Abstract
Purpose
To characterize patterns and determinants of normal and abnormal cognitive development in children with new onset epilepsy compared to healthy controls.
Methods
Longitudinal (2-year) cognitive growth was examined in 100 children, age 8-18 years, including healthy controls (n=48) and children with new onset epilepsy (n=52). Cognitive maturation was examined as a function of the presence/absence of two neurobehavioral comorbitiies (Attention Deficit Hyperactivity Disorder and/or Academic Problems) identified at the time of epilepsy diagnosis. Groups were compared across a comprehensive neuropsychological battery assessing intelligence, academic achievement, language, memory, executive function and psychomotor speed.
Results
Children with new onset epilepsy without neurobehavioral comorbidities were comparable to healthy controls at baseline, rate of cognitive development, and follow-up assessment across all neuropsychological domains. In contrast, the presence of neurobehavioral comorbidities were associated with significantly worse baseline and prospective cognitive trajectories across all cognitive domains, especially executive functions.
Conclusion
The presence of neurobehavioral comorbidities at the time of epilepsy onset is a major marker of abnormal cognitive development both prior to and after the onset of epilepsy.
Keywords: epilepsy, cognition, prospective, development
INTRODUCTION
Cognitive abnormalities are an important potential comorbidity of childhood epilepsy (Hommet et al., 2006; Lassonde et al., 2000; Macallister and Schaffer 2007; Williams et al., 1998a,b). An extensive cross-sectional descriptive literature has identified a number of seizure-related factors associated with cognitive disruption in pediatric epilepsy including epilepsy etiology and syndrome (Besag 2006; Elger et al., 2004; Hommet et al., 2006; Lendt et al., 1999; Reijs et al., 2006; van Rijckevorsel 2006), degree of seizure control (Nolan et al., 2003; Smith et al., 2002), antiepilepsy medications (Bourgeois 1998; Loring et al., 2007; Vermeulen and Aldenkamp 1995), and underlying EEG abnormalities (Aldenkamp and Arends 2004a,b; Aldenkamp 1997; Koop et al., 2005; Tedrus et al., 2006; Wolff et al., 2005).
In contrast to adults with epilepsy, where a developed and reasonably stable cognitive substrate is the target of adverse epilepsy factors, children present with a dynamic pattern of cognitive and brain development (Gogtay et al., 2004; Sowell et al., 2003; 2004; Thompson et al., 2000). Cognitive abnormalities in childhood epilepsy identified by cross-sectional designs may represent any one of a number of effects including abject loss of function from a previously higher level of ability, slowed rate of normal cognitive development, static and fixed impairments that antedate epilepsy onset, transient postictal cognitive abnormalities due to seizures and treatment factors, or other influences.
Controlled prospective investigations initiated at epilepsy onset are best suited to characterize the nature, timing, and course of neuropsychological abnormalities in pediatric epilepsy, or in other words, their natural history. There are at least 18 prospective studies dating from 1924 that examined cognitive change in children and adolescents with epilepsy (Dodrill 2004; Seidenberg et al., 2007). Limitations of prior studies include lack of control groups, excessive reliance on broad IQ measures with under-investigation of important cognitive domains (e.g., language, memory, executive function), variable test-retest intervals, and arbitrary definitions of meaningful cognitive change (Dodrill 2004; Seidenberg et al., 2007). Most importantly, only two investigations examined prospective cognitive development from the time of epilepsy onset (Bourgeois et al., 1983; Oostrom et al., 2005), with one of the studies focused solely on IQ. It is clear that much remains to be learned about the natural history of cognitive impairment and general cognitive development in pediatric epilepsy. Notably, a small number of studies have demonstrated the important point that at the time of seizure onset, and even prior to administration of antiseizure mediations, cognitive impairment may be evident (Fastenau et al., 2005; Hermann et al., 2006; Kolk et al., 2001; Oostrom et al., 2003; Stores et al., 1992). These findings suggest the presence of antecedent anomalies in cognitive development with their cause and course remaining to be determined.
Although rarely discussed in the epilepsy literature, patterns of cognitive maturation may be influenced by co-occurring conditions known to independently affect neuropsychological status in the population at large, conditions that are in fact known to be overrepresented in childhood epilepsy. Examples of such conditions include learning disorders (Aldenkamp et al., 1990; Beghi et al., 2006; Berg et al., 2005; McNelis et al., 2007) and psychiatric diagnoses with known cognitive consequences such as Attention Deficit Hyperactivity Disorder (ADHD) (Dunn et al., 2003; Dunn and Kronenberger 2003; 2005; Gonzalez-Heydrich et al., 2007; Hesdorffer et al., 2004; Sherman et al., 2006). Differences in the cognitive trajectory associated with these conditions have not been characterized in pediatric epilepsy, and importantly, the cognitive development of children with epilepsy without these co-morbid conditions has not been reported.
Examining a cohort of children with new onset idiopathic epilepsy, we identified two groups of children who presented with comorbid conditions that significantly affected cognitive status, children with ADHD and children with academic problems (Hermann et al., 2006, 2007). These two groups demonstrated significant cognitive abnormalities at baseline, associated neuroimaging abnormalities, and evidence that these co-occurring conditions or comorbidities antedated seizure onset in a subset of children suggesting an antecedent neurobiological effect. In this controlled prospective cohort study, we report patterns of cognitive development over a fixed 2-year interval. Children with new onset epilepsy and healthy controls underwent comprehensive neuropsychological re-assessment and prospective cognitive trajectories were examined taking into consideration the effects of two major comorbidities diagnosed at baseline evaluation (ADHD, AP).
METHODS
Subjects
The research sample included 100 children between the ages of age 8 and 18, including 52 children with new/recent onset epilepsy and 48 healthy first-degree cousin controls, all attending regular schools. All children underwent neuropsychological evaluation at time of enrollment and at a fixed follow-up time point (2 years later).
Children with epilepsy were recruited from pediatric neurology clinics at Midwestern medical centers (University of Wisconsin-Madison, Marshfield Clinic, Dean Clinic) and initial selection criteria included: 1) diagnosis of epilepsy within the past 12 months, 2) chronological age between 8-18 years, 3) no other developmental disabilities (e.g., autism), 4) no other neurological disorder, and 5) normal clinical MRI. Epilepsy participants met criteria for classification of idiopathic epilepsy in that they had normal neurological examinations, no identifiable lesions on MR imaging, and no other signs or symptoms indicative of neurological abnormality (Engel 2001). Control participants were age and gender-matched first-degree cousins. Criteria for controls included no histories of: 1) any initial precipitating event (e.g., simple or complex febrile seizures), 2) any seizure or seizure-like episode, 3) diagnosed neurological disease, 4) loss of consciousness greater than 5 minutes, or 5) other family history of a first-degree relative with epilepsy or febrile convulsions. This study was reviewed and approved by the Institutional Review Boards of both institutions and on the day of study participation families and children gave informed consent and assent and all procedures were consistent with the Declaration of Helsinki (1991). 94% of the baseline cohort was retained and retested two years later.
