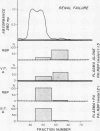
Abstract
The effects of diseases of the liver, the thyroid, and the kidneys on the retinol-binding protein (RBP)-prealbumin (PA) system responsible for the transport of vitamin A in plasma were examined, using a radial gel diffusion immunoassay for PA and the previously described radioimmunoassay for RBP. Measurements were made on plasma samples from 118 normal subjects, 31 patients with cirrhosis, 5 with chronic active hepatitis, 27 with acute viral hepatitis, 14 patients with hyperthyroidism, 7 with hypothyroidism, and 26 patients with chronic renal disease of varying etiologies. In the patients with liver disease, the levels of vitamin A, RBP, and PA were all markedly decreased and were highly significantly correlated over a wide range of concentrations. Serial samples were available in 19 patients with acute hepatitis; as the disease improved the plasma concentrations of vitamin A, RBP, and PA all increased. In patients with acute hepatitis RBP concentrations correlated negatively with the levels of plasma bilirubin, glutamic-oxaloacetic transaminase, and alkaline phosphatase. In the hyperthyroid patients both RBP and PA concentrations were significantly lower than normal; in hypothyroidism, neither protein showed levels significantly different from normal. In both hyper- and hypothyroidism and in liver disease, the molar ratios of RBP:PA and of RBP:vitamin A were not significantly different from normal.
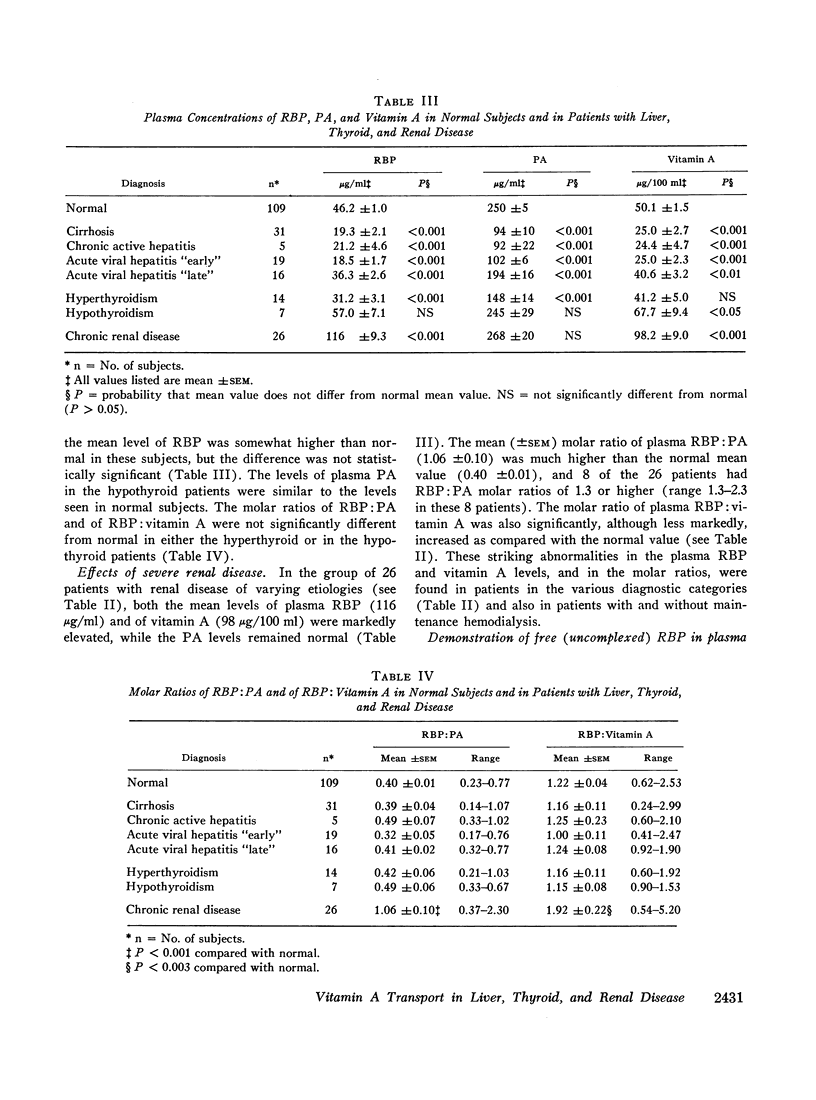
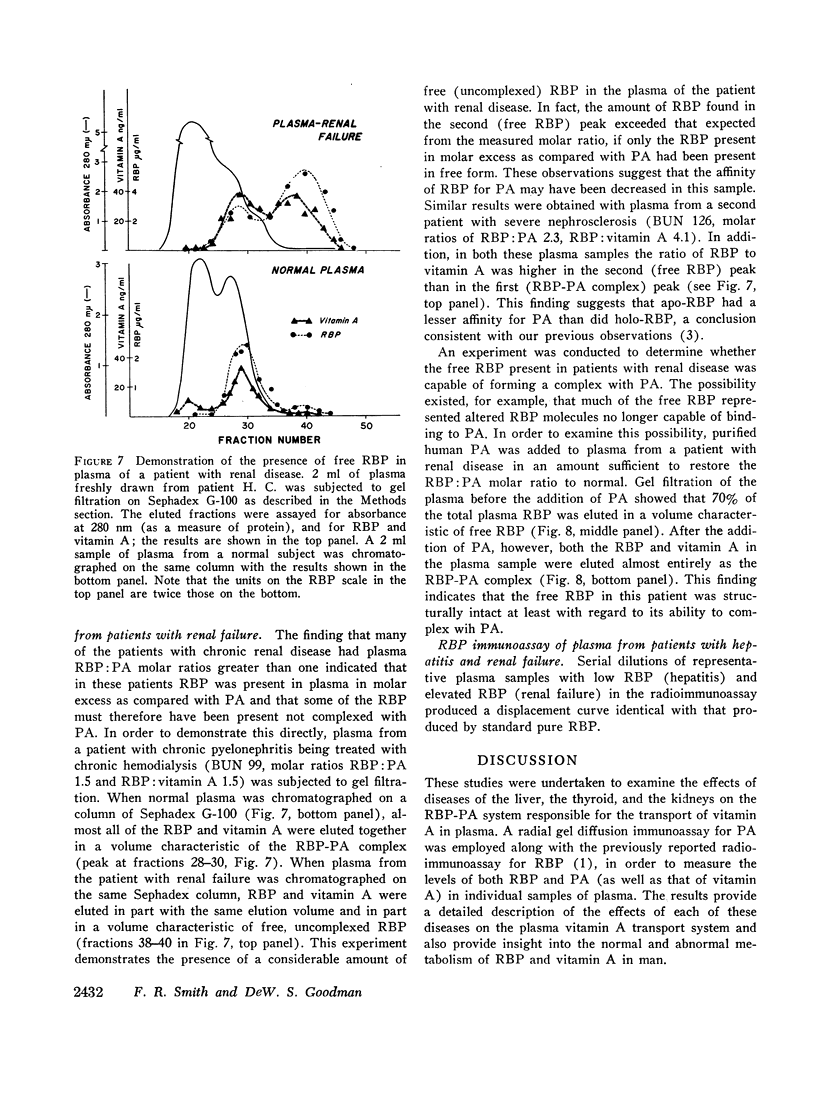
