Abstract
Thimet oligopeptidase (EC3.4.24.15, also called EP24.15 and TOP and henceforth referred to as TOP) is a neuropeptidase involved in the regulation of several physiological functions including reproduction. Among its substrates is gonadotropin-releasing hormone (GnRH), an important hypothalamic hormone that regulates the synthesis and release of oestradiol and facilitates female sexual behaviour. Using immunohistochemistry, we found that TOP is expressed in the nucleus of cells throughout the female mouse brain, and in high levels in steroid-sensitive regions of the hypothalamus, which is consistent with previous findings in male rats. Furthermore, dual-label immunofluorescence revealed that TOP and oestrogen receptor α (ERα) coexpress in several reproductively-relevant brain regions, including the medial preoptic area (mPOA), arcuate nucleus (ARC), ventrolateral portion of the ventromedial hypothalamic nucleus (VMNvl), and the midbrain central grey (MCG). Previous studies in rats have shown that oestradiol decreases hypothalamic TOP levels or activity, possibly potentiating GnRH effects. In the present study, analysis by immunohistochemistry revealed that oestradiol decreased TOP immunoreactivity in the VMNvl, while no differences were detected in the mPOA, ARC, or median eminence. Overall, the present findings indicate that TOP is coexpressed with ERα, and oestradiol regulates TOP expression in a brain region-specific manner in female mice, providing neuroanatomical evidence that TOP may function in reproductive physiology and/or behaviour.
Keywords: Thimet oligopeptidase, oestradiol, oestrogen receptor α, ventromedial hypothalamic nucleus
Introduction
The neuropeptidase thimet oligopeptidase (EC3.4.24.15, also called EP24.15 and TOP and henceforth referred to as TOP) catalyzes the degradation of many peptides involved in essential physiological processes, such as regulation of blood pressure, perception of pain, the immune response, and signaling between brain and endocrine organs (reviewed in 1). Several lines of evidence indicate that TOP also regulates reproduction. For example, studies in humans and rodents demonstrate that TOP is expressed in tissues throughout the body (2), but is most active in endocrine tissues associated with reproduction, including the hypothalamus, pituitary and gonads (e.g. 3, 4–7), suggesting that TOP may function both centrally and peripherally to regulate reproduction. Immunohistochemistry studies in male rats demonstrate that TOP is found in high concentrations in the hypothalamus and specifically in areas associated with the production and release of gonadotropin-releasing hormone (GnRH) (8, 9). GnRH, which is a substrate for TOP (1), regulates the production and release of gonadal steroid hormones such as testosterone and oestradiol (10). In addition, GnRH has been shown to facilitate sexual behavior in female rodents (11, 12).
Recent studies provide evidence that one of the breakdown products of GnRH degradation by TOP, GnRH1–5, is bioactive and functions in GnRH auto-regulation as well as female sexual behaviour in rodents (reviewed in 13). For example, GnRH promotes lordosis, the hormone-dependent sexually receptive posture the female assumes in order to facilitate intromission by the male (14–16). Oestradiol-primed ovariectomized rodents treated with GnRH display lordosis behaviour (11, 12). However, Wu et al. (17) demonstrated that central administration of GnRH1–5 also induced lordosis in oestradiol-primed ovariectomized rats. In fact, the evidence suggests that GnRH-facilitated lordosis may partially occur via its metabolism to GnRH1–5 by TOP. Central administration of a TOP antibody (the same antibody used in the current study) 60 min prior to GnRH administration in oestradiol-primed ovariectomized rats substantially reduced GnRH-facilitated lordosis, presumably due to the prevention of the metabolism of GnRH to GnRH1–5 by the antibody (17). Consequently, hydrolysis of GnRH by TOP may affect oestradiol-dependent female sexual behaviour.
Additionally, several studies have demonstrated that ovarian steroids control TOP levels and/or activity in brain (e.g. 9, 18, 19). The degree of GnRH degradation at the Tyr5-Gly6 bond in rat brain has been shown to change over the course of the oestrous cycle (20, 21). Furthermore, Pierotti et al. (18) showed that TOP enzymatic activity decreases in the medial preoptic area (mPOA) and median eminence (ME), brain regions involved in GnRH production and release respectively, as well as in the anterior pituitary in ovariectomized rats treated with oestradiol compared to controls. Taken together, these results suggest that ovarian steroid hormones regulate TOP activity in a brain-region-specific manner. In contrast to TOP activity in rats, the enzyme activity in ewe brain remains consistent throughout the estrous cycle (19, 22). It is not known if oestradiol regulates TOP levels in the female mouse brain.
The present study investigated the localization of TOP in the hypothalamus of female mice. This study focused on reproductively-relevant brain regions, including those involved with the synthesis and release of GnRH (mPOA, arcuate nucleus (ARC), and ME), as well as the ventrolateral portion of the ventromedial hypothalamic nucleus (VMNvl) and the midbrain central grey (MCG), two regions important for the control of oestradiol-dependent female reproductive behaviour (16, 23–25). While there are two subtypes of oestrogen receptor (ER) (26), oestrogen regulation of reproductive behaviour appears to be mediated mostly by ERα (27–31). Consequently, the present study used dual-label immunofluorescence to investigate the coexpression of TOP and ERα. In addition, the present study also tests the hypothesis that oestradiol regulates TOP levels in female mouse brain. In particular, Western blot analysis and immunohistochemistry were used to examine whether oestradiol regulates TOP levels in whole hypothalamic samples, and specific reproductively-relevant brain regions, respectively.