Neuropsychological Assessment
Subjects were administered a comprehensive test battery that included standard clinical measures of intelligence, language, immediate and delayed verbal memory, executive functions, speeded fine motor dexterity, and academic achievement. Table 1 provides details regarding the target cognitive domains, the specific abilities assessed within each domain, the test measures and the nature of the dependent measure (i.e., number correct, errors, or time). The initial age range studied here was broad (ages 8-18 years) and particular attention was paid to the use of tests that allowed administration of identical items across the entire age range. For safety reasons, the research assistants were aware of each participant's group membership (epilepsy versus controls), but blinded to co-morbidity status.
Table 1.
Neuropsychological Test Battery
| Domain | Ability | Tests |
|---|---|---|
| Intelligence | Verbal | Wechsler Abbreviated Scale of Intelligence (Verbal IQ) (Wechsler 1999) 1 |
| Nonverbal | Wechsler Abbreviated Scale of Intelligence (Performance IQ) (Wechsler 1999) 1 |
|
| Academic achievement |
Letter/word recognition | Wide Range Achievement Test-3 (Reading) (Wilkinson 1993) 1 |
| Name and letter writing | Wide Range Achievement Test-3 (Spelling) (Wilkinson 1993) 1 | |
| Basic arithmetic | Wide Range Achievement Test-3 (Arithmetic) (Wilkinson 1993) 1 | |
| Language | Confrontation naming | Boston Naming Test (Kaplan et al. 2001) 2 |
| Expressive naming | Expressive Vocabulary Test (Williams 1997) 1 | |
| Receptive language | Peabody Picture Vocabulary Test-III (Dunn & Dunn 1997) 1 | |
| Generative naming | Delis-Kaplan Executive Function System (Letter Fluency) (Delis et al. 2001) 3 |
|
| Memory | Verbal memory | Children's Memory Scale (Word Lists Learning) (Cohen 1997) 3 |
| Children's Memory Scale (Word Lists Delayed) (Cohen 1997) 3 | ||
| Executive function | Problem solving | Delis-Kaplan Executive Function System (Confirmed Correct Sorts) (Delis et al. 2001) 3 |
| Response inhibition | Delis-Kaplan Executive Function System (Color-Word Interference Test-Inhibition) (Delis et al. 2001) 3 |
|
| Divided attention | Delis-Kaplan Executive Function System (Category Switching Accuracy) (Delis et al. 2001) 3 |
|
| Inattentiveness | Connors' Continuous Performance Test-II (Omission and Commission errors) (Conners 1995) 4 |
|
| Motor function | Speeded fine motor dexterity |
Grooved Pegboard (Trites 1977) 5 |
| Psychomotor speed | Wechsler Intelligence Scale for Children-III (Digit Symbol- Coding) (Wechsler 1991) 3 |
standard scores.
raw scores.
scaled scores.
T-scores.
seconds.
Parents were interviewed and medical records were reviewed to determine interval seizure history and seizure course. Three research staff, blinded to cognitive, psychiatric and social information, classified outcomes over the two-year prospective period using a slightly modified version of Berg et al.'s (2001) outcome classification scheme. The three categories, and their respective criteria, are as follows: Good: Children had at least a 1-year remission as of their 2-year follow-up visit. Remission was defined as being aura and seizure free, and included children taking antiepileptic drugs (AEDs). Intractable: Children who met the criteria for intractability from the time of enrollment to the 2-year follow-up time point. Intractability was defined as (a) failure or lack of seizure control on at least two appropriate AEDs or failure of one AED for seizure control and two other AEDs with adverse side effects; and (b) an average of at least one seizure per month over the 2-year interval. Drug failure was determined based on a review of the medical records including documentation by the treating physician that doses were increased,; the AED was not effective; or the AED resulted in adverse side effects. Indeterminate: Children who were either not in remission at the 2-year follow-up or did not meet the criteria for intractability during the 2-year interval.
Definition of Comorbidities
DSM-IV ADHD
Lifetime-to-date psychiatric status was assessed using the Kiddie-SADS-PL (K-SADS) (Ambrosini 2000), the semi-structured diagnostic interview designed to assess current and past episodes of psychopathology in children and adolescents according to DSM-IV criteria. The K-SADS was completed separately with the child and parent(s) and summary ratings included all sources of information in arriving at a diagnosis. Children and adolescents were interviewed first followed by interview with parents. Administration of the K-SADS included completion of the Diagnostic Screening Interview and the appropriate Diagnostic Supplements. Interviews were videotaped with patient/family consent and IRB approval. 15% of subjects were randomly selected for independent review with an outside consultant to insure reliability of diagnoses and prevent rater drift. Impairment and/or distress criteria were evaluated in order to accurately reflect the diagnostic criteria of the DSM-IV. The primary dependent measures were the rate of lifetime to date ADHD and the specific ADHD subtypes (predominantly inattentive, hyperactive, combined, or NOS). An important concern in assessing symptoms of inattention, and ADHD in particular, is the possible confounding effect of postical or frank ictal activity. Parents were specifically instructed to ignore anything that could be construed as seizure related symptoms and this was reconfirmed during the interviews. In addition, one purpose of the independent review of 15% of the interviews was to guard such potential errors and no diagnostic changes were made through this process. The interviewer and outside consultant were not blinded to group membership as seizure history often arose spontaneously during the interview.
Academic Problems
History of academic problems (AP) was obtained through a structured interview with the child's parent(s) by an interviewer blinded to all cognitive and psychiatric information. The categories of AP were based on actual academic modifications/responses instituted by the child's educational system to identified difficulties. These categories of services were developed by an independent pediatric neuropsychologist with considerable experience with educational systems and the ways in which academic difficulties were handled other than the traditional IEP process. There were 6 overall categories of services: a) birth to 3 programs, 2) early childhood programs, 3) IEP, 4) repeating a grade, 5) required summer school, and 6) miscellaneous school based assistance (tutor, homework club, reading/math groups, Title 1). If a parent indicated a positive response on any item or more than one item they were coded as positive for AP.
Analyses
A mixed 3 × 2 MANOVA was conducted for each of the 6 cognitive domains shown in Table 1 (intelligence, academic achievement, language, memory, executive function, psychomotor speed). Raw test scores (as opposed to standard scores) were used for all analyses in order to permit assessment of cognitive change/development from baseline to 2-year follow up examination. For each MANOVA the between subject variable was group (controls, epilepsy with comorbidity [comorbidity+] and epilepsy without comorbidity [comorbidity−]) and the within subject variable was time of assessment (baseline, 2-year follow-up). Bonferroni correction was used to adjust for testing across multiple domains using MANOVA, with strict significance achieved if p-value <.05/6=.008.
Most neuropsychological measures are known to be multifactiorial in nature and assignment to a priori cognitive domains (Table 1) should be viewed with caution. Our sample size precludes the likelihood that a stable factor structure for the administered cognitive tests would be derived. However, the selected tests have been validated to assess the cognitive constructs under investigation. The administered tests within each domain serve to provide a multi-dimensional measure of that particular cognitive area. Though each test provides incremental information regarding the cognitive domain under investigation, more information would be gained per test if the measures were completely independent, which is known not to be the case. Overall, the statistical approach utilized here takes into consideration the correlated nature of the tests within each domain by jointly analyzing statistical significance within major cognitive areas rather than evaluating each test separately, thereby significantly reducing the number of analyses and reducing the probability of Type I error with further corrections for multiple comparisons.