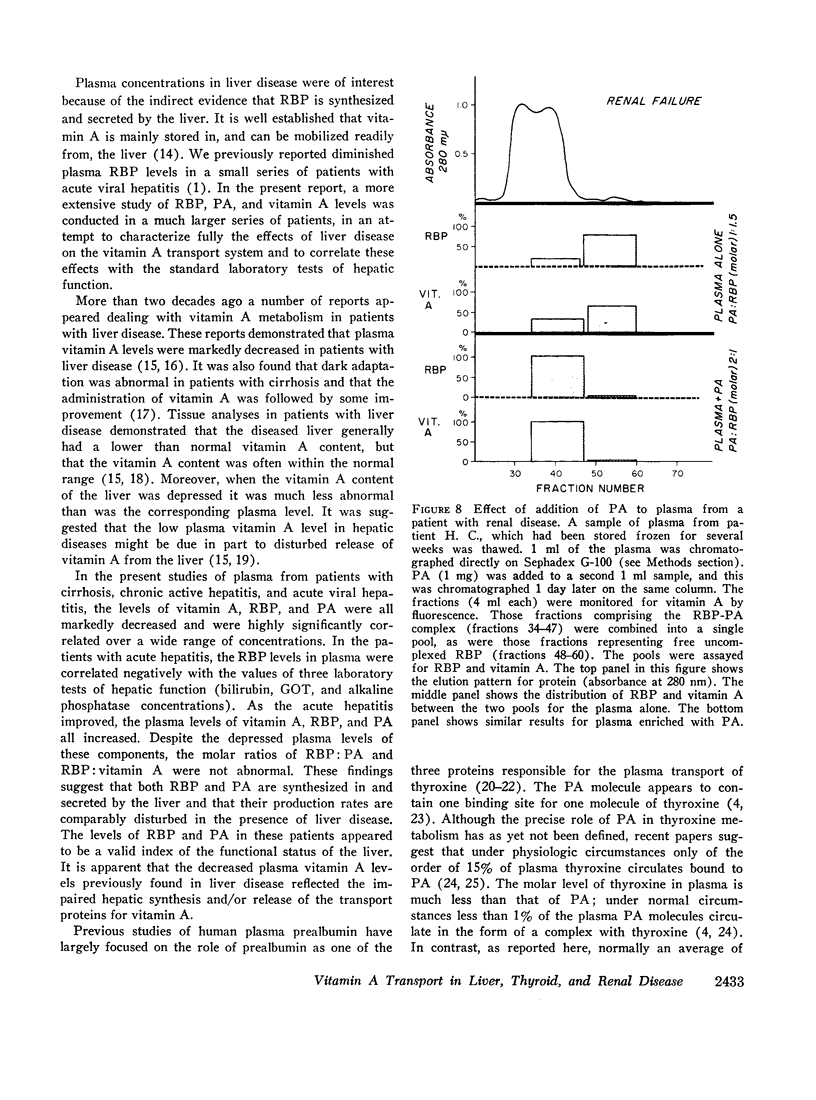
Patients with chronic renal disease had marked abnormalities in the plasma concentrations of RBP and vitamin A and in the molar ratios examined. In renal disease the levels of both RBP and vitamin A were greatly elevated, while the PA levels remained normal. The molar ratios of RBP:PA and of RBP:vitamin A were both markedly elevated. In many patients RBP was present in molar excess as compared with PA. The presence of a relatively large proportion of free RBP, not complexed to PA, in some patients with chronic renal disease was confirmed by gel filtration. The free RBP, present in molar excess, was capable of forming a complex with additional purified PA added to the plasma. The kidneys appear to play an important role in the normal metabolism of RBP.
