Abstract
This paper describes work aimed at combining 3D ultrasound with full-field digital mammography via a semi-automatic prototype ultrasound scanning mechanism attached to the digital mammography system gantry. Initial efforts to obtain high x-ray and ultrasound image quality through a compression paddle are proving successful. Registration between the x-ray mammogram and ultrasound image volumes is quite promising when the breast is stably compressed. This prototype system takes advantage of many synergies between the co-registered digital mammography and pulse-echo ultrasound image data used for breast cancer detection and diagnosis. In addition, innovative combinations of advanced US and X-ray applications are being implemented and tested along with the basic modes. The basic and advanced applications are those that should provide relatively independent information about the breast tissues. Advanced applications include x-ray tomosynthesis, for 3D delineation of mammographic structures, and non-linear elasticity and 3D color flow imaging by ultrasound, for mechanical and physiological information unavailable from conventional, non-contrast x-ray and ultrasound imaging.
Introduction
Breast ultrasound (US) is a valuable diagnostic adjunct to x-ray mammography for characterization of breast lesions such as cysts and solid masses, and evaluation of palpable masses that are obscured radiographically by dense breast tissue.1,2,3 Recently, there have been several studies suggesting the potential emerging role of ultrasound as a screening adjunct to x-ray mammography.4,5,6,7,8,9 For example in a study of 11,130 asymptomatic women, Kolb et al9 recently reported that the combined sensitivity of x-ray mammography and radiologist performed free-hand 3D breast ultrasound for women with dense breasts (BI-RADS10 density category 4) improved to 94% from 48% for x-ray mammography alone. When mammographic findings indicate the need for follow up imaging with ultrasound, the specific regions in the breast requiring further interrogation must be anatomically identified for subsequent positioning and manipulation of an ultrasound probe. The critical step of accurately localizing the regions of interest however can be challenging to implement for a number of reasons. First, mammograms and sonograms are acquired with the patient in different positions - upright for the mammogram and supine for the US examination. This requires the sonographer to estimate the approximate 3D location of the region of interest from a 2D x-ray projection of a deformable breast. Second, US imaging is performed predominantly through free-hand manual manipulation of ultrasound probes in direct contact with the breast. The experience and skills of the operators may impact the accuracy of locating the region of interest. Radiologist hand scanning, although preferable, is expensive. Scanning by a technologist may suffer from inadequate communication of clinically needed imaging locations and orientations and from a lower level of skill in detection and discrimination of lesions. Third, mammograms and sonograms may not be acquired on the same day. Therefore normal fibrocystic changes occurring over a period of time may lead to confusion in localizing the desired region of interest. Fourth, since mammography and ultrasound are different modalities, structures within the breast may be occult on one modality and visible on the other. Finally, the presence of multi-focal and/or multi-centric disease may cause confusion in correctly identifying the corresponding regions on both image sets.
The potential impact of incorrectly registering mammograms with sonograms was reported by Conway et al in a study comprising 50 patients with masses or cysts.11 In this study standard mammograms and free-hand sonograms thought to correspond with mammographically visible regions of interest were acquired. A special compression paddle with a localization grid and a cutout was used to acquire mammograms and free-hand sonograms with the patient in the same position for both image acquisitions. The authors found that in 10% of the cases, the mammographic regions of interest did not correspond to those identified with free-hand ultrasound. This study illustrates that the potential for incorrect localization of regions of interest is real.
A solution to these issues of localization and operator dependence is to have a dedicated breast imaging system for 3D ultrasound breast imaging with the breast compressed in a manner similar to that in the x-ray mammography set up. Several researchers have proposed and constructed such dedicated breast scanning systems.12,13,14,15,16,17,18,19 These systems consisted of a compression paddle or similar breast immobilization device with the patient either seated upright or lying prone with the pendant breast immersed in a water bath. In these systems, the breast was scanned through a plastic plate. Kelly-Fry, et al., have been leaders in this effort over the past decade.16,17,18 They scanned the breast with a modest-quality ultrasound linear array through a moderately thin, rigid plastic mammographic compression paddle. Richter12,19 performed extensive studies involving scanning through a thick, rigid plate. In an earlier study at the University of Michigan, 3D ultrasound imaging, including vascular imaging was evaluated using a mammographic type system with a thin Mylar membrane.20 In general, improvements in localization were obtained with these approaches even though they were not fully developed for ease of use, image quality and appearance. In the Indianapolis system (Kelly-Fry), reverberations and defocusing effects in the rigid plate were not solved well. The Richter system suffered unnecessary attenuation of the ultrasound beam by the thick plastic compression plate. This forced the use of low ultrasound frequencies, which degraded spatial resolution. In all systems, ultrasound scanning with the breast in oblique and lateral views, and acoustic coupling to the breast were less than ideal. Other limitations included older imaging technologies, the need to display film from the two modalities and difficulty with interfacing bulky scanning systems on a mammography system. These early efforts did not use many of the advanced ultrasound imaging techniques that are beginning to show much promise for contributing to breast cancer detection and diagnosis. They also did not employ full-field digital mammography (FFDM) systems.
In this work, a prototype compression paddle system has been developed that enables co-registered, sequential acquisition of digital mammograms and 3D US images of the breast within a single examination (Figure 1). The intent is to provide operator-independent, intrinsic registration of the two image sets through hardware design and therefore improved localization of regions of interest between the two modalities without adversely compromising image quality. It is expected that improved localization combined with the use of current state-of-the-art imaging systems will overcome many of the issues affecting image quality and registration reported in previous work.
Figure 1.
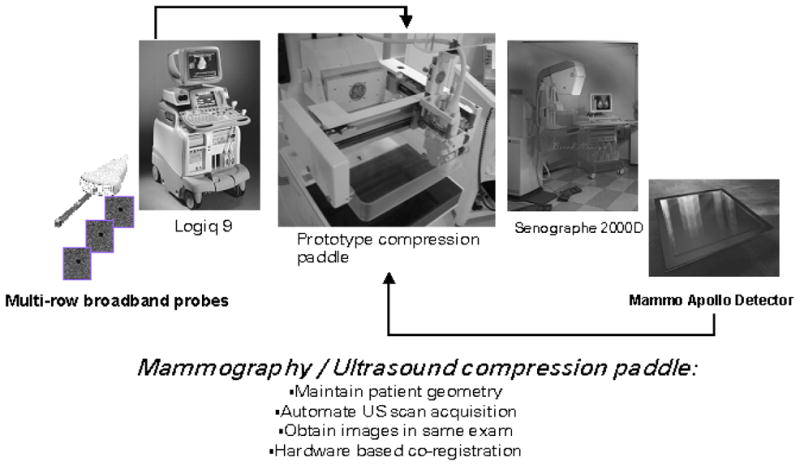
Dual-modality prototype compression paddle is a system to interface the 19×23cm2 full-field GE digital mammography x-ray detector on the Senographe 2000D FFDM system with the Logiq 9 ultrasound system using a M12L transducer array.
