Abstract
Sleep is hypothesized to play a functional role in the consolidation of memory, with more robust findings for implicit, than explicit memory. Previous studies have observed improvements on an explicit motor task after a sleep period. We examined the role of massed practice and sleep on implicit and explicit learning within a motor task. Controlling for non-sleep factors (e.g. massed practice, circadian confounds) eliminated both explicit and implicit learning effects that have been attributed to sleep.
Keywords: SLEEP, NAPPING, FATIGUE, CONSOLIDATION, MOTOR MEMORY, PURSUIT MOTOR LEARNING
Performance improvements following an inter-session sleep episode have been interpreted as sleep having a necessary role in consolidation [1], although some argue against the sleep-memory consolidation hypothesis [2]. Evidence of sleep benefits for explicit [3] and implicit [4] memory have been found, though enhancements in implicit memory are typically larger and more robust [5]. One explicit memory task frequently used to study the benefits of sleep is sequential motor learning. In this motor task, participants repeatedly enter a specific number sequence (e.g. 4-1-3-2-4) during training and then are tested 12 or 24 hrs later for speed and accuracy. Participants allowed to sleep between training and testing have shown improved performance, compared to awake controls [6].
Recently, several studies have shed light on uncontrolled factors present in many sequential motor learning study designs. Factors, such as averaging artifacts, time-of-day confounds, and fatigue [7], may account for the observed benefits independent of sleep-related processes. Importantly, studies that controlled for these factors reported the elimination of sleep effects [7–9]. Furthermore, morning performance has been shown to be significantly better than evening performance on simple motor tasks [7], indicating that time-of-day confounds inherent in nocturnal sleep studies may produce an illusory sleep effect. Consistent with this observation, training and testing at the same time of day eliminates between session benefits [7, 8]. Lastly, massing effects, due to long training blocks, create a false measurement of learning when fatigue has dissipated by the subsequent session [9]. Accordingly, inserting rest breaks while maintaining the same global time-on-task eliminates observed sleep enhancement [9].
These results contrast with sleep improvements on implicit memory tasks that have been observed even while controlling for these factors. For example, studies implementing a napping paradigm, which controls for circadian factors by testing nappers and non-nappers at the same time of day, show sleep-specific improvements on implicit memory tasks [4, 10–12]. Thus, while an emerging literature suggests that careful controls must be used to study and interpret the role of sleep on explicit motor memory, there is strong evidence supporting the benefit of sleep in consolidating implicit visual memories.
The present study compares implicit and explicit motor learning in a pursuit motor task. Two other studies have shown sleep-specific improvements on this task using a sleep deprivation paradigm that controls for time-of-day confounds by having all participants test simultaneously; one group sleeps overnight while the control group remains awake [13, 14]. In the pursuit motor task, the participant controls a cursor and follows a target moving along a specified path. Performance is measured by how closely the participant is able to follow the target, either in terms of distance to the target or the proportion of time spent within a specified range of the target. Unlike motor sequence learning, pursuit motor learning necessitates the integration of visual information with motor planning. In traditional pursuit motor learning, the target’s path is explicitly circular. However, Maquet et al. [13] have used a non-repeating target trajectory, where the path was determined by a combination of several sine waves of different fixed frequencies. They found that learning in this more implicit, procedural version of the task was better in people who had a full night’s sleep compared with those who spent the night awake. Unlike learning on an explicit motor sequence task, implicit learning on a pursuit motor task may depend on sleep for performance enhancement. We note, however, that fatigue effects due to massed practice have not been investigated in this motor task, and therefore, could confound a true sleep effect.
The current study attempts to reconcile the findings of negative fatigue results [9] with positive sleep results [13] by investigating explicit and implicit motor learning in a single task. To do this, we manipulated three factors. First, we varied target patterns between an explicit, repeating pattern and an implicit, non-repeating pattern. Second, to examine fatigue, we tested a massed condition (based on Maquet et al. [13]) and a spaced condition (based on Rickard et al. [9]). Lastly, we compared napping to a quiet rest control group [11, 15] to eliminate circadian confounds and the possible negative effects of sleep deprivation, waking interference, on-task fatigue, or uncontrolled forms of inhibition in the non-sleep group.
