Abstract
Objectives
Surgical resection is the preferred treatment in selected patients with pulmonary metastases. In high-risk patients, radiofrequency ablation (RFA) may offer an alternative option. RFA may be used either alone or in combination with surgical resection as a lung parenchymal-sparing approach. Our objectives were to evaluate the intermediate term outcomes after RFA and to determine the prognostic variables associated with outcome in patients with pulmonary metastases
Methods
Thoracic surgeons evaluated and performed RFA under computed-tomography (CT) guidance or in combination with surgical resection as a lung parenchymal-sparing modality. Patients were monitored in the thoracic surgery clinic for recurrence and survival.
Results
Twenty-two patients {10 men, 12 women; median age 63 years (37-88)} underwent RFA. The primary cancer was colorectal in 9 (41%), renal in 2 (9%), sarcoma in 4 (18%) and other in 7 (32%) patients. CT-guided RFA was performed as a sole modality of treatment in 17 patients (77%), and in combination with surgical resection in 5 (23%) patients. There were no procedure-related mortalities. At a mean follow-up of 27 months (13.3-53.6 months), 9 patients are alive. The median survival was 29 months (CI 9.1-33.8). Size of the lesion was an important prognostic variable associated with overall and disease-free survival (P<0.05).
Conclusions
Our experience indicates that RFA is safe in this group of pulmonary metastases patients with reasonable results. Surgery remains the standard for resectable patients, but RFA offers an alternative option in selected patients or may be used as a parenchymal-sparing approach in combination with surgical resection in selected patients.
Introduction
Surgical resection has been shown to be beneficial in selected patients with pulmonary metastases. The potential benefits of resection of pulmonary metastases in selected patients have been demonstrated in several series (1-4). The criteria used for selection of patients for surgical resection include complete control of primary tumor, no extrathoracic disease, long disease-free interval and limited pulmonary metastases. In these patients, surgical resection is the preferred treatment and offers the best results in patients with metastatic disease (1). In medically inoperable or unresectable patients with pulmonary metastases, there are few effective options. Many of these patients are treated with chemotherapy and the long term survival is not encouraging (5).
One of the factors associated with a survival benefit is complete resection of all pulmonary metastases. Further, even patients who have undergone a complete resection have a high incidence of re-recurrence and may need a redo-thoracotomy with resection (6,7). Radiofrequency ablation (RFA) may be potentially applicable in this group of patients who are high-risk, require repeated thoracotomies, or as a parenchymal-sparing adjunct to surgery to completely treat all lesions.
Radiofrequency ablation is a thermal ablative technique and is a relatively new modality of treatment, which may be applicable in high-risk patients. Although RFA has been shown to be feasible in several studies, there are few reports on longer term outcomes (8,9). In addition, the intermediate term results of a combination of RFA as an adjunct to surgical resection for pulmonary metastases have not been reported. The primary objectives of this study were to a) evaluate the outcomes after Radiofrequency Ablation (RFA) either alone or as an adjunct to surgical resection as a lung parenchymal-sparing approach in selected patients with pulmonary metastases and b) to evaluate the prognostic variables associated with both overall survival (OS) and disease-free survival (DFS).
Methods
We retrospectively reviewed our experience with Radiofrequency Ablation for the treatment of pulmonary metastases at the University of Pittsburgh over a four year period from 2001 to 2005. The study was approved by the Institutional Review Board (IRB) at the University of Pittsburgh. Since this was a retrospective study, individual consent was waived.
Patients with metastatic lung cancer who underwent RFA were identified. We excluded patients who had recurrent or metastatic primary non-small cell lung cancer. All patients underwent chest computed-tomography (CT) scan and 17 (77%) patients also underwent a positron emission tomography (PET) scan. The selection criteria for RFA treatment for pulmonary metastases included complete control of the primary tumor, extrathoracic disease which was controlled or being treated, and completely treatable pulmonary metastases. The inclusion criteria for image-guided RFA in the treatment of these patients were (1) patients who were considered medically inoperable due to poor pulmonary function (predicted post-operative FEV1 or DLCO less than 40%), high cardiac risk, or other co-morbidities; (2) failed prior treatments; or (3) patients who refused surgery. Exclusion criteria included central tumors. In some patients who were operable, in addition to surgical resection, RFA was used as an adjunct parenchymal-sparing procedure. All patients were evaluated by a thoracic surgeon to determine suitability for RFA either alone or as an adjunct to surgical resection.