Full text
PDF
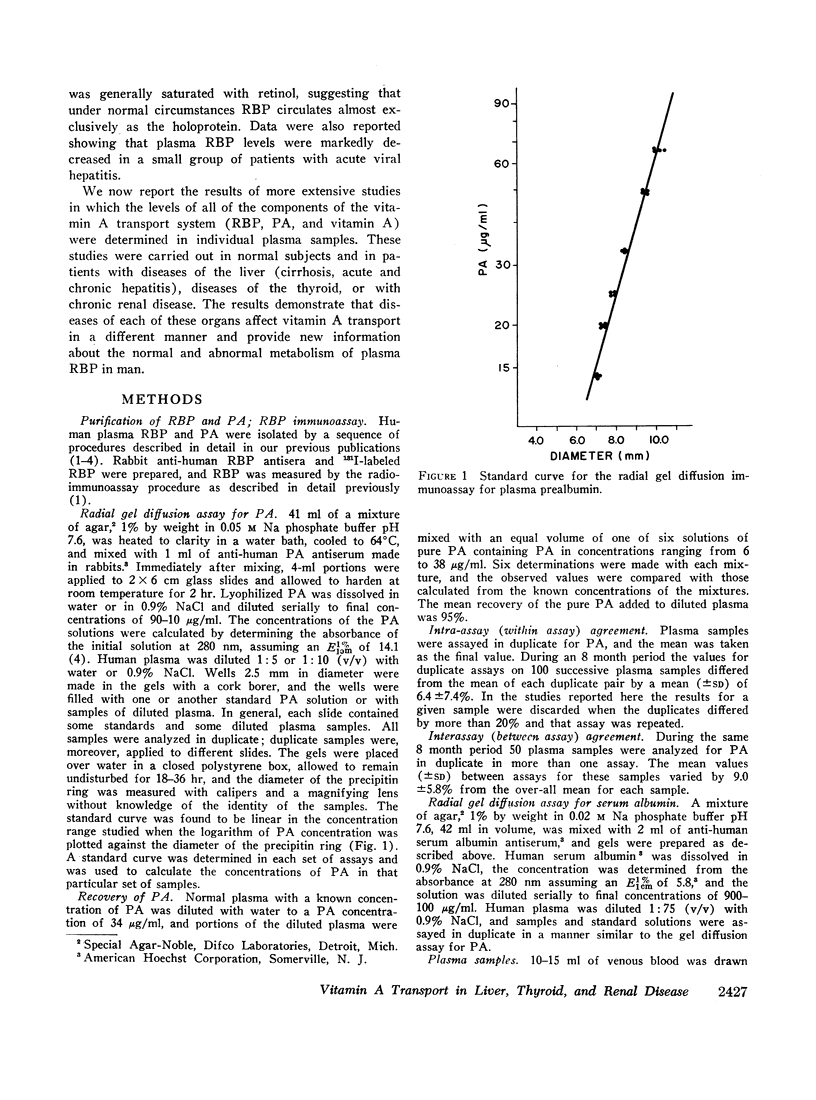

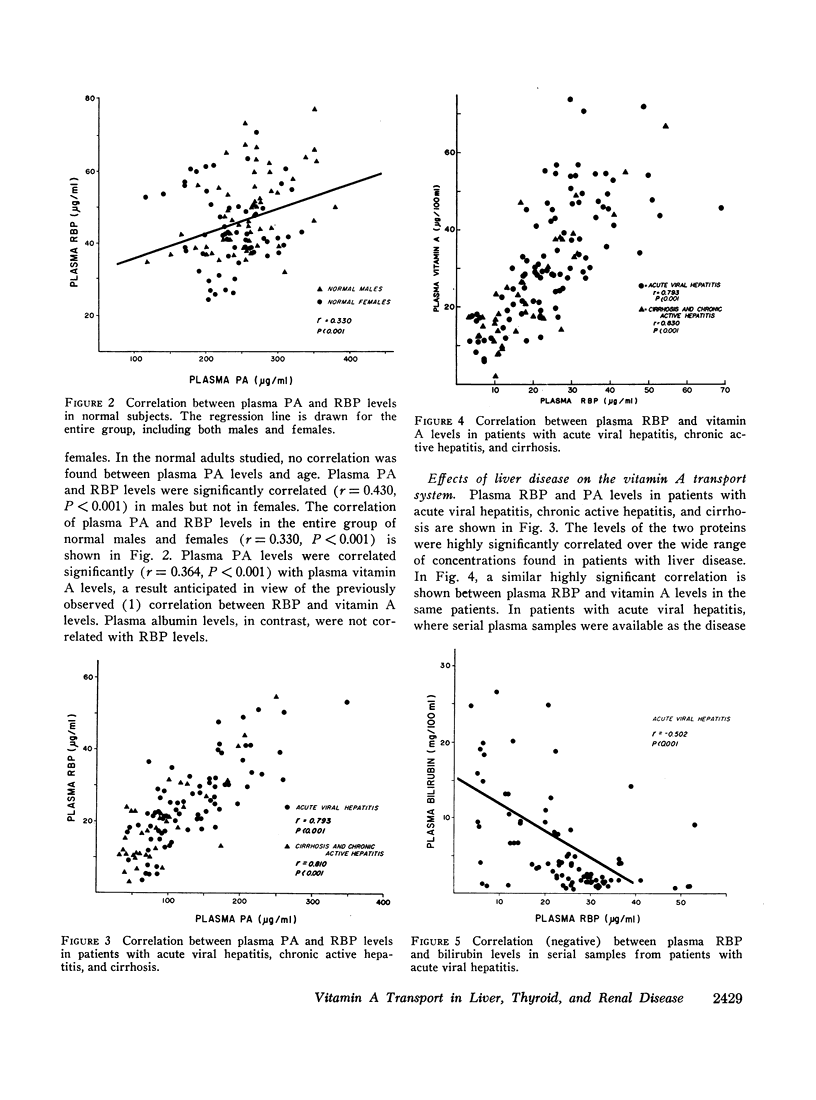
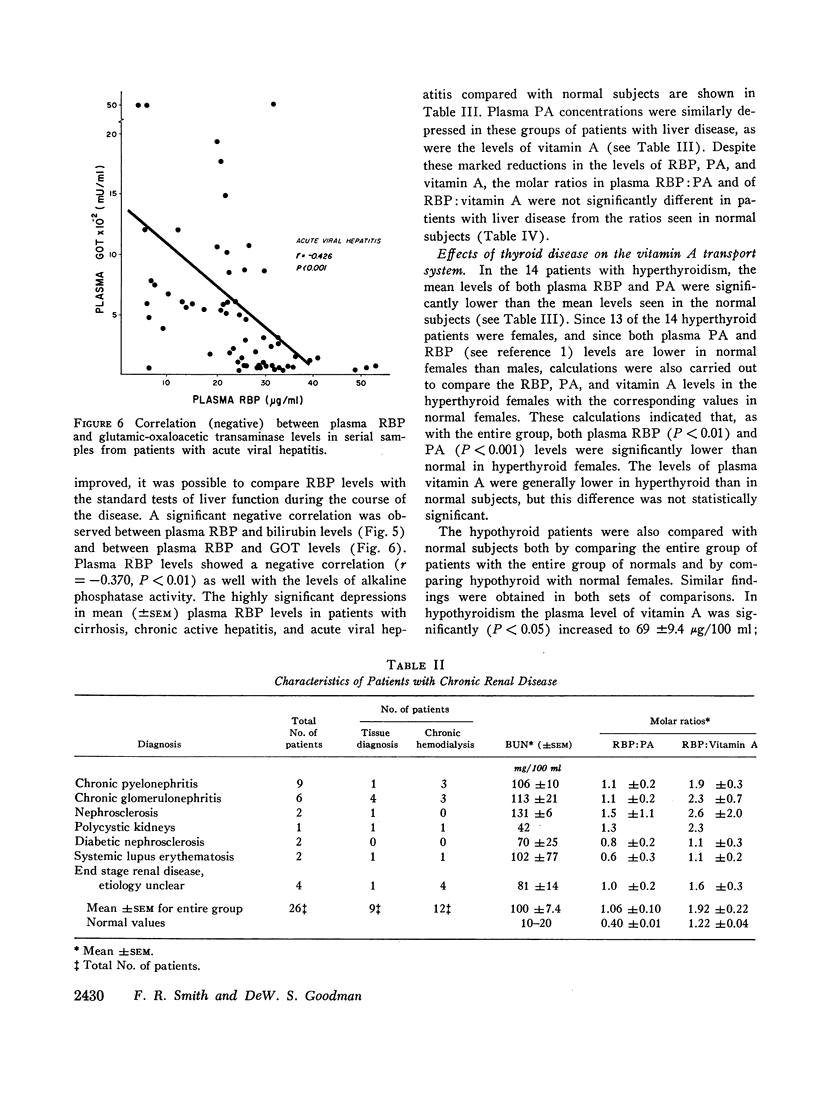



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostoni A., Vergani C., Stabilini R., Petrella A. Thyroxine-binding prealbumin in alcoholic cirrhosis. Lancet. 1968 Apr 27;1(7548):926–927. doi: 10.1016/s0140-6736(68)90289-4. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Inada M., Varsano-Aharon N., Sterling K. Thyroxine transport and turnover in major nonthyroidal illness. J Clin Endocrinol Metab. 1968 Jul;28(7):1023–1030. doi: 10.1210/jcem-28-7-1023. [DOI] [PubMed] [Google Scholar]
- Chamberlain M. J., Stimmler L. The renal handling of insulin. J Clin Invest. 1967 Jun;46(6):911–919. doi: 10.1172/JCI105597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS A. D., MOORE T. Vitamin A in infective hepatitis. Br Med J. 1947 Apr 26;1(4503):553–559. doi: 10.1136/bmj.1.4503.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUCK J. C., BERMAN L. B. Serum ribonuclease activity. J Appl Physiol. 1958 May;12(3):473–476. doi: 10.1152/jappl.1958.12.3.473. [DOI] [PubMed] [Google Scholar]
- INGBAR S. H., FREINKEL N. Regulation of the peripheral metabolism of the thyroid hormones. Recent Prog Horm Res. 1960;16:353–403. [PubMed] [Google Scholar]
- INGBAR S. H. Observations concerning the binding of thyroid hormones by human serum prealbumin. J Clin Invest. 