Since the introduction of cyclosporine (CsA)-steroid therapy for our liver transplant recipients in March 1980, the survival rates have increased significantly as shown in Fig 1. The effective and relatively safe immunosuppression provided by CsA is undoubtedly the most important factor in the improved success rate.1
Fig 1.
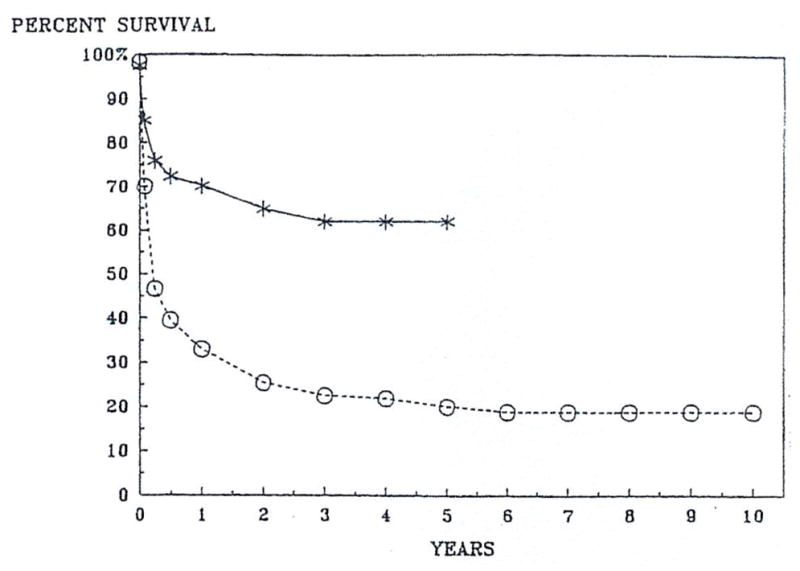
Actuarial survival after liver transplantation.
Nephrotoxicity is a troublesome side effect of CsA. The distinction between nephrotoxicity and rejection poses a major diagnostic dilemma in renal transplantation,2 but not after extrarenal organ transplantation. However, end-stage liver diseases often predispose to renal dysfunction, and extensive surgical intervention also can cause acute renal impairment. Thus, a different kind of “artifact” may exist in the assessment of drug nephrotoxicity.
Even so, liver transplantation provides a reasonable model to study some aspects of the nephrotoxicity of CsA. In this report, acute and chronic renal dysfunction among our liver transplant recipients will be described.
CsA-STEROID THERAPY
A brief description of our management practices is in order. If there are several hours with which to prepare a recipient for liver transplantation, an initial loading dose of 17.5 mg/kg of CsA is administered orally. If the oral loading is possible, CsA is usually not given again until the operation is completed at which time the venous route is used. If there is not enough time before surgery for oral loading, all therapy is withheld until 2 mg/kg of intravenous (IV) CsA is administered over a period of two to three hours after revascularization of the graft. If the recipient becomes hemodynamically stable and is producing an adequate amount of urine, the IV dose is repeated every eight to 12 hours in the early postoperative period. The IV medication is continued, even after the resumption of oral intake.
Although oral CsA (17.5 mg/kg/d in two divided doses) is administered as soon as can be tolerated postoperatively, the intestinal absorption of the drug is usually poor for a few weeks after liver transplantation. Therefore, during the early postoperative period, CsA is administered IV as well as orally (double-route therapy) to maintain adequate trough levels of the drug (usually 800 ± 200 ng/mL of whole blood measured by radioimmunoassay [RIA]). When an adequate blood level of the drug can be maintained by the double-route therapy, the IV doses of CsA are gradually tapered and discontinued at the same time that oral doses are increased accordingly.
Often, oral doses > 17.5 mg/kg/d are required during the early posttransplant period to maintain an adequate blood level. However, if renal dysfunction exists, the dose of CsA is reduced and the whole blood trough levels are allowed to drift to as low or lower than 300 ng/mL. Then, additional doses of steroids may be required to prevent the graft rejection. More recently, monoclonal antibody therapy has been used. With the improvement of renal function, the dose of CsA is gradually increased to reach a therapeutic drug level consistent in that patient with satisfactory renal function.
When satisfactory and stable graft function is obtained (usually a month or two after transplantation), long-term dose adjustments are directed by side effects of CsA, such as nephrotoxicity, tremor, gum hyperplasia, hirsutism, and hypertension, rather than relying solely on blood concentrations of the drug.