RESULTS
Patterns of Comorbidity
Comorbidities in the control sample included ADHD (n= 3), AP (n=7), or none (n=38); while comorbidities in the epilepsy group included ADHD (n=15), AP (n=13), or none (n=24). Children with new onset epilepsy exhibited a significantly elevated rate of both ADHD (p <.001) and AP (p <.001). A small number of control children had neurobehavioral comorbidities (n=10). To provide a rigorous test of the comparability of comorbidity− epilepsy children to healthy controls, these 10 control children with AP/ADHD were excluded from further consideration. Primary analyses combined the epilepsy ADHD and AP groups in the comorbidity+ group (n=28) for comparison to the epilepsy comorbidity− (n=24) and healthy controls (n=38) groups. A limited set of exploratory secondary analyses examined the unique effects associated with ADHD and AP in the children with epilepsy.
Table 2 provides information regarding the sociodemographic characteristics of the control and epilepsy groups. There were no significant group differences in overall age (p=.66), grade (p=.59), or gender (p=.49). There were no differences between the two epilepsy groups in the age of onset of epilepsy (p=.37), overall syndrome (idiopathic generalized vs. localization related, p=.16), number of epilepsy medications (p=.25), comorbid medical conditions (p=.26) or other medications (p=.27). The distribution of left handedness differed significantly across groups with more left-handed children in the epilepsy comorbidity+ group (21%) compared to both the epilepsy comorbidity− (4%) and control (0%) groups (X2=11.5, df=2, p<.001). There was no difference in the course of the epilepsy (Berg et al., 2001) over the two year interval (X2=1.5, df=4, p=.83). Time to baseline assessment from the diagnosis of epilepsy ranged from 1 to 12 months overall with no significant difference between the epilepsy comorbidity +/− groups (p= .33). We asked parents if, in retrospect, they believed their children may have experienced seizures prior to the event that led to evaluation and diagnosis of epilepsy. A total of 26 of the 52 families thought this might be the case. We of course have no way to prove that these prior events were indeed seizures. There was no difference between the epilepsy comorbidity +/− groups (p=.33) or the AP and ADHD groups in particular (p=.27) in the distribution of the presence/absence of possible prior seizures. Table 2 shows that 24 children were diagnosed with localization related epilepsies and 28 with idiopathic generalized epilepsies. In terms of specific syndrome types, there were 12 BECTS, 18 focal (temporal, frontal, nos), 15 JME, and 7 absence (child and juvenile). The number of children in these specific syndromes was felt to be too small to provide reliable findings and analyses were restricted to IGE vs. LRE.
Table 2.
Demographic Characteristics (baseline)
| Controls (n = 38) mean (SD) |
Comorbidity− (n = 24) mean (SD) |
Comorbidity+ (n = 28) mean (SD) |
|
|---|---|---|---|
| Age (years) | 12.7 (3.0) | 12.7 (2.8) | 12.3 (3.4) |
| Gender (M/F) | 17/21 | 12/12 | 17/11 |
| Grade | 6.6 (3.0) | 6.7 (3.1) | 6.2 (3.5) |
| Age on onset (years) | - | 11.8 (2.9) | 10.9 (3.8) |
| Time since diagnosis (months) | - | 8.0 (4.4) | 9.3 (4.4) |
| Localization-related epilepsies | - | 11 | 19 |
| Generalized epilepsies | - | 13 | 9 |
| Number of AEDs | |||
| 0 | - | 7 | 5 |
| 1 | - | 17 | 22 |
| 2 | - | 0 | 1 |
We examined the distribution of comorbid mood and anxiety disorders (the most commonly occurring lifetime to date K-SADS disorders), demographic and clinical seizure variables to determine whether there were any baseline differences between the epilepsy comorbidity +/− groups overall or the epilepsy ADHD and AP groups in particular. There were no significant differences between epilepsy comorbidity +/− groups, or between the AP and ADHD groups, in the rate of lifetime to date mood or anxiety disorders, core demographic variables (age, gender, or education), or clinical seizure variables (age of onset, time since diagnosis of epilepsy, number of medications) (p's ranging from .13 to .59). There was no difference in the distribution of associated medical illness (e.g., asthma) across the groups (p=.48). .
Neuropsychological performance
Table 3 provides the mean baseline and 2 year prospective retest data organized by cognitive domain. The effect of group was significant for five of the six cognitive domains including Intelligence (F=6.91, df=4,170, p <.0001), Academic Achievement (F=5.54, df=6,166, p<.0001), Language (F=3.53, df=8,166, p=.0008), Executive Function (F=2.57, df=10,156, p=.0067), and Psychomotor Speed (F=4.57, df=6,164, p=.0003). These results are significant after Bonferroni adjustment. A significant group effect was not evident for Memory (F=2.11, df=4,170, p=.082).
Table 3.
Raw Baseline and Prospective Neuropsychological Test Scores
| Controls (n = 38) |
Epilepsy Comorbidity− (n = 24) |
Epilepsy Comorbidity+ (n = 28) |
||||
|---|---|---|---|---|---|---|
| Time 1 mean (SD) |
Time 2 mean (SD) |
Time 1 mean (SD) |
Time 2 mean (SD) |
Time 1 mean (SD) |
Time 2 mean (SD) |
|
| Intelligence | ||||||
| WASI Verbal IQ | 79.8 (17.2) | 87.8 (15.4) | 80.5 (16.5) | 87.3 (11.2) | 66.3 (14.9) | 71.7 (14.3) |
| WASI Performance IQ | 68.3 (18.9) | 81.4 (15.5) | 69.3 (20.6) | 79.7 (15.1) | 45.1 (22.3) | 57.6 (19.2) |
| Academic Achievement | ||||||
| WRAT-III Arithmetic | 38.7 (8.0) | 42.8 (5.5) | 38.4 (8.0) | 42.8 (5.9) | 30.5 (6.6) | 34.7 (5.3) |
| WRAT-III Reading | 41.6 (6.7) | 44.1 (5.6) | 43.0 (6.2) | 46.0 (5.1) | 35.8 (6.6) | 39.1 (6.0) |
| WRAT-III Spelling | 35.8 (6.9) | 39.6 (5.9) | 37.9 (7.0) | 40.1 (5.5) | 31.0 (7.8) | 34.2 (8.5) |
| Language | ||||||
| Boston Naming Test (short form) | 12.7 (1.6) | 13.3 (1.5) | 12.5 (1.7) | 13.2 (1.1) | 10.8 (1.8) | 11.9 (1.5) |