While the combined system is designed as a practical implementation to combine the advantages of two proven breast imaging modalities with excellent spatial correlation, the system also can serve as a nearly ideal platform for developing, evaluating and comparing new x-ray and ultrasound imaging techniques. Tomosynthesis x-ray imaging offers the possibility of separating structures in the depth dimension, thereby providing a 3D x-ray delineation of the breast. It is expected to substantially improve visibility of calcifications and masses relative to 2D projection mammography due to the removal of structured noise. Ultrasound based elasticity imaging displays tissue properties related to the compressibility, but with greater sensitivity and resolution than palpation. Tumor vasculature and flow characteristics, obtained with color flow ultrasound imaging, provide additional discrimination of breast cancer, which is unavailable from x-ray mammography. Compound or Crossbeam ultrasound imaging offers the potential of increasing the signal to noise ratio of tumors and cysts by reducing the speckle noise of all the tissues and, to a lesser extent, by averaging out artifactual signals from beam side lobes and multiple scattering. Also, posterior borders of attenuating tumors generally are delineated much more clearly. The possibility of providing the first direct, 3D comparison of gray scale and advanced ultrasound breast tissue properties with X ray tissue properties from tomosynthesis also is extremely promising.
The research focuses on integrating breast ultrasound and digital mammography hardware and software, with the goal of providing the sensitivity of mammography and specificity of breast ultrasound in the same examination with real-time display. This improvement is expected to result in reduced callback rates, fewer unnecessary biopsies, fewer false negatives, reduced patient anxiety and increased efficiency and throughput in screening and diagnostic examinations. A brief description of the prototype system design, initial phantom studies and clinical cases follows.
Materials and Methods
Overview of System Design
A prototype dual modality mammography/ultrasound compression paddle system has been developed for use with a Senographe 2000D FFDM system in conjunction with a Logiq 9 ultrasound system (GE Health Care, Waukesha, WI) (Figure 2). Preliminary versions of this system developed using a prototype GE digital mammography system and GE Logiq 700 ultrasound system have been reported elsewhere.21,22 This system is designed to slide into the mounting bracket of a standard 19×23-cm2 mammography compression paddle on the Senographe2000D, so that it is exactly positioned above the flat-panel digital mammography detector. Using the positioning capabilities of the Senographe 2000D gantry, cranial-caudal, oblique or lateral views of the breast may be obtained.
Figure 2.

Prototype compression paddle mounted on the GE Senographe 2000D with an M12L ultrasound probe attached.
The prototype compression paddle system consists of a frame for holding the compression paddle and an automated ultrasound probe mover assembly. The latter translates the probe holder that houses a high-frequency GE M12L ultrasound transducer, over the compression paddle. Prior to patient scanning the probe positioning system is calibrated with respect to the compression paddle. This step is needed for the subsequent registration of the ultrasound images with the mammogram. The probe holder is initially removed from the compression paddle to allow unobstructed full-field x-ray exposure of the breast. It is then attached to the assembly and electro-mechanically scanned over the acoustically coupled breast through computerized control. The ability to position the probe near the chest wall is limited by the shape of the shell of the probe and the thickness of the mount that supports it. A dedicated shell design can bring the probe to within a few mm of the chest wall edge of a mammography paddle. Additional deeper layers of tissue near the chest wall, potentially missed on mammograms may be imaged ultrasonically by electronically steering the beams until grating lobes start to appear. In the initial design, the rounded regions of the breast that are not in direct physical contact with the compression paddle are only partially imaged with ultrasound. The probe sweeps across the paddle in a raster fashion until the breast has been fully scanned to obtain 3D gray scale US images. Alternatively, scans can be limited to smaller regions of interest in order to obtain additional views or advanced US mode images such as Doppler scans, compound images or elasticity images. The probe may be directed to a region of the breast based on the mammogram by using the x-ray co-ordinates of the center of the region of interest and the co-ordinate transformation indices obtained during initial calibration.
Efforts were made to minimize patient set-up and study acquisition time in order to improve patient comfort and reduce patient motion. A wide array transducer, rapid probe translation and data acquisition system with sufficient computer memory to accommodate the large number of 2D ultrasound frames needed to cover the full breast at a reasonable frame interval and spacing are required. The time to acquire a full mammogram is of the order of a few seconds or less (ignoring patient set up). Hand-held US scans may require several minutes. For 3D Ultrasound with the M12L matrix array, a frame spacing of 0.25 – 0.4 mm is suggested for highest image quality. As an example of the time required for the 3D ultrasound image acquisition, to cover a breast with 16 × 12 cm2 area using a ~ 4 cm long array probe at 0.4 mm spacing ~1200 frames are required. These are typically acquired in 2 minutes 30 seconds using an acoustic frame rate of 8 Hz. The exact scanning times and settings will vary for different numbers of focal zones, breast areas and thickness. The time taken to ultrasonically scan the breast may be significantly reduced to less than a minute if a higher acoustic frame rate is used. In this work, the intent was to initially use scan settings similar to those used for manual scanning for comparison of image quality. The ultrasonic scanning time is significantly longer than that required to acquire a mammogram, but less than or comparable to that required for a manual full breast ultrasound scan.
Image Quality and Associated System Design Considerations
To meet the goals of this research, several issues related to image quality were considered in the design of the prototype and are described below.