Corticosteroids are used in combination with CsA not only as rejection therapy, but also as a maintenance immunosuppressive therapy. Steroid therapy is begun immediately before the revascularization of the graft with a bolus of IV methylprednisolone. After the operation, a five-day burst of prednisone or methylprednisolone is started at 200 mg/d in adults. The dose is reduced by daily decrements of 40 mg to a maintenance dose of 20 mg/d for adults. In children, smaller doses of steroids are used. The maintenance dose of prednisone at one month is 15 to 20 mg/d for adults and 5 to 15 mg/d for children. Further reductions of prednisone depend on graft function.
ACUTE RENAL DYSFUNCTION DURING A ONE-YEAR SAMPLE
From Jan 1 to Dec 31, 1984, 135 patients with various end-stage liver diseases received their first liver grafts while receiving CsA-steroid therapy at the University Health Center of Pittsburgh (Pittsburgh). Seventy-one of the 135 patients were adults (18 years old or older), and the remaining 64 patients were children (under 18 years old). As of March 1, 1985, 58 (82%) of the 71 adults and 57 (89%) of the 64 children are alive.
Immediately before liver transplantation, three adult and two pediatric patients had abnormal renal functions (BUN level > 50 mg/100 mL and/or serum creatinine > 2 mg/100 mL). One of the adult and one of the pediatric patients were receiving hemodialysis (Table 1).
Table 1.
Incidence of Renal Dysfunction Before and After Liver Transplantation
| Abnormal Renal Function Before Liver Transplant (%) | Acute Renal Dysfunction After Liver Transplant (%) | |
|---|---|---|
| Adults (≥ 18 yr) | 3/71 (4) | 15/71 (21) |
| Children (<18 yr) | 2/64 (3) | 14/64 (22) |
| Total | 5/135 (4) | 29/135 (21) |
Before the introduction of CsA, acute renal dysfunction postoperatively was often observed. Massive transfusion during surgery, failure of prompt graft function, and infectious complications were predisposing factors. Without these complications, severe renal dysfunction was uncommon with conventional triple-drug immunosuppressive therapy (azathioprine, steroids, and antilymphocyte globulin [ALG]). In order to eliminate the transient and mild renal dysfunction inherent in liver transplant surgery, acute renal dysfunction postoperatively is defined as follows in this report: for adult recipients, BUN level of 100 mg/100 mL or greater and/or serum creatinine level of 5 mg/100 mL or greater; and for pediatric recipients, BUN level of 70 mg/mL or greater and/or serum creatinine level of 3 mg/mL or greater during the first three months after liver transplantation.
There were 15 (21%) of the 71 adult and 14 (22%) of the 64 pediatric liver recipients who developed severe acute renal dysfunction as just defined above (Table 1). In response to the complication, CsA was administered in quantities far less than the usual early maintenance doses, and the trough levels were kept <500 ng/mL of whole blood measured by RIA.
Among the 15 adult recipients, only three had abnormal renal function before transplantation and one was already receiving hemodialysis. Five of the 15 adult recipients required hemodialysis after liver transplantation, and all died within three months. These five patients had very poor liver graft function. Four of them received second grafts, and two of the four received the third grafts within two months.
Among the remaining ten adult patients (of the 15 with renal failure), only two had abnormal kidney function before transplantation and both regained near-normal renal function after liver transplantation. They are alive and well in the fourth and 12th months. The other eight patients had normal renal function before transplantation. Five of them died in three months with liver failure and infectious complications. In the end, ten (67%) of the 15 patients who developed severe renal dysfunction postoperatively died despite the drastic decrease of or temporary withdrawal of CsA. Liver failure and infectious complications were almost always present.
Among the 14 pediatric recipients with postoperative renal dysfunction, only two had abnormal renal function before transplantation. One patient was already receiving acute hemodialysis and the other was receiving chronic dialysis because of renal cystic disease. For the latter patient, both kidney and liver were transplanted from the same donor. The patient with acute preoperative renal failure (from Wilson’s disease) was dialyzed four times after liver transplantation. The liver-kidney recipient did not need dialysis. Both patients are living and well with good renal functions in the fourth and sixth months.
The remaining 12 children developed severe renal dysfunction after transplantation. Although all 12 patients had a BUN level of 70 mg/100 mL or greater, only four of them had a serum creatinine level of 2 mg/100 mL or greater. None of the 12 required hemodialysis after transplant and only three have died as of March 1, 1985. The three patients who died had very poor liver function, and two of them had severe infectious complications as well. The other nine patients are all alive and well with good renal as well as hepatic function.