We hypothesized that the explicit, repeating target pattern would show a between session benefit driven by fatigue in the first session rather than sleep. Second, we hypothesized that spacing practice in the explicit pattern condition would eliminate both massed practice fatigue and the between session performance increases previously ascribed to sleep-dependent processes. Third, we hypothesized that improvements in the implicit, non-repeating target pattern would be sleep-dependent, not observed in the quiet rest group, and independent of both massed and spaced learning.
Results were obtained from a cohort of 81 participants. Forty-seven participants (28 and 19 in the napping and quiet rest conditions, respectively) received massed block training. Thirty-four participants received spaced training (19 and 15 in the napping and quiet rest conditions, respectively). Participants were 18–35 years of age with no personal history of neurological, psychological, or other chronic illness. Participants provided informed consent to participate in the experiment, which was approved by the Institutional Review Board of the University of California at San Diego. Ensuring that participants were well rested and had typical sleep histories, participants completed sleep diaries and were monitored by actigraphy for 5–7 days before testing to assess sleep–wake activity. Participants were required to sleep an average of 6hrs per night for the 5 days leading up to the experiment and at least 6.5hrs the night before the experimental day. They were also restricted from consuming caffeine and alcohol 24hrs before and during the experimental day. While this is a light requirement on sufficient sleep and the caffeine restrictions may have resulted in withdrawal for some participants, any effects due to these restrictions would increase apparent sleep benefits. Typical caffeine consumption for these participants was reported to be between 1 and 2 caffeinated drinks per day.
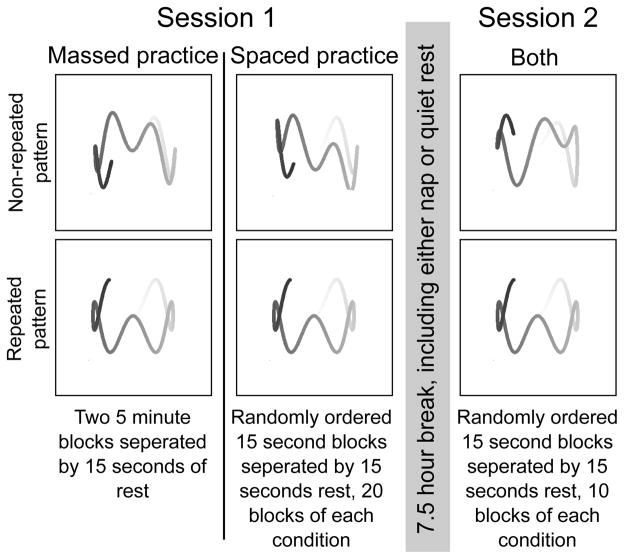
All participants performed two sessions of the pursuit motor task, once in the morning and then again in the afternoon (Fig. 1). At 09:00, participants completed a training session of the pursuit motor task that consisted of following the motion of a circular red target on a computer screen with a cursor controlled by a mouse operated by the participant’s non-dominant hand. Participants returned at 13:00, at which time they were randomly assigned to a nap or a quiet rest group. Those assigned to the nap received a polysomnographically recorded (PSG) nap of up to 90 min of sleep or 2hrs in bed. The mean sleep time of all participants was 59.9 min. Data were excluded from further analysis if their total sleep time was less than 20 min. Those in the quiet rest condition listened to instrumental music with PSG monitoring for 90 min. Participants in the quiet rest condition were monitored for sleep and alerted if the first signs of stage one sleep were observed. At 16:30, participants returned for session two of the pursuit motor task.
Figure 1.
The experimental paradigm with examples of the movement patterns used in the experiment. The “tracers” or “tails” in the figure are for illustrative purposes only; during the experiment the screen was blank, with the exception of a single dot target and the cursor.