For dual modality imaging, the choices of material composition and thickness dimensions of the compression paddle were restricted to meet specific criteria. The paddle was required to be radiolucent, sonolucent, acoustically matched with the components in the ultrasound beam path, sufficiently transparent for breast positioning, and mechanically capable of sustaining compression forces up to 20 daN with a maximum horizontal deflection below the 1-cm Mammography Quality Standards Act (MQSA) limit.23 Two broad types of compression paddles have been developed for evaluation. One is composed of a rigid plastic plate of thickness comparable to that of the standard mammography compression paddle. The second paddle is composed of a polymeric membrane less than 500 micron thick with a reinforcing frame and tensioning mechanism to satisfy the maximum deflection constraints. LEXAN® and Poly-4-methyl 1-pentene or PMP commercially available as TPX (Mitsui Plastics Inc., White Plains, NY) were chosen as the candidate materials for the membrane paddles and TPX was chosen for the thick plate compression paddle.
Acoustic coupling gel or fluid is placed between the transducer face and the compression plate, and between the compression plate and the breast to ensure transmission of ultrasound energy. Acoustic coupling media and methods of application were carefully developed so as to not degrade x-ray image quality or absorb the ultrasound beam significantly or non-uniformly or lubricate the breast so much as to allow it to slip out from the paddle.
Because the acoustic beam traverses the plastic plate, refraction occurs. To minimize refraction effects, the transmit and receive mode time delays of the beam formation process were adjusted in accordance with the laws of refraction to ensure that the beams fired from individual channels of the transducer reach the focal depths in phase and the beam summation of the received beams is accordingly corrected.24 Acoustic power testing was done in this special mode in order to ensure compliance with regulatory ultrasound power limits. A second consideration is that the net compression force, when applied to a breast of a given tissue composition, results in mechanical bowing of the plate. Since the probe rides above the paddle with a small gap for coupling media to be inserted, the ultrasound beams propagate through a non-uniform gap between the probe face and the bowed paddle. An increasing gap increases the ultrasound path between the transducer and the paddle and results in reverberations deep into the image. A non-parallel surface leads to variable beam refraction at different locations on the plate. Both of these factors adversely affect the ultrasound image quality. In order to maintain a uniform and parallel gap between the ultrasound probe face and the compression paddle, the probe holder supporting the M12L transducer was mechanically modified to permit probe self-reorientation during translation over the nominally deformed compression paddle. Spring loading was employed to allow the probe to conform to the bowed compression paddle surface. The probe tilts in the x and y-axes and translates along the vertical or z-axis. It has sensors that register probe position and orientation for correct slice-to-slice registration. These mechanical modifications enable the same time delays in the beam formation process to be used for all transducer positions within the allowed space of travel. In addition, the breast tissue loses contact with the compression plate in the sub-areolar, retro-mammary, medial and lateral breast regions, causing insufficient acoustic energy transmission in these regions. To compensate for this, changes in the ultrasonic beam formation process were incorporated to adjust the ultrasound beam steering to permit partial visualization of structures in the breast not directly below the transducer.
The spatial resolution of ultrasound probes is superior along the axial and lateral directions relative to the elevation directions. Hence linear structures aligned perpendicular to these directions, are well resolved. With a default probe orientation, the lateral direction is perpendicular to the chest wall. However, some structures within the breast, e.g. ducts, are usually aligned in the perpendicular direction. The compliant probe holder has been designed to permit probe orientations about its longitudinal axis and hence align the lateral direction normal to the direction of such tubes and other structures of interest.
Mechanical Considerations
Mechanical load tests at extreme compression forces of 20 daN indicate that the membrane and thick plate paddle do not deflect by more than 1 cm and that the paddle can sustain repeat load cycles. Mechanical damage in fatigue is non linear with stress. For many thermoplastics, including polycarbonate, the rate of crack propagation in fatigue, is related to the load range or stress excursion delta by a power law, with powers between 2 and 6, depending on material (approximately 4 for LEXAN® and slightly higher for TPX).25 Thus at 20 daN, the extreme compression condition, equivalent crack length or damage will occur in ~ 1/16 as many cycles as at 10 daN. In addition, this system is a research platform with expected use on a relatively small number of patients. Hence the paddles were subjected to only 100 load cycles at extreme conditions. No cracking or other deterioration of either the LEXAN® membrane material or the TPX paddle was observed following these tests. The thick TPX paddle did creep during the initial portion of its test, which resulted in a quasi-permanent deflection of 3 mm. No creep was observed in the LEXAN® or TPX membrane. Maximum deflections in both cases were less than the MQSA standards specification of 10 mm.24
Registration of mammography and ultrasound images
The image sets obtained with the prototype system are inherently co-registered, since the patient is virtually in the same position for the mammogram and ultrasound. The accuracy of positioning the probe through computer control is ± 30 microns, which is well within the dimensions of the x-ray pixels. With the breast in stable compression, a rigid transformation can be applied to the ultrasound volume for spatial registration with the mammogram. Matching regions of interest on either image set can be simultaneously viewed on side-by-side image displays. Co-registered images can be displayed off-line on a stand-alone PC with high-resolution video displays. Dedicated software, based on the Visualization Toolkit (VTK)26 has been developed to permit simultaneous display of mammographic and ultrasound images. It is important to note that the mammogram is obtained at a perspective view, with the x-ray tube focal spot placed vertically above the chest wall edge of the x-ray detector. On the other hand the 2D ultrasound images are obtained with the ultrasound probe positioned directly above the tissue. Furthermore the mammogram is a 2D projection image while the ultrasound images are a stack of parallel 2D slices, oriented perpendicular to the plane of the x-ray detector. During image review, when a cursor is positioned at a point on the mammogram, its corresponding position on the reconstructed C-plane ultrasound image can be estimated using the height of this plane, by simple back-projection. Similarly when a cursor is placed on an acquired or reformatted ultrasound image plane, its corresponding location on the mammogram may be estimated by forward projection. The accuracy of registration using this scheme is within 2 mm. During patient imaging procedures, non-rigid organ and internal tissue movements of course cannot be avoided. Feature-based registration and mutual information based registration schemes are being developed towards the quantification of these effects and but a description of these is beyond the scope of this paper.
Results and Discussion
Initial results of phantom and clinical studies for the combination of 2D mammography with 3D gray scale ultrasound through the prototype compression paddle system are reported below.
