Abstract
Background and Purpose
Most improvement from post-stroke aphasia occurs within the first three months, but there remains unexplained variability in recovery. Recently, we reported a strong correlation between initial impairment and change scores in motor recovery at 90 days. We wanted to determine whether aphasia recovery (defined as a change from baseline to 90 days) shows a comparably strong correlation, and whether the relationship was similar to that in motor recovery.
Design/Methods
Twenty-one stroke patients had aphasia scores on the Western Aphasia Battery (WAB) obtained on stroke admission (WABinitial) and at 90 days (WAB3mon). The relationship between actual change (Δ) scores (defined as WAB3mon - WABinitial) and WABinitial was calculated in multiple regression analysis.
Results
Regression analysis demonstrated that WABinitial was highly correlated with ΔWAB (R2 = 0.81, p<0.001) and that, in addition, the relationship between WABinitial and ΔWAB was proportional, such that patients recovered 0.73 of maximal potential recovery (WABmaximum - WABinitial).
Conclusions/Relevance
We show that, like motor recovery, there is a highly predictable relationship between aphasia recovery and initial impairment, which is also proportional in nature. The comparability of recovery from motor and language impairment suggests that common mechanisms may govern reduction of post-stroke neurological impairment across different functional domains, and that they could be the focus of therapeutic intervention.
Most improvement from post-stroke aphasia occurs in the first three months.1,2 The factors that account for variability in the degree of recovery over this period, however, remain largely unexplained.3
Recently, we found that when motor recovery is defined as a change between initial (baseline) and final impairment levels, initial severity is highly predictive of the magnitude of the change, accounting for almost 90% of the variance.4 Further, we then found that the relationship between the observed change and the maximal potential change (maximum score minus initial score) was proportional, such that patients recovered 70% of their maximal potential recovery. To begin addressing whether such predictable recovery is motor specific or a more generalized characteristic of stroke recovery, we applied the same analysis to stroke patients with language deficits.
Subjects and Methods
We used the Performance and Recovery in Stroke (PARIS) database of patients with image-verified, first-time ischemic strokes who underwent serial assessment with impairment measures for hemiparesis, aphasia and visual neglect.4,5 Between May 2002 and August 2007, eligible patients screened from the adult, inpatient stroke service as having a new clinical deficit in language, motor and/or visual spatial function, signed IRB-approved informed consent. Individuals with severe comprehension deficits were considered unable to provide consent and could not be enrolled. Initial assessment occurred 24–72 hours after stroke onset (mean = 2.1 days; SD = 1.3); the follow-up examination took place at 90 days (mean = 93.1 days; SD = 18.8) after the qualifying stroke because it is felt that most spontaneous recovery occurs by this point.6 The aphasia examination, derived from standardized subtests from the Western Aphasia Examination (WAB)7, consisted of the evaluation of comprehension (“Yes/No Questions”, “Auditory Word Recognition”, “Sequential Commands”), repetition, and naming (“Object Naming”, “Word Fluency”, “Responsive Speech”), and chosen because of their high, respective 88-, 97- and 92-percent intra-individual reliabilities.8 Each of the three spheres of function yielded a possible score of 10, with a composite perfect score of 30 (WABMax). Initial impairment (WABinitial) was defined as a composite score < 28. To determine if initial aphasia severity predicts change in aphasia scores (Achieved ΔWAB [WAB3mon - WABinitial]) and, if the relationship is proportional, we ran a regression analysis of aphasia recovery (Achieved ΔWAB), using WABinitial, age, and lesion volume as independent variables and the change score as the dependent variable. Lesion volume was estimated in cm3: lesion volume = [product of maximal perpendicular diameters of the diffusion weighted imaging (DWI) lesion in cm] × [number of 0.5-cm slices]/2, a reasonably reliable method compared with automated methods.9 Testing procedures and results for motor function, and the methods for the visual-spatial tasks have been described elsewhere.4,10
Results
There were 118 patients in the PARIS database during the study period, of whom 21 had aphasia on the baseline PARIS assessment, and had deficits in the mild-to-moderate range to allow them sufficient comprehension to sign consent. Table 1 displays demographic characteristics, lesion locations and lesion volumes. There were 13 males/8 females with a mean age of 59.4 years (SD = 14.9). All were right handed with first-time, left hemisphere ischemic strokes. The mean lesion volume was 19.8 cm3 (SD = 13.3): 16 cortical involving cortex and immediately subjacent white matter, 2 subcortical involving deep gray matter, and 3 mixed cortical and subcortical. (See Table 1 for specific structures involved.) Among the 21 patients, 9 received some form of speech-language therapy after stroke, 8 received no therapy, and we could not ascertain if language intervention occurred for the remaining 4 patients.