Materials and methods
Animals
Female C57BL/6J (19–22 g) were bred at Wellesley College, Wellesley, MA to 7–8 weeks and group-housed in a controlled environment of 25 °C with a reverse light cycle of (12L:12D). Food and water were provided ad libitum. Mice were anaesthetized using a combination of isoflurane and oxygen administered through a nose cone for bilateral ovariectomy. Animals were given buprenorphine (0.1 mg/kg) as an analgesic immediately following surgery. A one-week recovery period followed surgery to allow clearing of endogenous ovarian hormones. All animal procedures were approved by the Wellesley College Institutional Animal Care and Use Committee (Wellesley, MA, USA).
Western blot analysis of oestradiol regulation of TOP
Twenty-three mice were ovariectomized under isoflurane anaesthesia. The oestradiol group (n=8) was injected seven days following ovariectomy surgery with oestradiol benzoate (EB, s.c., 2 μg in vehicle of 0.1 ml sesame oil, n=16) and injected again two days later with 0.1 ml vehicle. The oestradiol plus progesterone group (n=8) was injected with 2 μg of EB in 0.1 ml vehicle seven days following ovariectomy surgery, and two days later injected with progesterone (100 μg, in 0.1 ml sesame oil containing 5% benzyl alcohol and 15% benzyl benzoate). Control animals (n=7) were injected twice with 0.1 ml of vehicle, first seven days after ovariectomy surgery and again two days later. All animals were euthanized eleven days following ovariectomy surgery by CO2 overdose followed by decapitation, and hypothalamic brain tissue was rapidly dissected out, snap frozen on dry ice, and stored immediately at −80 °C. Tissue was homogenized using a Teflon homogenizer in 25 mMTris-HCl, 125 mMKCl, 1 μM ZnCl2, 10 mMNaF, 1 mM PMSF added fresh, pH 7.8, conductivity 12.0 mS/cm2 per gram of tissue. Homogenized tissue was then centrifuged at 13,000 rpm for 30 min at 4 °C, after which the supernatant was removed and stored immediately at −80 °C. Samples were pooled (groups of two, except for one vehicle sample) for a total of 4 hypothalamic samples per treatment. Total protein concentration in each hypothalamic homogenate was determined by Bradford assay. One μg total protein of each hypothalamic homogenate sample was gel electrophoresed on 7.5% polyacrylamide gels containing 1% SDS (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to a polyvinylidenedifluoride membrane (Millipore, Bedford, MA, USA). Hypothalamic homogenate samples were analyzed for TOP levels by Western blot as described previously (32). Briefly, membranes were rinsed in 0.1 M TBS with 0.05% Tween-20 (TBS-T), then blocked in TBS-T and 5% nonfat milk at room temperature for 1 hr. Blots were cut at the 50 kDa marker. The upper blot was probed for TOP with a rabbit polyclonal antibody directed against rat TOP at two epitopes KPPAACAGD and LSKGLQVEGC (8) (1:20,000, generously provided by Marc Glucksman, Rosalind Franklin U. of Medicine & Science, Chicago, IL) the lower blot was probed for actin with a monoclonal antibody directed against the N-terminal peptide, Ac-Asp-Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-Gly-Lysof mouse actin (Chemicon International, Inc., Temecula, CA; 1:75,000) in TBS-T overnight at 4°C. The TOP antibody is highly specific. For example, the antibody does not recognize neurolysin (EC 3.4.24.16), an endopeptidase structurally similar to TOP (8). The following day the membranes were incubated in a horseradish peroxidase-linked donkey anti-rabbit secondary IgG (1:10,000, GE Healthcare UK Limited, Buckinghamshire, UK) or sheep anti-mouse secondary IgG (Amersham Biosciences, Buckinghamshire, UK; 1:10,000) for 1 hr at room temperature, and proteins were detected using an enhanced chemiluminescence kit (ECL; New England Biolabs, Ipswich, MA, USA). Purified recombinant rat TOP protein (a gift from Marc Glucksman) was run as a positive control for each Western blot.
Immunoreactive bands were visualized using a PhosphorImager (STORM Scanner 860, Molecular Dynamics) at an excitation wavelength of 450 nm. Images of each gel were taken and saved as 16-bit grayscale Tiff files and the area of each immunoreactive band was analyzed using ImageQuant software (v. 5.0). TOP levels were normalized to actin, which served as a loading control (33).
Immunohistochemistry
Dual-label immunofluorescence
For immunohistochemical analysis mice were ovariectomized and treated with either EB (2 μg, n=7) or vehicle (n = 7). Forty-eight hours later the mice were injected i.p. with an overdose of sodium pentobarbital (75 mg/kg) and perfused intracardially with saline (0.9%) for 1 minute followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.2) for 8 min at a flow rate of 8 ml/min. Brains were immediately removed, blocked, and incubated in 0.1 M sodium phosphate buffer (pH = 7.2) containing 20% sucrose at 4 °C overnight. Using a freezing rotary microtome, 40 μm coronal sections were collected from the preoptic area through the hypothalamus of each brain and stored in cryoprotectant at −20 °C.
Dual-label immunofluorescence was used to detect cells expressing TOP and ERα. All sections were run through the immunofluorescence protocol simultaneously. Brain sections were incubated in 0.05% TBS buffer (0.05 M Tris-HCl, 0.15 M NaCl, pH 7.6) followed by a 90 min incubation in 0.05% TBS buffer containing donkey anti-mouse IgG (0.02 μg/μl) to reduce non-specific background staining. The sections were incubated in 0.05% TBS containing 20% non-immune donkey serum and 1% w/v BSA for 20 minutes to reduce non-specific staining. Sections were then incubated at 4°C overnight in a cocktail containing rabbit anti-TOP (1:5,000) and a rat monoclonal antibody generated against the ligand binding domain of human ERα (H222, 1:50, Santa Cruz Biotechnology) in 0.05 M TBS/NaN3 buffer (0.02% Triton X-100, 0.02% NaN3, 0.1% gelatin, pH 7.6) with 1% non-immune donkey serum. The following day, sections were rinsed in 0.05M TBS/NaN3 buffer, then incubated for 90 min in 0.05M TBS/NaN3 buffer containing the secondary Alexa Flour 594 donkey anti-rabbit IgG (Invitrogen, A-21207, 1:100) used to visualize TOP and Alexa Flour 488 donkey anti-rat IgG (A-21208, 1:50) used to visualize ERα. Sections were then rinsed in 0.05M TBS/NaN3 buffer and then again in 0.05M TBS buffer. Sections were mounted on gelatin-coated slides, left to dry, cleared in distilled water and coverslipped with Gel/Mount mounting medium (Biomedia Foster City, CA).