Thus, severe acute renal dysfunction after liver transplantation was always associated with preexisting renal failure, difficult surgery, poor liver function, and/or infectious complications. In the absence of these problems, nephrotoxicity of CsA did not cause significant morbidity when the dose was adjusted properly during the first three months after liver transplantation.
CHRONIC RENAL DYSFUNCTION IN CHRONIC SURVIVORS
From March 1980 to February 1984, 178 patients with various end-stage liver diseases received the first liver grafts while receiving CsA-steroid therapy. One hundred thirty-six (76%) of the 178 recipients survived one to five years after transplant as of March 1985.
Sixty-nine of the 136 one-year survivors were adults; and remaining 57 were children. BUN and serum creatinine levels and the oral doses of CsA (mg/kg/d) were analyzed before transplantation; at 2, 6, and 12 months; and yearly up to five years to study chronic nephrotoxicity of CsA when the doses were adjusted by close monitoring of renal function as mentioned earlier. The mean values with SDs are shown in Table 2.
Table 2.
Renal Function and CsA Dose After Liver Transplantation in Adults and Children
| Before Transplant | 2 mo | 6 mo | 1 yr | 2 yr | 3 yr | 4 yr | |
|---|---|---|---|---|---|---|---|
| Adult (age ≥ 18 yr) | |||||||
| BUN mg/100 mL | 17.7 ± 17.0 | 37.0 ± 21.6 | 40.5 ± 43.9 | 37.7 ± 31.1 | 33.7 ± 12.9 | 26.1 ± 8.5 | 20.8 ± 5.1 |
| Creatinine mg % | 1.1 ±0.8 | 1.5 ± 0.6 | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.8 ± 0.6 | 1.5 ± 0.3 | 1.3 ± 0.4 |
| CsA mg/kg/d | 13.9 ± 7.4 | 8.2 ± 3.8 | 6.1 ± 2.0 | 5.0 ± 1.9 | 4.9 ± 1.9 | 6.2 ± 2.7 | |
| Pediatric (age < 18 yr) | |||||||
| BUN mg/100 mL | 18.4 ± 24.7 | 24.8 ± 16.3 | 24.2 ± 11.2 | 20.8 ± 9.5 | 22.2 ± 9.1 | 25.9 ± 10.2 | 21.5 ± 9.2 |
| Creatinine mg % | 0.8 ± 1.8 | 0.5 ± 0.3 | 0.7 ± 0.4 | 0.6 ± 0.4 | 0.7 ± 0.3 | 0.6 ± 0.4 | 0.8 ± 0.2 |
| CsA mg/kg/d | 23.3 ± 14.7 | 17.0 ± 9.2 | 14.6 ± 8.9 | 9.3 ± 2.3 | 9.0 ± 6.3 | 8.7 ± 1.8 | |
Among the 69 adult one-year survivors, there were five patients who had abnormal renal function (serum creatinine level 2 mg/100 mL or greater) before transplantation; none were on hemodialysis. In three of these five patients, renal function improved after liver transplantation to serum creatinine levels <2 mg/100 mL, but in two, the improvement was limited to serum creatinine levels of 2.5 mg/100 mL and 3.5 mg/100 mL under CsA-steroid therapy.
Sixty-five of the 69 adult patients had normal or near normal renal functions (serum creatinine level <2 mg/100 mL) before transplantation. Only one of the 65 patients developed severe renal failure after transplantation that necessitated hemodialysis for a month. This patient had been an insulin-dependent diabetic for 15 years before transplantation He received reduced doses of CsA for the first two days and for two weeks in the second month after transplantation. During the other postoperative times, he was given azathioprine-steroid therapy because of renal failure. The patient’s renal function slowly deteriorated on azathioprine-prednisone therapy but did not require hemodialysis other than during the immediate postoperative period. He died of a myocardial infarction after one year. The rest of adult survivors did not develop chronic progressive renal dysfunction under CsA-steroid therapy during one to five years.
The numbers of adult one-year survivors who had abnormal renal functions (BUN level >50 mg%, creatinine level >2 mg/100 mL) before and after liver transplantation are summarized in Table 3. The incidence of renal dysfunction increased after transplantation under CsA-steroid therapy. However, it gradually decreased with reduction of CsA dosage during the observation of up to five years.
Table 3.
