Abstract
Preterm birth continues to be a growing problem in the USA. Although approximately half of preterm births are caused by intrauterine infection, uterine over-distension is also a cause. In this study we have compared the effects of static stretch, cyclic stretch/release and an inflammatory stimulus alone and in combination on the expression of Pre-B cell colony-enhancing factor (PBEF) and IL-8 in primary amniotic epithelial cells (AEC). We then sought to identify some of the mechanism(s) by which these cells respond to stretching stimuli. We show that cyclic stretch/release is a more robust stimulus for both PBEF and IL-8 than static stretch. Cyclic stretch/release increased both intracellular and secreted PBEF and a combination of both types of stretch was a more robust stimulus to PBEF that IL-8. However, when an inflammatory stimulus (IL-1β) was added to either kind of stretch, the effect on IL-8 was much greater than that on PBEF. Thus, different kinds of stretch affect the expression of these two cytokines from AEC, but inflammation is a much stronger stimulus of IL-8 than PBEF, agreeing with its primary role as a chemokine. Although the AEC showed morphological signs of increased cellular stress during stretching, blocking reactive oxygen species (ROS) had little effect. However, blocking integrin binding to fibronectin significantly reduced the responses of both PBEF and IL-8 to cyclic stretch/release. The increased PBEF, both intracellularly and secreted, suggests that it functions both to increase the metabolism of the cells, at the same time as stimulating further the cytokine cascade leading to parturition.
Keywords: Pre-B-cell colony-enhancing factor, IL-8, stretch, inflammation, fetal membranes
1. Introduction
Preterm birth continues be a growing problem as its rate has risen by 20% in the USA in the last ten years [1]. Approximately 50% of premature births are associated with intrauterine infection/inflammation [2], which triggers an immune response involving the proinflammatory cytokines, which then cause the synthesis and release of uterotonic prostaglandins [3]. However, normal term parturition also involves local cytokine signaling [4]. During normal pregnancy, the fetal membranes are subjected to a combination of chronic distension, allowing their accommodation to the growing fetus [5] and acute cyclical stretch/release both during Braxton-Hicks contractions, and during the major uterine contractions of labor. The stretching of the fetal membranes is known to increase a number of cytokines [6] which are associated with preterm birth [7]. In addition, inflammation caused by local infection or sterile inflammatory mediators such as thrombin also induce localized cytokine production. Therefore, in this study we attempted to mimic the in vivo situation where different stretch stimuli can occur simultaneously with inflammatory stimuli.
Pre-B-cell colony-enhancing factor (PBEF/visfatin) is a cytokine involved in the events of parturition [8, 9]. It is expressed in all cellular layers of the fetal membranes [10] and both labor and sterile distension cause its increased expression [8, 9]. Indeed, a number of stimuli associated with inflammation and infection; LPS, TNFα, IL-1β and IL-6 all up-regulate its expression [10] while in severe chorioamnionitis, it is produced by both the endogenous cells of the fetal membranes as well as the infiltrating neutrophils [9]. Although originally identified as a cytokine [12], more recently it was shown to be an adipokine and re-named Visfatin [13]. Although PBEF lacks a classical secretion sequence, its secretion has been demonstrated [12, 14, 15, 13], although the mechanism is currently unknown [16]. The treatment of several different cell types with PBEF increases production of pro-inflammatory cytokines and matrix metalloproteinases (MMPs) [17, 18, 19, 20], showing it to be an important modulator of the immune response [21], [15]. However, PBEF also functions intracellularly as the enzyme nicotinamide phosphoribosyltranferase (Nampt) and increases the amount of NAD+ available for metabolism [22]. Thus, the static stretch-induced increased production of PBEF [8, 23] may be a mechanism for providing the extra energy needed for the cell to successfully alter its cytoskeleton and gene expression profile required for adaptation to the stimulus and accommodation for the extra work required during labor. At the same time, its action to induce some key cytokines, such as TNFα [24] clearly involved in the parturition process [2], would further assist in the progression of labor.
Distension of the fetal membranes also increases expression of the IL-8 gene [8, 23, 25]. IL-8 has potent chemotactic and neutrophil activation properties [26]. It is constitutively expressed by the endogenous cells of the fetal membranes [27] and its expression is increased during normal gestation, resulting in accumulation in amniotic fluid during the third trimester [7]. However, its expression is increased in acute infection [28] and IL-8 is therefore involved in both the initiation of normal term parturition and in infection-induced preterm birth.
The aims of this study were (1) to compare the effects of static stretch, cyclic stretch/release, and inflammation (alone and in combination) on the expression of PBEF and IL-8 in primary amniotic epithelial cells (AEC). (2) To show which pathways are activated and cause their up-regulation, focusing on the roles of reactive oxygen species (ROS) and integrins.
2. Methods and Materials
2.1. Tissue collection and amniotic epithelial cell culture
Fetal membranes (n=33) were collected from patients having elective Cesarean sections before labor (38-40 weeks gestation) at Kapiolani Medical Center for Women and Children (Honolulu, HI, USA) with approval from the University Committee on Human Experimentation and the Hospital Institutional Review Board. All tissues were examined by a pathologist for histological evidence of infection, and if positive were excluded.
Primary AEC were isolated as previously described [29] and as used in our prior studies [30]. In brief, the amnion was stripped from adjacent choriodecidua and the epithelial cells isolated by consecutive trypsin (0.2%) digestion (Sigma, St. Louis, MO). The purity of epithelial cells obtained from each patient was similar to that previously reported [30]. The cells were utilized without passage and were seeded at a density of 2 million per well in a 6 cm culture plate in DMEM:F12 supplemented with heat inactivated 10% FBS (Invitrogen), penicillin (50U/ml)-streptomycin (50μg/ml) and incubated at 37°C in 95% air/5% C02 for 4 days. Media was changed every 3 days until the cells were 70-80% confluent (7-10 days).
2.2. Culture of amniotic epithelial-like cells (WISH)
Human amnion-derived WISH cell (ATCC CCL25) were obtained from the American type Culture Collection (ATCC, Manassas, VA) and grown in Dulbecco’s Modified Eagle Medium: Ham F-12 (DMEM:F12) (1:1) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Life technologies, Carlsbad, CA), penicillin (50U/ml)-streptomycin (50μg/ml) and incubated at 37°C in 95% air/5% CO2. When confluent, cells were plated onto Flexcell collagen IV (Flexcell International Corp, Hillsborough, NC) coated plates, at a density of 500,000 per well in DMEM:F12 medium containing 10% FBS and always used between passages 5-9.