Treatment Protocol
The technique of RFA has been discussed in detail previously (10-12). In brief, percutaneous CT-guided approach was used in most patients (n=17, with 1 open conversion) and, as described previously, all procedures were performed by thoracic surgeons. RFA was performed as an adjunct to surgical resection in 5 patients.
RFA was administered using two different radiofrequency generators and needle electrodes. One system was composed of the radiofrequency generator (RF3000, Boston Scientific, MA) and needle electrodes (LeVeen Needle Electrode, RadioTherapeutics Corporation, Sunnyvale, CA). A two-phase, impedance-based algorithm was used according to the protocol suggested by the manufacturer. The second system comprised a RF generator, the RITA Starburst XL Electrosurgical Device (RITA Medical Systems, Inc., Moutainview, CA). Based upon the size of the target tumor, the multi-tined expandable array (Starburst XL Electrosurgical Device, RITA Medical Systems, Inc., Moutainview, CA) was deployed and a temperature based algorithm was followed.
Post-procedure follow-up
Follow-up of patients and Assessment of response
Patients were followed in the thoracic surgery clinic. Our current protocol is follow-up every three month intervals with clinical examinations, CT scans and selectively with PET scans. A modified Response Evaluation Criteria in Solid Tumors (RECIST) criterion was utilized to assess initial response to treatment as described previously (10,12) (Table 1). Patients were evaluated for initial response rate, time to progression and overall survival.
Table 1. Modified Response Evaluation Criteria in Solid Tumors (RECIST) Criteria.
| RESPONSE | CT MASS SIZE | CT MASS QUALITY | PET * |
|---|---|---|---|
|
COMPLETE (Two of the following) |
Lesion disappearance (scar) or less than 25% original size | Cyst cavity formation Low density | SUV<2.5 |
|
PARTIAL (One of the following) |
More than 30% decrease in the sum LD of target lesions | Mass central necrosis or central cavity with liquid density | Decreased SUV or area of FDG uptake |
|
STABLE LESION (One of the following) |
Less than 30% decrease in the sum LD of target lesions | Mass solid appearance, no central necrosis or cavity | Unchanged SUV or area of FDG uptake |
|
PROGRESSION (Two of the following) |
Increase of more than 20% in sum LD of target lesions | Solid mass, invasion adjacent structures | Higher SUV or larger area of FDG uptake |
PET done selectively; SUV: Standardized uptake value
LD: Lesion diameter
FDG: Fluorodeoxyglucose
Data Collection and Statistical Analysis
The objectives of the study were to determine the outcomes of RFA in the treatment of pulmonary metastases and to evaluate the prognostic variables predictive of outcome. Information on patient demographics, tumor characteristics, and treatment was collected. Specific endpoints studied were clinical response rates, progression-free survival (PFS) and overall survival (OS). The prognostic variables evaluated were cell type, approach (RFA alone or in combination with surgical resection), size of metastases, number of metastases treated, disease-free interval from treatment of primary to first metastases (any site), disease-free interval from treatment of primary site to lung metastases, age and sex.
The overall survival was calculated as the time interval between date of surgery and date of death. OS was censored by the last follow-up date if a patient was still alive. The PFS was calculated as the time interval between date of surgery and date of any progression or death. For alive and progression-free patients, PFS was censored by the last follow-up date when patient was known as progression free. OS and PFS were estimated by Kaplan-Meier method. Greenwood formula was used to estimate the 95% confidence interval (CI) for OS and PFS (13). A log rank test was used to assess the association between OS, PFS and categorical covariates, such as primary cancer cell type, approach, nodal disease, and site of first metastasis. Univariate Cox regression model was used to assess the association between OS, PFS and continuous covariates such as age, disease-free interval between the treatment of the primary and date of first recurrence, the disease-free interval (DFI) between the treatment of the primary and treatment of lung metastases, and the size of the largest lesion, site of first metastases, and gender.
