Abstract
In this study, the histocompatibility match of a group of patients treated at least one and a half years ago was calculated by a modification of the net histocompatibility ratio (NHR) formula of Rapaport and Dausset.1 Correlations were then made with the outcome as judged by patient and kidney survival, homograft function, the magnitude of maintenance immunosuppression, and the extent of histopathologic abnormalities in the transplants.
Materials and Methods
The cases were those recently reported in detail2 and started with unculled groups of 131 consecutive recipients whose first kidneys were given by blood relatives and of 58 consecutive recipients of nonrelated kidneys (35 volunteers and 23 cadavers). The related cases were compiled 2½–7⅚ years ago and the nonrelated cases from 1½–7½ years ago. The immunosuppression for 112 of the patients was azathioprine and prednisone; the last 77 were also given heterologous ALG.
Of the 189 cases, all were included if the raw lymphocyte antigen typing data were available for both donor and recipient. There was sufficient information with transplantations from 56 siblings, 49 parents, 8 more distant relatives (aunts, uncles, and cousins which were included for the various statistical analyses with the siblings) and 38 nonrelatives (total 151). Correlations of match with survival were made in all 151 typed cases. For the related recipients, matches were correlated with steroid dosage and homograft function at 1 and 2 years in the event of survival for these periods; the same applied in nonrelated cases except that survival periods for sampling were 1 and 1½ years.
Correlations of the matches expressed by the NHR with histopathology were done only if the homografts sampled had been in residence for at least 2½ months, thereby excluding 8 of the 151 cases because of death before this time. Twenty-one specimens included in the analysis were obtained at autopsy or at homograft nephrectomy from 2½ to 27 months after transplantation. Most of the tissues studied (113 total) were biopsies taken after 15–33 months. Nine of the homografts have never become available under any of the foregoing circumstances. The tissues were examined with light microscopy, and in most instances by electron microscopy and immunofluorescence (IF) as described elsewhere.2 Insofar as the method of tissue collection permitted, the presence or absence of the 13 features listed in Table 2 were determined and graded in severity from 0 to 4.
Table 2. NHR vs. Histopathology and Immunopathology in 134 Renal Transplantations.
| NHR | No. of Cases |
Average Time Specimen (Mos.) |
Histopathology* | Immunopathology | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | IgG | IgM | BlC | Fibrinogen | ||||
| Siblings † | ||||||||||||||||
| >0.5 | 24 | 23.9 | 1.08 | 0.38 | 0.38 | 0.83 | 1.00 | 1.25 | 1.00 | 0.96 | 0.96 | 0.80 | 0.95 | 0.70 | 0.55 | |
| 0.5 | 27 | 23.5 | 1.63 | 0.56 | 0.48 | 1.07 | 1.22 | 1.44 | 0.89 | 1.48 | 1.26 | 0.81 | 0.90 | 1.00 | 0.57 | |
| <0.5 | 8 | 21.9 | 1.00 | 0.25 | 0.13 | 1.13 | 1.75 | 1.88 | 1.88 | 1.00 | 1.50 | 0.83 | 1.00 | 1.17 | 0.0 | |
| p ‡ | >0.5/ 0.5 | NS | < 0.0005 | < 0.0125 | < 0.0005 | < 0.05 | < 0.0005 | < 0.01 | NS | < 0.0005 | < 0.0125 | NS | NS | 0.0025 | NS | |
| p ‡ | 0.5/< 0.5 | NS | (< 0.0025) | (< 0.025) | (< 0.01) | NS | NS | NS | 0.0005 | NS | NS | NS | NS | NS | (<.0125) | |
| Parental: | ||||||||||||||||
| >0.5 | 16 | 22.2 | 1.19 | 0.19 | 0.38 | 0.56 | 1.44 | 1.62 | 1.25 | 1.00 | 1.31 | 0.28 | 0.86 | 1.00 | 0.43 | |