| Expressive Vocabulary Test | 124.6 (29.2) | 131.2 (26.2) | 123.1 (26.6) | 133.3 (25.9) | 98.9 (21.4) | 110.4 (21.4) |
| Peabody Picture Vocabulary Test- III | 157.6 (22.3) | 169.4 (16.9) | 162.2 (21.8) | 170.2 (17.4) | 144.0 (24.4) | 155.3 (20.5) |
| Letter Fluency (D-KEFS) | 29.9 (10.5) | 35.4 (10.0) | 32.0 (11.5) | 35.7 (9.8) | 25.1 (8.9) | 29.0 (10.5) |
| Memory | ||||||
| Word Lists Learning (CMS) | 36.2 (5.9) | 38.5 (5.3) | 36.1 (5.2) | 36.9 (4.7) | 32.0 (8.6) | 34.8 (6.7) |
| Word Lists Delayed Recall (CMS) | 8.7 (2.3) | 9.3 (2.5) | 7.8 (2.5) | 9.3 (2.0) | 7.4 (3.2) | 8.8 (2.3) |
| Executive Function | ||||||
| Color-Word Interference (Inhibition; D-KEFS) | 63.3 (21.5) | 50.1 (16.1) | 66.9 (21.6) | 54.5 (17.7) | 87.2 (34.2) | 74.0 (30.4) |
| Confirmed Correct Sorts (D-KEFS) | 9.6 (1.7) | 10.6 (2.4) | 9.7 (2.3) | 10.5 (2.7) | 7.5 (3.1) | 8.4 (3.1) |
| Category Switching Accuracy (D-KEFS) | 11.5 (3.5) | 12.7 (2.7) | 12.0 (3.4) | 13.9 (3.5) | 9.4 (3.3) | 10.9 (3.3) |
| Omission Errors (CPT) | 5.5 (8.1) | 3.3 (7.4) | 6.8 (9.8) | 3.8 (5.2) | 11.9 (18.1) | 12.8 (18.2) |
| Commission Errors (CPT) | 20.6 (7.9) | 16.5 (7.8) | 20.5 (7.7) | 16.7 (8.7) | 19.7 (9.4) | 21.0 (9.4) |
| Motor Function | ||||||
| Total Grooved Pegboard | 147.7 (25.7) | 136.8 (19.1) | 149.4 (25.3) | 142.5 (18.5) | 184.8 (56.8) | 165.4 (28.3) |
| Digit Symbol-Coding (WISC-III) | 60.8 (17.3) | 70.3 (17.3) | 54.5 (18.2) | 62.2 (15.6) | 45.4 (17.6) | 52.4 (15.9) |
The simple effects of group were examined across all cognitive domains (except Memory). There were no significant differences between controls and the epilepsy comorbidity– groups across any cognitive domain including Intelligence (p=.93), Academic Achievement (p=.32), Language (p=.67), Executive Function (p=.23) and Psychomotor Speed (p=.31). The epilepsy comorbidity+ group performed significantly worse than both the epilepsy comorbidity− and control groups across all cognitive domains (all p's < .0025), these results remain significant after Bonferroni adjustment.
For every cognitive domain there was a significant effect of time indicating that subjects' scores improved across the two year interval; this was the case for Intelligence (F=73.7, df=2,85, p<.0001), Academic Achievement (F=67.9, df=3,83, p<.0001), Language (F=39.2, df=4,83, p< .0001), Memory (F=11.71, df=2,85, p< .0001), Executive Function (F=18.4, df=5,78, p<.0001), and Psychomotor speed (F=19.1, df=3,82, p<.0001). These results are significant after Bonferroni adjustment.
For most cognitive domains the differences between groups remained constant over time. This was the case for Intelligence (p=.56), Academic Achievement (p=.33), Language (p=.48), Memory (p=.16), Executive Function (p=.11), and Psychomotor Speed (p=.35). There was a significant group by time interaction for Executive Function (F=1.99, df=10,128, p=.03). This interaction was driven by two of the five tests included in this domain, CPT errors of omission (inattention) and commission (impulsive) errors. For each measure the number of errors for the control and comorbidity− groups decreased over time reflecting improved performance, while the comorbidity group+ increased slightly reflecting poorer performance. The remaining three tests in the Executive Function domain followed the same pattern seen in the other five cognitive domains and improved over time.
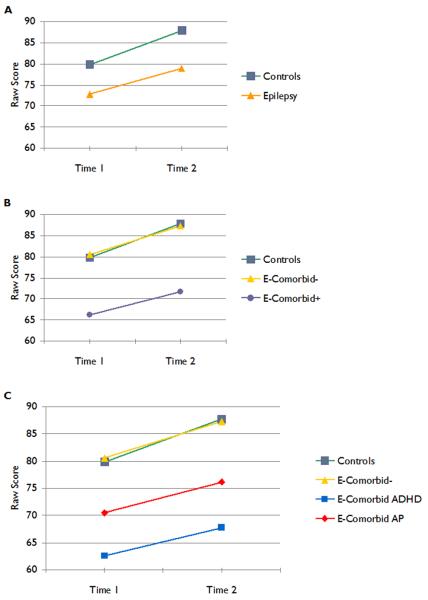
The figures characterize the main findings for two representative specific test measures. Figure 1 provides the results for raw score Verbal IQ performance. The top panel of Figure 1 depicts a traditional comparison of children with epilepsy versus controls. The middle panel of Figure 1 depicts the results of the analyses when the presence/absence of neurobehavioral comorbidities is considered. As can be seen, epilepsy children without comorbidities perform identically to healthy controls at baseline, follow-up, and rate of cognitive development. The bottom panel of Figure 1 depicts the relative performance of children with epilepsy with ADHD or AP to the other groups. The results in Figure 1 depict the predominant pattern observed across cognitive domains (except memory) and individual tests (Table 3).
Figure 1.
Raw Verbal IQ scores. The top (a) panel provides a traditional comparison of control versus epilepsy groups. The middle (b) panel presents the results of the main analyses where children categorized by comorbidity+/− are contrasted to controls. The bottom (c) panel presents the results with the epilepsy comorbidity+ group subdivided into the ADHD and AP groups.
Secondary Analyses
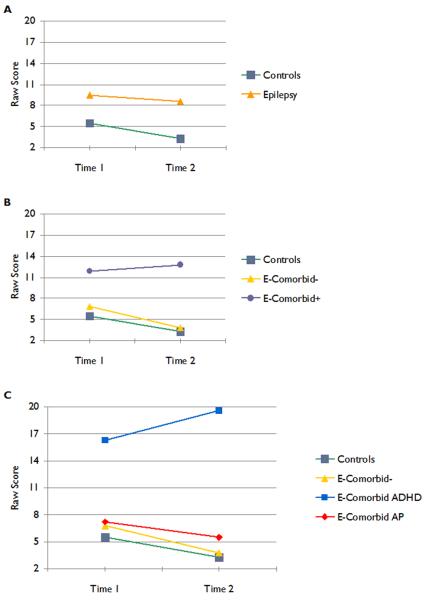
Two sets of secondary analyses were performed. First, in order to confirm the robustness of results across individual tests within each cognitive domain, repeated-measures ANOVAs were examined separately for each test and p-values were adjusted to account for the comparison of multiple tests (n=18). Similar patterns were observed with three exceptions, two in executive function (CPT omission and commission errors) where a group by time interaction indicated that scores for the control and epilepsy comorbidity− group improved over time while performance of the comorbidity+ group did not. This effect was due specifically to poorer attention performance in the ADHD group compared to all others (Figure 2). The final exception was delayed verbal memory where none of the differences between groups reached statistical significance.
Figure 2.
Raw CPT omission errors. The top (a) panel shows a traditional comparison of control versus epilepsy groups. The middle (b) panel presents the results of the main analyses where children categorized by comorbidity+/− are contrasted to controls. The bottom (c) panel presents the results with the epilepsy comorbidity+ group subdivided into the ADHD and AP groups.