Results
1. Patient Characteristics
Twenty-two patients underwent RFA in patients over a four year period for pulmonary metastases. There were 10 men and 12 women; the median age was 63 years (range 37-88). The primary cancer was colorectal in 9 (41%), renal in 2 (9%), sarcoma in 4 (18%) and others in 7 (32%) patients. The mean size of the lesion was 2.5 cms (median 2.4 cm; range 0.6 cm-5.8cm). Patient characteristics are summarized in Table 2, 3. The median disease-free interval (DFI) from the treatment of the primary lesion to first metastasis (any site) and to lung metastases was 17. 5 months and 35 months respectively. A total of seven patients had prior treatment for intrathoracic metastatic disease.
Table 2. Patient Characteristics: Summary.
| Gender | ||
| • | Male | 10 (45.4%) |
| • | Female | 12 (54.6%) |
| Age | ||
| • | Median | 63 yrs |
| • | Range | 37- 88 yrs |
| Type of Primary Cancer | ||
| • | Colon | 9 (40.9%) |
| • | Sarcoma | 4 (18.2%) |
| • | Other | 9 (40.9%) |
| Renal Cell | 2 (9%) | |
| Breast | 1 (4.5%) | |
| Esophageal | 1(4.5%) | |
| Head and Neck | 1(4.5%) | |
| Melanoma | 1(4.5%) | |
| Pheochromocytoma | 1(4.5%) | |
| Germ Cell | 1(4.5%) | |
| Cervical | 1(4.5%) | |
| Size of Lesion | ||
| • | Mean | 2.9 cm |
| • | Median | 2.5 cm |
| Site of first metastases | ||
| • | Lung | 16 (72.7%) |
| • | Other | 6 (27.3%) |
Table 3. Individual Patient Characteristics.
| ID | Sex | Age | Primary | RFA Approach | # Lesions treated | Site of first metastases | Progression after RFA | Vital status | Survival time (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 80 | colon | CT-guided | 1 | LIVER | yes | alive | 16 |
| 2 | m | 69 | squamous cell tongue | CT-guided | 2 | LUNG | no | dead | 6 |
| 3 | f | 88 | colon | CT-guided | 1 | LUNG | yes | alive | 15 |
| 4 | m | 68 | renal | CT-guided | 1 | BRAIN | yes | alive | 13 |
| 5 | f | 82 | breast | CT-guided | 1 | LUNG | no | dead | 60 |
| 6 | f | 75 | colorectal | CT-guided | 1 | LUNG | yes | alive | 27 |
| 7 | f | 62 | colon | CT-guided | 1 | ADRENAL | yes | dead | 18 |
| 8 | f | 57 | sarcoma | CT-guided | 1 | LUNG | yes | dead | 34 |
| 9 | m | 63 | renal | CT-guided | 1 | RENAL(LOCAL) | yes | dead | 7 |
| 10 | m | 59 | colon | CT-guided | 1 | LIVER | yes | alive | 35 |
| 11 | f | 37 | pheochromocytoma | CT-guided | 1 | PARASPINAL | no | alive | 54 |
| 12 | f | 75 | colon | CT-guided | 1 | LUNG | yes | dead | 21 |
| 13 | m | 50 | testicular | CT-guided | 1 | LUNG | yes | dead | 8 |
| 14 | m | 51 | esophageal | CT-guided | 3 | LUNG | yes | dead | 7 |
| 15 | f | 43 | cervical | CT-guided | 1 | LUNG | yes | dead | 25 |
| 16 | m | 84 | colon | CT-guided | 1 | LUNG | yes | alive | 30 |
| 17 | m | 68 | colon | CT-guided | 2 | LUNG | no | dead | 8 |
| 18 | f | 41 | sarcoma | Thoracotomy with adjunct RFA | 1 | LIVER AND LUNG | no | alive | 18 |
| 19 | m | 74 | melanoma | Thoracotomy with adjunct RFA | 1 | LUNG | yes | dead | 12 |
| 20 | f | 63 | colorectal | Thoracotomy with adjunct RFA | 1 | LIVER (LOCAL) | yes | dead | 39 |
| 21 | f | 61 | leiomyosarcoma | Thoracotomy with adjunct RFA | 1 | LUNG, NECK | yes | alive | 36 |
| 22 | f | 48 | uterine leiomyosarcoma | Thoracotomy with adjunct RFA | 2 | LUNG | yes | dead | 9 |
A total of 1-3 lesions (median 1) were treated with RFA per patient in each setting. There were a total of 5 patients with bilateral lesions. In these patients with contralateral lesions, one patient was treated with surgical resection and adjunct RFA; 3 patients were treated with RFA and one patient who was on home O2 declined further therapy.