1963 Feb;42:143–160. doi: 10.1172/JCI104701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGAN B. M., THOMAS E. M. Serum vitamin A and total plasma lipid concentrations as influenced by the oral administration of vitamin A to children with the nephrotic syndrome. J Clin Invest. 1950 Feb;29(2):141–145. doi: 10.1172/JCI102239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J. H., Gregerman R. I. pH dependence of the binding of thyroxine to prealbumin in human serum. J Clin Endocrinol Metab. 1969 Apr;29(4):487–495. doi: 10.1210/jcem-29-4-487. [DOI] [PubMed] [Google Scholar]
- Mogielnicki R. P., Waldmann T. A., Strober W. Renal handling of low molecular weight proteins. I. L-Chain metabolism in experimental renal disease. J Clin Invest. 1971 Apr;50(4):901–909. doi: 10.1172/JCI106562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SQUEF R., SURKS M. I., HAUER H. BINDING OF THYROXINE BY SERUM PROTEINS EVALUATED BY EQUILIBRUM DIALYSIS AND ELECTROPHORETIC TECHNIQUES. ALTERATIONS IN NONTHYROIDAL ILLNESS. J Clin Invest. 1963 Nov;42:1769–1782. doi: 10.1172/JCI104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SURKS M. I., SMITH J. C., SQUEF R. ISOLATION AND CHARACTERIZATION OF HUMAN THYROXINE-BINDING PREALBUMIN. J Biol Chem. 1965 Jan;240:173–180. [PubMed] [Google Scholar]
- Oppenheimer J. H., Martinez M., Bernstein G. Determination of the maximal binding capacity and protein concentration of thyroxine-binding prealbumin in human serum. J Lab Clin Med. 1966 Mar;67(3):500–509. [PubMed] [Google Scholar]
- Oppenheimer J. H. Role of plasma proteins in the binding, distribution and metabolism of the thyroid hormones. N Engl J Med. 1968 May 23;278(21):1153–1162. doi: 10.1056/NEJM196805232782107. [DOI] [PubMed] [Google Scholar]