Table 1.
Patient Demographics and Lesion Locations. Lesions designated as those involving the frontal, parietal and temporal lesions always involved cortical regions and in some cases subjacent white-matter areas; subcortical lesions in the right-most column did not involve the cortex.
| P Sex | Age | Lesion Volume | Lesion Location | |||
|---|---|---|---|---|---|---|
| Frontal | Parietal | Temporal | Sub-Cortical | |||
| M | 76 | 49.1 | X | X | ||
| M | 69 | 35.3 | X | X | ||
| F | 65 | 3.4 | X§ | |||
| M | 60 | 8.5 | X | X | ||
| M | 64 | 36.7 | X | X | ||
| M | 57 | 19.7 | X | X | ||
| F | 40 | 26.3 | X‡ | |||
| F | 60 | 24.7 | X¶ | X | X | |
| M | 51 | 16.1 | X* | |||
| M | 52 | 1.0 | X | |||
| M | 61 | 1.1 | X | |||
| M | 81 | 19.4 | X | X | ||
| M | 77 | 1.0 | X | |||
| F | 27 | 11.2 | X | X | ||
| F | 71 | 21.2 | X | X | ||
| M | 52 | 19.1 | X | X | ||
| F | 57 | 24.1 | X | X | ||
| M | 72 | 47.6 | X | |||
| M | 65 | 5.8 | X | |||
| F | 67 | 30.2 | X@ | |||
| F | 24 | 13.9 | X | X | ||
Corona radiata and caudate
Includes corona radiata and internal capsule
Includes insula
Includes insula and corona radiata
Thalamic
The mean composite aphasia score at baseline (WABinitial) was 20.0 (SD = 7.7). The mean composite aphasia score at 90 days (WAB3mon) was 27.5 (SD = 3.7). A t-test for paired samples showed a statistically significant improvement from baseline to follow-up (p <.001). For patients who did not receive speech-language therapy, mean WABinitial was 24.2 (SDS = 6.1); for those receiving therapy the mean WABinitial was 17.7 (SD = 5.5), a difference which was statistically significant (p = 0.03). Within the three language spheres at baseline across all patients, the mean naming score was 6.4 (SD = 3.5), the mean repetition score was 6.2 (SD = 3.7), and the mean comprehension score was 6.9 (SD = 3.9), which were not statistically different from each other.
A linear regression model to predict Achieved ΔWAB based on WABinitial, lesion volume and age was highly predictive, with an overall R2 = 0.83, as shown in Table 2. The regression coefficient was significant for WABinitial; the estimated regression coefficients of the lesion volume and age, however, were not significant. WABinitial, alone, accounted for 81% of the variance. That the coefficient for the y-intercept was near WABmax, implied a proportional relationship between Achieved ΔWAB and Potential ΔWAB (WABMax - WABInitial), as we previously found for motor recovery5 (see Figure 1), with a y-intercept near 0.0 and a slope near 1.0. The mean Potential ΔWAB was 9.96 (SD = 7.6) and the mean Achieved ΔWAB was 7.44 (SD = 6.2), yielding an overall 0.73 proportional relationship. This predictability in recovery held both for those who received speech-language therapy (R2=0.76, p=.005) and those who did not (R2=0.90, p<.001). Among the three spheres of language function that were assessed, the mean proportions of recovery were 0.68 (SD = .29) for naming, 0.70 (SD = .46) for repetition and 0.83 (SD = .25) for comprehension, respectively.