Controls for dual-label immunohistochemistry included the omission of primary or secondary antibodies. Furthermore, the TOP and H222 primary antibodies were each preadsorbed with recombinant TOP and ERα proteins, respectively.
Immunofluorescence imaging and analysis
Brain sections were matched to the mPOA (plate 31); ARC, VMNvl and ME (plate 46); and the MCG (plate 56) (34). One representative matched section per mouse was used for each brain region. Immunofluorescent images of each brain region for each mouse were captured at 200× magnification using a Leica DM IRBE laser confocal microscope, equipped with Ar/Kr/HeNe lasers that excite at 488 nm (ERα) and 561 nm (TOP), and Leica software was used to capture images. A FieldMaster (Coherent) was used to standardize the output of each laser in mV before use. For each brain region, sections from all animals were imaged on the same day using the same gain and offset settings for each laser. All images with scale bars were saved as JPEG files.
Images were then analyzed using NIS-Elements AR 2.30 software by an investigator blind to treatment group. The scale generated from the confocal was used to convert pixels to microns in NIS element. A rectangular region of interest (ROI) was created for analysis of the mPOA (14566 μm2), ARC (3226 μm2), ME (5105μm2), VMNvl (9966 μm2), and MCG (10210μm2). Cells identified within each ROI were restricted by circularity (0.2–1), area (> 15 μm) and intensity (>90 for TOP-immunoreactive cells and >30 for ERα-immunoreactive cells). For the intensity measure, an intensity value was assigned by the program for each pixel within a cell based on a 0–256 scale for each 8 bit JPEG file. This provided an intensity value for each pixel in each cell. The sum of the intensity values for each cell in each region (optical integrated density) was then measured for each animal. This provided a measure of the optical integrated density of TOP staining within each region. The intensity cut-off of each protein was determined as the minimum intensity above background staining. The number of cells immunoreactive for both TOP and ERα in each area examined of each brain region of control mice was counted. To determine whether oestradiol alters TOP expression, the number of immunoreactive cells, total area of immunoreactivity (total area of immunoreactive cells (μm2) within the ROI), and average intensity (average optical integrated density) for TOP within the ROI was measured in each brain area of interest for EB and control mice.
Statistical analyses
JMP 7.0 (SAS Institute Inc., Cary, NC) was used for all statistical analyses. A two-way t-test was used to analyze differences between groups for each experiment. A Shapiro-Wilk W goodness of fit test was used to test for normal distributions and Levene’s test was used to test for homogenous variances in all variables. For variables with non-normal distributions and unequal variances, a nonparametric Mann-Whitney U test was used. All means are presented ± their standard errors, and p < 0.05 was considered statistically significant.
Results
Expression of TOP in female hypothalamus
Analysis by Western blot using the TOP polyclonal antibody revealed that TOP protein is expressed in the female mouse hypothalamus at a molecular mass of 78 kDa (Fig. 1), which is consistent with reports from other species (e.g. 8).
Fig. 1.
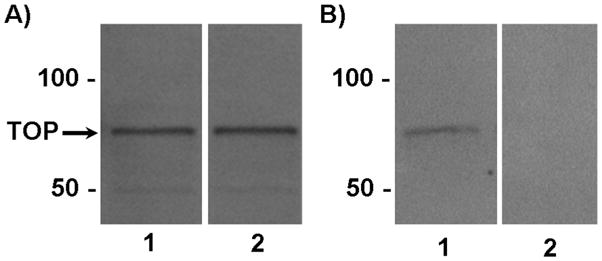
Western blot analysis ofTOP in the hypothalamus of female mice. A)TOP expressed in the hypothalamus of mice treated with vehicle (lane 1) or 2μg of estradiol benzote (lane 2) at the expected molecular mass of 78 kDa. The position of TOP (78 kDa) is indicated. B) TOP expressed in the hypothalamus of mice treated with vehicle (lane 1). Preadsorption of the TOP antibody with a 20-fold excess of recombinant full-length TOP protein resulted in no visible immunoreacitve bands of lanes containing hypothalamic whole cell extracts (lane 2).
Localization of TOP in Female Mouse Hypothalamus
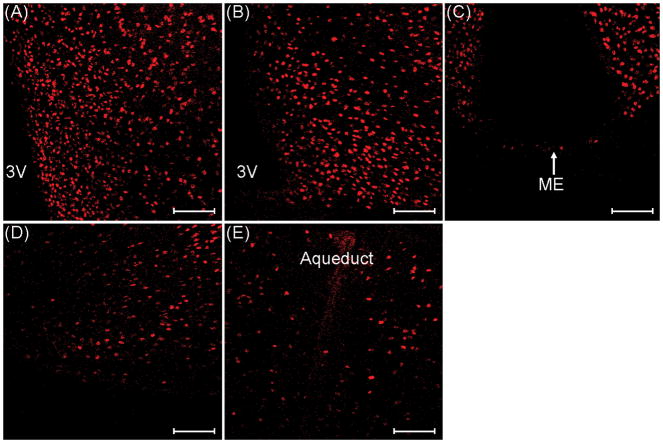
Immunohistochemical staining was used to determine TOP expression in reproductively-relevant brain regions of ovariectomized mice. TOP-immunoreactive (TOP-IR) cells were found throughout the hypothalamus. High levels of TOP-IR cells were observed in the mPOA, ARC, VMNvl, and MCG, while few to no TOP-IR cells were observed in the ME (Fig. 2). We found that TOP was expressed in the nucleus of the cell exclusively (Fig. 3).