- PILEGGI V. J., LEE N. D., GOLUB O. J., HENRY R. J. Determination of iodine compounds in serum. I. Serum thyroxine in the presence of some iodine contaminants. J Clin Endocrinol Metab. 1961 Oct;21:1272–1279. doi: 10.1210/jcem-21-10-1272. [DOI] [PubMed] [Google Scholar]
- Patek A. J., Haig C. THE OCCURRENCE OF ABNORMAL DARK ADAPTATION AND ITS RELATION TO VITAMIN A METABOLISM IN PATIENTS WITH CIRRHOSIS OF THE LIVER. J Clin Invest. 1939 Sep;18(5):609–616. doi: 10.1172/JCI101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS J., RALL J. E. Proteins associated with the thyroid hormones. Physiol Rev. 1960 Jul;40:415–489. doi: 10.1152/physrev.1960.40.3.415. [DOI] [PubMed] [Google Scholar]
- Rabkin R., Simon N. M., Steiner S., Colwell J. A. Effect of renal disease on renal uptake and excretion of insulin in man. N Engl J Med. 1970 Jan 22;282(4):182–187. doi: 10.1056/NEJM197001222820402. [DOI] [PubMed] [Google Scholar]
- Raz A., Goodman D. S. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J Biol Chem. 1969 Jun 25;244(12):3230–3237. [PubMed] [Google Scholar]
- Raz A., Shiratori T., Goodman D. S. Studies on the protein-protein and protein-ligand interactions involved in retinol transport in plasma. J Biol Chem. 1970 Apr 25;245(8):1903–1912. [PubMed] [Google Scholar]
- SURKS M. I., OPPENHEIMER J. H. POSTOPERATIVE CHANGES IN THE CONCENTRATION OF THYROXINE-BINDING PREALBUMIN AND SERUM FREE THYROXINE. J Clin Endocrinol Metab. 1964 Aug;24:794–802. doi: 10.1210/jcem-24-8-794. [DOI] [PubMed] [Google Scholar]