Table 2.
Estimated Regression Coefficients for Achieved Delta WAB as the dependent variable (N=21)
| Coefficient | t | Signif. | 95% Confidence Interval for B | |||
|---|---|---|---|---|---|---|
| B | Std. Error | Lower Bound | Upper Bound | |||
| y-intercept | 19.526 | 3.223 | 6.058 | <.001 | 12.726 | 26.326 |
| WABinitial | −.691 | .087 | −7.972 | <.001 | −.873 | −.508 |
| Age | .010 | .043 | .234 | .818 | −.081 | .101 |
| Lesion Volume | .059 | .048 | 1.228 | .236 | −.042 | .160 |
Figure 1.
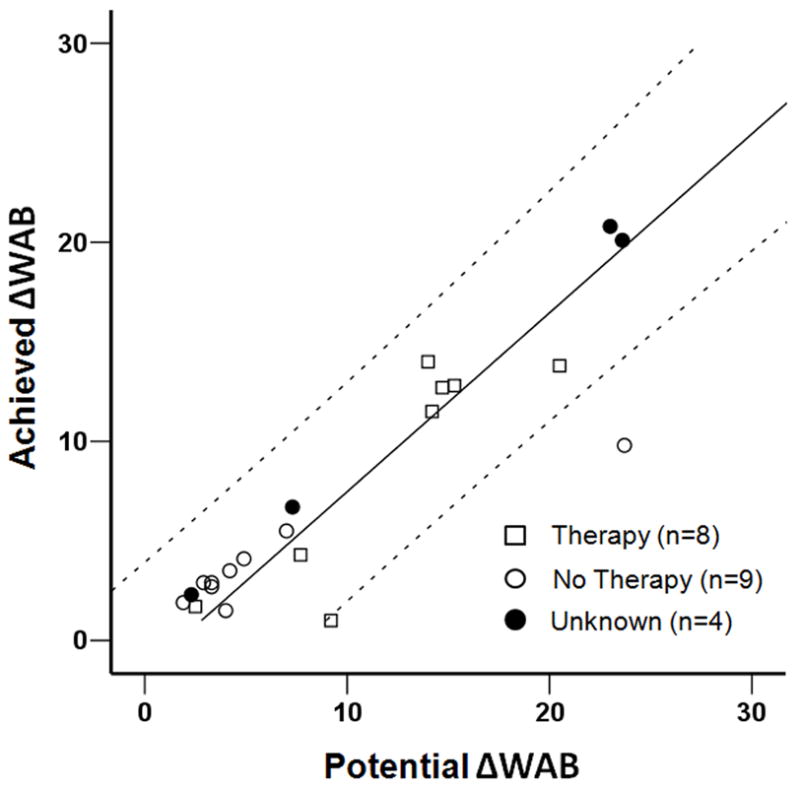
The relationship between the achieved change score on the Western Aphasia Battery (WAB) from baseline to follow-up at 90 days (Achieved ΔWAB) to the maximum possible change score (Potential ΔWAB) for patients receiving therapy, not receiving therapy, and unknown therapy status, respectively. The 95% confidence intervals are displayed above and below. The equation for the curve is y = 1.11x + 1.6834.