X-ray Image Quality
Half value layer (HVL) and x-ray transmission measurements taken for a range of thicknesses of TPX plates using Mo/Mo, Mo/Rh and Rh/Rh target/filter combinations over the range of 27 to 32 kVp indicated that 4.5 mm of TPX is equivalent to a conventional mammography compression paddle made of ~2.5 mm of LEXAN®. Hence in order to use TPX plates or membranes the total path length traversed by x-rays should be 4.5 mm. This requires the use of a dedicated filter for a specific TPX thickness. For example, for a 1 mm thick TPX compression paddle, a 3.5 mm thick filter should be added to the mammography unit.
MTF measurements were made using the edge response function method 27 using a tungsten edge phantom placed after either a TPX paddle or a LEXAN® paddle. The MTF measurements were found to coincide to within 1 % for all frequencies of interest. The addition of visco-elastic gel on the top of the compression paddle without bubbles or nonuniform thickness was difficult, precluding its use during the mammographic procedure. Therefore, the mammogram was acquired prior to the ultrasound scan or the gel or fluid for coupling to the transducer was wiped out of the paddle before the mammogram.
Ultrasound Image Quality
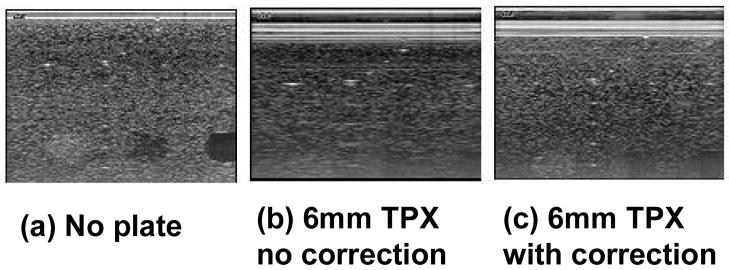
Preliminary experiments indicate that for paddle thickness less than 3.2 mm, ultrasound attenuation is less than 4 dB and contrast loss is less than 1 dB when appropriate refraction correction algorithms are implemented (Table 1). Images were acquired using the RMI 404 GS phantom with and without a TPX plate. The experimental plate thickness used was 6 mm so that the effects of refraction could be exaggerated. Figure 3a shows the image acquired without any TPX material. Figure 3b shows the image acquired through a 6 mm-TPX plate, uncorrected for refraction effects. Figure 3c shows the image acquired through the TPX plate with the correct beam formation code implemented in order to correct for refraction effects. Blurring due to refraction and losses through the TPX plate is evident from the appearance of the wire targets when compared with the direct contact baseline conditions. This partially accounts for the poor image quality observed in previous attempts to image the breast through thick plastic plates with ultrasound. With appropriate corrections in the beam formation time delays, image sharpness is restored and attenuation losses are small relative to the full dynamic range of the gray scale images. The latter may be partially restored by reducing the plate thickness, increasing the gain, increasing the acoustic output power, choosing an impedance-matched material and steering beams to further minimize reverberations.
Table 1.
Impact of refraction corrections for through plate transmission on (lateral) resolution, contrast and signal attenuation, measured with a custom tissue mimicking ultrasound phantom. The baseline measurements were made with direct contact (no through plate) scans of the phantom.28
| Window | Lateral Resolution (FWHM)Reduction | Contrast Reduction (−30 dB void) | Signal Loss (4 cm depth) |
|---|---|---|---|
| 3.2 mm TPX No Correction | 86% | 3.5 dB | 8 dB |
| 3.2 mm TPX Corrected | 7% | 0.5 dB | 3 dB |
Figure 3.
Correction for refraction effects. (a) RMI 404 GS phantom image acquired without any TPX plate at 9 MHz using an M12L probe. (b) The image is acquired using the same US settings with 6 mm of TPX placed on top of the phantom. (c) The imaging conditions are the same as (b), but refraction corrections were implemented in the beam forming code.
In order to ensure that the beam forming calculations do not modify acoustic power levels within any region in the breast that is insonified to levels which exceeding regulations, phantom experiments were performed in a water bath using a hydrophone. The experimental parameters were chosen to bracket the range of frequencies, depths and materials that may be used for constructing the compression paddles. Mechanical and thermal indices were measured and found to be within regulatory guidelines.29,30 All measurements were compared with standard beam formation calculations in a water bath in superficial, intermediate and deeper regions. When a plate was used without refraction corrections, the images appeared defocused and the power was seen to decrease significantly. The indices for all scenarios (including those with refraction corrections) where a plate or membrane was used were lower than those with no plate. Hence it may be concluded that the beam forming algorithms are safe for use with patients.
The impact of ultrasound beam transmission through a plate or membrane was studied on patient images acquired with the prototype. All known cysts were detected with both direct contact hand imaging and automated scanning. Mean cyst contrast in automated US scans was within 2.2 dB of contrast with direct contact, hand held US scans. Major differences in contrast were not detectable between cysts imaged with a compression plate thickness of 1 mm and of 2.5 mm.
With the automated system, relative in vivo contrast loss relative to direct contact hand scanning is apparently from increased reverberation and multiple scattering signals in the essentially echo free cysts, signal attenuation in the compression plate and in the greater depth of the cysts from the transducer. These contrast losses are consistent with the signal loss and increased reverberation levels measured when imaging line targets through the compression paddle. Half of the signal loss through the paddle can be compensated with no increased ultrasound exposure levels to the patient by increasing the transmitted power. The small contrast loss is consistent with radiologist impressions that the image quality was somewhat reduced when compared with expert direct contact scanning. The differences may well be worth the advantages of whole breast automated scanning and can be reduced by employing a transducer of lower frequency (reducing signal losses and multiple scattering) and by use of a stretched membrane instead of a solid paddle.
Clinical Images
Forty-eight patients have been imaged with the prototype compression paddle system. The clinical images have adequate image quality and patients tolerate the procedure without substantial discomfort. There was a high degree of patient satisfaction regarding the comfort and time involved.
In Figure 4, co-registered images are shown for a dense breast of a 36-year-old woman. A 10 mm × 7 mm × 7 mm circumscribed oval hypoechoic mass (indicated with white arrow) with slightly increased through transmission and multiple internal echoes was clearly seen on the sonogram but was relatively occult on the mammogram. It was assessed as a very low suspicion, likely complex, and vaguely palpable mass in the right lateral breast. Biopsy indicated benign mammary epithelium. Benign microcalcifications were seen on the mammogram but were occult on the sonograms. With the combined images, both the mass and the calcifications were seen. The reconstructed ultrasound planes orthogonal to the acquired plane are also shown corresponding to the region of the mass.