There were 3 patients with extrathoracic disease and in all these patients the extra-thoracic disease was controlled. One patient underwent resection and RFA of liver metastases prior to RFA; two patients had surgical resection (one adrenalectomy and another hysterectomy) following RFA of the lung lesion
2. Approach to RFA
RFA was performed as a sole modality of treatment in 17 patients (77%). In these patients, RFA was planned to be performed under CT guidance, with one patient requiring an open conversion (mini-thoracotomy) because of difficulty in placement of the probe due to hard consistency of the lesion. RFA was performed in combination with surgical resection (via thoracotomy) in 5 (23%) of patients. In patients who had a CT-guided RFA, the most common complication was pneumothorax requiring a pigtail catheter in 12 patients (12/17;70%) patients.
3. Response
Initial response was determined by the modified RECIST criteria (Table 1). An initial complete response was observed in 2 patients (9%), partial response in 8 (36.4%), and stable disease in 8 (36.4%) and was not evaluable in 1 (4.5%). Early progression occurred in 3 patients (13.6%). The one patient who was not evaluable, did not want to follow-up after one month, and the response could not be evaluated. She lived for 5 years after the procedure.
4. Analysis of Survival and Progression
A total of 9 patients were alive at a mean follow-up was 27 months (median 26.9 months; range 13.3-53.6 months). The median overall survival for the entire group was 29.4 months and the estimated two year overall survival rate is 68% {95% confidence interval (CI) 44%-83%} (Figure 1). There were a total of 13 patients who died during follow-up. Of these deaths, 10 were cancer related, one was not cancer related and the cause of death was not determined in two patients.
Figure 1.
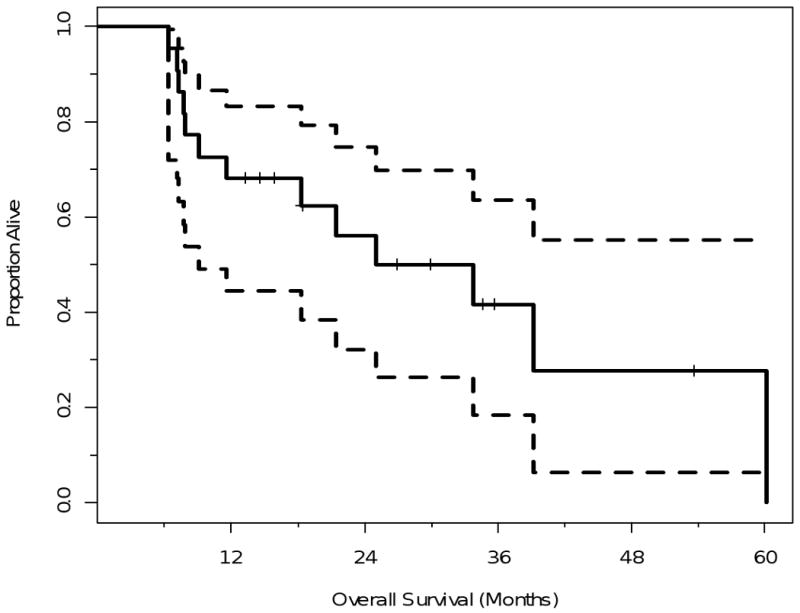
Kaplan-Meier Plot illustrating the overall survival for the entire group with confidence limits. The time shown is in x axis is in months from RFA.
The dotted lines are 95% confidence bands for the probability of overall survival.
During follow-up, overall progression (any site) occurred in 77% of patients and the median PFS was 5.8 months (3.2-8 months). Two patients whose lesions treated progressed locally were resected. The pathology examination showed primarily necrosis, but also residual tumor.
5. Analysis of Prognostic Factors associated with survival and progression
We analyzed the following covariates associated with overall survival and disease free survival: size, number of metastases, disease-free interval to first recurrence (any site), and to lung recurrence, approach (RFA alone or RFA as adjunct to surgical resection), primary cell type, single vs. multiple lesions, site of first metastases, age, and gender.