Fig. 2.
TOP-immunoreactive cells in the A) medial preoptic area, B) arcuate nucleus, C) median eminence (ME), D) ventrolateral portion of the ventromedial nucleus of the hypothalamus, and E) midbrain central grey of ovariectomized control mice. 3V, third ventricle. Images were taken at 200× magnification. Scale bars = 100 μm.
Fig. 3.
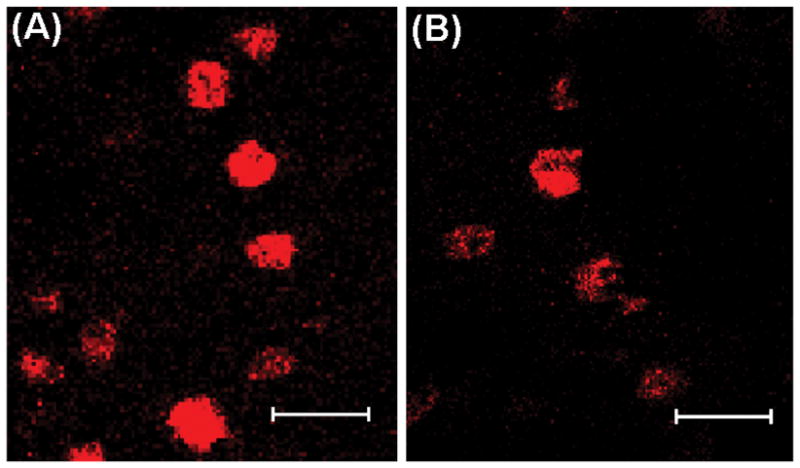
Nuclear TOP-immunoreactivity in the ventrolateral portion of the ventromedial hypothalamic nucleus (VMNvl) from females treated with A) vehicle or B) estradiol benzoate (2 ug). Images were taken at 400× magnification (bars = 20 μm).
Female mouse hypothalamic cells coexpress TOP and ERα
ERα immunoreactive (ERα-IR) cells were observed in the mPOA, ARC, and VMN, which is consistent with previous studies in mice (35) and rats (36). Prior studies using in-situ hybridization histochemistry in female rats demonstrate that oestradiol treatment down-regulates ER in the ARC and VMNvl (37, 38). Furthermore, Blaustein(39) showed that immunolabeling of ERα in the cell nucleus of ovariectomized oestradiol treated rats was reduced with the H222 antibody (generated against the C-terminal ligand binding domain) compared to a polyclonal antibody raised against the N-terminus of the receptor. Consistent with these previous findings, immunofluorescence staining for ERα in the cell nucleus was substantially reduced in EB-treated mice compared to controls in the mPOA, ARC, VMNvl, and MCG (data not shown). Thus, dual-label immunofluorescence was used in control animals only.
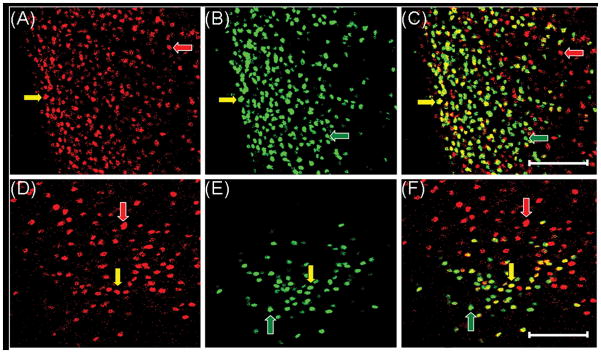
The majority of cells containing ERα in the mPOA, ARC and VMNvl express TOP (Table 1). The region with the greatest percentage of ERα-IR cells that also expressed TOP was the mPOA (95% ± 3.9; see Fig. 4), and the region with the lowest percentage was the VMNvl (46% ± 8.9; see Fig. 4). In contrast, the greatest percentage of TOP-IR cells that also expressed ERα was in the ARC (67% ± 5.7), and the region with the lowest percentage was the MCG (9%± 2.0). Although, most TOP-IR cells did not express ERα in the MCG, a large percentage of the few ERα-IR cells in this region expressed TOP (88% ± 4.8).
Table 1.
Cells Immunostained for thimet oligopeptidase (TOP), oestrogen receptor α (ERα), or Both in the Medial Preoptic Nucleus (MPO), Arcuate Nucleus (ARC), ventrolateral portion of the Ventral Medial Nucleus of the Hypothalamus (VMHVL), and Periaqueductal Grey (PAG).
| Brain region | TOP and ERα coexpression in control mice |
|||
|---|---|---|---|---|
| Total TOP cells | Total ERα cells | % TOP cells expressing ERα | % ERα cells expressing TOP | |
| MPO | 183.9 ± 36.0 | 86 ± 21.9 | 61 | 95 |
| ARC | 68.8 ± 6.9 | 68 ± 7.5 | 67 | 67 |
| VMHVL | 57.2 ± 7.3 | 76 ± 5.6 | 59 | 46 |
| PAG | 54.0 ± 7.5 | 6 ± 1.7 | 9 | 88 |
Total cells are presented as means ± their standard errors
Fig. 4.
Cells in the medial preoptic area (mPOA, panels A–C) and ventrolateral portion of the ventromedial hypothalamic nucleus (VMNvl, panels D–F) coexpress TOP (red) and ERα (green) from a control animal. TOP alone (A and D), ERα alone (B and E) and overlaid image of cells expressing both TOP and ERα (C and F). Cells in the mPOA and VMNvl expressing TOP only (red arrows), ERα only (green arrows) and both TOP and ERα (yellow arrows). Images were taken at 200× magnification. Magnification bars = 100 μm. 3V, third ventricle.