2.3. Cell stretching experiments
When primary AEC (or WISH cells) reached sub-confluency, the medium was replaced with 0.5% FBS DMEM/F12 for 12-16 h prior to their use. In addition, fresh DMEM:F12 containing 0.5% FBS was added before exposure to any stimulation. Stretching was performed using a Flexcell instrument (Flexcell tension PlusT, FX-4000T) that simulates biological strain conditions using a vacuum to deform the cells cultured on a silastic flexible growth surface. This was set up and used based on the parameters designed by the manufacturer. The 20% static stretching of these cells was performed as described in our previous studies [23] and represents the maximum amount of stretch achievable with this instrument. Cyclic stretch/release was performed using repetition of 23 secs of 20% stretch followed by 7 secs of release to 0% stretch. In order to block integrin binding, the RGD peptide GRGDNP, 50 μM (Biomol International, Plymouth Meeting, PA) was pre-incubated with the cells for 2 h before stretching. Integrins bind to ligands with an exposed arginine-glycine-aspartate (RGD) sequence. Therefore, RGD peptides mimic this adhesion and can be used to block this interaction of the integrin with its usual extracellular matrix substrate. In order to inhibit reactive oxygen species, N-acetyl-L-cysteine (NAC, 30μM) (Sigma) was added to the cells 2 h before stretching.
2.4. RNA isolation and Quantitative Real-time PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) and DNase (Ambion, Austin, TX) treated, in accordance with the manufacturer’s instructions. Reverse transcription was performed with random hexamers. The resulting complementary DNA was used as a template for quantitative real-time PCR. Fluorescence resonance energy transfer (FRET) probes and primers for PBEF, IL-8 and 18S, were purchased from Applied Biosystems Taq Man Assays on Demand (ABI, Foster City, CA) and the ABI protocol used; one cycle for 10 mins at 95°C and 40 cycles of 15 secs at 95°C and 1 min at 60°C. Each 96-well plate included a water blank and a reverse transcriptase blank. cDNA dilutions were used to generate standard curves for each gene. Real-time PCR was carried out on a MJ Research Opticon 2 Continuous Fluorescence Detector (MJ Research, Waltham, MA). The contents of the PCR mix were; primers (200 nmol/L), FRET probe (100 nmol/L), magnesium (5.5 mmol/L) and Amplitaq Gold (0.025 U/μl). Each reaction was performed in triplicate and the results normalized to the expression of 18S in each sample.
2.5. Immunofluorescence localization of PBEF and the integrin β1 subunit
After stretching, the cells were fixed in 3.7 % formaldehyde in 1X PBS followed by three washes in 1X PBS for 10 mins total. Non-specific binding was blocked with 1 % BSA (Sigma) in 1X PBS for 45 min. The cells were again washed and PBEF antibody at 1/500, (Phoenix Pharmaceuticals) or Integrin β1 subunit antibody at 1/500 (Chemicon) added to each well. After incubation for 1h, the cells were again washed and secondary antibody; anti-rabbit or anti-mouse alexafluor 546 at 1/10,000 (Molecular Probes, Eugene, OR), was added for 45 min. The cells were washed again and Flexcell membranes, with cells still attached, were cut around the edges with a scalpel to release them from the plastic tissue culture plate. These were then mounted in Mowiol/glycerol mounting media (Sigma) containing 1,4-Diazabicyclo[2.2.2]octane (DABCO) (Sigma) and viewed under fluorescence microscopy.
2.6. Phalloidin-TRITC staining for Actin cytoskeleton
After stretching, cells adherent to the Flexcell plates were fixed in 4% formaldehyde in 1X PBS containing calcium (0.901mM) and magnesium (0.493) followed by three washes in 1X PBS for 10 mins total. The cells were then permeabilized in 0.2% Triton x-100 in 1X PBS for 5 mins and washed three times with 1X PBS followed by blocking in 10% FBS (Invitrogen) in 1X PBS for 40 mins. The cells were washed again and then stained with Phalloidin-TRITC (Sigma) at 1/2500 in 1X PBS for 1 h. The cells were washed again before the Flexcell membranes were cut around the edges with a scalpel to release the membrane with the adherent cells. These were then mounted in Mowiol/glycerol mounting media (Sigma) containing 1,4-Diazabicyclo[2.2.2]octane (DABCO) (Sigma) and viewed under fluorescence microscopy.
2.7. Western blotting for PBEF, phoshorylated FAK and paxillin
Cell lysates (20 μl) were boiled for 5 min in 4X SDS loading buffer with 5mM DTT and loaded into a 10% SDS polyacrylamide gel. The electrophoretically separated proteins were then transferred onto nitrocellulose membranes. Immunoblotting was performed by overnight blocking with 5% non-fat milk (BioRad) 0.1% Tween-20 (Fisher) in 1X PBS. The membranes were then incubated with primary antibodies for 1h in blocking solution. Antibodies were used at; 1/1000 PBEF (Phoenix Pharmaceuticals, Belmont, CA), 1/300 phosphorylated FAK (Try 576), 1/500 phosphorylated Paxillin (Try 118) (Upstate). After washing, the membranes were incubated with secondary anti-rabbit antibody conjugated to horseradish peroxidase at 1/3000 (BioRad) for 45 min. The membranes were again washed and developed using an enhanced chemiluminescence kit (Amersham Inc, Pitscataway, NJ). The blots were immediately exposed to hyperfilm-enhanced chemiluminescence (Amersham) and the signals quantitated with a densitometer (Kodak EDAS290 System: Eastman Kodak Co, Rochester, NY). The membranes were washed overnight with PBS 0.1% tween and then re-probed with GAPDH antibody (Abcam, Cambridge, MA) conjugated to HRP at 1/1000 at room temperature for 1h as a protein loading control. The membranes were again washed and developed using an enhanced chemiluminescence kit, the blots exposed and the signals quantitated with a densitometer, the relative levels of each protein were normalized to the amount of GAPDH in each sample.
2.8. Statistical analysis
The Mann-Whitney U test was performed and only p values <0.05 were considered significant. All statistics were calculated with GraphPad software (GraphPad Software Inc, San Diego, CA).