Second, epilepsy ADHD and AP groups were compared to examine group differences within the comorbidity+ group. These analyses were conducted as exploratory secondary analyses because of small sample sizes. While there was a trend of poorer performance in the ADHD group across virtually all cognitive domains, there were no significant ADHD vs. AP differences across any cognitive domain including intelligence (p=.93), Academic Achievement (p=.60), Language (p=.35), Executive Function (p=.74), or Psychomotor Speed (p=.32). In part, this is due to limited power secondary to sample size constraints as a total of 26 cases were included in this analysis. Figure 2 shows the results for CPT omission errors where there was a group by time interaction. The top panel of Figure 2 depicts a traditional comparison of children with epilepsy versus controls. The middle panel of Figure 2 depicts the results of the analyses when the presence/absence of neurobehavioral comorbidities is considered. As can be seen, epilepsy children without comorbidities perform identically to healthy controls at baseline, follow-up, and rate of cognitive development, while the comorbidity+ group presents a distinct pattern of test-retest change. The bottom panel of Figure 2 provides details regarding relative performance of the children with epilepsy with ADHD and AP to the other groups where the ADHD group shows the most disparate interval cognitive change. In the supplemental file section a data table has been added providing specific test score data for the ADHD, AP, healthy controls and epilepsy comorbidity− groups for interested readers.
DISCUSSION
Three core findings derive from this investigation. First, the presence/absence of two primary comorbidites (ADHD, AP) at the time of epilepsy diagnosis is linked to patterns of abnormal cognitive development and remains predictive of the subsequent two year trajectory of cognitive maturation. Second, after accounting for these two major neurobehavioral comorbidities, the remaining children with epilepsy (representing about half the epilepsy sample) exhibit entirely normal neuropsychological status both at baseline and 2-years later with a rate of cognitive development identical to the healthy controls. Finally, presence or absence of the comorbidities in children with epilepsy were unassociated with biological variables including syndrome (LRE, IGE), age of onset, number of medications and the two year clinical course.
Essential comorbidites and cognitive development
We have previously shown that this cohort of healthy children and children with new onset idiopathic epilepsy are characterized by significant differences from controls in the presence, frequency, clinical presentation and neuroimaging characteristics of two comorbidities, ADHD and academic problems (AP) (Hermann et al., 2006, 2007). The fact that important neurobehavioral comorbidites may be present at epilepsy onset and even antedate the first recognized seizure is now well appreciated, with such effects reported in the areas of behavioral adjustment (Austin et al., 2001), cognition (Oostrom et al., 2003), school difficulties (Berg et al., 2005; McNelis et al., 2007; Oostrom et al., 2003), ADHD (Hesdorffer et al., 2004; Jones et al., 2007); depression (Hesdorffer et al., 2006, 2007), and other DSM-IV Axis I disorders (Jones et al., 2007).
Berg (2007) referred to the neurobehavioral complications evident at or before the onset of seizures in children with epilepsy who are otherwise neurologically “normal” (i.e., normal neurological exam, intelligence and imaging) as essential comorbidities. Cortez et al. (2006) pointed out that the onset of recurrent spontaneous seizures is the end result of the complex process of epileptogenesis involving a cascade of transcriptional changes in the brain triggered by an interaction of genetic and environmental factors. The neurobiological results of these transcriptional changes include plasticity, apoptosis and further neurogenesis, all of which could conceivably affect behavior or cognition prior to the appearance of overt seizures.
A major finding of this investigation is that children with epilepsy with the two essential comorbidities examined here exhibit a distinct pattern of cognitive development over the two year period. These findings are consistent with and extend the report of Oostrom et al (2005) who demonstrated that a particular problem in the early school career of children with epilepsy (i.e., repeating a grade) were associated with an abnormal trajectory in some cognitive abilities. Using a broader definition of problematic school career, we found that children with epilepsy with such histories exhibited significant cognitive growth over time across most but not all (executive function) cognitive domains, but remained significantly below healthy controls and children with epilepsy without comorbidities over the two year interval. Interestingly, it is in the area of executive function that a significant group by time interaction was detected, indicating a divergent cognitive course. Epilepsy children without essential comorbidities and healthy controls exhibited improvement in these skills over the two year period while the epilepsy comorbidity+ group exhibited a flat or worsening course. In order to understand the specifics of this divergent course, the tests within the Executive Function domain were examined and a specific pattern of change was found in sustained attention and concentration with increasing errors of commission (impulsive behavior) and omission (passive inattention) in the epilepsy comorbidity+ group, specifically among children with epilepsy with ADHD (bottom panel Figure 2). Executive functions are critically important in everyday function (Jurado and Rosselli 2007), demonstrated to be adversely affected in childhood epilepsy (Parrish et al., 2007; Sherman et al., 2006), and linked to the core symptom complex of ADHD (Barkley 2006; Doyle 2006). In summary, two specific neurobehavioral comorbidities, each easily identified at or shortly after the time of the diagnosis of epilepsy, are indicative of atypical antecedent as well as subsequent cognitive development. The importance of early recognition and intervention is readily apparent.
Children with epilepsy without essential comorbidities
Especially interesting is the finding that after accounting for these two specific comorbidities (ADHD and AP), there was a substantial percentage of children with epilepsy (46%) that were not different from healthy controls. Children with epilepsy without these two essential comorbidities were comparable and equivalent to healthy controls in all tested areas of cognitive and academic achievement, both at baseline and follow-up, with a comparable rate of cognitive development. These findings confirm that some epilepsies may indeed be benign not only in terms of epilepsy syndrome, but also their impact on cognition, but it is clear that neurobehavioral comorbidities are a critical consideration in this equation.
These findings have implications for an important related literature. Long term prospective investigations of individuals with childhood onset epilepsy have reported considerably poorer psychosocial outcomes in adulthood. These outcomes involve salient areas of life performance and quality of life including employment, marriage and family characteristics, psychiatric status, income and socioeconomic status (Camfield et al., 1993; Jalava and Sillanpää 1997; Jalava et al., 1997; Kokkonen et al., 1997; Koponen et al., 2007; Lindsay et al., 1979a,b,c; Shackleton et al., 2003; Sillanpaa et al., 1998; Wirrell et al., 1997). This general pattern of outcome has been reported even among individuals whose childhood onset epilepsy remitted and medications stopped (Sillanpaa et al., 1998). Similar findings have been reported in other population based studies involving children with “benign epilepsies” such as absence epilepsy (Wirrell et al., 1997). Especially noteworthy is the report that a history of unspecified “learning disorder” appeared significantly predictive of poorer long term psychosocial outcomes, more predictive than so-called biological factors (e.g., age of onset, seizure control) (Camfield et al., 1993). The results of this investigation would predict that the observed divergent patterns of cognitive development associated with ADHD and AP may have direct implications for successful transition to adulthood, particularly since it is known that these comorbidities are known to have a divergent life course in the general population (McKay and Halperin 2001; Seidman 2006; Spencer et al., 2007; Wilens and Dodson 2004). If this proves to be the case, the epilepsy comorbidity+ cohort might represent a critically important target for a variety of early cognitive, social, and transition programs in order to maximize opportunities for successful outcomes in adulthood.