Figure 4.
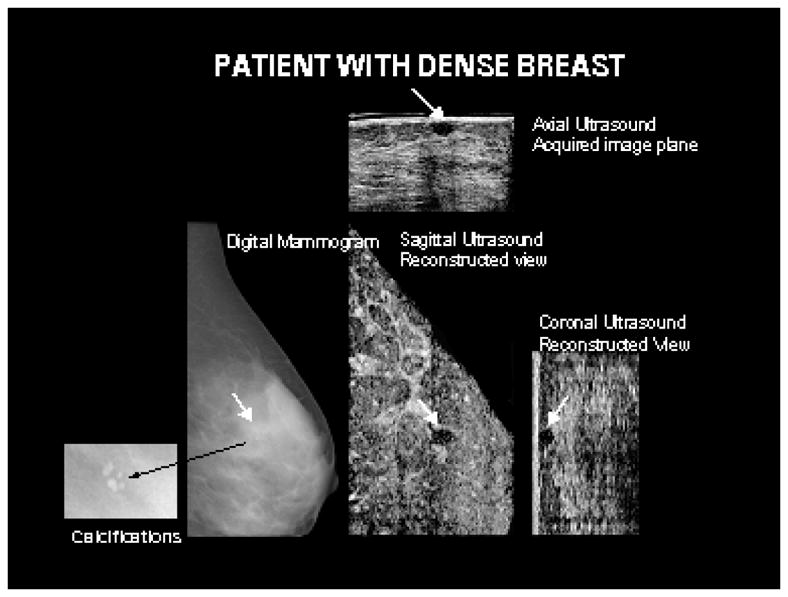
A benign circumscribed oval hypoechoic mass in a 36 year old patient with dense breast tissue, identified by white arrows, is seen on the acquired and reconstructed ultrasound planes. The mass was occult on the mammogram. Shown in the insert corresponding to the mammogram are benign calcifications not visible on the ultrasound images.
In Figure 5, the mammogram and corresponding ultrasound images of a patient with two cysts are shown. A 5 mm simple cyst was clearly visible on the sonogram but occult on the mammogram. Another mass seen deeper and more lateral was visible on both the hand held ultrasound scan and the prototype dual-modality compression paddle; but, not on the mammogram. This mass was evaluated to be a simple cyst on both hand-held ultrasound and with the prototype system.
Figure 5.
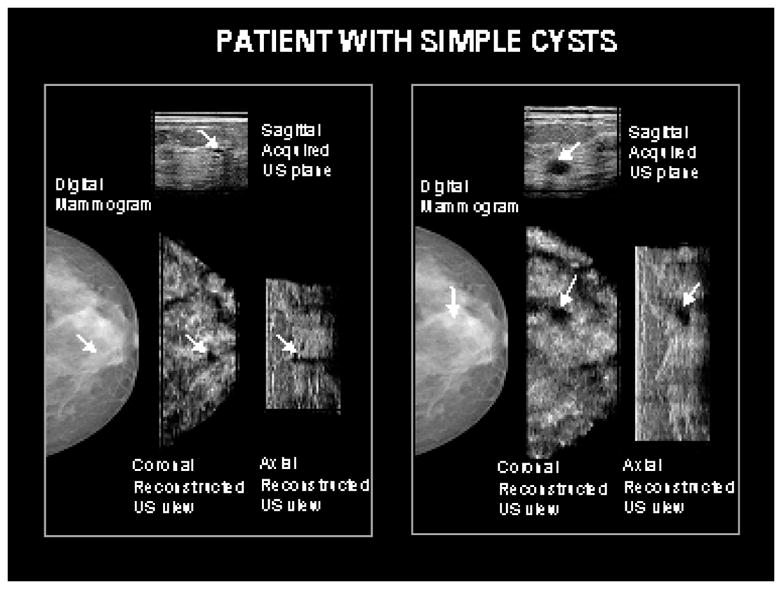
Two cysts seen with ultrasound are relatively occult on the corresponding mammogram. The cysts were similar in appearance on the hand-held direct contact scans as well as those obtained with the prototype system.
The ultrasound image quality of the prototype system appears to be similar, to that of hand-held scans. The small losses in image quality are minor when the full dynamic range of the ultrasound image is considered and are significantly less than those seen in previous studies of imaging through compression paddles. While these have been partially quantified in the previous section, the direct clinical impact remains to be determined. It is expected that with further technique optimization and the incorporation of advanced modes of imaging, the additional information that will be gleaned about the breast tissues will significantly exceed that attained through non co-registered imaging. The imaging techniques have not been sufficiently optimized to correct for minor signal loss, as other challenges are being addressed first. One of these challenges pertains to the issue of full breast coverage. In the sub-areolar, lateral and medial portions of the compressed breast, the rounded edges of the breast cause a loss of physical and hence acoustic contact with the face of the ultrasound transducer. Currently, electronic beam steering, trapezoidal scans, dedicated acoustic coupling and minor design modifications are being developed to address this issue. Patient comfort and tolerance of procedures as assessed through questionnaires and direct observation of the patients that have participated in these IRB approved research studies is very favorable.
Preliminary Results with Advanced Modes and Future Developments
The current prototype compression paddle system has been installed on a Senographe 2000D digital mammography system. An earlier version of this system was installed on a dedicated digital mammography-tomosynthesis system that was developed at GE Global Research (Figure 6). 3D clinical digital mammograms obtained on this tomosynthesis prototype have been very encouraging and efforts are in progress to develop the next generation tomosynthesis prototype system based on the Senographe 2000D system with substantial improvements expected in system performance.31 In tomosynthesis, low dose projection images of the breast are acquired over a curved trajectory of the x-ray tube relative to a stationary patient and detector.32 Initial phantom studies of co-registered tomosynthesis – ultrasound imaging have been previously reported.20,21
Figure 6.