3. Results
3.1. PBEF and IL-8 are increased by cyclic stretch/release in primary AEC
The effect of cyclic stretch/release has not previously been investigated using cells of the fetal membranes. Preliminary experiments were therefore performed with amniotic epithelial-like cells (WISH) (data not shown) and the Flexcell instrument using a protocol previously devised to simulate the dynamic effects of stretching myometrial cells [31]. Once the technique was established, primary AEC were then used to show the PBEF and IL-8 gene responses. PBEF gene expression (Fig. 1A) was significantly (p<0.05) increased by 60% after 4 h of cyclic stretch/release. IL-8 gene expression (Fig. 1B) increased more rapidly, reaching significance after only 20 mins of stimulation (p<0.05) and continued to rise over the 4 h, at which time, it was significantly increased (p<0.05) representing a 15-fold increase compared to the control. After 24 h of stretch/release stimulation, media were collected and the secreted PBEF and IL-8 proteins measured to confirm that the changes in gene expression were translated into protein. Secreted PBEF (Fig. 1C) was significantly increased (p<0.005) and IL-8 was significantly increased (p<0.05) (Fig. 1D).
Fig. 1.
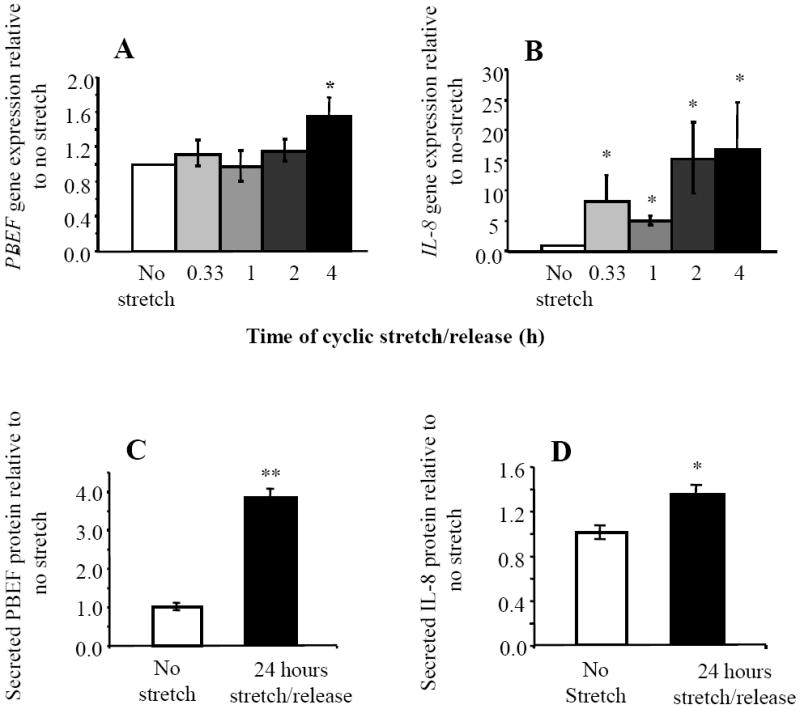
Effects of cyclic stretch/release on expression of PBEF and IL-8 in primary AEC (n=4 cells from different patients). (A) PBEF and (B) IL-8 gene expression during stimulation by cyclic stretch/release for 4 h. (C) PBEF and (D) IL-8 proteins secreted into medium after 24 h of cyclic stretch/release. Values are mean +/-SEM. *p<0.05, ** p<0.005 compared to control (no cyclic stretch/release).
PBEF is also active intracellularly and the effects of stretch/release on its intracellular pool were investigated. Immunofluorescence for intracellular PBEF in AEC showed increases in PBEF during 4 h of stretch/release stimulation (Fig. 2A-D). After 1 h, areas of very high density PBEF immunofluorescence were evident (Fig. 2C). The reason(s) for this are currently unknown. After 4 h of stretch/release, cytoplasmic staining of PBEF was generally more intense, and diffuse than at 1 h, although still punctate in its distribution. This PBEF increase was confirmed by quantitative Western blotting using cell lysates. An example Western blot is shown (Fig. 2E) showing the characteristic two PBEF bands [23] and the GAPDH protein loading control below. Quantitation (n=4) showed a significant increase (p<0.05) in intracellular PBEF after 4 h of cyclic stretch/release. These results show that both intracellular and secreted PBEF were increased by the cyclic stretch/release regimen.
Fig. 2.
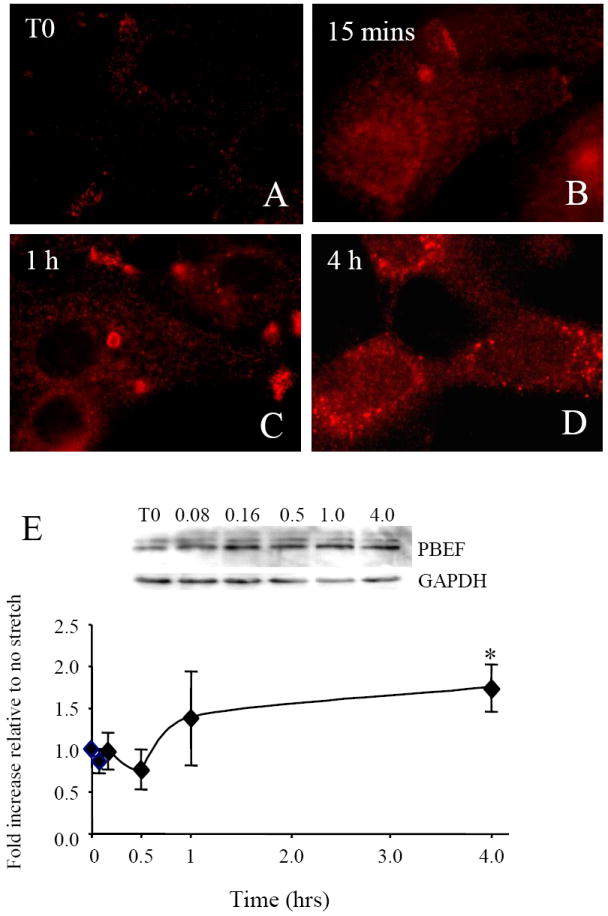
Effects of cyclic stretch/release on expression of intracellular PBEF protein. Immunofluorescence labeling of PBEF in AEC during 4 h of cyclic stretch/release stimulation. (A) T0 shows diffuse cytoplasmic labeling of PBEF, (B) at 15 mins there was increased intensity of PBEF labeling which continued during (C) 1 h and (D) 4 h of stimulation. (E) An example of a Western blot of PBEF with GAPDH as control using AEC lysates. Below, quantitation of Western data from n=4 (cells from different patients). Values are mean +/-SEM. *p<0.05 compared to control (no cyclic stretch/release).