The massed training condition consisted of two 5-min blocks of the task. In one block, the target followed a non-repeating trajectory. In the other, the target trajectory followed a repeating pattern. In the second session, participants performed the rotary pursuit task in 15s blocks. The order of blocks for both sessions was counterbalanced between participants and each block was separated by 15s of rest. These block durations replicate those used by Maquet et al. [13], where sleep effects were found in a similar non repeating pursuit motor task. The spaced training condition was identical to the massed training condition with the exception that in the first session, twenty 15s blocks of non-repeated and pattern trajectories were interleaved with 15s of rest. Rickard et al. [9] found that spacing practice in 15s blocks as opposed to 30s blocks eliminated both fatigue in the first session and between session improvements.
During the task, the target’s path was determined independently for horizontal and vertical directions using sine functions of different periods. The repeated and non-repeated pattern conditions were created by using paths which either repeated both within and between blocks or did not repeat during the entire experiment, and were therefore unpredictable. In both conditions, horizontal movement was determined by a single sine function with a 3/15 Hz period. Vertical movement was more complex and guided by the product of three sine functions of different periods. In non-repeated patterns, the periods were randomly chosen from a list of potential patterns for which the combined vertical and horizontal movement of the target would never repeat. In the repeated pattern condition, the global movement was determined by the product of sine functions with 3/15, 6/15 and 9/15 Hz periods. Given these parameters, the target movement pattern repeated every 5s. For all conditions, the target started in the center of the screen at the beginning of every block. Target position was indicated with a 10-pixel diameter red circle, and the position of the mouse cursor was indicated by crosshairs. The target position ranged in the center 400 × 400 pixel area of a 640 × 480 pixel resolution display.
The position of the mouse cursor was sampled at approximately the screen refresh rate (60 Hz). The mean of the Euclidian distance from the cursor to the target was computed and recorded every 200 ms. We obtained identical results analyzing our data using the distance from the target and the performance adjusted time-on-target (TOT) used by Maquet et al. [13] (where the target size is defined as half the standard deviation of each participant’s score in the training session). However, due to limitations in TOT as a dependent measure [16], we present our results using the mean of the distance to the target. Six participants were excluded from the analysis because their mean distance to the target for at least one of the two sessions was greater than 1.5 times the inter-quartile range over all participants. Two napping participants were excluded because their total sleep time was under 20min.