Size (<3cms vs. >3 cms) was a significant factor in both overall and DFS (P<0.05). The median survival for patients with lesion diameter less than ≤3 cms was 39.1 months (95% confidence limit 18.3- NR) versus. 7.9 months (95% confidence limit 6.3 months-NR) for patients with lesion size >3cm (P=0.002). Similarly the PFS was better in patients with lesion less than 3 cm. The median overall PFS (all sites) for patients with size of largest lesion ≤3cm was 7.15 months versus 3.2 months for patients with size of largest lesion >3cm (P=0.01) (Figures 2,3). Similarly the progression interval in the treated nodule site was significantly increased in patients with lesion size ≤3 cms (P<0.05).
Figure 2.
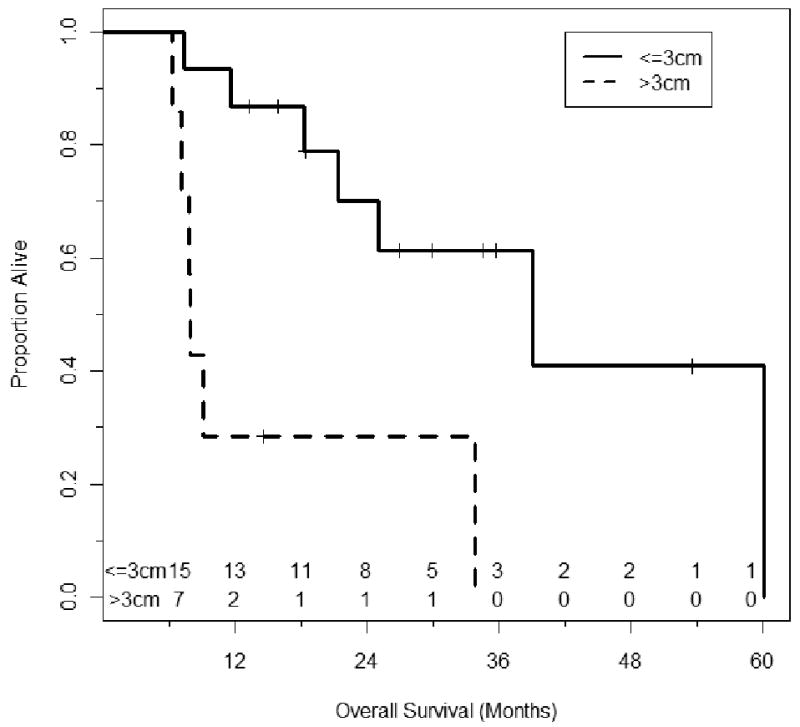
Kaplan-Meier Plot illustrating the overall survival stratified by size. The time shown is in x axis is in months from RFA (Log Rank P=0.002). The number of patients at risk is shown above the X-axis.
Figure 3.
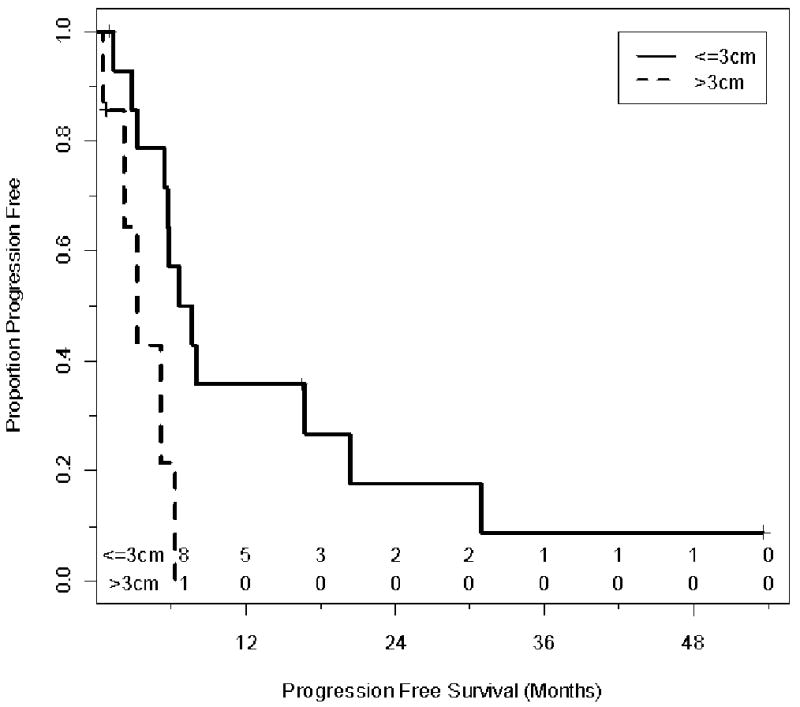
Kaplan-Meier Plot illustrating the progression free survival stratified by size. The time shown is in x axis is in months from RFA (Log Rank=0.01). The number of patients at risk is shown above the X-axis.