3.2. Comparison of the effects of cyclic and static stretch on PBEF and IL-8 expression
The fetal membranes are subjected to different patterns of distension/stretching in the third trimester of pregnancy and different in vitro stretch protocols have been shown to cause differential effects on stretch-responsive genes [32, 33]. We therefore compared the effects of different types of stretch on PBEF and IL-8 gene expression. First, AEC were subjected to 2 and 4 h of 20% static stretch or 20% cyclic stretch/release, allowing a direct comparison of their effects in cells isolated from the same patients. Both static and cyclic stretch significantly up-regulated PBEF gene expression (p<0.05) compared to the unstretched controls (Fig. 3A). The cyclic stretch/release caused a significantly greater response (p<0.05) compared to static stretch at 2 h but not after 4 h (Fig. 3A). However, when the cells were subjected to a protocol of 2 h of static stretch followed by 2 h of cyclic stretch/release, the response was significantly greater (p<0.05) than for either type of stretch alone for 4 h (Fig. 3A). Expression of the IL-8 gene was also measured (Fig. 3B). After 2 h both stretch protocols caused a significant increase in IL-8 gene expression (p<0.05) compared to the unstretched controls (Fig. 3B). After 4 h the static stretch response had declined to control levels (Fig. 3B) confirming our previous observation [23]. However, the cyclic stretch protocol caused a further significant increase in IL-8 (p<0.05). The 2 h static stretch followed by 2 h of cyclic stretch also caused a significant increase in IL-8 expression (p<0.05), but this was not as robust as that caused by 4 h of cyclic stretch/release alone (Fig. 3B). These data suggest that cyclic stretch/release is more robust at stimulating both PBEF and IL-8 gene expression than static stretch and that the response of IL-8 was more attenuated than that of PBEF. In addition, PBEF was more responsive to the consecutive effects of static stretch and cyclic stretch/release than IL-8.
Fig. 3.
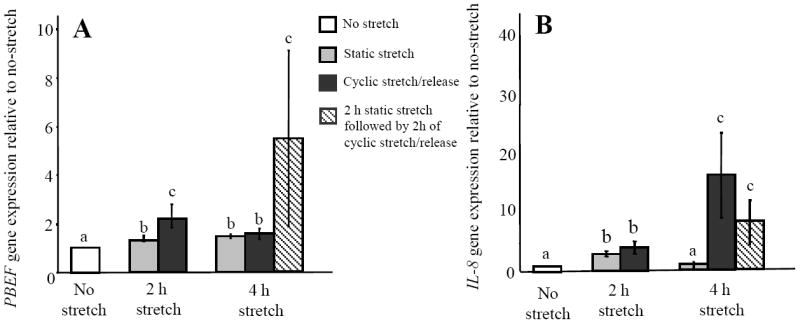
Effects of static stretch and cyclic stretch/release on PBEF and IL-8 gene expression in AEC (n=4) from different patients. (A) PBEF (B) IL-8. Values are mean +/-SEM. P<0.05 for dissimilar letters.
3.3. The combined effect of stretch and inflammation on PBEF and IL-8 expression
Inflammation often occurs simultaneously with stretching in the fetal membranes. Therefore, we determined if the effect(s) of these stimuli were additive. Cells were stretched by either 20% static stretch or 20% cyclic stretch/release for 2 h, with or without the addition of IL-1ß (1ng/ml) to mimic a mild inflammatory response. 2 h was chosen as both the PBEF and IL-8 genes were shown to be responsive to both static and cyclic stretch/release at this time. IL-1ß alone caused a significant increase in PBEF gene expression (p<0.05) (Fig. 4A). However, the response of PBEF in cells either statically stretched or exposed to cyclic stretch/release for 2 h was significantly greater (p<0.05) when IL-1ß was present during these stretching protocols (Fig. 4A). This was also the case for IL-8 (Fig. 4B) although the magnitude of its response to IL-1ß was two orders more than that caused by either type of stretching alone (Fig. 4B). Thus, although both the PBEF and IL-8 genes were responsive to stretch, an inflammatory stimulus (IL-1ß) caused a much more robust response in IL-8 than in PBEF, and this was markedly greater when combined with static stretch.
Fig. 4.
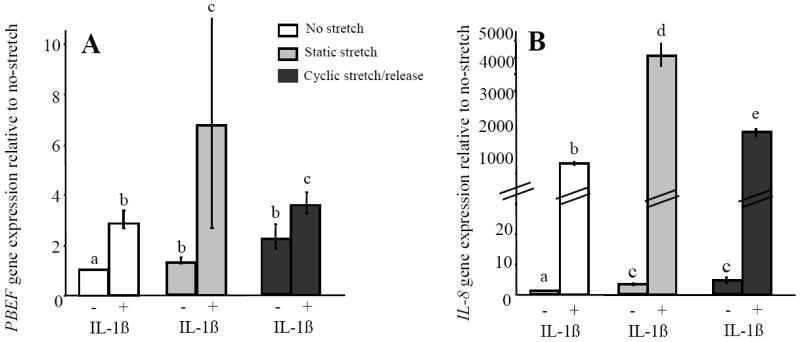
The combined effect(s) of stretch and an inflammatory stimulus (IL-1β (1ng/ml)) on PBEF and IL-8 gene expression in AEC. (A) PBEF (B) IL-8. Values are mean +/- SEM. P<0.05 for dissimilar letters.