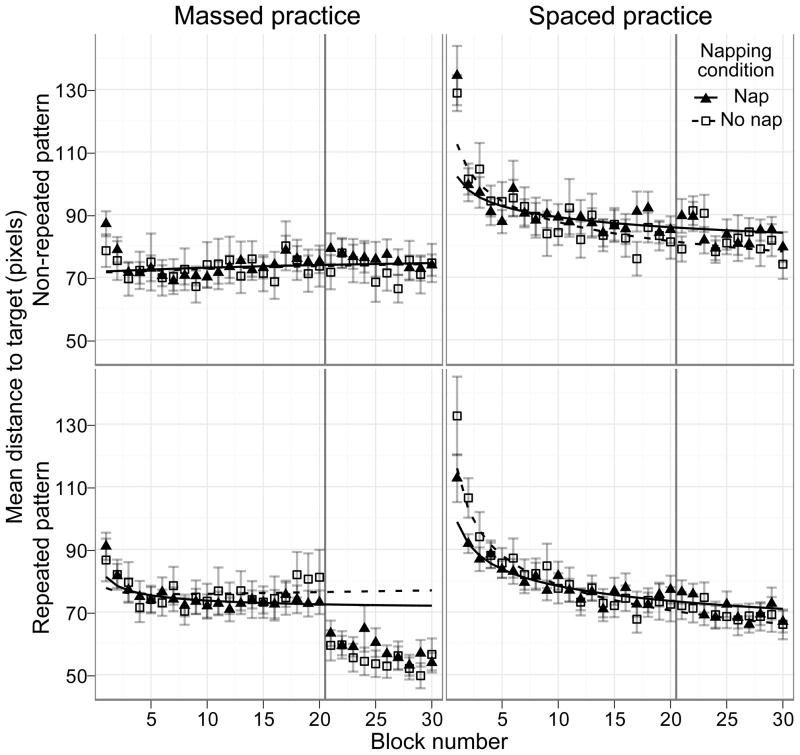
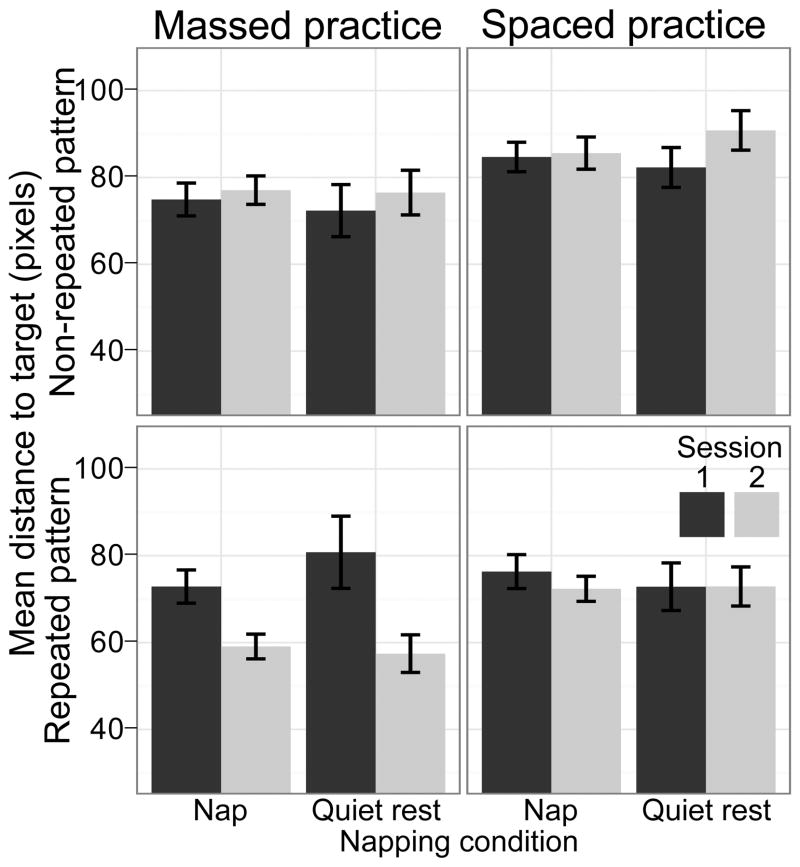
For plotting, dependent measures were averaged over blocks of trials, defined as 15-s divisions of on-task time. This equates the analyses between massed and spaced training, resulting in 20 blocks during the training session, and 10 blocks during the test session for both massed and spaced training. Fig. 2 plots distance as a function of block for each condition. As visualized in Fig. 3, the between session improvement was analyzed by averaging the data from blocks 19–20 (the last two blocks of session one) and comparing this average to the average of blocks 22–23 (the second and third blocks of session two). Block one was eliminated to compensate for warm up effects.
Figure 2.
Distance to the target over blocks for practice and sleep conditions. A smaller distance indicates better performance. The vertical line indicates the between session break. Error bars are +/−1 standard error of the means across participants. Curves are the average of individual fits to each participant’s data from blocks 2 to 20 of session one.
Figure 3.
Distance to the target averaged over blocks 19 and 20 of session one and 22 and 23 of session two for each condition. Error bars are +/−1 standard error of the means across participants.
To test our hypotheses, we used a repeated measures ANOVA to examine the factors of target pattern (repeating versus non-repeating), training type (massed versus spaced), nap group (nap versus quiet rest) and session on the average distance from the target. Fatigue effects were expected for explicit repeated patterns, which would produce a between session effect selectively for massed practice. This was confirmed by the presence of a three way interaction between target pattern, training type and session (F(1,69) = 10.641, p =.002, ηp2 = .12, see Fig. 3), as well as all main effects and two-way interactions between pairs of those three factors. The interaction was driven by a significant between session increase in performance for only the massed practice with repeated target patterns (t(43) = 6.45, p < .001), in agreement with the predictions of fatigue effects for explicit patterns. Further breaking down the three way interaction between target pattern, training type and session revealed that in the second session, performance was better for the repeated patterns, and this effect was larger after massed practice (training type and target pattern interaction during the second session, F(1,71) = 21.91, p <.001, ηp2 = .12). No significant effects or interactions were found in the first session.