Epilepsy variables and comorbidity status
The presence or absence of the neurobehavioral comorbidities in children with epilepsy was not associated with a variety of epilepsy factors (idiopathic generalized vs. localization related epilepsy, presence or number of medications, age of onset of epilepsy, or two year clinical course. Further, the neurobehavioral comorbidities were not associated with the presence of other medical conditions (e.g. asthma) or use of medications for associated medical conditions. These findings are consistent with prior reports of weak relationships between seizure related variables and cognitive/psychosocial outcomes in children with idiopathic or uncomplicated epilepsy (Camfield et al., 1993; Oostrom et al., 2005). Only the distribution of left handedness separated the groups (epilepsy comorbidity+ group = 21%, epilepsy comorbidity− group = 4%, and control group = 0%).
Limitations and areas for future research
Comorbidity subtypes: lumping versus splitting
Both essential comorbidities examined here (ADHD, AP) reflected composite groupings. The major subtypes of both ADHD (inattentive, hyperactive, combined) and AP (e.g., language-based, nonverbal) should be independently investigated as there may be meaningful differences in baseline cognition and developmental course among specific comorbidity subtypes. The fact that the composite groupings used here were associated with major effects underscores their clinical significance and suggests that investigation of more specific categories of ADHD and AP will prove informative.
Case ascertainment and subject sample
Subject selection was not based on the presence or absence of ADHD or AP comorbities. Case ascertainment was determined by the onset of spontaneous unprovoked seizures, normal clinical imaging, attendance at regular school, and a diagnosis of epilepsy—children characterized by Oostrom et al (2003) as “epilepsy only”. Cohorts of this type provide the advantage of investigating cognition in the absence of severe etiological insults, brain lesions, and other complicating factors associated with other common but more severe childhood epilepsies that are known to affect cognition and cognitive development. The relative contribution of these more severe epilepsies and their underlying effects relative to the impact of the comorbidities examined here is an open question. It is entirely possible that neurobehavioral comorbidities may have less independent influence compared to the fundamentals of epilepsy cause, course, and treatment. In point of fact, the presence of even subtle MR abnormalities identified at the onset of epilepsy (Doescher et al., 2006) have been found to be associated with baseline cognitive abnormalities (Byars et al., 2007). In addition, the epilepsy centers represented here are major catchment sites for specialty care. It is possible that community based investigations may reveal lower rates of comorbidities in new onset epilepsy, but the relationships between cognition and cognitive course should be comparable in similar groups of children with idiopathic epilepsies. However, the children investigated here did not suffer from severe epilepsy. In fact at baseline approximately 23% of children were not treated with antiepilepsy medications, and only 5.7% (3 of 52) were found to have intractable epilepsy over the prospective two year period as defined by a contemporary rating system (Berg et al., 2001). Given the modest sample size of children with epilepsy we restricted our analyses to broad syndromes of LRE and IGE. It would be of interest to examine comparative cognitive developmental issues in discrete syndromes (JME, BECTS), but that will require a larger sample size. Finally, the sample was quite broad in age range (8-18). This provides the advantage of yielding a broad view of cognitive development, but a more restricted age range might prove useful in yielding a finer characterization of cognitive change in more defined developmental epochs.
Duration of follow-up
While very different prospective cognitive trajectories were evident across the epilepsy comorbidity+/− groups and controls, the duration of the follow-up period was modest (2 years). Longer follow-up would be of particular interest as a dynamic pattern of cognitive maturation appears to be taking place in some areas of cognition. The distinct pattern of change for executive function raises the question of associated social and behavioral development given the importance of this cognitive domain for those areas of maturation (Jurado and Rosselli 2007). In addition, longer follow up would allow formal growth curve modeling, something heretofore not available in this literature, and a determination of whether some epilepsy comorbidity+ children might demonstrate later accelerated neurocognitive growth and eventually match controls in cognitive performance as described by others in this literature (Shaw et al., 2007).
Linking cognitive and brain development
Significant changes are occurring in the brain development of children within the age range represented in this investigation (8-18 years), with region-specific patterns of reduction in gray matter volume and increasing volume/myelination of white matter (Gogtay et al., 2004; Sowell et al., 2003, 2004; Thompson et al., 2000). Very little is known about patterns of brain maturation in children with epilepsy and how they may differ from normal developmental trajectories. There should be a degree of symmetry between patterns of neurocognitive and age-appropriate brain development, and this area of research remains to be addressed in childhood epilepsy.
Comorbidities, transition to adulthood, and long term implications
As inferred previously, investigations of this type may inform the long term psychosocial outcome literature. A related consideration, not examined here, is the effect of important moderating factors, such as those that have been shown to be related to family integrity. Family mastery (Austin et al., 2004; Fastenau et al., 2004) and family related impact and reactions to epilepsy (so-called contextual variables) (Oostrom et al., 2005) have been shown influence, moderate or “buffer” cognitive performance in both cross-sectional and longitudinal epilepsy investigations. The effects of these factors on longer term transitions to adulthood remain to be determined.
The umbrella effect of comorbid conditions
A tendency in the epilepsy literature has been to examine cognitive, social, psychological and other complications in isolation. It is possible, and indeed probable, that some comorbidities may subsume a number of these problems under one “diagnosis”. This is of course best demonstrated in ADHD with this diagnosis encompassing symptoms of cognition, parent and teacher ratings of behavior, other psychiatric comorbidities, and social function. These relationships suggest that specific comorbidities may provide an organizing influence through which diverse neurobehavioral symptoms might be investigated.
Conclusion
The presence or absence of two essential comorbidities, readily identifiable early in the course of childhood epilepsy, have a very significant relationship with immediate and prospective (2-year) cognitive maturation. The ways in which this information may be used to improve long term psychosocial outcomes of childhood onset epilepsy needs to be explored.
Supplementary Material
ACKNOWLEDGEMENTS
This project was supported by NIH NINDS RO1-44351, NCRR/NIH 1KL2RR025012-01, and MO1 RR 03186 (GCRC). We thank Michelle Szomi for overall project coordination; Dr. Ryann Watson for direction regarding classification of educational services; Adan Myers y Gutierrez for manuscript preparation, Chase Allen for cognitive testing; and Drs. Jason Doescher , Fred Edelman, David Hsu and Carl Stafstrom for referring patients to this investigation. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
The authors have provided full disclosure of any conflict(s) of interest and have no conflicts of interest to disclose.