| 0.5 | 26 | 23.3 | 1.00 | 0.08 | 0.62 | 0.58 | 1.38 | 1.46 | 1.31 | 1.16 | 1.58 | 0.73 | 1.05 | 0.73 | 0.41 | |
| <0.5 | 2 | 23.5 | 0.5 | 1.0 | 0 | 0.5 | 1.0 | 1.0 | 1.5 | 1.0 | 1.5 | 0 | 2.0 | 0 | 0 | |
| p ‡ | >0.5/0.5 | NS | NS | NS | <.0125 | NS | NS | NS | NS | NS | NS | < 0.0005 | NS | NS | NS | |
| Unrelated: | ||||||||||||||||
| ≥0.5 | 12 | 20.1 | 1.00 | 0.17 | 0.33 | 0.50 | 1.08 | 1.00 | 0.83 | 0.67 | 1.08 | 1.22 | 1.33 | 1.33 | 0.22 | |
| <0.5 | 19 | 16.5 | 2.10 | 0.26 | 0.79 | 1.42 | 2.11 | 2.11 | 1.74 | 1.37 | 1.89 | 0.85 | 1.77 | 1.54 | 1.00 | |
| p ‡ | < 0.05 | < 0.0005 | NS | < 0.0005 | < 0.0005 | < 0.0005 | < 0.0005 | < 0.0005 | < 0.0005 | < 0.0005 | NS | NS | NS | < 0.025 | ||
Average histopathology scores are tabulated in each of the following categories: 1 = subendothelial glomerular capillary basement membrane thickening; 2 = subepithelial glomerular capillary basement membrane thickening; 3 = increased cellularity of glomerular tufts; 4 = increased amount of mesangial matrix; 5 = tubular atrophy; 6 = interstitial fibrosis; 7 = mononuclear cell infiltration of the interstitium; 8 = “Hyaline” in arteriolar walls; 9 = thickening of intima of interlobular arteries. Each patient was graded from 0 to 4 according to severity in each of the categories.
Includes seven transplantations from uncles (three), cousins (two), an aunt, and a niece.
Designation NS signifies a p value >0.05. p values in parentheses indicate a negative correlation (in the reverse direction).
The NHR calculation1 was based upon the hypotheses that the major histocompatibility factors are on two loci of a single (HL-A) chromosome, that each locus governs the expression of two histocompatibility antigens, and that the measurement of either more or less than two antigens at one or the other locus is by definition probably a methodologic artifact. In the formula, NHR = ¼ (donor-recipient antigen identities/antigen incompatibilities), adjustments were made by the designation of “potential relations” if a full complement of alleles could not be defined in either the donor or recipient, particularly the former. The result was to depreciate the NHR value in situations with transplantation of no antigen to antigen by considering this as a “potential” rather than as an identity and by therefore preventing it from contributing to a high NHR score. A second adjustment was to consider the following three families of cross reacting antigens to be operationally identical*: HL-A 1 (HL-A 3 if present as a third antigen); HL-A 5 (Te 6, Te 55, Te 58); and HL-A 7 (Te 51, Te 60). This latter adjustment tended to improve the NHR scores by changing a number of incompatibilities to compatibilities; in no instance was the NHR worsened. In some cases in which typing was carried out several years ago with a multispecific antiserum called “Old 3,” a positive reaction could have been due to HL-A 9 or HL-A 10 on the first sublocus or HL-A 5, HL-A 12, or Te 60 on the second sublocus. The interpretation of the “Old 3” reactions was on a highly individual basis. All NHR scores were computed by Rapaport* without knowledge of the outcome in the cases under scrutiny. To those monitoring these calculations, it was obvious that an element of judgment and intelligence was introduced which rendered the determinations much more than a technical exercise, and which would make difficult a duplication of the scores by the simple insertion of data into a strict mathematical formula.