In contrast with our hypothesized improvements with sleep in the implicit condition, we found no benefits after napping compared to quiet rest in any condition (all F ratios < 2.78, p values >.1). Direct comparison of between session improvement for the nap and quiet rest groups without consideration of training type and target pattern additionally showed no effect of napping (t(97) = .10, two-tailed p = .46). Individual one-tailed comparisons of the between session difference between napping and non-napping participants for each training type and target pattern, correcting for the number of comparisons (αadj = .0125), found no significant effects. Given the observed variability, the power to detect a just 5-pixel effect (the observed massing effect was 17 pixels) was greater than .97 for every comparison, even after adjusting for multiple comparisons in the calculation. The largest positive napping effect (t(26) = 1.581, one-tailed uncorrected p = .06) was for participants in the spaced and non-repeating conditions, and was in the form of less between session detriment for the nap group. This is suggestive of prior findings of napping restoring perceptual deterioration [17], however the effect is small.
In conclusion, contrary to prior studies reporting benefits from sleep using a sleep deprivation control, we found no sleep-specific improvements on pursuit motor performance compared with a quiet rest control even using an implicit motor task. For all pattern and spacing conditions, performance either did not change between sessions, or changed identically for sleeping and resting participants.
Previous studies reporting sleep improvements on the pursuit motor task utilized a sleep deprivation control group, which may have led to an illusion of a sleep effect. Sleep deprivation, even with up to a week of recovery sleep, may have long lasting negative effects on tasks learned prior to deprivation [18]. Our quiet rest condition may have controlled for some of these negative effects on performance. Similarly, Mednick and colleagues [15] found the same rates of learning between napping and quiet rest on an implicit visual memory task, compared to an active wake control. Taken together, these results suggest two important points. First, it is possible that quiet rest may mimic sleep conditions by reducing possible waking interference, which allows previously learned information to consolidate without competition for new memory formation. Indeed, offline persistence of memory-related cerebral activity has been show during sleep, and wake in both humans [19, 20] and animals [21, 22]. This neural replay has been argued to be the mechanism for memory consolidation [23, 24]. Second, studies of sleep and memory may have increased rates of illusory sleep effects due to comparing sleep to sleep deprivation or active wake control groups. This is an important consideration that should be experimentally investigated.
The most parsimonious explanation of the between session improvement in the explicit condition is that massing practice causes a build up of fatigue or inhibition, which is alleviated during rest. Similar fatigue-related decreases in performance with as short as 30s blocks have been shown in a motor sequence task [9] and a visual texture discrimination task [17, 25, 26]. Research on general learning in pursuit motor tasks report benefits after a period of rest, known as ‘reminiscence,’ that occur with or without sleep [27, 28]. With massed practice, there appears to be an improvement after 24hrs, but this putative effect was due to the combination of the build up of fatigue at the end of training and a release from fatigue at the beginning of test [27]. When the fatigue was substantially reduced with spaced training, there was no benefit after a 24hr delay. It was experimentally verified that this was due to the build up of negative inhibition opposing learning [29], as previously hypothesized [30], and not to consolidation during rest or changes in arousal. This characterization is in line with our findings. The between session difference is explained as the result of masked performance in the first session. This negative effect is relieved during rest, with or without sleep, resulting in a between session increase in performance.
Interestingly, we found no massing effect for the non-repeated patterns. One possibility is that the non-repeated pattern is more difficult to master due to the occurrence of a new pattern with each trial. This novelty might have attenuated the build-up of fatigue across training. Previously [13], the same generating frequencies were used throughout the experiment, leaving open the possibility of build up of massing effects to specific kinds of motion. It is also possible that circadian effects similar to those seen in other motor learning studies [7, 29] dampened performance in the second afternoon session, potentially masking or decreasing between session improvements in our results. In combination, these factors would explain why we observed a between session effect for the repeated and not non-repeated pattern.
The present findings are of potential interest to motor training regimes for athletes and occupations that require extended periods of repeated motor actions. We show that even brief bouts of 5min training sessions can significantly decrease immediate performance, and judgment of true learning requires a rest interval of either quiet rest or sleep to dissipate the fatigue. Most occupations and athletic training intervals extend beyond 5min, and therefore are even more vulnerable to these fatigue effects. Experimentation with a variety of training-to-rest ratios for different types of tasks would further elucidate the dynamics of this process.