There were no significant differences in overall or progression free survival when patients who had RFA alone were compared to patients who had RFA as an adjunct to surgical resection {median survival 25 months vs. 33.8 months (p=0.99); median PFS 5.5 months vs. 7.0 months (P=0.43}. There were no significant differences noted when the following factors were analyzed: disease-free interval, approach, primary cell type, single vs. multiple lesions, site of first metastases, age, and gender. The results of analysis of association of individual covariates between overall survival and PFS are summarized in Table 4.
Table 4. Analysis of Prognostic Factors: Association between overall survival and progression free survival.
| Overall Survival P value | Progression Free Survival P value | |
|---|---|---|
|
Approach (RFA alone vs. RFA + Thoracotomy) |
0.99 | 0.43 |
|
Cancer type (Colon vs. Sarcoma vs. Others) |
0.57 | 0.92 |
|
Site of primary metastasis (Lung vs. Others) |
0.85 | 0.8 |
|
Gender (Male vs. Female) |
0.08 | 0.3 |
|
Largest size of lesion * (<=3cm vs. >3cm) |
0.003 | 0.02 |
| Age | 0.53 | 0.55 |
| Largest size of lesion (continuous variable)* | 0.02 | 0.04 |
| Disease-free interval to first metastases (any site) | 0.5 | 0.2 |
| Disease-free interval to metastases to lung | 0.26 | 0.59 |
P value < 0.05
Comment
Surgery is the treatment of choice for selected patients with pulmonary metastases. In particular, patients who have a single metastases, prolonged disease free interval (>36 months), complete control of the primary tumor and no evidence of extrathoracic disease are good candidates for resection and surgery offers good results in these patients with metastatic disease (1). In the medically inoperable or unresectable patients with pulmonary metastases, there are few effective options. Many of these patients, for instance patients with inoperable colorectal metastases are treated with chemotherapy and the long term survival is not encouraging (5). RFA may offer an alternative in these patients.
The International Registry for lung metastases (IRLM) reported the results in 5206 patients, of which 4572 had complete resection (1). The estimated 5 year survival after complete resection was 36% and the median survival was 35 months. In multivariate analysis, disease-free interval of more than 36 months, single metastases and germ cell primary were independent predictors of better outcome. Recurrences occurred in 53% of patients and the median time to recurrence was 10 months. Complete resection of all lesions was an important prognostic factor. When the metastases were resectable and when all factors were favorable the median survival was 61 months; when they were unresectable, the median survival was 14 months with an estimated 5 year overall survival of 5%.
Potential role of Radiofrequency Ablation in Pulmonary Metastases
With complete resection being an important predictor of outcome, RFA may be utilized in combination with surgical resection as a parenchymal-sparing approach or in lesions which are not resectable. In the current series, five patients had RFA as an adjunct to surgical resection as a parenchymal-sparing procedure. This may be applicable in patients who have peripheral lesions which can be resected; more central lesions can be treated with RFA during a thoracotomy with care taken to be away from the bronchus and pulmonary vessels. This use of RFA as an adjunct may be helpful in avoiding a lobectomy in some instances and may serve as a parenchymal-sparing procedure.
Further, the IRLM study showed that recurrences occurred in 53% of patients, and the median time to recurrence was 10 months. Many of these recurrences present in the ipsilateral chest even after open thoracotomy. The morbidity of a redo thoracotomy is higher and about 60% of patients may re-present with recurrent disease even after a redo thoracotomy (14). RFA in particular under CT-guidance may offer an alternative in some of these patients particularly as a parenchymal-sparing procedure.