Discussion
We found among patients with mild to moderate aphasia after acute stroke that recovery, defined as a change between baseline and 90 days, is very well predicted by initial severity. Furthermore, this relationship can be expressed as a proportion of the maximal remaining recovery possible. We have reported similar predictability and proportionality (0.7) for recovery from motor impairment.4 The extension of these previous motor findings to recovery of language suggests that spontaneous recovery may have similar biological mechanisms, related to initial severity, across modalities. The proportionality relationship, which need not be present in order to have a high correlation between initial impairment and recovery, suggests a first order process, which could be common to stroke recovery from injury, regardless of location. We have recently shown that there is an fMRI pattern of brain activation in the first few days after stroke which correlates with changes in motor function after 90 days, but appears to be anatomically independent of contralateral and ipsilateral M1.11 This finding, in addition to the similarity in predictability of motor and language recovery over the first 90 days after stroke, raises the interesting possibility that multimodal brain areas could influence recovery for both hemiparesis and aphasia.
The high predictability of recovery at the time of acute stroke raises several alternative hypotheses regarding treatment in the first three months after stroke. The first, and least likely, is that treatment itself induces the predictable relationship, with the therapists providing intervention in direct proportion to impairment. While it was the case that it was the more impaired patients who received treatment, it would be unlikely that proportionality would be the same for self-recovery in the untreated as it is for the treated (but see interpretation 3). In addition, the same proportional recovery was seen for motor recovery.4 One would have to posit that therapists have a “0.7” target for both language and motor rehabilitation. The second possibility is that treatment is not having any effect on language recovery. We could not directly address this notion here since the direct comparison was not made, nor would we propose the unethical experiment to deny patients therapy. The third possibility, which we believe is most consistent with our data, is that treatment acts to trigger or enable spontaneous, biological recovery mechanisms. If this hypothesis is correct, then the patient who did not receive therapy and whose recovery was an outlier might have achieved an outcome predicted by our model had therapy been given. Thus, our data provide support for the notion that the degree of language recovery at 90 days post-stroke is a proportion of maximum potential improvement in patients with moderate aphasia and who have at least some language therapy. Our findings suggest that if a new therapy is to be considered more effective than current modalities within the initial 90 days after stroke onset, patients who receive it should show a greater change in the WAB composite score than that predicted by the model.
Our findings should not be taken to mean that comprehension, naming and repetition as assessed here represent the full range of language functions that can be affected by stroke, exclusive of functions such agrammatism and paragraph-length comprehension. We chose these functions because: 1.) They are those frequently assessed as elemental components of clinical examinations; 2.) They have excellent inter-rater reliability on the Western Aphasia Battery; and 3.) They are sufficiently brief that they could be part of our evaluation battery in our PARIS database that included other neurological components. Speech fluency was not included because it has among the lowest rates of inter-observer agreement12, especially by non-specialist examiners. The rationale for combining them into a single composite measure lies in the matrix in Shewan and Kertesz for subtests on the WAB showing significant correlations among these three spheres of language evaluation.8 These skills do not appear to be functionally independent. Indeed, the internal consistency (co-efficient theta) on the overall WAB was 0.97, demonstrating how well the overall WAB score represents its components. Nevertheless, it will be interesting to determine whether other aspects of linguistic function that have low measurement error also demonstrate proportional recovery.
We were not able to address the question regarding recovery from severe aphasia because of consent restrictions imposed by local law. In our previous study of recovery from motor impairment, prediction broke down for patients with severe hemiparesis; some showed proportional recovery but others did not.4 Whether this occurs for patients with more severe aphasia deficits will have to be addressed in future studies. It would also be of interest, with respect to the question of common mechanisms, to see if patients who do not recover from severe hemiparesis also do not recover from concomitant severe aphasia, correcting for lesion volume. We also did not have information regarding type or intensity of therapy. It is possible that any therapy (intense or not) might differentially affect the proportion of recovery, since 8 of 21 did not receive therapy (and we had no information on 4 cases). Furthermore, there might be other therapies that alter the path of natural recovery. While our method for calculating lesion volumes is considered reliable, there is the possibility that small errors in absolute measurement of small lesions can result in larger measurement error, which could have produced a lack of impact in our regression model. We also recognize that there can be dynamic changes in DWI volume after we obtained our images at 24 – 72 hours post-onset; decreasing in size because of recovery or re-perfusion of the ischemic penumbra, or expanding because the penumbra can go on to infarction.13 Nevertheless, the relationship between actual and potential recovery accounted for more than 80% of the variance so that there was relatively little residual variance that might be accounted for by other factors. Correcting any potential volume measurement errors is therefore unlikely to alter our findings.