Results
Survival
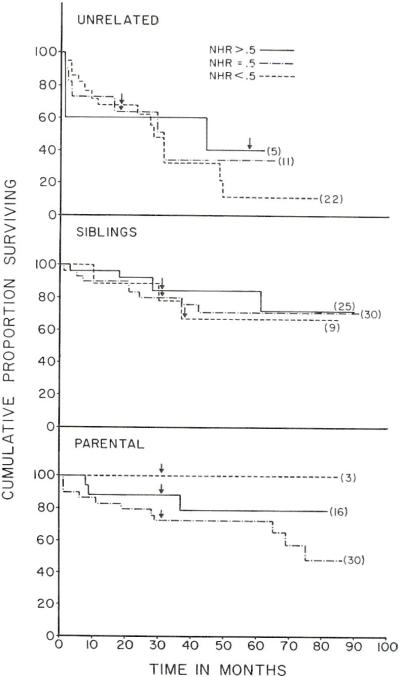
Cumulative proportional survivals were calculated by the life table method developed for cancer statistics by Cutler and Ederer.3 The recipients of related kidneys were at potential risk for 31–92 months post-transplantation. In the sibling cases and in the parent to offspring transplantations (Fig. 1), the three patient groups defined with NHR scores did not belong to significantly different populations at any stage of followup as judged by Mantel’s Chi-square procedures4 for comparing two sets of life table data in their entirety.
Fig. 1.
Cumulative proportional survival curves of recipients of related and unrelated renal homografts, divided according to the net histocompatibility ratio (NHR) scores. The minimum followup for each curve is indicated by an arrow (see text for details).
The same general conclusions applied with the series of nonrelated transplantations in which complete potential followup of 38 typed recipients was available out to 18 months with maximum followups of as long as 78 months. Long after operation (in the third to sixth postoperative years) there seemed to be an advantage of a good NHR score (Fig. 1) but with the Chi-square procedure mentioned above this never approached statistical significance. The maximum Chi-square value of 1.28 was at 75 months; significance would have required a figure of 3.84 or greater. It was of interest that all the patients with an NHR score > 0.5 were operated upon 57 months or longer ago. The donors in these cases were not available for retyping, making it necessary to compute the NHR values with relatively fragmentary serologic data and with a consequent need for guess work. Thus, a form of inadvertent bias may have been introduced of the kind that could ultimately have affected other statistical analyses such as those of pathologic abnormalities (see below).
Function and Immunosuppression
In the sibling, parent, and nonrelated cases, there was no apparent consistent correlation between the NHR score and either the quality of renal function or the prednisone dose used to maintain this function (Table 1). For example, in the sibling cases at 2 years the best creatinine clearances were in patients with the best NHR scores, but in the parental transplantations this correlation was inverse. Other examples of such incongruities are obvious in Table 1.
Table 1. NHR vs. Function and Immunosuppression in 124 Renal Transplantations.
| NHR | No. of Patients* |
Average Creatinine Clearance † (cc. min.) |
Average BUN † (mg. per cent) |
Average Prednisone Dosage † (mg./Kg./day) |
||||
|---|---|---|---|---|---|---|---|---|
| 1 Yr. | 2 Yr. | 1 Yr. | 2 Yr. | 1 Yr. | 2 Yr. | |||
| Siblings ‡ | > 0.5 | 24 (22) | 84 | 87 | 23 | 25 | 0.20 | 0.21 |
| 0.5 | 27 (24) | 84 | 84 | 28 | 23 | 0.27 | 0.18 | |
| < 0.5 | 8 | 92 | 71 | 26 | 28 | 0.24 | 0.21 | |
| Parentals | > 0.5 | 14 | 69 | 67 | 27 | 26 | 0.32 | 0.25 |
| 0.5 | 24 (22) | 72 | 71 | 24 | 28 | 0.23 | 0.18 | |
| < 0.5 | 3 | 58 | 80 | 45 | 42 | 0.44 | 0.19 | |
| Unrelated | ≥ 0.5 | 11 ( 9) | 79 | 64 | 25 | 27 | 0.38 | 0.36 |
| < 0.5 | 13 | 67 | 61 | 40 | 30 | 0.40 | 0.31 | |
In parenthesis is the number of patients available for analysis at 2 years in related and at 1½ years in unrelated cases if different than that of one year.
Averages calculated at 1 and 1½ years in the unrelated cases.
Includes six recipients of homografts from uncles (two), cousins (two), an aunt, and a niece.