REFERENCES
- Aldenkamp A, Arends J. The relative influence of epileptic EEG discharges, short nonconvulsive seizures, and type of epilepsy on cognitive function. Epilepsia. 2004a;45:54–63. doi: 10.1111/j.0013-9580.2004.33403.x. [DOI] [PubMed] [Google Scholar]
- Aldenkamp AP. Effect of seizures and epileptiform discharges on cognitive function. Epilepsia. 1997;38(Suppl 1):S52–55. doi: 10.1111/j.1528-1157.1997.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Aldenkamp AP, Alpherts WC, De Bruine-Seeder D, Dekker MJ. Test-retest variability in children with epilepsy--a comparison of WISC-R profiles. Epilepsy Res. 1990;7:165–172. doi: 10.1016/0920-1211(90)90102-2. [DOI] [PubMed] [Google Scholar]
- Aldenkamp AP, Arends J. Effects of epileptiform EEG discharges on cognitive function: is the concept of “transient cognitive impairment” still valid? Epilepsy Behav. 2004b;5(Suppl 1):S25–34. doi: 10.1016/j.yebeh.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ambrosini PJ. Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS) J Am Acad Child Adolesc Psychiatry. 2000;39:49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- Austin JK, Dunn DW, Johnson CS, Perkins SM. Behavioral issues involving children and adolescents with epilepsy and the impact of their families: recent research data. Epilepsy Behav. 2004;5(Suppl 3):S33–41. doi: 10.1016/j.yebeh.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit/hyperactivity disorder, third edition: a handbook for diagnosis and treatment. Guilford; New York: 2006. [Google Scholar]
- Beghi M, Cornaggia CM, Frigeni B, Beghi E. Learning disorders in epilepsy. Epilepsia. 2006;47(Suppl 2):14–18. doi: 10.1111/j.1528-1167.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- Berg A. Presented at NINDS Conference entitled Curing Epilepsy 2007. Natcher Conference Center, National Institutes of Health; Bethesda, Maryland: 2007. Cognitive and psychological issues in epilepsy: the scope of the problem. [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B, Ebrahimi N. Defining early seizure outcomes in pediatric epilepsy: the good, the bad and the in-between. Epilepsy Res. 2001;43:75–84. doi: 10.1016/s0920-1211(00)00184-4. [DOI] [PubMed] [Google Scholar]
- Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, Shinnar S. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47:749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- Besag FM. Cognitive and behavioral outcomes of epileptic syndromes: implications for education and clinical practice. Epilepsia. 2006;47(Suppl 2):119–125. doi: 10.1111/j.1528-1167.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois BF. Antiepileptic drugs, learning, and behavior in childhood epilepsy. Epilepsia. 1998;39:913–921. doi: 10.1111/j.1528-1157.1998.tb01440.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois BF, Prensky AL, Palkes HS, Talent BK, Busch SG. Intelligence in epilepsy: a prospective study in children. Ann Neurol. 1983;14:438–444. doi: 10.1002/ana.410140407. [DOI] [PubMed] [Google Scholar]
- Byars AW, deGrauw TJ, Johnson CS, Fastenau PS, Perkins SM, Egelhoff JC, Kalnin A, Dunn DW, Austin JK. The association of MRI findings and neuropsychological functioning after the first recognized seizure. Epilepsia. 2007;48:1067–1074. doi: 10.1111/j.1528-1167.2007.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield C, Camfield P, Smith B, Gordon K, Dooley J. Biologic factors as predictors of social outcome of epilepsy in intellectually normal children: a population-based study. J Pediatr. 1993;122:869–873. doi: 10.1016/s0022-3476(09)90009-9. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Perez Velazquez JL, Snead OC., 3rd Animal models of epilepsy and progressive effects of seizures. Adv Neurol. 2006;97:293–304. [PubMed] [Google Scholar]
- Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(Suppl 1):S21–24. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Doescher JS, deGrauw TJ, Musick BS, Dunn DW, Kalnin AJ, Egelhoff JC, Byars AW, Mathews VP, Austin JK. Magnetic resonance imaging (MRI) and electroencephalographic (EEG) findings in a cohort of normal children with newly diagnosed seizures. J Child Neurol. 2006;21:491–495. [PMC free article] [PubMed] [Google Scholar]
- Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(Suppl 8):21–26. [PubMed] [Google Scholar]
- Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Dev Med Child Neurol. 2003;45:50–54. [PubMed] [Google Scholar]
- Dunn DW, Kronenberger WG. Attention-deficit/hyperactivity disorder in children and adolescents. Neurol Clin. 2003;21:933–940. doi: 10.1016/s0733-8619(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Dunn DW, Kronenberger WG. Childhood epilepsy, attention problems, and ADHD: review and practical considerations. Semin Pediatr Neurol. 2005;12:222–228. doi: 10.1016/j.spen.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Fastenau PS, Johnson CS, Byars AW, Dunn DW, Austin JK, Perkins SM. Abstracts from the joint annual meeting of the American Epilepsy Society and the American Clinical Neurophysiology Society; Epilepsia; Washington DC, USA. December 2-6, 2005; 2005. p. 76. (Abst. 71.180) [Google Scholar]
- Fastenau PS, Shen J, Dunn DW, Perkins SM, Hermann BP, Austin JK. Neuropsychological predictors of academic underachievement in pediatric epilepsy: moderating roles of demographic, seizure, and psychosocial variables. Epilepsia. 2004;45:1261–1272. doi: 10.1111/j.0013-9580.2004.15204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Heydrich J, Dodds A, Whitney J, MacMillan C, Waber D, Faraone SV, Boyer K, Mrakotsky C, DeMaso D, Bourgeois B, Biederman J. Psychiatric disorders and behavioral characteristics of pediatric patients with both epilepsy and attention-deficit hyperactivity disorder. Epilepsy Behav. 2007;10:384–388. doi: 10.1016/j.yebeh.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, Jones J, Dabbs K, Allen CA, Sheth R, Fine J, McMillan A, Seidenberg M. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain. 2007;130:3135–3148. doi: 10.1093/brain/awm227. [DOI] [PubMed] [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Ludvigsson P, Hauser WA, Olafsson E, Kjartansson O. Co-occurrence of major depression or suicide attempt with migraine with aura and risk for unprovoked seizure. Epilepsy Res. 2007;75:220–223. doi: 10.1016/j.eplepsyres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61:731–736. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- Hommet C, Sauerwein HC, De Toffol B, Lassonde M. Idiopathic epileptic syndromes and cognition. Neurosci Biobehav Rev. 2006;30:85–96. doi: 10.1016/j.neubiorev.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Jalava M, Sillanpää M. Physical activity, health-related fitness, and health experience in adults with childhood-onset epilepsy: a controlled study. Epilepsia. 1997;38:424–429. doi: 10.1111/j.1528-1157.1997.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Jalava M, Sillanpaa M, Camfield C, Camfield P. Social adjustment and competence 35 years after onset of childhood epilepsy: a prospective controlled study. Epilepsia. 1997;38:708–715. doi: 10.1111/j.1528-1157.1997.tb01241.x. [DOI] [PubMed] [Google Scholar]