In summary, both non-severe language and motor dysfunction after stroke seem to show highly predictable recovery during the first 90 days that is related to initial impairment in a very specific way, as a proportion of maximum potential recovery. This similar predictability suggests that there are spontaneous recovery mechanisms operating in the first three months that are common to patients with mild to moderate stroke, regardless of domain of dysfunction. These mechanisms, however, might be augmented with biologically-focused intervention early on after stroke, perhaps with non-invasive brain stimulation, pharmacology, or targeted behavioral methods to improve function beyond what is currently predicted.
Acknowledgments
Acknowledgments and Funding
This research was supported by NIH Grants 5R01-HD43249 (RML), 1P50-NS049060 (RSM), K02-048099 (JWK), and the Tananbaum Family Foundation.
References
- 1.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998 Feb;41(1):172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 2.Berthier ML. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging. 2005;22(2):163–182. doi: 10.2165/00002512-200522020-00006. [DOI] [PubMed] [Google Scholar]
- 3.Lazar RM, Antoniello D. Variability in recovery from aphasia. Curr Neurol Neurosci Rep. 2008 Nov;8(6):497–502. doi: 10.1007/s11910-008-0079-x. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008 Jan-Feb;22(1):64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 5.Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. Variability in language recovery after first-time stroke. J Neurol Neurosurg Psychiatry. 2008 May;79(5):530–534. doi: 10.1136/jnnp.2007.122457. [DOI] [PubMed] [Google Scholar]
- 6.Laska AC, Hellblom A, Murray V, Kahan T, Von Arbin M. Aphasia in acute stroke and relation to outcome. J Intern Med. 2001 May;249(5):413–422. doi: 10.1046/j.1365-2796.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 7.Kertesz A. Western Aphasia Battery. San Antonio, TX: Harcourt; 1982. [Google Scholar]
- 8.Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB) J Speech Hear Disord. 1980 Aug;45(3):308–324. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- 9.van der Worp HB, Claus SP, Bar PR, et al. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke. 2001 Feb;32(2):424–430. doi: 10.1161/01.str.32.2.424. [DOI] [PubMed] [Google Scholar]
- 10.Lazar RM, Fitzsimmons BF, Marshall RS, et al. Reemergence of stroke deficits with midazolam challenge. Stroke. 2002 Jan;33(1):283–285. doi: 10.1161/hs0102.101222. [DOI] [PubMed] [Google Scholar]
- 11.Marshall RS, Zarahn E, Alon L, Minzer B, Lazar RM, Krakauer JW. Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol. 2009 May;65(5):596–602. doi: 10.1002/ana.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonville S, van der Worp HB, Maat P, Aldenhoven M, Algra A, van Gijn J. Accuracy and inter-observer variation in the classification of dysarthria from speech recordings. J Neurol. 2008 Oct;255(10):1545–1548. doi: 10.1007/s00415-008-0978-4. [DOI] [PubMed] [Google Scholar]
- 13.Barrett KM, Ding YH, Wagner DP, Kallmes DF, Johnston KC. Change in diffusion-weighted imaging infarct volume predicts neurologic outcome at 90 days: results of the Acute Stroke Accurate Prediction (ASAP) trial serial imaging substudy. Stroke. 2009 Jul;40(7):2422–2427. doi: 10.1161/STROKEAHA.109.548933. [DOI] [PMC free article] [PubMed] [Google Scholar]