Oestradiol regulation of TOP in the VMNvl
Western blots were used to determine whether oestradiol regulates TOP expression in whole hypothalamus. Since TOP levels in EB-treated mice were statistically similar to TOP levels in EB + P animals [t(1,6) = 1.11, p = 0.31], these mice were grouped together. TOP levels in hypothalamic samples of EB-treated and control animals were similar [t(1,10) = 0.39, p = 0.7] (data not shown).
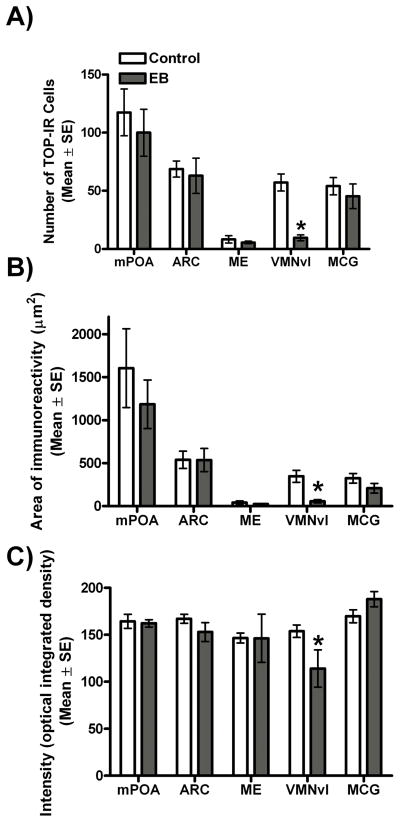
Immunofluorescence staining of hypothalamic brain sections from ovariectomized mice treated with either EB or an oil control was used to determine whether oestradiol alters TOP expression in reproductively-relevant brain regions. In the VMNvl, EB mice had fewer TOP-IR cells [t(1,11) = 6.5, p < 0.0001] and a smaller area of TOP immunoreactivity [t(1,11) = 4.3, p = 0.001] (Figs.5 and 6.). In contrast, no differences in cell number or area of immunoreactivity were found in any other brain region examined [all p > 0.05] (Fig. 6). Consistent with data for cell number and area of immunoreactivity, intensity of TOP immunofluorescence [Z(1,11) = 2.1, p = 0.04] in the VMNvl of EB-treated animals was lower than for control animals; however, no differences in intensity were detected in any other brain region examined [all p > 0.05].
Fig. 5.
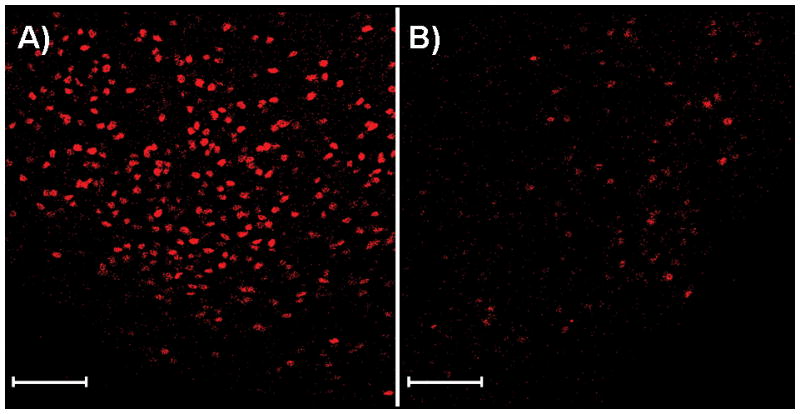
TOP-immunoreactive cells in the ventrolateral portion of the ventromedial hypothalamic nucleus (VMNvl) from a female treated with A) vehicle or B) 2μg of estradiol benzoate. Images were taken at 200× magnification at the same laser power and the same gain and offset on the same day. Magnification bars = 100 μm.
Fig. 6.
TOP-immunoreactivity in the medial preoptic area (mPOA), arcuate nucleus (ARC), median eminence (ME), ventrolateral portion of the ventromedial nucleus of the hypothalamus (VMNvl), and midbrain central grey (MCG) of ovariectomized animals treated with vehicle (control; white bars) or 2 μg of oestradiol benzoate (EB; grey bars). A) Mean number of TOP-immunoreactive cells, (B) Area of TOP-immunoreactivity measured as the sum of the number of pixels divided by the area examined, and (C) Intensity (optical integrated density) of TOP immunoreactivity * p < 0.05.
Controls for immunohistochemistry
Omission of the primary TOP antibody or preadsorption with recombinant TOP protein resulted in no observable TOP-IR cells, but many detectable ERα-IR cells. Likewise, excluding the H222 antibody and preadsorption with recombinant ERα protein resulted in no ERα-IR cells, but many visible TOP-IR cells. In further confirmation of the specificity of the double-label immunofluorescent technique, intensely labeled TOP-IR cells devoid of ERα-IR cells were observed, as well as ERα-IR cells that lacked TOP-IR.
Discussion
Previous immunohistochemical studies in male rats reveal that TOP is found throughout the brain, and in high levels in the hypothalamus (4, 8). The present study demonstrated that TOP is also expressed throughout the hypothalamus and localized in many steroid-sensitive regions of the female mouse. More specifically, TOP-IR cells were found in a variety of reproductively-relevant brain regions, including the mPOA, ARC, ME, VMNvl, and MCG (Fig. 2). Moreover, we found that cells in the mPOA, ARC, VMNvl, and MCG coexpress TOP and ERα (Table 1 and Fig. 4). These regions regulate hormone-dependent reproductive physiology and sexual behaviour in female mice (40). In addition, given that oestradiol regulation of reproductive physiology and behaviour is predominantly mediated by ERα (28, 41), our present data suggest a role for TOP regulation of hormone-dependent female reproduction.