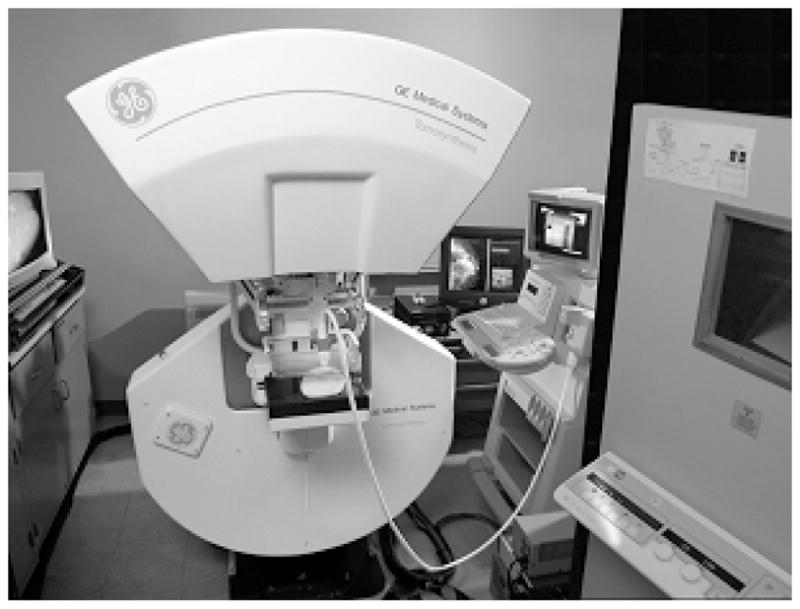
An early version of a combined tomosynthesis – ultrasound compression paddle system installed on a prototype tomosynthesis system developed at GE Global Research.
The ability to merge 3D datasets is realized through the common prototype compression paddle system. In Figure 7, some of the potentials of the combined approach are shown. A triple modality CIRS biopsy-training phantom was modified to incorporate microcalcification clusters in a 50% fat/50% fibroglandular mimicking material. A 2D digital mammogram is shown in panel (a) of Figure 7 where an arrow has been placed on a region showing overlapping masses. Three dimensional tomosynthesis images remove the overlapping structures in the 2D mammogram as seen in panel (b) and provide the depth of masses that were collapsed in the two dimensional projection of panel (a). The masses and microcalcification clusters embedded in the phantom are clearly shown in the tomosynthesis images. Differentiation between mass and cyst like structures is not as clear in the tomosynthesis images as they are on the corresponding ultrasound image shown in panel (c). Microcalcifications are not visible in this ultrasound image. A fused image, shown on panel (d) blends the tomosynthesis and ultrasound slices so that visibility of the masses, microcalcifications and three-dimensional localization is retained. In addition, a clear difference in contrast distinguishes a cyst like structure on which the cross hairs are shown centered.
Figure 7.
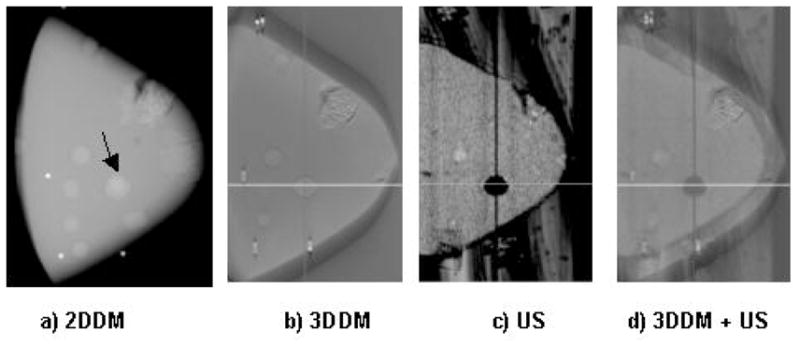
Phantom images of a modified CIRS triple modality biopsy phantom. Panel (a) is a 2D digital mammogram obtained on an early prototype 18×24 cm2 digital x-ray detector. Panel (b) shows a slice of the tomosynthesis (3DDM) image, which shows masses and a cluster of microcalcifications. Panel (c) shows a corresponding reconstructed ultrasound slice of the same phantom. Panel (d) is the same tomosynthesis slice merged with the reconstructed ultrasound slice, showing cyst-mass differentiation as well as microcalcifications.
The challenge of full breast coverage with ultrasound due to the rounded extremities is shown in Figure 7, where the total volume of the imaged phantom is less than that imaged with tomosynthesis. Much of the volume loss is on the sides of the breast, which are mostly subcutaneous fat and less likely to contain cancer than the central breast tissues. Also in the background of the ultrasound image, the gel applied on the paddle is seen which was not present during the tomosynthesis image acquisition. The gel will introduce artifacts in the tomosynthesis images if present during tomosynthesis acquisition. This points to the need for further development of homogeneous acoustic coupling agents. This phantom study demonstrates the potential for gaining mammographic information in the depth dimension while retaining the high x-ray resolution imaging capabilities of masses and microcalcifications and associated features as well as providing improved characterization of the regions of interest.
The ability to rapidly change the separation distance of the compression paddle and breast support provides a near ideal geometry for elasticity imaging. Elasticity imaging, one of the most promising approaches to breast diagnosis, is usually performed in an approximate way in two dimensions. The parallel plate-constrained breast in 3D scanning should allow tracking of 3D motion and more accurate and robust imaging of the elastic modulus than done previously, as well as allow for a new nonlinear type of elasticity imaging. While this process is slower than gray scale or even color flow imaging, it will be possible to perform advanced elasticity imaging33,34 under more controlled conditions than has been available in the past. Frame rates are practical for 3D or region of interest imaging. Imaging the density of the breast and surrounding tissues is extremely promising for characterizing lesions in procedures close to that of current practice.35
A key factor in developing tissue tracking and elasticity reconstruction algorithms is to have ultrasound data for the deformation of objects with a known distribution of elastic properties. Breast tissues have an elastic modulus that depends strongly on the strain level within the tissue.36 By collecting data over a large deformation range while accounting for the non-linearities, an improvement in tissue differentiation can be obtained.37 A direct mechanical measurement method was developed to determine the nonlinear elastic properties of biological tissues, and nonlinear finite element simulations in Abaqus (Hibbit, Karson and Sorensen, Inc., Pawtucket, RI) were used to simulate the deformation of 3D axisymetric geometries using such tissue types. The resulting displacement fields and measured point-spread functions of transducer arrays are used to generate synthetic RF datasets with known underlying elastic properties distributions
In Figure 8 a B-scan cross section through an intra-ductal carcinoma inclusion is shown where the imaging beams go from left to right. This structure was deformed along the imaging direction, and the right side shows the axial strain image as measured by speckle tracking. Simulations allow exact calculation of elasticity imaging contrast to noise ratios, and quantitative analysis of the influence of different system parameters and patient motion. The main test with these simulations will be evaluation of imaging nonlinear elasticity and the need for 3D data and reconstructions as opposed to the usual 2D elasticity imaging.