3.4. The effect of reactive oxygen species (ROS) on the expression of PBEF and IL-8 after stretching
A morphological study of the effect of static stretching on the AEC was carried out. These were grown and statically stretched for up to 4 h and labeled for actin, in order to examine the cytoskeleton morphology. This demonstrated the formation of stress fibers after only 15 mins of static stretching (Fig. 5A). These fibers were visualized throughout static stretching for up to 4 h (Fig. 5A). By 4 h the cells also produced increased numbers of filapodia, indicating their increased levels of cellular stress. One of the typical consequences of this is the production of reactive oxygen species (ROS), known to be produced by cellular stretching [34] and previously shown to be involved in the production of some other cytokines [35]. Therefore, we blocked the production of ROS by the addition of N-acetyl-L-cysteine (NAC). WISH cells were used to avoid the patient variability intrinsic to AECs, these were pre-incubated with NAC (30μM) for 3 h before stretching. Again there were significant increases in PBEF and IL-8 (p<0.05) gene expression after both static and cyclic stretch/release (Fig. 5B and C). However, there were no significant effects on either gene when NAC was added to either stretch protocol (Fig. 5 B-C). Therefore, taken together with the morphological changes, it is likely that ROS is produced during stretching of AEC but from these results it does not appear to be of major importance for the response of PBEF or IL-8 to stretching.
Fig. 5.
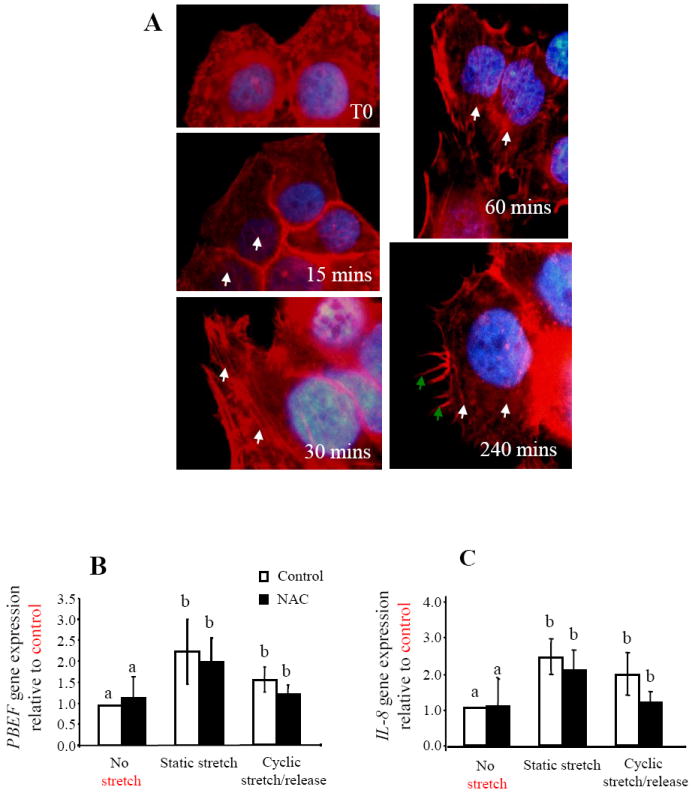
The role of reactive oxygen species (ROS) in the responses of PBEF and IL-8 to stretching was shown by the specific blocking of ROS by NAC (30μM). In AEC the localization of stress fibers (white arrows) and filapodia (green arrows) in primary AEC during 4 h of static stretching. In WISH cells (n=6) static and cyclic stretch/release increased both PBEF (B) and IL-8 (C) gene expression. Values are mean +/-SEM.
3.5. The effect of stretch on integrin clustering and signaling
Integrins are transmembrane molecules that mediate cell attachment and transduce mechanical forces. Therefore, we investigated the effect of stretch on their activation in AEC. As integrins change in number, location or strength of adhesive contacts to alter cell harmonics, we investigated their expression pattern by immunofluorescence localization. The β1 integrin subunit is present at the basal surface of AEC [36] and has been shown to cluster in keratinocytes facilitating adhesion after mechanical stretch [37]. Therefore, primary AEC were stretched using both stretch protocols for up to 4 h and the cells then labeled with anti-β1 antibody. Clustering of the β1 integrin subunit was visualized on the basal surface of the AEC after 15 mins of cyclic stretch/release and remained over the 4 h tested (Fig. 6A-D). In Fig. 5A the normal cell surface expression of β1 integrin expression was localized between cells at their membranous interface. However, after stretching for 15 mins and longer, β1 clustering was also visible at the basal surface of the AEC where these cells adhere to the Flexcell plate (Fig. 6B-D). This clustering was not evident after static stretching (data not shown). Integrin receptors are known to function through the activation of a number of signaling molecules [38], we therefore showed that the stretch/release of these cells also caused the activation of the integrins by examining two of the signaling molecules involved; focal adhesion kinase (FAK) and paxillin. Primary AEC were cyclically stretch/released for 5, 10, 30 and 60 mins, cell lysates prepared and Western blots performed. Example Western blots for phosphorylated FAK and Paxillin are shown with GAPDH protein loading controls below each blot (Fig. 6E and F). Quantitation of Western blots (n=4) for both phophorylated FAK (Fig. 6E) and phosphorylated paxillin (Fig. 6F), showed significantly increased levels of phosphorylation after 5 and 5-10 mins respectively (p<0.05), confirming integrin signaling events occurred subsequent to the cyclic stretch/release stimulus.
Fig. 6.
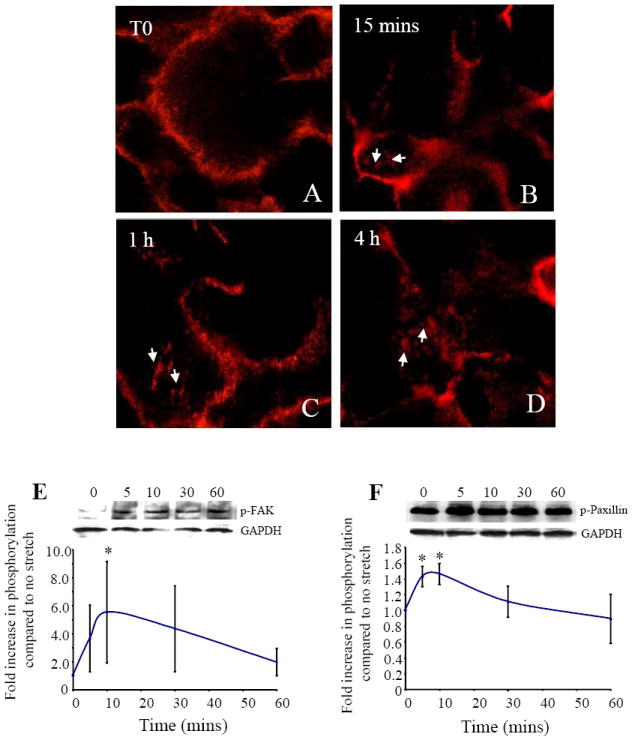
Integrins were activated in AEC by the cyclic stretch/release stimulus, shown by immunofluorescent localization of integrin β1 subunit (A-D). Clustering on the basal surface of the AEC (white arrows) can be seen after 15 mins of stimulation, maintained at both 1 h and 4 h of continued cyclic stretch/release. (E) Western blot of p-FAK with AEC lysates during 1 h of cyclic stretch/release, with GAPDH as control and quantitation (n=4) cells from different patients shown below. (F) Western blot of p-Paxillin of the same cell lysates shown during 1 h of cyclic stretch/release, with GAPDH as control and quantitation (n=4) shown below. Values are mean +/-SEM. *p<0.05 compared to no stretch control.
