effects of Ach in a NO dependent manner in rat peripheral vessels without altering eNOS levels (Sobey et al 2004; Woodman et al 2004; Baluchnejadmojarad and Roghani 2008).
It bears noting that many previous studies with chronic treatment of rats with isoflavones, control rats were not fed soy free diets. Many commercial chows use soy as a protein source, and thus, the isoflavone treatments were likely given to animals with already high circulating isoflavones (Thigpen et al 1999). The absence of detailed information regarding the soy content of the diets makes the interpretation of many of these studies difficult. In the present study we utilized a soy free diet for the control rats and the E2-treated rats allowing us to remove any underling effects of soy provided by the regular rat chow. This key difference in experimental design may be the underling cause of the differences in the results obtained in previous reports and those obtained here.
The role played by NO in the cerebral vasculature is of particular importance as NO controls basal tone and blood flow and is produced not only by the endothelium but also by neurons and glia (Faraci 1993). The ability of E2 to enhance BK-mediated relaxation in the MCA in the present study agrees well with previously published results. Rats (Geary et al 1998; Geary et al 2000; Sobey et al 2004; Woodman et al 2004) and mice (Geary et al 2000) show enhanced NO-dependent relaxations in the MCA and pial vessels (Pelligrino et al 2000) when treated chronically with E2. E2 also reverses ovariectomy-induced enhancement of cerebrovascular tone (Tsang et al 2004), and HT in postmenopausal women induces vasodilation in the MCA (Bain et al 2004). Importantly, as we observed here, these NO-dependent effects are not associated with increased eNOS expression (Sobey et al 2004; Woodman et al 2004).
The BK-mediated dilation of the MCA has previously been reported to be completely NO dependent (Faraci and Brian 1994; Zimmermann et al 1997; Lagaud et al 1999). However, in the current study, in the presence of L-NAME a small amount of dilation was still present in response to BK. This presents the possibility that under the conditions used in the present study BK also stimulates other vasodilators such as endothelium derived hyperpolarizing factor. Surprisingly, the results observed with BK were not replicated with Ach, another endothelium dependent dilator. The reason for this is unclear, but are potentially related to differences in cellular signaling through the muscarinic versus BK receptors. The addition of L-NAME to the bath caused a marked increase in vascular tone in vessels from the E2 treated rats, suggesting that NOS activity was increased in these vessels. The absence of an effect of E2 on the response to Ach in the MCA relative to control rats may relate to the relatively poor dilation obtained in response to Ach in most cerebral vessels. Several other studies have failed to show significant dilation of the MCA with Ach (Lagaud et al 1999).
The trend towards greater contraction in response to L-NAME alone is of interest. This reflects basal NO production by the vessel, and a larger contraction indicates greater basal production of NO with E2. The absence of an effect of HS or E2 on myogenic tone, however, suggests that other vasoregulatory mechanisms may balance any small effects of the diets on unstimulated NO mediated dilation. The absence of an effect of either diet on vascular structure suggests that the results observed for the vasodilators are not due to alterations in the smooth muscle mass in the vessel. Other studies have suggested that E2 alters vessel structure in the periphery. It should be noted however that most of the studies documenting an effect E2 on vessel structure require some type of vessel injury (Liu et al 2002; Yuan et al 2002; Francisco et al 2005), hypertension (Garcia et al 2005; Garcia et al 2006) or aging (Zhang et al 2000).
In this study we investigated the effects of HS and E2 on the expression of eNOS and caveolin-1. It is possible that the treatments used affected the expression and activity of the other NOS isoforms. For example, some studies suggest that neuronal NOS is expressed in the cerebral vasculature of Wistar Kyoto rats (Briones et al 2002). Although rapid and long-term induction of eNOS phosphorylation in cerebral vessels has been observed with E2 treatment, (Stirone et al 2005), we did not observe such an increase in the present study on the estrogen-sensitive phosphorylation site serine 1177. This may result from the fact that we used the MCA and large pial vessels for immunoblot analysis rather than the whole brain vasculature. Indeed, both E2 and HS had a larger effect on eNOS mRNA in microvascular cells than on the large cerebral vessels.
The effects of E2 on the cerebrovasculature have been attributed primarily to ERα activation (Stirone et al 2003), while isoflavones are considered to be primarily ERβ ligands (Turner et al 2007). However, we and others have shown that ERα is activated by soy isoflavones at concentrations similar to those achieved in the current study (Lovekamp-Swan et al 2007; Kuiper et al 1998; An et al 2001; Schreihofer 2005). We chose a soy dose in the present study that is consistent with dietary or supplement intake in human populations (Setchell et al 2001; Whitten and Patisaul 2001; Nagata et al 2002). Previous studies with these dietary levels of soy have demonstrated beneficial effects on gene expression in the brain (Lephart et al 2000; Bu and Lephart 2005; Lovekamp-Swan et al 2007), vasculature (Mahn et al 2005), and kidney (Trujillo et al 2005). The HS diet increased ERα expression in the brain parenchyma and microvascular cells, but the same effect was not observed in cerebral resistance vessels. This result might underlie the lack of effect on vascular reactivity.