Several studies have shown that TOP enzymatic activity in brain is attenuated by ovarian steroid hormones (reviewed in 1). The current study found no differences in TOP expression in whole hypothalamic tissue by Western blot; however, this was most likely due to the lack of cellular resolution of this technique that is required to detect effects in specific brain regions. In fact, cellular analysis by immunohistochemistry of specific brain regions revealed that oestradiol influenced TOP expression in a region-specific manner. Oestradiol reduced the number of TOP-IR cells in the VMNvl, but not in the ARC, ME, or MCG of female mice. Furthermore, TOP and ERα coexpressed in these reproductively-relevant brain regions. While both TOP and ERα are expressed in neurons and glia(4, 42, 43); these cell types were not distinguished in the present study. It will be important for future studies to determine the proportion of TOP and ERα coexpression in neuronal versus glial cells.
Although not statistically significant, there appeared to be a decrease in TOP-IR cells in the mPOA of EB-treated mice, which is consistent with previous studies in rats. Pierotti et al. (18) found that oestradiol treatment in ovariectomized rats significantly reduced the catalytic activity of TOP in the mPOA and other specific brain regions. The VMNvl, however, was not examined in the Pierotti study (19). The data presented here are measurements of TOP expression rather than activity and, because expression may not directly indicate activity, it will be important in future studies to investigate the activity of TOP in the VMNvl and other reproductively-relevant brain regions.
Because GnRH is a substrate for TOP, we examined brain regions associated with the production and release of GnRH. In fact, Cleverly et al. (44) present evidence in a recent review demonstrating that TOP is the predominant enzyme metabolizing GnRH. Within the brain, the mPOA contains the most cell bodies of GnRH neurons(45–48), many of which terminate in the ARC and ME (49). ME is the site of GnRH secretion into the hypophysial portal system (47). While the present data suggest that oestradiol does not regulate TOP levels in the mPOA, ARC or ME, it may be that the immunohistochemical technique was not sensitive enough to detect changes in TOP levels in these regions. Alternatively, oestradiol may indirectly affect TOP concentrations after it is secreted into the extracellular space.
The present study found that TOP is localized to the nucleus of cells within the hypothalamus, which is consistent with studies in rat brain (4, 8). Although these studies (4, 8) showed a small amount of cytoplasmic TOP staining in male rat brain, we found TOP exclusively in the nucleus of female mouse brain (Fig. 3). Nuclear localization of TOP is to be expected since the protein contains a nuclear-targeting sequence (50). Although the mechanism is unknown, there is evidence that TOP moves from the nucleus to the cytoplasm, where TOP concentrations in the cytoplasm are negatively correlated with TOP concentrations in the nucleus (42). TOP may hydrolyze GnRH within the cell or in the extracellular space. TOP action within the cell might also be targeted at other cytosolic or nuclear substrates. Within the nucleus, TOP may function to degrade transcription factors and/or interact with proteins in the proteasome (42).
TOP is also thought to hydrolyze peptides in the extracellular space. Once in the cytoplasm, TOP can be secreted to the extracellular space by one of several pathways. While TOP is secreted partially via the classical pathway in AtT-20 cells(51), there is compelling evidence that TOP is secreted via an alternate non-classical pathway in brain (42). Wu et al. (9) have demonstrated that TOP is present in the perivascular space of the ME and hypophysial portal blood, and others have suggested different mechanisms by which TOP may degrade GnRH extracellularly(13). Although not investigated in the current study, TOP secreted from neurons in the ME may affect GnRH concentrations. Therefore, it will be important in the future to investigate the function and activity of extracellular TOP as well as the mechanisms by which TOP is secreted into the extracellular space.
Neuropeptidases play an important role in regulating physiological functions. It has been hypothesized that the GnRH-degrading peptidase TOP mediates reproduction. Immunohistochemical studies from other groups have demonstrated that TOP is expressed in reproductively-relevant brain regions of male rodents (4, 8). Our data provide neuroanatomical evidence that oestradiol reduced both the number of TOP-IR cells and the intensity of TOP immunofluorescence in the VMNvl, a region considered to be an important site for the expression of hormone-dependent female sexual behaviours (15, 16, 52). Our results support other recent findings in rats (17), which suggest that TOP may play a role in regulation of sexual behaviors in female rodents. Future studies using TOP inhibitors injected into specific brain regions will be useful in elucidating the precise role that TOP plays in reproductive physiology and behaviour.
Acknowledgments
We thank Stefany Acosta-Torres, Maria Bisquera, Yi Dai, SadafSaeed, and Michelle Song for technical assistance. We also thank Dr. Marc Glucksman for providing the TOP antibody and the recombinant TOP protein and for helpful discussions, Dr. Shaila Mani for helpful comments on the manuscript, and Pat Carey and Valerie LePage for animal care. This study was supported by (MJT) National Institutes of Health R01 DK61935, and a Howard Hughes Medical Institute grant for Science Education at Wellesley College.