Acknowledgments
This work was funded by Dr. Mednick’s National Institutes of Health Grant K01 MH080992 and UCSD Interdisciplinary Collaboratory Grant (DJC). Cory Rieth was additionally supported through NSF IGERT Grant #DGE-0333451 to GW Cottrell/VR de Sa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stickgold R, et al. Sleep, learning and dreams: Off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 2.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellenbogen JM, et al. Human relational memory requires time and sleep. Proc Natl Acad Sci U S A. 2007;104(18):7723–8. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6(7):697–8. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- 5.Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Medicine Reviews. 2001;5(6):491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 6.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 7.Keisler A, Ashe J, Willingham DT. Time of day accounts for overnight improvement in sequence learning. Learn Mem. 2007;14(10):669–72. doi: 10.1101/lm.751807. [DOI] [PubMed] [Google Scholar]
- 8.Cai DJ, Rickard TC. Reconsidering the role of sleep for motor memory. Behav Neurosci. 2009;123(6):1153–7. doi: 10.1037/a0017672. [DOI] [PubMed] [Google Scholar]
- 9.Rickard TC, Cai DJ, Rieth CA, Jones J, Ard MC. Sleep does not enhance motor sequence learning. J Exp Psychol Learn Mem Cogn. 2008;34(4):834–42. doi: 10.1037/0278-7393.34.4.834. [DOI] [PubMed] [Google Scholar]
- 10.Mednick SC, et al. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav Brain Res. 2008;193(1):79–86. doi: 10.1016/j.bbr.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009;106(25):10130–4. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gais S, et al. Visual-procedural memory consolidation during sleep blocked by glutamatergic receptor antagonists. J Neurosci. 2008;28(21):5513–8. doi: 10.1523/JNEUROSCI.5374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maquet P, Schwartz S, Passingham R, Frith C. Sleep-related consolidation of a visuomotor skill: Brain Mechanisms as assessed by functional magnetic resonsance imaging. The Journal of Neuroscience. 2003 Feb 15;23(4):1432–1440. doi: 10.1523/JNEUROSCI.23-04-01432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following Stage 2 sleep loss in college students. J Sleep Res. 1994;3(4):206–213. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Mednick SC, et al. Sleep and rest facilitate implicit memory in a visual search task. Vision Res. 2009 doi: 10.1016/j.visres.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahrick HP, Fitts PM, Briggs GE. Learning curves; facts or artifacts? Psychological Bulletin. 1957;54(3):256–268. [PubMed] [Google Scholar]
- 17.Mednick SC, Arman AC, Boynton GM. The time course and specificity of perceptual deterioration. Proc Natl Acad Sci U S A. 2005;102(10):3881–5. doi: 10.1073/pnas.0407866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna BS, Meloy MJ, Wetherell L, Stricker J, Drummond SPA. Change in neural networks following total sleep deprivation and recovery sleep. Sleep. 2010 (in press) [Google Scholar]
- 19.Peigneux P, et al. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4(4):e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, Luxen A, Maquet P. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12(7):913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 24.Stickgold Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 25.Mednick SC, et al. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5(7):677–81. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- 26.Mednick SC, et al. Sleep-dependent learning and practice-dependent deterioration in an orientation discrimination task. Behav Neurosci. 2008;122(2):267–72. doi: 10.1037/0735-7044.122.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams JA. Warm-up decrement in performance on the pursuit-rotor. Am J Psychol. 1952;65(3):404–14. [PubMed] [Google Scholar]
- 28.Ammons RB. Acquisition of motor skill: III. Effects of initially distributed practice on rotary pursuit performance. J Exp Psychol. 1950;40(6):777–87. doi: 10.1037/h0061049. [DOI] [PubMed] [Google Scholar]
- 29.Coppage SJ, Payne RB. An experimental test of current theories of psychomotor reminiscence. Perceptual and Motor Skills. 1981;52:343–352. [Google Scholar]
- 30.Hull CL. Principles of behavior. New York: Appleton-Century-Crofts; 1943. [Google Scholar]