Rolle and colleagues also demonstrated the importance of complete resection in using the 1318 nm Nd: YAG laser (2). The 5 year overall survival after complete resection was 40% and 7% after incomplete resection. The overall survival after complete resection of single, 10 or more, and more than 20 resected metastases was 55%, 28% and 26% respectively. These authors stressed the importance of complete resection even when multiple lesions were present as a very important determinant of survival. The recurrence rate was 60% and the median time to recurrence was 9 months. Among local pulmonary recurrences, only 32% underwent redo surgery.
Radiofrequency ablation may play a role in the treatment of re-recurrent metastases when the risks of redo surgery are high. Further redo-resection is associated with a high re-recurrence rate and may point to a more aggressive behavior of the primary tumor (14). In these circumstances, RFA may be a reasonable choice particularly in patients who have had one or more previous resections to avoid the morbidity of redo thoracotomy. In this series about a third of patients had a previous resection, and RFA was used to treat re-recurrences.
Prognostic Factors associated with survival after treatment with RFA
In addition to complete resection other prognostic factors have been examined after surgical resection of pulmonary metastases (3-4). Pfannschmidt and colleagues reported the results in 167 patients who underwent resection for colorectal metastases (3). These investigators reported that single vs. multiple lesions, and lymph node involvement were among the significant factors, while disease free interval,, resection of hepatic metastases, age, and sex were not significant factors.
In the current study, we examined the prognostic factors associated with survival and progression. Size was a significant predictor of both overall survival and disease free survival. The median overall survival was 39.1 months when the lesion size was less than 3 cm vs. 7.9 months when the lesion was more than more than 3 cm. We did not find a significant association for covariates such as cell type of primary, disease-free interval, site of first metastases, and single vs. multiple lesions, and survival; however this may be a limitation of a small group of patients.
In summary, with a mean follow-up of 27 months, the median overall survival was 29.4 months with an estimated two year overall survival rate is 68%, and the median time to progression was 5.8 months. These results while not equivalent to the reported results of complete surgical resection are better than reported results when the metastases are not completely resectable. Further, in this series several patients had failed other therapies prior to RFA, including previous surgical resection. Thus this group of patients treated may represent a group with biologically more aggressive tumors.
Other investigators have reported the results of RFA in the treatment of primary and metastatic lung tumors (5, 11,15,16,17). Recently Yan and colleagues reported the results of RFA for the treatment of pulmonary metastases in 55 patients with colorectal neoplasm (5, 15). The median follow-up in this series was 24 months. The median survival in these patients was 33 months and the estimated 2 year survival was 64%. On multivariate analysis the only factor which was associated with survival and overall progression was size (>3 cm). Interestingly, we also found that the size of the largest lesion was a significant factor in both overall and PFS in patients treated with RFA.
Strengths and Limitations
This study has its strengths and limitations. One of the novel aspects of this study which is applicable to thoracic surgeons is the combination of surgical resection in conjunction with RFA as a parenchymal-sparing procedure. There is minimal data in the literature where the intermediate term results of a combination of RFA in conjunction with surgical resection have been presented. In addition, this study represents one of the longest follow-up period (mean 27 months) reported in the literature on RFA for the treatment of pulmonary metastases. Further, we have evaluated prognostic variables which are associated with progression and survival with size of the lesion being significantly associated. This information may lead to better patient selection and prospective studies with development of protocols for larger lesions such as RFA with stereotactic radiosurgery and/or adjuvant therapy in these patients.
The current study however has the limitations which are inherent to retrospective studies, such as selection bias. The patients treated in this study comprise a very heterogeneous group, encompassing patients with different primary tumors who had metastatic disease. Finally, longer follow-up is required for greater maturity of time-to-event data. There are several factors which merit further investigation. These include optimal patient selection, and the role of combination therapy or adjuvant therapy.
Conclusion
Our experience indicates RFA is safe in this group of patients with pulmonary metastases with reasonable results. Surgery remains the standard for resectable patients, but RFA offers an alternative option in selected patients with pulmonary metastases or may be used as a parenchymal-sparing approach in combination with surgical resection in selected patients. Thoracic surgeons should continue to evaluate new technology and add these techniques to their armamentarium in the treatment of lung neoplasm. Prospective studies are necessary, and are ongoing at our institution and others, to define the role of RFA in the treatment of metastatic lung neoplasm.