References
- 1.Shrimpton CN, Smith AI, Lew RA. Soluble metalloendopeptidases and neuroendocrine signaling. Endocrine Reviews. 2002;23(5):647–64. doi: 10.1210/er.2001-0032. [DOI] [PubMed] [Google Scholar]
- 2.Chu TG, Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin- containing peptides and other bioactive peptides. Endocrinology. 1985;116(4):1418–25. doi: 10.1210/endo-116-4-1418. [DOI] [PubMed] [Google Scholar]
- 3.Chu TG, Orlowski M. Active site directed N-carboxymethyl peptide inhibitors of a soluble metalloendopeptidase from rat brain. Biochemistry. 1984;23(16):3598–603. doi: 10.1021/bi00311a005. [DOI] [PubMed] [Google Scholar]
- 4.Healy DP, Orlowski M. Immunocytochemical localization of endopeptidase 24.15 in rat brain. Brain Research. 1992;571(1):121–8. doi: 10.1016/0006-8993(92)90517-d. [DOI] [PubMed] [Google Scholar]
- 5.Smith AI, Tetaz T, Roberts JL, Glucksman M, Clarke IJ, Lew RA. The role of EC 3.4.24.15 in the post-secretory regulation of peptide signals. Biochimie. 1994;76(3–4):288–94. doi: 10.1016/0300-9084(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 6.Pineau C, McCool S, Glucksman MJ, Jegou B, Pierotti AR. Distribution of thimet oligopeptidase (EC 3.4.24.15) in human and rat testes. Journal of Cell Science. 1999;112(20):3455–62. doi: 10.1242/jcs.112.20.3455. [DOI] [PubMed] [Google Scholar]
- 7.Molineaux CJ, Yu B, Ayala JM. Distribution of endopeptidase-24.15 in rat brain nuclei using a novel fluorogenic substrate: comparison with endopeptidase-24.11. Neuropeptides. 1991;18(1):49–54. doi: 10.1016/0143-4179(91)90163-d. [DOI] [PubMed] [Google Scholar]
- 8.Massarelli EE, Casatti CA, Kato A, Camargo ACM, Bauer JA, Glucksman MJ, Roberts JL, Hirose S, Ferro ES. Differential subcellular distribution of neurolysin (EC 3.4. 24.16) and thimet oligopeptidase (EC 3.4. 24.15) in the rat brain. Brain Research. 1999;851(1–2):261–5. doi: 10.1016/s0006-8993(99)02135-6. [DOI] [PubMed] [Google Scholar]
- 9.Wu TJ, Pierotti AR, Jakubowski M, Sheward WJ, Glucksman MJ, Smith AI, King JC, Fink G, Roberts JL. Endopeptidase EC 3.4.24.15 presence in the rat median eminence and hypophysial portal blood and its modulation of the luteinizing hormone surge. Journal of Neuroendocrinology. 1997;9(11):813–22. doi: 10.1046/j.1365-2826.1997.00637.x. [DOI] [PubMed] [Google Scholar]
- 10.Schally AV, Kastin AJ, Coy DH. LH-releasing hormone and its analogs - recent basic and clinical investigations. International Journal of Fertility. 1976;21(1):1–30. [PubMed] [Google Scholar]
- 11.Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science. 1973;182(4117):1148–9. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- 12.Moss RL, McCann SM. Induction of Mating Behavior in Rats by Luteinizing Hormone-Releasing Factor. Science. 1973;181(4095):177–9. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JL, Mani SK, Woller MJ, Glucksman MJ, Wu TJ. LHRH-(1–5): a bioactive peptide regulating reproduction. Trends in Endocrinology and Metabolism. 2007;18(10):386–92. doi: 10.1016/j.tem.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Pleim ET. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124(4):1807–12. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 15.Barfield RJ, Chen JJ. Activation of estrous behavior in ovariectomized rats by intracerebral implants of estradiol benzoate. Endocrinology. 1977;101(6):1716–25. doi: 10.1210/endo-101-6-1716. [DOI] [PubMed] [Google Scholar]
- 16.Davis PG, Krieger MS, Barfield RJ, McEwen BS, Pfaff DW. The site of action of intrahypothalamic estrogen implants in feminine sexual behavior: an autoradiographic analysis. Endocrinology. 1982;111(5):1581–6. doi: 10.1210/endo-111-5-1581. [DOI] [PubMed] [Google Scholar]
- 17.Wu TJ, Glucksman MJ, Roberts JL, Mani SK. Facilitation of lordosis in rats by a metabolite of luteinizing hormone releasing hormone. Endocrinology. 2006;147(5):2544–9. doi: 10.1210/en.2005-1646. [DOI] [PubMed] [Google Scholar]
- 18.Pierotti AR, Lasdun A, Ayala JM, Roberts JL, Molineaux CJ. Endopeptidase-24.15 in rat hypothalamic/pituitary/gonadal axis. Molecular and Cellular Endocrinology. 1991;76(1–3):95–103. doi: 10.1016/0303-7207(91)90264-s. [DOI] [PubMed] [Google Scholar]
- 19.Smith AI, Shrimpton CN, Norman UM, Clarke IJ, Wolfson AJ, Lew RA. Neuropeptidases regulating gonadal function. Biochemical Society Transactions. 2000:28430–4. [PubMed] [Google Scholar]
- 20.Advis JP, Krause JE, McKelvy JF. Luteinizing hormone-releasing hormone peptidase activities in discrete hypothalamic regions and anterior pituitary of the rat: apparent regulation during the prepubertal period and first estrous cycle at puberty. Endocrinology. 1982;110(4):1238–45. doi: 10.1210/endo-110-4-1238. [DOI] [PubMed] [Google Scholar]
- 21.Lapp CA, Oconner JL. Hypothalamic and pituitary enzymatic degradation of luteinizing hormone-releasing hormone during the 4-day estrous cycle of the rat. Neuroendocrinology. 1986;43(2):230–8. doi: 10.1159/000124531. [DOI] [PubMed] [Google Scholar]
- 22.Lew RA, Cowley M, Clarke IJ, Smith AI. Peptidases that degrade gonadotropin-releasing hormone: Influence on LH secretion in the ewe. Journal of Neuroendocrinology. 1997;9(9):707–12. doi: 10.1046/j.1365-2826.1997.00628.x. [DOI] [PubMed] [Google Scholar]
- 23.Pleim ET, Brown TJ, Maclusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124(4):1807–12. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 24.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. The Journal of Physiology. 1979:288203. [PMC free article] [PubMed] [Google Scholar]