3.6. The effect of integrin binding on PBEF and IL-8 gene expression after stretching in WISH cells
In order to determine if the activation of integrins is important for the increases in both PBEF and IL-8 gene expression after stretching, WISH cells were again used. These were pre-incubated with an RGD peptide (50μM) for 3 h before stretching, in order to inhibit the binding of integrin subunits to fibronectin [33] and then stretched for 2 h. We also established that the RGD peptide alone had no effect on the expression of either PBEF or IL-8 in the absence of stretching (Fig. 7A and 6B respectively). Static stretching and cyclic stretch/release both significantly (p<0.05) increased the expression of both PBEF (Fig. 7A and B) and IL-8 (Fig. 7C and D respectively), as shown previously for primary AEC (Fig. 3A and B). The RGD peptide marginally but not significantly reduced the expression of PBEF and IL-8 during static stretch (Fig. 7A and C). However, blocking integrin binding to fibronectin significantly (p<0.05) inhibited the up-regulation of both PBEF and IL-8 caused by cyclic stretch/release (Fig. 7B and D). These results were confirmed with primary AEC, but because of large inter-patient variability in the responses of these cells, results with cells from one patient are shown for PBEF and IL-8 respectively (Fig. 7E and F). The RGD peptide only reduced the expression of PBEF (Fig. 7E) and IL-8 (Fig. 7F) when the cyclic stretch/release protocol was used.
Fig. 7.
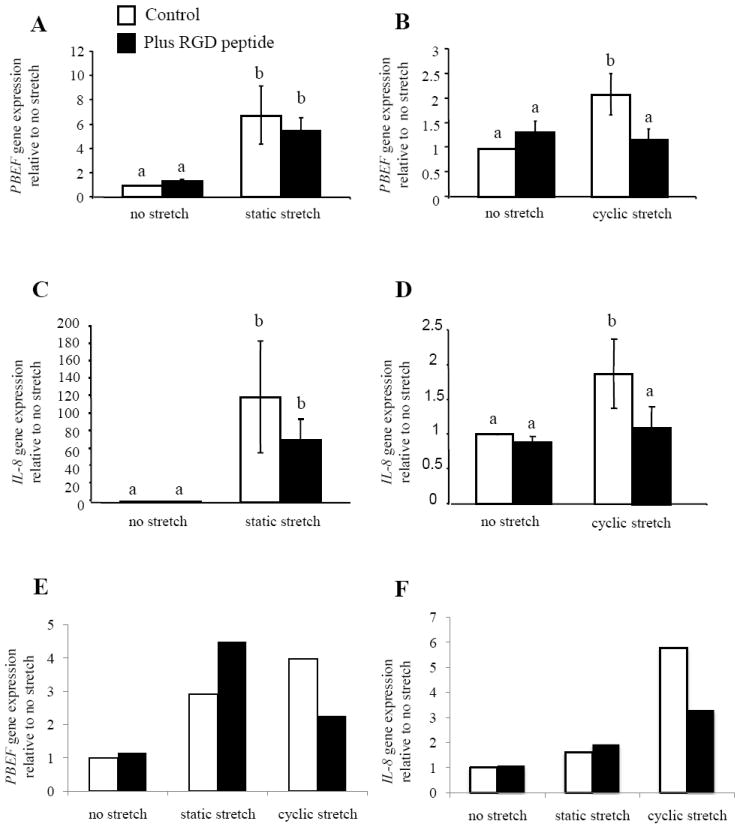
The role of integrins in PBEF and IL-8 responses to stretching was shown by addition of a blocking peptide (RGD) to WISH cells during static and cyclic stretch/release (n=5). (A) PBEF (C) IL-8 gene expression with static stretch and (B) PBEF and (D) IL-8 with 2 h of cyclic stretch/release. An example experiment completed with primary isolated AEC from one patient showing gene expression for (E) PBEF and (F) IL-8. Values are mean +/-SEM. P<0.05 for dissimilar letters.
4. Discussion
In this study we have compared the effects of static stretch, cyclic stretch/release and an inflammatory stimulus alone, and in combination, on the expression of PBEF and IL-8 in primary AEC. We then sought to identify the mechanism(s) by which these cells responded to the stretching stimuli. Thus, we hoped to gain insights into how cellular stretching affects these two cytokines and how concurrent stretching and inflammation may alter their responses.
We have shown that cyclic stretch/release of AEC up-regulates both the PBEF and IL-8 genes and that these were translated into increases in their secreted proteins. We used the maximum amount of stretch (20%) possible with the Flexcell system as fetal membranes are stretched to as much as 70% in vivo at term [5]. PBEF increased by almost 400% compared to an increase of approximately 50% for IL-8. Thus, although IL-8 gene expression was robustly increased, this was not translated into high levels of secreted IL-8. This is similar to our previously published data where we showed that transiently increased IL-8 gene expression following static stretch was not translated into protein [23]. Indeed, it may be that the modest increase in secreted IL-8 caused by stretching is merely a mechanism to assure its constitutive expression during gestation [27]. This is the first time that the effects of cyclic stretch/release have been shown for PBEF expression, although it has been shown to increase IL-8 expression in smooth muscle cells [39], cervical fibroblasts [40] and myometrial cells [41]. As PBEF is an intracellular enzyme as well as a secreted cytokine, we also measured its intracellular changes after cyclic stretch/release. We show that this rapidly increased after only 15 min, and was maintained over the 4 h tested. Some of this intracellular pool was likely to be stored PBEF but it probably also represents de novo production. PBEF in its intracellular form is identical to that of its secreted form [42] and functions as the metabolic enzyme ‘Nampt’ which sequesters NAD+ by its salvage pathway [22]. Thus, by cyclically stretching these cells, their increased metabolic needs may also be met.