Figure 8.
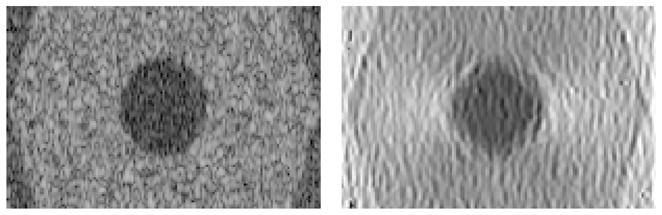
B-scan (60 dB scale) and measured axial strain image (0–2.5% scale) of simulated intra-ductal carcinoma (5 mm radius) surrounded by glandular and fatty tissue
Color flow imaging of vasculature, with and without contrast agents has been shown to contribute to characterization of breast masses.38 With this system, the ease of performing the exam may push color flow imaging past the barriers to adding additional studies for frequent use in breast imaging. To ensure adequate blood flow for vascular imaging, or to minimize discomfort associated with full mammographic compression for extended periods of time, most if not all ultrasound modes will probably employ slightly less compression than that of x-ray mammography. The stability of the geometry and controlled scan rate will be particularly useful in contrast imaging where there is limited infusion time and often limited tolerance by the patient to an inconsistent search. The contrast agent gas bubbles are usually disrupted by the ultrasound and will give inconsistent results if the ultrasound beam is backtracked rapidly or even scanned at variable speeds. Both elasticity and blood motion imaging could be potentially used in a multi-modality screening system along with CAD to evaluate many more lesions that are marginally suspicious than could be done with methods even as innocuous as call back for additional noninvasive studies.
Summary
Forty-eight patients have been dual imaged with a prototype x-ray/ultrasound compression paddle. Exceptional image quality has been achieved for imaging through a compression paddle. Prospective clinical trials with the system are scheduled to evaluate discrimination of benign and malignant lesions as well as prediction of response to chemotherapy. The core dual modality imaging system with stable breast compression is an ideal platform for evaluating and selecting the most promising advanced applications currently being evaluated at the author-affiliated institutions. Improvements in the acquisition, display and analysis of breast ultrasound and x-ray mammography images are anticipated. Several of the techniques most relevant to a combined system have and will continue to be developed and evaluated to assess their potential to achieve enhanced breast cancer diagnosis.
Depiction and discrimination of breast cancer remains challenging because of cancer’s diverse–manifestations on mammography. The synergism of ultrasound and x-ray mammography in discriminating features of cancer is accepted in an increasing number of breast imaging situations. The combined system will increase the number of tissue features that can be assessed, particularly as advanced imaging modes are implemented. Along with precise spatial registration of those multiple features, the system should increase the efficiency and accuracy of breast cancer diagnosis.
Acknowledgments
This work was supported in part by NIH grant RO1 CA 91713 and in part by the Office of Naval Research grant MDA905-00-10041. The authors would like to gratefully acknowledge the contributions of Anne Hall and Anupam Dattamajumdar (GE Healthcare, Waukesha, MI) in acoustic power testing and Ralph Hoctor (GE Global Research) in the implementation of beam formation correction algorithms.
References
- 1.Jackson VP. The role of US in breast imaging. Radiology. 1990;177:305–311. doi: 10.1148/radiology.177.2.2217759. [DOI] [PubMed] [Google Scholar]
- 2.Bassett LW, Kimme-Smith C. Breast sonography. AJR. 1991;156:449–455. doi: 10.2214/ajr.156.3.1899737. [DOI] [PubMed] [Google Scholar]
- 3.Jackson VP, Hendrick RE, Feig SA, Kopans DB. Imaging of the radiographically dense breast. Radiology. 1993;188:297–301. doi: 10.1148/radiology.188.2.8327668. [DOI] [PubMed] [Google Scholar]
- 4.Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening ultrasound – diagnostic yield and tumor characteristics. Radiology. 1998;207:191–199. doi: 10.1148/radiology.207.1.9530316. [DOI] [PubMed] [Google Scholar]
- 5.Berg W. Rationale for a trial of screening breast ultrasound. AJR. 2003;180:1225–1228. doi: 10.2214/ajr.180.5.1801225. [DOI] [PubMed] [Google Scholar]
- 6.Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound: a retrospective review. Cancer. 1995;76:626–630. doi: 10.1002/1097-0142(19950815)76:4<626::aid-cncr2820760413>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dunser M. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000;21:325–336. doi: 10.1016/s0887-2171(00)90027-1. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001;221:641–649. doi: 10.1148/radiol.2213010364. [DOI] [PubMed] [Google Scholar]
- 9.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 10.American College of Radiology. Illustrated Breast Imaging Reporting and Data System (BI-RADS) 3. Reston, VA: American College of Radiology; 1998. [Google Scholar]
- 11.Conway WF, Hayes CW, Brewer WH. Occult breast masses: Use of mammographic localizing grid for US evaluation. Radiology. 1991;181:143–146. doi: 10.1148/radiology.181.1.1653441. [DOI] [PubMed] [Google Scholar]
- 12.Richter K. Technique for detecting and evaluating breast lesions. J Ultrasound Med. 1994;13:797–802. doi: 10.7863/jum.1994.13.10.797. [DOI] [PubMed] [Google Scholar]
- 13.Bassett LW, Kimme-Smith C, Sutherland LK, Gold RH, Sarti D, King W. Automated and hand-held breast US: effect on patient management. Radiology. 1987;165:103–108. doi: 10.1148/radiology.165.1.3306779. [DOI] [PubMed] [Google Scholar]