- Sakurada T., Saito S., Inagaki K., Tayama S., Torikai T. Concentration and binding capacity of thyroxin-binding prealbumin in pregnancy, hyper- and hypothyroidism. Tohoku J Exp Med. 1968 Nov;96(3):259–266. doi: 10.1620/tjem.96.259. [DOI] [PubMed] [Google Scholar]
- Samaan N. A., Freeman R. M. Growth hormone levels in severe renal failure. Metabolism. 1970 Feb;19(2):102–113. [PubMed] [Google Scholar]
- Smith F. R., Raz A., Goodman D. S. Radioimmunoassay of human plasma retinol-binding protein. J Clin Invest. 1970 Sep;49(9):1754–1761. doi: 10.1172/JCI106393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolow E. L., Woeber K. A., Purdy R. H., Holloway M. T., Ingbar S. H. Preparation of I-131-labeled human serum prealbumin and its metabolism in normal and sick patients. J Clin Invest. 1965 Oct;44(10):1600–1609. doi: 10.1172/JCI105266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani C., Stabilini R., Agostoni A. Thyroxine-binding prealbumin in thyrotoxicosis and hypothyroidism. Clin Chem. 1969 Mar;15(3):216–218. [PubMed] [Google Scholar]
- Wochner R. D., Strober W., Waldmann T. A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967 Aug 1;126(2):207–221. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A., Ingbar S. H. The contribution of thyroxine-binding prealbumin to the binding of thyroxine in human serum, as assessed by immunoadsorption. J Clin Invest. 1968 Jul;47(7):1710–1721. doi: 10.1172/JCI105861. [DOI] [PMC free article] [PubMed] [Google Scholar]