- Jones JE, Watson R, Sheth R, Caplan R, Koehn M, Seidenberg M, Hermann B. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493–497. doi: 10.1111/j.1469-8749.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kokkonen J, Kokkonen ER, Saukkonen AL, Pennanen P. Psychosocial outcome of young adults with epilepsy in childhood. J Neurol Neurosurg Psychiatry. 1997;62:265–268. doi: 10.1136/jnnp.62.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A, Beilmann A, Tomberg T, Napa A, Talvik T. Neurocognitive development of children with congenital unilateral brain lesion and epilepsy. Brain Dev. 2001;23:88–96. doi: 10.1016/s0387-7604(01)00180-2. [DOI] [PubMed] [Google Scholar]
- Koop JI, Fastenau PS, Dunn DW, Austin JK. Neuropsychological correlates of electroencephalograms in children with epilepsy. Epilepsy Res. 2005;64:49–62. doi: 10.1016/j.eplepsyres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Koponen A, Seppala U, Eriksson K, Nieminen P, Uutela A, Sillanpaa M, Hyvarinen L, Kalviainen R. Social functioning and psychological well-being of 347 young adults with epilepsy only--population-based, controlled study from Finland. Epilepsia. 2007;48:907–912. doi: 10.1111/j.1528-1167.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- Lassonde M, Sauerwein HC, Jambaque I, Smith ML, Helmstaedter C. Neuropsychology of childhood epilepsy: pre- and postsurgical assessment. Epileptic Disord. 2000;2:3–13. [PubMed] [Google Scholar]
- Lendt M, Helmstaedter C, Elger CE. Pre- and postoperative neuropsychological profiles in children and adolescents with temporal lobe epilepsy. Epilepsia. 1999;40:1543–1550. doi: 10.1111/j.1528-1157.1999.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Ounsted C, Richards P. Long-term outcome in children with temporal lobe seizures. I: Social outcome and childhood factors. Dev Med Child Neurol. 1979a;21:285–298. doi: 10.1111/j.1469-8749.1979.tb01621.x. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Ounsted C, Richards P. Long-term outcome in children with temporal lobe seizures. II: Marriage, parenthood and sexual indifference. Dev Med Child Neurol. 1979b;21:433–440. doi: 10.1111/j.1469-8749.1979.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Ounsted C, Richards P. Long-term outcome in children with temporal lobe seizures. III: Psychiatric aspects in childhood and adult life. Dev Med Child Neurol. 1979c;21:630–636. doi: 10.1111/j.1469-8749.1979.tb01677.x. [DOI] [PubMed] [Google Scholar]
- Loring DW, Marino S, Meador KJ. Neuropsychological and Behavioral Effects of Antiepilepsy Drugs. Neuropsychol Rev. 2007 doi: 10.1007/s11065-007-9043-9. [DOI] [PubMed] [Google Scholar]
- Macallister WS, Schaffer SG. Neuropsychological deficits in childhood epilepsy syndromes. Neuropsychol Rev. 2007;17:427–444. doi: 10.1007/s11065-007-9048-4. [DOI] [PubMed] [Google Scholar]
- McKay KE, Halperin JM. ADHD, aggression, and antisocial behavior across the lifespan. Interactions with neurochemical and cognitive function. Ann N Y Acad Sci. 2001;931:84–96. doi: 10.1111/j.1749-6632.2001.tb05774.x. [DOI] [PubMed] [Google Scholar]
- McNelis AM, Dunn DW, Johnson CS, Austin JK, Perkins SM. Academic performance in children with new-onset seizures and asthma: a prospective study. Epilepsy Behav. 2007;10:311–318. doi: 10.1016/j.yebeh.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MA, Redoblado MA, Lah S, Sabaz M, Lawson JA, Cunningham AM, Bleasel AF, Bye AM. Intelligence in childhood epilepsy syndromes. Epilepsy Res. 2003;53:139–150. doi: 10.1016/s0920-1211(02)00261-9. [DOI] [PubMed] [Google Scholar]
- Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- Oostrom KJ, van Teeseling H, Smeets-Schouten A, Peters AC, Jennekens-Schinkel A. Three to four years after diagnosis: cognition and behaviour in children with ‘epilepsy only’. A prospective, controlled study. Brain. 2005;128:1546–1555. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- Parrish J, Geary E, Jones J, Seth R, Hermann B, Seidenberg M. Executive functioning in childhood epilepsy: parent-report and cognitive assessment. Dev Med Child Neurol. 2007;49:412–416. doi: 10.1111/j.1469-8749.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- Reijs RP, van Mil SG, van Hall MH, Arends JB, Weber JW, Renier WO, Aldenkamp AP. Cryptogenic localization-related epilepsy with childhood onset: The problem of definition and prognosis. Epilepsy Behav. 2006;8:693–702. doi: 10.1016/j.yebeh.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Pulsipher DT, Hermann B. Cognitive progression in epilepsy. Neuropsychol Rev. 2007;17:445–454. doi: 10.1007/s11065-007-9042-x. [DOI] [PubMed] [Google Scholar]
- Seidman LJ. Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev. 2006;26:466–485. doi: 10.1016/j.cpr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Shackleton DP, Kasteleijn-Nolst Trenite DG, de Craen AJ, Vandenbroucke JP, Westendorp RG. Living with epilepsy: long-term prognosis and psychosocial outcomes. Neurology. 2003;61:64–70. doi: 10.1212/01.wnl.0000073543.63457.0a. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman EM, Slick DJ, Eyrl KL. Executive dysfunction is a significant predictor of poor quality of life in children with epilepsy. Epilepsia. 2006;47:1936–1942. doi: 10.1111/j.1528-1167.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- Smith ML, Elliott IM, Lach L. Cognitive skills in children with intractable epilepsy: comparison of surgical and nonsurgical candidates. Epilepsia. 2002;43:631–637. doi: 10.1046/j.1528-1157.2002.26101.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. Ambul Pediatr. 2007;7:73–81. doi: 10.1016/j.ambp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Stores G, Williams PL, Styles E, Zaiwalla Z. Psychological effects of sodium valproate and carbamazepine in epilepsy. Arch Dis Child. 1992;67:1330–1337. doi: 10.1136/adc.67.11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedrus GM, Fonseca LC, Tonelotto JM, Costa RM, Chiodi MG. Benign childhood epilepsy with centro-temporal spikes: quantitative EEG and the Wechsler intelligence scale for children (WISC-III) Clin EEG Neurosci. 2006;37:193–197. doi: 10.1177/155005940603700306. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- van Rijckevorsel K. Cognitive problems related to epilepsy syndromes, especially malignant epilepsies. Seizure. 2006;15:227–234. doi: 10.1016/j.seizure.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Vermeulen J, Aldenkamp AP. Cognitive side-effects of chronic antiepileptic drug treatment: a review of 25 years of research. Epilepsy Res. 1995;22:65–95. doi: 10.1016/0920-1211(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65:1301–1313. doi: 10.4088/jcp.v65n1003. [DOI] [PubMed] [Google Scholar]
- Williams J, Bates S, Griebel ML, Lange B, Mancias P, Pihoker CM, Dykman R. Does short-term antiepileptic drug treatment in children result in cognitive or behavioral changes? Epilepsia. 1998a;39:1064–1069. doi: 10.1111/j.1528-1157.1998.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Williams J, Griebel ML, Dykman RA. Neuropsychological patterns in pediatric epilepsy. Seizure. 1998b;7:223–228. doi: 10.1016/s1059-1311(98)80040-x. [DOI] [PubMed] [Google Scholar]
- Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheeps' clothing. Arch Pediatr Adolesc Med. 1997;151:152–158. doi: 10.1001/archpedi.1997.02170390042008. [DOI] [PubMed] [Google Scholar]
- Wolff M, Weiskopf N, Serra E, Preissl H, Birbaumer N, Kraegeloh-Mann I. Benign partial epilepsy in childhood: selective cognitive deficits are related to the location of focal spikes determined by combined EEG/MEG. Epilepsia. 2005;46:1661–1667. doi: 10.1111/j.1528-1167.2005.00255.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.