Incidence of Renal Dysfunction Among 69 Adult One-Year Survivors After Liver Transplantation With CsA-Steroid Therapy
| Before Transplant | 2 mo | 6 mo | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | |
|---|---|---|---|---|---|---|---|---|
| Serum creatinine ≥ 1 mg/100 mL | 5/69 | 19/69 | 18/69 | 18/69 | 8/34 | 2/17 | 1/6 | 0/1 |
| % | 7 | 28 | 26 | 26 | 24 | 12 | 17 | 0 |
| BUN ≥ 30 mg/dL | 3/69 | 12/69 | 8/69 | 9/69 | 3/34 | 0/10 | 0/6 | 0/1 |
| % | 4 | 17 | 12 | 13 | 9 | 0 | 0 | 0 |
Among the 57 pediatric one-year survivors, seven children had abnormal renal functions (creatinine level >1 mg/100 mL) before transplantation, but only one of them was receiving hemodialysis. None required hemodialysis after transplant. In all of these seven children, renal function improved after liver transplantation under CsA-prednisone therapy. In the remaining 50 children, the serum creatinine level was <1 mg/100 mL before transplant. None of the 57 children developed chronic, progressive renal dysfunction while receiving CsA-steroid therapy during one to five years.
The numbers of pediatric one-year survivors who had abnormal (BUN level >30 mg/100 mL, creatinine level >1.0 mg/100 mL) before and after liver transplantation are summarized in Table 4. The incidence of renal dysfunction increased after transplant under CsA-steroid therapy, but it gradually decreased with reduction of CsA dosage during the observation of up to five years.
Table 4.
Incidence of Renal Dysfunction Among 57 Pediatric One-Year Survivors After Liver Transplantation With CsA-Steroid Therapy
| Before Transplant | 2 mo | 6 mo | 1 yr | 2yr | 3yr | 4 yr | |
|---|---|---|---|---|---|---|---|
| Serum creatinine ≥ 1 mg/100 mL | 7/57 | 4/57 | 12/57 | 8/57 | 2/28 | 1/11 | 0/2 |
| % | 12 | 7 | 21 | 14 | 7 | 9 | 0 |
| BUN ≥ 30 mg/dL | 6/57 | 17/57 | 16/57 | 8/57 | 3/28 | 1/11 | 0/2 |
| % | 11 | 30 | 28 | 14 | 11 | 9 | 0 |
Discussion
Acute and reversible nephrotoxicity of CsA was recognized soon after the introduction of this effective and safe immunosuppressive agent in clinical organ transplantation.3–7 Severe renal dysfunction during the early postoperative period of liver transplantation is an ominous prognostic sign often accompanied by unstable hemodynamics, poor liver function, and/or infectious complications. In our study of acute renal dysfunction, 13 (45%) of the 29 recipients who had severe renal failure died within three months after transplantation.
CsA is an additional cause of acute renal dysfunction in liver transplantation, in which hemodynamic instability, massive operative intervention, and very poor liver function are major causes of renal impairment. The dose of CsA must be carefully adjusted to avoid nephrotoxicity. Daily monitoring of the drug level is helpful in adjusting the dose particularly during the immediate and early posttransplant period.
Recently, chronic and irreversible renal damages were reported both in heart8 and kidney9 transplantation under long-term CsA therapy. Histologic changes characterized by tubulointerstitial fibrosis were thought to be progressive. In the present clinical study of chronic renal dysfunction in 136 liver transplants receiving CsA therapy for one to five years, we could neither find an example of chronic, progressive renal dysfunction, nor could we find any evidence of increasing incidence of chronic, renal impairment (Tables 2 through 4). Although recipients of different organs were studied, the lack of chronic, progressive renal dysfunction in our liver recipients was probably due to our policy of dose adjustment to avoid nephrotoxicity rather than to maintain any specific trough level of blood CsA.
References
- 1.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Hepatology. 1982;2:614. [Google Scholar]
- 2.Starzl TE, Hakala TR, Rosenthal JT, et al. Surg Gynecol Obstet. 1982;154:819. [PMC free article] [PubMed] [Google Scholar]
- 3.Calne RY, White DJG, Thiru S, et al. Lancet. 1978;2:1323. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, Rolles K, White DJG, et al. Lancet. 1979;2:1033. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Weil R, III, Iwatsuki S, et al. Surg Gynecol Obstet. 1980;151:17. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Klintmalm GBG, Weil R, III, et al. Surg Gynecol Obstet. 1981;153:486. [PMC free article] [PubMed] [Google Scholar]
- 7.Calne RY, White DJG, Evans DB, et al. Br Med J. 1981;282:934. [Google Scholar]
- 8.Myers BD, Ross J, Newton L, et al. N Engl J Med. 1984;311:699. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 9.Klintmalm GBG, Bohman S, Sundolin B, et al. Lancet. 1984;2:950. doi: 10.1016/s0140-6736(84)91166-8. [DOI] [PubMed] [Google Scholar]