Histopathology
In sibling cases with an NHR ≥ 0.5, certain histopathologic changes were less common and less severe than with an NHR equal to 0.5. These included lesions affecting the glomeruli, the tubules and the large and small arteries (Table 2). However, the eight siblings with very poor NHR’s (< .5) actually had significantly fewer glomerular lesions (categories 1 and 2, Table 2) and less fibrinogen than those with NHR’s equal to 0.5. The demonstration of the latter inverse correlations (identified by enclosure in parentheses, Table 2) dealt a serious blow to the credibility of the positive correlations in a subdivision of the same collection of sibling cases. In the parent to offspring transplantations, only 2 of the 13 categories of histopathologic and immunopathologic abnormalities were less if there was a high NHR (Table 2).
In view of the foregoing findings, it was surprising in the unrelated cases to find a rather striking advantage of an NHR > .5 in comparison to an NHR < .5. The former homografts were spared from structural damage to a statistically significant degree in eight of the nine categories defined by light and electron microscopy and in one of the 4 immunofluorescent columns (Table 2). It was mentioned earlier under the section on survival that an accidental bias could have been introduced by the circumstances of case selection and serologic analysis.
Discussion and Conclusions
The results underscore the need for continued evaluation of histocompatibility testing employing different systems of analysis. Recently, these cases were examined using an alphabetical (A-D, F) method of phenotype match expression.2 A poor correlation of outcome with match was obtained except with siblings. The NHR’s in the present study were calculated using the same serologic raw data in an attempt to convert phenotypes into genotypes. An improved correlation was not obtained and in fact the discrimination within sibling cases was lost except when the NHR’s were 0.88 or greater. Ten sibling recipients in the latter category (presumably double haplotype identity of HL-A chromosome) all lived for at least 31 months; the only kidney lost in the group functioned for more than 5 years before failing.
In order for decisive correlations to be obtained with any system of matching, it would be expected that incompatibilities would consistently cause failure and that uniform compatibilities would assure success. Obviously, neither premise has been very completely fulfilled. Successful transplantation has often been achieved despite frank antigenic mismatches. There have even been numerous instances of proven or probable multiple incompatibilities with an excellent result. For example, the four most badly matched sibling transplantations (NHR below .16) eventuated in perfect and continuing renal function after 38, 44, 47, and 88 months. At 2-year biopsy, only one of these kidneys had significant structural abnormalities. It should also be noted that none of the major HL-A antigens presently detectable were neither uniquely hazardous nor especially safe.
In addition, it is necessary to explain failures despite apparently good HL-A matches. The presence of such cases in every large series means that other factors may significantly effect the results after renal transplantation. These could include other antigens within or outside the HL-A system, surgical technical considerations, and original host disease2 to mention only three. Consequently, while good HL-A matching should be a desirable condition in performing organ transplantation, the HL-A system may only be the tip of an enormous and as yet poorly understood biological iceberg.
ACKNOWLEDGMENTS
The statistical analyses of life survival were carried out by Dr. Strother Walker and by Mr. Gary Zerbe, Professor and Instructor, respectively, in the Division of Biometrics, Department of Preventive Medicine, University of Colorado.
Supported by USPHS Grants AM-12148, AI-AM-08898, AM-07772, AI-04152, RR-00051, RR-00069, HE-09110, and GM-01686.
Footnotes
Data on cross reacting antigens were developed at the workshops of the Fourth Histocompatibility Conference convened in Los Angeles, January 24-26, 1970.
Using Dr. Terasaki’s serologic data.
REFERENCES
- 1.Rapaport FT, Dausset J. Science. 1970;167:1260. doi: 10.1126/science.167.3922.1260. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Porter KA, Halgrimson CG, Andres G, Hurwitz R, Giles G, Terasaki PI, Penn I, Lilly J, Starkie SJ, Schroter GPJ, Putnam CW. Ann. Surg. 1970;172:437. doi: 10.1097/00000658-197009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutler SJ, Ederer F. J. Chron. Dis. 1958;8:699. doi: 10.1016/0021-9681(58)90126-7. [DOI] [PubMed] [Google Scholar]
- 4.Mantel N. Cancer Chemother. Rep. 1966;50:163. [PubMed] [Google Scholar]