We have compared the effects of cyclic stretch/release with static stretching and shown that the former stimulated these genes more than static stretch. In addition, we showed that if these two types of stretch were applied consecutively, they had a marked additive effect on the expression of PBEF. PBEF is responsive to static stretching over longer periods of time [23], thus it is likely that in this case the static stretch ‘primed’ the cells for the subsequent response to cyclic stretch/release, resulting in a greatly enhanced up-regulation of PBEF compared to either stretch stimulus alone for the same amount of time. This is important because the cells of the fetal membranes in utero are in a state of constant stretch, as they accommodate the growing fetus, but as the pregnancy progresses they are also subjected to fetal movements and Braxton Hicks contractions. Therefore our data suggests that static stretching, followed by cyclic stretch/release in vivo would cause a robust up-regulation of PBEF.
The effect(s) of an inflammatory stimulus, at the same time as stretching was also studied, because in vivo these tissues are often subjected to both stimuli together. IL-1β is often used to induce a milder (non-infection driven) inflammatory response compared to the addition of LPS, which is used to mimic bacterial infection. IL-1β is able to induce endogenous expression of other inflammatory cytokines and is an acceptable way to mimic non-infection driven inflammation. We showed that IL-1β had a marked additive effect on the expression of PBEF and IL-8 when used with either stretching protocol. However, this effect was much greater for IL-8, than PBEF.
During static stretching, actin stress fibers and filapodia became visible suggesting an increased production of ROS due to cellular stress. However, blocking the production of ROS with NAC failed to significantly alter the stretch-induced increases in PBEF and IL-8. Although NAC has anti-oxidant properties and functions as a free radical scavenger blocking ROS and decreasing cytokine production [35] the lack of any effects here suggests that it contributes little to the effects of stretch on these genes. However, other ROS inhibitors need to be investigated for further clarification of the importance of ROS in the production of other cytokines after stretch.
The role of integrins in the stretch-induced increases in PBEF and IL-8 was also investigated, as the αvβ6 integrin has been localized to the amnion of the fetal membranes and shown to increase near term [44]. We confirmed their activation by clustering and downstream signaling by FAK and paxillin phosphorylation after cyclic stretch/release. When the binding of integrins to their fibronectin substrate was blocked and the cells stretched, the increases in PBEF or IL-8 after cyclic stretch/release was prevented. To do this a previously successful strategy in which we pre-treated our cells with an RGD peptide was used [33]. Although, this inhibited the increases in PBEF and IL-8 by cyclic stretch/release, it had no effect on their increased expression after static stretching. Integrins are mechanoreceptors sensing changes between the cell surface and the surrounding extracellular matrix. Thus during the static stretch stimulus, only a single change upon the initial stretching stimulus would be registered. However, during cyclic stretch/release, repeated changes between the cell and the extracellular matrix upon each stretch/release cycle would be registered. We conclude that integrin activation is central to the increases in these two cytokine genes caused by cyclic stretch/release. Endogenous changes in integrin expression in the fetal membranes from patients in preterm labor, with or without PPROM, have not been studied to date, but would be of interest in light of these results.
In conclusion, we have shown that cyclic stretch/release is a more robust stimulus for both PBEF and IL-8 than static stretch. Cyclic stretch/release increased both intracellular and secreted PBEF, suggesting that its dual functions as an enzyme and secreted cytokine are important. A combination of static stretch followed by cyclic stretch/release was a more robust stimulus for PBEF than for IL-8. However, when IL-1β was added to either kind of stretch, the effect on IL-8 was very much greater than for PBEF, in line with its major role as a chemokine [26]. Blocking the integrin binding to fibronectin significantly reduced the responses of both PBEF and IL-8 to cyclic stretch/release. Therefore, we have shown that different kinds of stretching affect the expression of these two cytokines produced by AEC and although inflammation was a much stronger stimulus for IL-8 than PBEF, had an additive effect on the expression of both cytokines.
Acknowledgments
Supported by NIH grant #U54RR14607-08 to The Pacific Research Center for Early Human Development, University of Hawaii (CEKW), NIH grant #HD-24314 (G.B.G), and grants from the Hawaii Community Foundation and the Research Centers in Minority Institutions Program of NIH (RRIA1-03061 and RR-11091).