In conclusion, the present study does not support a vasodilatory role for HS diet in the cerebrovasculature under basal conditions. Four weeks of HS diet in ovariectomized female rats did not mimic the effects of physiological E2 levels on vascular reactivity in MCA and did not result in any significant changes in basal caveolin-1, eNOS levels, or eNOS phosphorylation. It remains to be determined whether soy can mimic E2 under pathophysiological conditions such as stroke, where soy diets confer a beneficial effect (Schreihofer et al 2005; Burguete et al 2006; Lovekamp-Swan et al 2007).
Acknowledgments
Portions of this project were presented at the 90th Annual Meeting of The Endocrine Society. The authors would like to acknowledge the technical assistance of Dr. Kelly Hyndman in performing the NOx measurements.
This work was supported by NIH R01AT001882 (DAS), NIH RO1 HL0077385 (AMD), and AHA0625626B (TLS), AHA0840122N (AMD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52(2):119–32. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- An J, Tzagarakis-Foster C, Scharschmidt TC, Lomri N, Leitman DC. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276(21):17808–14. doi: 10.1074/jbc.M100953200. [DOI] [PubMed] [Google Scholar]
- Bain CA, Walters MR, Lees KR, Lumsden MA. The effect of HRT on cerebral haemodynamics and cerebral vasomotor reactivity in post-menopausal women. Hum Reprod. 2004;19(10):2411–4. doi: 10.1093/humrep/deh396. [DOI] [PubMed] [Google Scholar]
- Baluchnejadmojarad T, Roghani M. Chronic administration of genistein improves aortic reactivity of streptozotocin-diabetic rats: Mode of action. Vascul Pharmacol. 2008;49(1):132–5. doi: 10.1016/j.vph.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Brass LM. Hormone replacement therapy and stroke: clinical trials review. Stroke. 2004;35(11 Suppl 1):2644–7. doi: 10.1161/01.STR.0000143218.20061.ac. [DOI] [PubMed] [Google Scholar]
- Briones AM, Alonso MJ, Hernanz R, Miguel M, Salaices M. Alterations of the nitric oxide pathway in cerebral arteries from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;39(3):378–88. doi: 10.1097/00005344-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Bu L, Lephart ED. Soy isoflavones modulate the expression of BAD and neuron-specific beta III tubulin in male rat brain. Neurosci Lett. 2005;385(2):153–7. doi: 10.1016/j.neulet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Burguete MC, Torregrosa G, Perez-Asensio FJ, Castello-Ruiz M, Salom JB, Gil JV, Alborch E. Dietary phytoestrogens improve stroke outcome after transient focal cerebral ischemia in rats. Eur J Neurosci. 2006;23(3):703–710. doi: 10.1111/j.1460-9568.2006.04599.x. [DOI] [PubMed] [Google Scholar]
- Catania MA, Crupi A, Firenzuoli F, Parisi A, Sturiale A, Squadrito F, Caputi AP, Calapai G. Oral administration of a soy extract improves endothelial dysfunction in ovariectomized rats. Planta Med. 2002;68(12):1142–4. doi: 10.1055/s-2002-36349. [DOI] [PubMed] [Google Scholar]
- Colacurci N, Chiantera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiantera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause. 2005;12(3):299–307. doi: 10.1097/01.gme.0000147017.23173.5b. [DOI] [PubMed] [Google Scholar]
- Cuevas AM, Irribarra VL, Castillo OA, Yanez MD, Germain AM. Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur J Clin Nutr. 2003;57(8):889–94. doi: 10.1038/sj.ejcn.1601622. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34(8):801–8. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- Evans M, Njike VY, Hoxley M, Pearson M, Katz DL. Effect of soy isoflavone protein and soy lecithin on endothelial function in healthy postmenopausal women. Menopause. 2007;14(1):141–9. doi: 10.1097/01.gme.0000227404.83686.1b. [DOI] [PubMed] [Google Scholar]
- Faraci FM. Endothelium-derived vasoactive factors and regulation of the cerebral circulation. Neurosurgery. 1993;33(4):648–58. doi: 10.1227/00006123-199310000-00014. discussion 658-9. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Brian JE., Jr Nitric oxide and the cerebral circulation. Stroke. 1994;25(3):692–703. doi: 10.1161/01.str.25.3.692. [DOI] [PubMed] [Google Scholar]
- File SE, Hartley DE, Alom N, Rattray M. Soya phytoestrogens change cortical and hippocampal expression of BDNF mRNA in male rats. Neurosci Lett. 2003;338(2):135–8. doi: 10.1016/s0304-3940(02)01391-5. [DOI] [PubMed] [Google Scholar]