- 14.Hilton SW, Leopold GR, Olson LK, Wilson SA. Real-time breast sonography: application in 300 consecutive patients. AJR. 1986;147:479–486. doi: 10.2214/ajr.147.3.479. [DOI] [PubMed] [Google Scholar]
- 15.Shmulewitz A. Methods and apparatus for performing sonomammography and enhanced x-ray imaging. 5,479,927. US Patent. 1996 January 2;
- 16.Kelly-Fry E, Jackson VP. Adaptation development and expansion of x-ray mammography techniques for ultrasound mammography. Ultrasound Med. 1991;10:S–16. [Google Scholar]
- 17.Kelly-Fry E, Dines KA, Romilly-Harper AP. In: Jellins J, Madjar H, editors. Mammography instrumentation for combined x-ray and ultrasound imaging; Ninth International Congress on the Ultrasonic Examination of the Breast, International Assoc. Breast Ultrasound; Sept. 28-Oct. 1; Indianapolis, IN, USA. 1995. pp. 40–42. Abstract Booklet, Int’l Assoc. Breast Ultras. [Google Scholar]
- 18.Dines KA, Kelly-Fry E, Romilly-Harper P. Automated three-dimensional ultrasound breast scanning in the craniocaudal mammography position. Ninth International Congress on the Ultrasonic Examination of the Breast; Indianaplis, IN. 1995. pp. 43–44. [Google Scholar]
- 19.Richter K. Technique for detecting and evaluating breast lesions. J Ultrasound Med. 1994;13:797–802. doi: 10.7863/jum.1994.13.10.797. [DOI] [PubMed] [Google Scholar]
- 20.Carson PL, Moskalik AP, Govil A, Roubidoux MA, Fowlkes JB, Normolle D, Adler DD, Rubin JM, Helvie M. The 3D and 2D color flow display of breast masses. Ultrasound in Medicine & Biology. 1997;23:837–849. doi: 10.1016/s0301-5629(97)00073-2. [DOI] [PubMed] [Google Scholar]
- 21.Kapur A, Krücker J, Astley O, Buckley D, Eberhard JW, Alyassin A, Claus B, Thomenius K, Myers H, Rumsey M, Johnson R, Karr S. Fusion of Digital Mammography with Breast Ultrasound – a Phantom Study, Medical Imaging 2002: Image Processing. Proc. SPIE 4684; 2002. [Google Scholar]
- 22.Kapur A, Carson PL, Thomenius K, Eberhard JW, Goodsitt M, Krücker JL, Roubidoux MA, Helvie M, Astley O, Yamrom B, Claus B, Alyassin A. Co-registered breast imaging with 3D X-rays and 3D ultrasound. Procs., 6th International Workshop on Digital Mammography; June 24–26, 2002; Bremen, Springer Ferlag, Heidelberg. [Google Scholar]
- 23.Department of Health and Human Services, Food and Drug Administration. Mammography quality standards final Rules. Federal Register. 1997 Oct 28;68(208):55852–55994. [Google Scholar]
- 24.Hoctor RT, Thomenius KE. Focus correction for ultrasound imaging through mammography compression plate. 6,607,489. US Patent. 2003 Aug 19;
- 25.Hertzberg RW, Manson JA. Fatigue of Engineering Plastics. Academic Press; New York: 1990. p. 131. [Google Scholar]
- 26.Schroeder W, Martin K, Lorensen W. The Visualization Toolkit. Prentice Hall; 1996. [Google Scholar]
- 27.Samei E, Flynn MJ, Reimann DA. A method for measuring the presampled MTF of digital radiographic systems using an edge test device. Medical Physics. 1998;25 (1):102–1138. doi: 10.1118/1.598165. [DOI] [PubMed] [Google Scholar]
- 28.Krucker J, Hoctor R, Kapur A, Goodsitt MM, Carson PL, Thomenius KE. Ultrasound image quality obtainable through a mammography compatible compression paddle. Presented at the IEEE International Ultrasonics Symposium; October 2003; Abstract p168. [Google Scholar]
- 29.FDA. Guide for measuring and reporting output of diagnostic ultrasound medical devices. Center for Devices and Rad. Health; Rockville: 1995. [Google Scholar]
- 30.Thomenius KE. Estimation of the potential for bioeffects. In: Ziskin MC, Lewin PA, editors. Ultrasonic Exposimetry. CRC Press; Boca Raton: Nov, 1992. pp. 372–407. [Google Scholar]
- 31.Rafferty E, Moore R, Niklason L, Eberhard JW, Kopans D. Clinical Evaluation of Digital Tomo-synthesis of the Breast. RSNA. 2002 [Google Scholar]
- 32.Niklason LT, Christian BT, Niklason LE, Kopans DB, Castleberry DE, Opsahl-Ong BH, Landberg CE, Slanetz PJ, Giardino AA, Moore R, Albagli D, DeJule MC, Fitzgerald PF, Fobare DF, Giambattista BW, Kwasnick RF, Liu J, Lubowski SJ, Possin GE, Richotte JF, Wei CY, Wirth RF. Digital tomosynthesis in breast imaging. Radiology. 1997;205:399–406. doi: 10.1148/radiology.205.2.9356620. [DOI] [PubMed] [Google Scholar]
- 33.Emelianov SY, Erkamp RQ, Lubinski MA, Skovoroda AR, O’Donnell M. Non-linear tissue elasticity: adaptive elasticity imaging for large deformations. 1998 IEEE Ultrasonics Symposium; 1998. pp. 1753–1756. [Google Scholar]
- 34.Erkamp RQ, Skovoroda AR, Emelianov SY, O’Donnell M. Measuring the Nonlinear Elastic Properties of Tissue-Like Phantoms. IEEE Trans Ultras Ferr. 2004;51:410–419. doi: 10.1109/tuffc.2004.1295426. [DOI] [PubMed] [Google Scholar]
- 35.Hall TJ, Zhu Y, Spalding CS. In vivo real-time freehand palpation imaging. Ultrasound Med Biol. 2003;29:427–435. doi: 10.1016/s0301-5629(02)00733-0. [DOI] [PubMed] [Google Scholar]
- 36.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. The elastic moduli of breast and prostate tissues under compression. Ultrasonic Imaging. 1998;20:151–159. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 37.Erkamp RQ, Emelianov SY, Skovoroda AR, O’Donnell M. Nonlinear Elasticity Imaging. IEEE Ultrasonics Symposium. 2002;2:1891–1894. [Google Scholar]
- 38.Forsberg F, Goldberg BB, Merritt CRB, et al. Diagnosing breast lesions with contrast -enhanced 3-dimensional power Doppler imaging. J Ultrasound Med. 2004;23:173–182. doi: 10.7863/jum.2004.23.2.173. [DOI] [PubMed] [Google Scholar]