- 25.Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behavioural Brain Research. 1998;92(2):169–80. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper G, Carlsson BO, Grandien KAJ, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors a and β. Endocrinology. 1997;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 27.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(27):10456–60. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine Reviews. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of Estrogen Receptor-a Gene Expression in Reproduction-Related Behaviors in Female Mice 1. Endocrinology. 1998;139(12):5070–81. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (beta ERKO) male and female mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(22):12887–92. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudwa AE, Rissman EF. Double oestrogen receptor a and β knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. Journal of neuroendocrinology. 2003;15(10):978–83. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 32.Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor-and progestin receptor-dependent aspects of sexual behavior in female rats. Hormones and Behavior. 2006 doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiology & Behavior. 2007;92(5):1010–8. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Franklin KBJ. The Mouse Brain in Steriotaxic Coordinates. Boston: Academic Press; 2004. [Google Scholar]
- 35.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor a. Endocrinology. 2003;144(5):2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 36.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-a and-β mRNA in the rat central nervous system. The Journal of comparative neurology. 1997;388(4) doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Lauber AH, Romano GJ, Mobbs CV, Pfaff DW. Estradiol regulation of estrogen receptor messenger ribonucleic acid in rat mediobasal hypothalamus: an in situ hybridization study. Journal of Neuroendocrinology. 1990;2(5):605–11. doi: 10.1111/j.1365-2826.1990.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 38.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Molecular Endocrinology. 1991;5(3):424–32. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- 39.Blaustein JD. Estrogen receptor immunoreactivity in rat brain: rapid effects of estradiol injection. Endocrinology. 1993;132(3):1218–24. doi: 10.1210/endo.132.3.7679973. [DOI] [PubMed] [Google Scholar]
- 40.Pfaff DW, Sakuma Y, Kow LM, Lee AWL, Easton A. Hormonal, neural, and genomic mechanisms for female reproductive behaviors, motivation, and arousal. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Amsterdam: Academic Press; 2006. pp. 1825–920. [Google Scholar]
- 41.Mazzucco CA, Walker HA, Pawluski JL, Lieblich SE, Galea LAM. ERa, but not ERβ, mediates the expression of sexual behavior in the female rat. Behavioural Brain Research. 2008;191(1):111–7. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Fontenele-Neto JD, Massarelli EE, Gurgel Garrido PA, Beaudet A, Ferro ES. Comparative Fine Structural Distribution of Endopeptidase 24.15 (EC3. 4.24. 15) and 24.16 (EC3. 4.24. 16) in Rat Brain. The Journal of Comparative Neurology. 2001;438(4):399–410. doi: 10.1002/cne.1323. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Segura LM, Lorenz B, DonCarlos LL. The role of glia in the hypothalamus: implications for gonadal steroid feedback and reproductive neuroendocrine output. Reproduction. 2008;135(4):419–29. doi: 10.1530/REP-07-0540. [DOI] [PubMed] [Google Scholar]
- 44.Cleverly K, Wu TJ. Is the metalloendopeptidase EC 3.4.24.15 (EP24.15), the enzyme that cleaves luteinizing hormone-releasing hormone (LHRH), an activating enzyme? Reproduction (Cambridge, England) 139(2):319–30. doi: 10.1530/REP-09-0117. [DOI] [PubMed] [Google Scholar]
- 45.Sternberger L, Hoffman G. Immunocytology of luteinizing-hormone-releasing hormone. Neuroendocrinology. 1978;25(2):111–28. doi: 10.1159/000122734. [DOI] [PubMed] [Google Scholar]
- 46.Witkin J, Paden C, Silverman A. The luteinizing-hormone-releasing hormone (LHRH) systems in the rat-brain. Neuroendocrinology. 1982;35(6):429–38. doi: 10.1159/000123419. [DOI] [PubMed] [Google Scholar]
- 47.Krey L, Silverman A. Lutenizing hormone releasing hormone. In: Krieger D, Brownstein M, Martin J, editors. Brain Peptides. New York: Wiley; 1983. pp. 687–709. [Google Scholar]
- 48.Liposits Z, Setalo G, Flerko B. Application of the silver gold intensified 3,3′-diaminobenzidine chromogen to the light and electron microscopic detection of luteinizing-hormone-releasing hormone system of the rat-brain. Neuroscience. 1984;13(2):513–25. doi: 10.1016/0306-4522(84)90245-8. [DOI] [PubMed] [Google Scholar]
- 49.Ibata Y, Watanabe K, Kinoshita H, Kubo S, Sano Y, Sin S, Hashimura E, Imagawa K. The location of LH-RH neurons in the rat hypothalamus and their pathways to the median eminence. Cell and Tissue Research. 1979;198(3):381–95. doi: 10.1007/BF00234184. [DOI] [PubMed] [Google Scholar]
- 50.Ferro E, Tullai J, Glucksman M, Roberts J. Secretion of metalloendopeptidase 24.15 (EC 3.4.24.15) DNA and cell biology. 1999;18(10):781–9. doi: 10.1089/104454999314926. [DOI] [PubMed] [Google Scholar]
- 51.Thompson A, Huber G, Malherbe P. Cloning and functional expression of a metalloendopeptidase from human brain with the ability to cleave a beta-APP substrate peptide. Biochemical and biophysical research communications. 1995;213(1):66–73. doi: 10.1006/bbrc.1995.2099. [DOI] [PubMed] [Google Scholar]
- 52.Pfaff DW, Sakuma Y, Kow LM, Lee AWL, Easton A. Hormonal, Neural, and Genomic Mechanisms for Female Reproductive Behaviors, Motivation, and Arousal. Knobil and Neill’s Physiology of Reproduction. 2006 [Google Scholar]