- Foster JM, Carmines PK, Pollock JS. PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol. 2009;297(2):F471–80. doi: 10.1152/ajprenal.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco YA, Dantas AP, Carvalho MH, Laurindo FR. Estrogen enhances vasoconstrictive remodeling after injury in male rabbits. Braz J Med Biol Res. 2005;38(9):1325–9. doi: 10.1590/s0100-879x2005000900006. [DOI] [PubMed] [Google Scholar]
- Garcia MP, Gimenez J, Serna M, Salom MG, Bonacasa B, Carbonell LF, Quesada T, Hernandez I. Effect of estrogen and angiotensin-converting enzyme inhibitor on vascular remodeling in ovariectomized spontaneously hypertensive rats. Menopause. 2006;13(3):397–403. doi: 10.1097/01.gme.0000222472.08593.e4. [DOI] [PubMed] [Google Scholar]
- Garcia PM, Gimenez J, Bonacasa B, Carbonell LF, Miguel SG, Quesada T, Hernandez I. 17beta-estradiol exerts a beneficial effect on coronary vascular remodeling in the early stages of hypertension in spontaneously hypertensive rats. Menopause. 2005;12(4):453–9. doi: 10.1097/01.GME.0000151654.10243.01. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275(1 Pt 2):H292–300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000;279(2):H511–9. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26(9):1103–13. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- Jackman KA, Woodman OL, Chrissobolis S, Sobey CG. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007;1141:99–107. doi: 10.1016/j.brainres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Katz DL, Evans MA, Njike VY, Hoxley ML, Nawaz H, Comerford BP, Sarrel PM. Raloxifene, soy phytoestrogens and endothelial function in postmenopausal women. Climacteric. 2007;10(6):500–7. doi: 10.1080/13697130701750123. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Kaufman DW, Rosenberg L, Kelley K, Cooper SG, Mitchell AA. Use of postmenopausal hormone therapy since the Women's Health Initiative findings. Pharmacoepidemiol Drug Saf. 2005;14(12):837–42. doi: 10.1002/pds.1103. [DOI] [PubMed] [Google Scholar]
- Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr. 2005;81(1):189–95. doi: 10.1093/ajcn/81.1.189. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, vanderSaag PT, vanderBurg B, Gustafsson JÅ. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor ß. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kurzer MS. Phytoestrogen supplement use by women. J Nutr. 2003;133(6):1983S–1986S. doi: 10.1093/jn/133.6.1983S. [DOI] [PubMed] [Google Scholar]
- Lagaud GJ, Skarsgard PL, Laher I, van Breemen C. Heterogeneity of endothelium-dependent vasodilation in pressurized cerebral and small mesenteric resistance arteries of the rat. J Pharmacol Exp Ther. 1999;290(2):832–9. [PubMed] [Google Scholar]
- Lephart ED, Porter JP, Hedges DW, Lund TD, Setchell KD. Phytoestrogens: implications in neurovascular research. Curr Neurovasc Res. 2004;1(5):455–64. doi: 10.2174/1567202043361974. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Thompson JM, Setchell KD, Adlercreutz H, Weber KS. Phytoestrogens decrease brain calcium-binding proteins but do not alter hypothalamic androgen metabolizing enzymes in adult male rats. Brain Res. 2000;859(1):123–31. doi: 10.1016/s0006-8993(00)01968-5. [DOI] [PubMed] [Google Scholar]
- Lissin LW, Oka R, Lakshmi S, Cooke JP. Isoflavones improve vascular reactivity in post-menopausal women with hypercholesterolemia. Vasc Med. 2004;9(1):26–30. doi: 10.1191/1358863x04vm531oa. [DOI] [PubMed] [Google Scholar]
- Liu WL, Guo X, Guo ZG. Estrogen prevents bovine aortic endothelial cells from TNF-alpha-induced apoptosis via opposing effects on p38 and p44/42 CCDPK. Acta Pharmacol Sin. 2002;23(3):213–8. [PubMed] [Google Scholar]
- Lovekamp-Swan T, Glendenning M, Schreihofer DA. A high soy diet reduces programmed cell death and enhances bcl-x(L) expression in experimental stroke. Neuroscience. 2007;148(3):644–52. doi: 10.1016/j.neuroscience.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Glendenning ML, Schreihofer DA. A high soy diet enhances neurotropin receptor and Bcl-XL gene expression in the brains of ovariectomized female rats. Brain Res. 2007;1159:54–66. doi: 10.1016/j.brainres.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Rhees RW, Setchell KD, Lephart ED. Altered sexually dimorphic nucleus of the preoptic area (SDN-POA) volume in adult Long-Evans rats by dietary soy phytoestrogens. Brain Res. 2001;914(1-2):92–9. doi: 10.1016/s0006-8993(01)02779-2. [DOI] [PubMed] [Google Scholar]
- Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Vina J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. Faseb J. 2005;19(12):1755–7. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- Matthan NR, Jalbert SM, Ausman LM, Kuvin JT, Karas RH, Lichtenstein AH. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85(4):960–6. doi: 10.1093/ajcn/85.4.960. [DOI] [PubMed] [Google Scholar]
- Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Soy product intake and serum isoflavonoid and estradiol concentrations in relation to bone mineral density in postmenopausal Japanese women. Osteoporos Int. 2002;13(3):200–4. doi: 10.1007/s001980200014. [DOI] [PubMed] [Google Scholar]
- Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet Gynecol. 2002;100(1):18–25. doi: 10.1016/s0029-7844(02)02005-7. [DOI] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Clarkson TB. Evidence for up-regulation of brain-derived neurotrophic factor mRNA by soy phytoestrogens in the frontal cortex of retired breeder female rats. Neurosci Lett. 1999;261(1-2):17–20. doi: 10.1016/s0304-3940(98)00994-x. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Ye S, Tan F, Santizo RA, Feinstein DL, Wang Q. Nitric-oxide-dependent pial arteriolar dilation in the female rat: effects of chronic estrogen depletion and repletion. Biochem Biophys Res Commun. 2000;269(1):165–71. doi: 10.1006/bbrc.2000.2206. [DOI] [PubMed] [Google Scholar]
- Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73(3):198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer DA. Transcriptional regulation by phytoestrogens in neuronal cell lines. Mol Cell Endocrinol. 2005;231(1-2):13–22. doi: 10.1016/j.mce.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Do KD, Schreihofer AM. High-soy diet decreases infarct size after permanent middle cerebral artery occlusion in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R103–8. doi: 10.1152/ajpregu.00642.2004. [DOI] [PubMed] [Google Scholar]
- Setchell KD. Soy isoflavones--benefits and risks from nature's selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001;20(5 Suppl):354S–362S. doi: 10.1080/07315724.2001.10719168. discussion 381S-383S. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4 Suppl):1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Weiler JM, Boujaoude M, Woodman OL. Effect of short-term phytoestrogen treatment in male rats on nitric oxide-mediated responses of carotid and cerebral arteries: comparison with 17beta-estradiol. J Pharmacol Exp Ther. 2004;310(1):135–40. doi: 10.1124/jpet.103.063255. [DOI] [PubMed] [Google Scholar]
- Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am J Clin Nutr. 2003;78(1):123–30. doi: 10.1093/ajcn/78.1.123. [DOI] [PubMed] [Google Scholar]
- Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol. 2005;67(1):105–13. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284(1):E184–92. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Tang YB, Wang QL, Zhu BY, Huang HL, Liao DF. Phytoestrogen genistein supplementation increases eNOS and decreases caveolin-1 expression in ovariectomized rat hearts. Sheng Li Xue Bao. 2005;57(3):373–8. [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci. 1999;49(5):530–536. [PubMed] [Google Scholar]
- Trujillo J, Ramirez V, Perez J, Torre-Villalvazo I, Torres N, Tovar AR, Munoz RM, Uribe N, Gamba G, Bobadilla NA. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am J Physiol Renal Physiol. 2005;288(1):F108–16. doi: 10.1152/ajprenal.00077.2004. [DOI] [PubMed] [Google Scholar]
- Tsang SY, Yao X, Chan FL, Wong CM, Chen ZY, Laher I, Huang Y. Estrogen and tamoxifen modulate cerebrovascular tone in ovariectomized female rats. Hypertension. 2004;44(1):78–82. doi: 10.1161/01.HYP.0000131659.27081.19. [DOI] [PubMed] [Google Scholar]
- Turner JV, Agatonovic-Kustrin S, Glass BD. Molecular aspects of phytoestrogen selective binding at estrogen receptors. J Pharm Sci. 2007;96(8):1879–85. doi: 10.1002/jps.20987. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Patisaul HB. Cross-species and interassay comparisons of phytoestrogen action. Environ Health Perspect. 2001;109 1:5–20. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta-estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol. 2004;44(2):155–63. doi: 10.1097/00005344-200408000-00003. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Liao L, Tulis DA, Xu J. Steroid receptor coactivator-3 is required for inhibition of neointima formation by estrogen. Circulation. 2002;105(22):2653–9. doi: 10.1161/01.cir.0000018947.95555.65. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stewart KG, Davidge ST. Estrogen replacement reduces age-associated remodeling in rat mesenteric arteries. Hypertension. 2000;36(6):970–4. doi: 10.1161/01.hyp.36.6.970. [DOI] [PubMed] [Google Scholar]
- Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81(6):996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]