Acknowledgments
This research was funded in part by the National Institutes of Health (NIH) Specialized Program of Research Excellence in Lung Cancer (P50 CA090440), and in part by research grants from RITA Medical Inc/Angiodynamics to the University of Pittsburgh
Footnotes
Presented at the Southern Thoracic Surgical Association, Bonita Springs, November 2007
References
- 1.Pastorino U, Buyse M, Friedel G, et al. The International Registry of Lung Metastases, Long-term results of lung metastasectomy prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 2.Rolle A, Pereszlenyi A, Koch R, Richard M, Baier B. Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1318-nm Nd:YAG laser. J Thorac Cardiovasc Surg. 2006 Jun;131(6):1236–42. doi: 10.1016/j.jtcvs.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Pfannschmidt J, Muley T, Hoffmann H, Dienemann H. Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg. 2003 Sep;126(3):732–9. doi: 10.1016/s0022-5223(03)00587-7. [DOI] [PubMed] [Google Scholar]
- 4.Okumura S, Kondo H, Tsuboi M, et al. Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg. 1996 Oct;112(4):867–74. doi: 10.1016/S0022-5223(96)70085-5. [DOI] [PubMed] [Google Scholar]
- 5.Yan TD, King J, Sjarif A, et al. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006 Nov;13(11):1529–37. doi: 10.1245/s10434-006-9101-1. [DOI] [PubMed] [Google Scholar]
- 6.Ketchedjian A, et al. Minimally invasive techniques for managing pulmonary metastases: video-assisted thoracic surgery and radiofrequency ablation. Thorac Surg Clin. 2006;16:157–65. doi: 10.1016/j.thorsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Weiser MR, Downey RJ, et al. Repeat resection of pulmonary metastases on patients with soft-tissue sarcoma. J Am Coll Surg. 2000;191:184–190. doi: 10.1016/s1072-7515(00)00306-9. [DOI] [PubMed] [Google Scholar]
- 8.Gadaleta C, Mattioli V, Colucci G, et al. Radiofrequency ablation of 40 lung neoplasms preliminary results. AJR Am J Roentgenol. 2004;183:361–369. doi: 10.2214/ajr.183.2.1830361. [DOI] [PubMed] [Google Scholar]
- 9.Yasui K, Kanazawa S, Sano Y, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231:850–857. doi: 10.1148/radiol.2313030347. [DOI] [PubMed] [Google Scholar]
- 10.Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency Ablation for Stage I non small cell lung neoplasm. J Thorac Cardiovasc Surg. 2007;134(4):857–64. doi: 10.1016/j.jtcvs.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Ambrogi MC, Lucchi M, Dini P, et al. Percutaneous radiofrequency ablation of lung tumours: results in the mid-term. Eur J Cardiothorac Surg. 2006;30(1):177–83. doi: 10.1016/j.ejcts.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 12.Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg. 2005;129:639–44. doi: 10.1016/j.jtcvs.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood M. Reports on public health and medical subjects. Vol. 33. His Majesty's Stationery Office; London: 1926. A report on the natural duration of cancer; pp. 1–26. [Google Scholar]
- 14.Fernando HC. Radiofrequency ablation to treat non-small cell lung cancer and pulmonary metastases. Ann Thorac Surg. 2008 Feb;85(2):S780–4. doi: 10.1016/j.athoracsur.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 15.Yan TD, King J, Sjarif A, et al. Treatment Failure after Percutaneous Radiofrequency Ablation for Nonsurgical Candidates with Pulmonary Metastases from Colorectal Carcinoma. Ann Surg Oncol. 2007;14(5):1718–26. doi: 10.1245/s10434-006-9271-x. [DOI] [PubMed] [Google Scholar]
- 16.Hiraki T, Sakurai J, Tsuda T, et al. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors. Evaluation based on a preliminary review of 342 tumors. Cancer. 2006;107(12):2873–2880. doi: 10.1002/cncr.22333. [DOI] [PubMed] [Google Scholar]
- 17.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007 Apr;243(1):268–75. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]