We thank the nurses and staff of the labor and delivery ward of Kapiolani Medical Center for Women and Children for their help with the tissue collection for the present study. We would also like to acknowledge that the Flexcell instrument was purchased with funds from an award from the IDeA Networks of Biomedical Research Excellence (INBRE) grant number; PO RR16467. This publication was made possible by a grant from the NIH #U54 RR014607-10 “Pacific Research Center for Early Human Development” from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.March of Dimes. 2000 http://marchofdimes.com/peristats/
- 2.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J of Reprod Immunol. 2008;79:50–7. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Keelan JA, Blumenstein M, Helliwell RJA, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 4.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet and Gynecol. 1999;181:1530–6. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 5.Millar LK, Stollberg J, DeBuque L, Bryant-Greenwood G. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol. 2000;182:128–34. doi: 10.1016/s0002-9378(00)70501-1. [DOI] [PubMed] [Google Scholar]
- 6.Kendal-Wright CE. Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci. 2007;14:35–41. doi: 10.1177/1933719107310763. [DOI] [PubMed] [Google Scholar]
- 7.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–8. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. Am J Obstet Gynecol. 2000;182:60–7. doi: 10.1016/s0002-9378(00)70491-1. [DOI] [PubMed] [Google Scholar]
- 9.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human Pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–17. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 10.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–8. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 11.Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, et al. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am J Obstet Gynecol. 2005;193:404–13. doi: 10.1016/j.ajog.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human Pre-B-cell colony-enhancing factor. Mol Cel Biol. 1994;14:1431–7. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 14.Ognjanovic S, Ku TL, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am J Obstet Gynecol. 2005;193:273–82. doi: 10.1016/j.ajog.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–27. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Nozaki M, Fukuhara A, Segawa K, Aoki N, Matsuda M, et al. Visfatin is released from 3T3-l1 adipocytes via a non-classical pathway. Biochem Biophys Res Comm. 2007;359:194–201. doi: 10.1016/j.bbrc.2007.05.096. [DOI] [PubMed] [Google Scholar]
- 17.Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of Pre-B-cell colony-enhancing factor on the human fetal membranes by microarray analysis. Am J Obstet Gynecol. 2003;189:1187–95. doi: 10.1067/s0002-9378(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 18.Adya R, Tan BK, Chen J, Randeva HS. Pre-B cell colony enhancing factor (PBEF)/visfatin induces secretion of MCP-1 in human endothelial cells: role in visfatin-induced angiogenesis. Atheroscl. 2009;205(1):113–9. doi: 10.1016/j.atherosclerosis.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signaling pathways: novel insights into visfatin-induced angiogenesis. Cardiovas Res. 2008;78:356–65. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- 20.Gosset M, Berenbaum F, Salvat C, Sautet A, Pigenet A, Tahiri K, et al. Crucial role of visfatin/Pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arth Rheu. 2008;58:1399–409. doi: 10.1002/art.23431. [DOI] [PubMed] [Google Scholar]
- 21.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171:361–370. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 22.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic stretching of amniotic epithelial cells increases Pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta. 2008;29:255–65. doi: 10.1016/j.placenta.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Van Gool F, Gallí M, Gueydan C, Kruys V, Prevot P, Bedalov A, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206–210. doi: 10.1038/nm.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maehara K, Kanayama N, Maradny EE, Uezato T, Fujita M, Terao T. Mechanical stretching induces interleukin-8 gene expression in fetal membranes: a possible role for the initiation of human parturition. Eur J Obstet Gynecol Reprod Biol. 1996;70:191–6. doi: 10.1016/s0301-2115(95)02602-9. [DOI] [PubMed] [Google Scholar]
- 26.Matsushima K, Baldwin ET, Mukaida N. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol. 1992;51:236–265. [PubMed] [Google Scholar]
- 27.Dudley DJ, Trautman MS, Mitchell MD. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J ClinEndocrinol Met. 1993;76:404–10. doi: 10.1210/jcem.76.2.8432783. [DOI] [PubMed] [Google Scholar]
- 28.Harada A, Mukaida N, Matsushima K. Interleukin 8 as a novel target for intervention therapy in acute inflammatory diseases. Mol Med Today. 1996;2:482–9. doi: 10.1016/1357-4310(96)10042-3. [DOI] [PubMed] [Google Scholar]
- 29.Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol Reprod. 1996;55:1253–1260. doi: 10.1095/biolreprod55.6.1253. [DOI] [PubMed] [Google Scholar]
- 30.Millar LK, Reiny R, Yamamoto SY, Okazaki K, Webster L, Bryant-Greenwood GD. Relaxin causes proliferation of human amniotic epithelium by stimulation of insulin-like growth factor-II. Am J Obstet Gynecol. 2003;188:234–241. doi: 10.1067/mob.2003.80. [DOI] [PubMed] [Google Scholar]
- 31.Harada M, Osuga Y, Hirota Y, Koga K, Morimoto C, Hirata T, et al. Mechanical stretch stimulates interleukin-8 production in endometrial stromal cells: possible implications in endometrium-related events. J Clin Endocrinol Met. 2005;90:1144–8. doi: 10.1210/jc.2004-1089. [DOI] [PubMed] [Google Scholar]
- 32.Kanefsky J, Lenburg M, Hai C. Cholinergic receptor and cyclic stretch-mediated inflammatory gene expression in intact ASM. Am J Respir Cell Mol Biol. 2006;34:417–425. doi: 10.1165/rcmb.2005-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasamoto A, Nagino M, Kobayashi S, Naruse K, Nimura Y, Sokabe M. Mechanotransduction by integrin is essential for IL-6 secretion from endothelial cells in response to uniaxial continuous stretch. Am J Physiol. 2005;288:C1012–22. doi: 10.1152/ajpcell.00314.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ali MH, Mungai PT, Schumacker PT. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol. 2006;291:L38–45. doi: 10.1152/ajplung.00287.2004. [DOI] [PubMed] [Google Scholar]
- 35.Lappas M, Permezel M, Rice GE. N-acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88:1723–9. doi: 10.1210/jc.2002-021677. [DOI] [PubMed] [Google Scholar]
- 36.Behzad F, Jones CJ, Ball S, Alvares T, Aplin JD. Studies of hemidesmosomes in human amnion: the use of a detergent extraction protocol for compositional and ultrastructural analysis and preparation of a hemidesmosome-enriched fraction from tissue. Acta Anat. 1995;152:170–184. doi: 10.1159/000147695. [DOI] [PubMed] [Google Scholar]
- 37.Knies Y, Bernd A, Kaufmann R, Bereiter-Hahn J, Kippenberger S. Mechanical stretch induces clustering of beta1-integrins and facilitates adhesion. Exp Dermatol. 2006;15:347–55. doi: 10.1111/j.0906-6705.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Knox AJ, Boriek AM. CCAAT/enhancer-binding protein and activator protein-1 transcription factors regulate the expression of interleukin-8 through the mitogen-activated protein kinase pathways in response to mechanical stretch of human airway smooth muscle cells. J Biol Chem. 2003;278:18868–76. doi: 10.1074/jbc.M212777200. [DOI] [PubMed] [Google Scholar]
- 40.Takemura M, Itoh H, Sagawa N, Yura S, Korita D, Kakui K, et al. Cyclic mechanical stretch augments both interleukin-8 and monocyte chemotactic protein-3 production in the cultured human uterine cervical fibroblast cells. Mol Hum Reprod. 2004;10:573–80. doi: 10.1093/molehr/gah077. [DOI] [PubMed] [Google Scholar]
- 41.Sooranna SR, Engineer N, Loudon JA, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: the differential effect of stretch and interleukin-1beta. J Clin Endocrinol Met. 2005;90:3517–27. doi: 10.1210/jc.2004-1390. [DOI] [PubMed] [Google Scholar]
- 42.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Met. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antiox Redox Signal. 2009;11:1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed N, Riley C, Oliva K, Barker G, Quinn MA, Rice GE. Expression and localization of alphavbeta6 integrin in extraplacental fetal membranes: possible role in human parturition. Mol Hum Reprod. 2004;10:173–9. doi: 10.1093/molehr/gah025. [DOI] [PubMed] [Google Scholar]


