Abstract
Empirical gaps remain regarding infant mandibular kinematics observed during naturally occurring episodes of chewing and pre-linguistic vocalizations during the first 2-years of life. Vertical jaw displacement was measured from a typically developing infant from 8 to 22 months. Infant jaw kinematics was measured for vowel babble, non-variegated and variegated babble, and chewing. Results indicated that measures of kinematic variability were significantly less for chewing than all babble categories. These measures changed across age for chewing: (a) peak vertical jaw elevation decreased in variability, while (b) jaw displacement and (c) speed of movement increased in variability. Kinematics for vowel babble were characterized as exhibiting less jaw displacement with higher average vertical jaw position than other babble types and chewing. Developmentally, jaw kinematics for babble changed for jaw displacement and average vertical jaw position. These changes were related to decreased episodes for vowel babble productions and increased episodes for variegated babble and reduplicative syllables. These results suggest that developmental processes such as non-overlapping task-demands likely differentiate trajectories of jaw movement for infant chewing and babble. Infant jaw kinematics for babble cannot be predicted from observations of adult speakers or from non-speech behaviors observed for infants or adults.
Keywords: Speech, Development, Kinematics, Mandible, Human, Infant
1. INTRODUCTION
An understanding of typical developmental processes underlying emerging speech is important for establishing theoretical representations of disordered speech and therapeutic procedures for its remediation. A crucial element in understanding typical development is to accurately measure and describe early jaw kinematics associated with pre-linguistic vocalizations. Characterizations of early jaw movement associated with infant and toddler prelinguistic vocalizations may be based on a premise regarding how motor control for speech develops. For example, a commonly discussed theoretical perspective regarding speech development proposes that jaw kinematics for early, multisyllabic babble and first words are constrained to rhythmical cyclic movement patterns observed for alimentary oromandibular behaviors such as chewing and sucking (”ingestive cyclicities”, MacNeilage, 1998) or nonspeech motor stereotypies (Thelen, 1991) such as mandibular oscillation (Meier, McGarvin, Zakia, & Willerman, 1997). However, physiologic investigations of speech development are unsupportive of this premise and support the idea that coordination among mandibular muscle groups and associated jaw trajectories are dissimilar among early forms of speech and non-speech oromandibular behaviors, which suggests that early mandibular kinematics for emerging speech cannot be predicted from jaw movement observed for non-speech behaviors (Green, Moore, Ruark, Rodda, Morvée, & VanWitzenburg, 1997; Moore & Ruark, 1996; Steeve, Moore, Green, Reilly, & Ruark McMurtery, 2008). Notwithstanding of these different perspectives for speech development, empirical gaps exist in our understanding of direct measures for jaw kinematics that characterize early, naturally occurring vocalizations and chewing during the first two-years of life.
1.1 Neural control: Task-dependent differences
Proponents who model jaw kinematics as similar between non-speech behaviors and early vocalizations have focused solely on the concept that control for developing speech arises specifically from ontogenetic and phylogenetic nonspeech control mechanisms (e.g., “ingestive cyclicities”, MacNeilage, 1998) such as specialized neural networks of pattern generation, also referred to as a central pattern generator, associated with alimentary behaviors or other non-speech oromandibular behaviors. In terms of motor control and its development in general, specific types of central pattern generators may in fact organize for control of complex motor behaviors such as speech (Grillner, 1991). These models of control are based on direct observations of neural control mechanisms for pattern generation in nonhuman preparations and have been used to support the suggestion that similar neural networks are active in humans (Delcomyn, 1980; Marder & Bucher, 2001; Marder & Calabrese, 1996).
Jürgens (2002, 2009) proposes that animal models (e.g., squirrel monkeys) provide evidence that there are central pattern generators located in different areas of the brain that function for specific oromandibular behaviors such as mastication and vocalization, and stimulation of these neural regions creates significantly different trajectories for jaw movement (Jürgens, see open peer commentary in MacNeilage, 1998). Task-specific differences for jaw movement is also observed in humans, for example, trajectory of jaw movement for chewing is characterized as being slower and sinusoidal in comparison to faster and complex movements observed in adult speakers (Gibbs & Masserman, 1972; see review by Smith, 2006). Models of vocal control based on nonhuman preparations support the concept that different and diffuse neural networks in the central nervous system underlie a continuum of basic and complex levels of vocal behaviors that entails coordination of the respiratory, laryngeal, and supralaryngeal vocal tract structures. Vocal behaviors include innate vocal reactions (e.g., cry), imitative vocalizations, and learned vocalizations, including those that require complex vocal tract modulation similar to that observed for human speech (Jürgens, 2002, 2009). These neural models of vocal behavior represent various forms of expressive control for infant vocalizations, which require control networks to extend beyond traditionally identified nonspeech mechanisms.
Physiologic investigations comparing mandibular control among speech and nonspeech behaviors suggest that coordinative infrastructures organize differently among specific oromandibular behaviors. Motor organization for a given oromandibular behavior is influenced by factors such as distinct non-overlapping task demands (Fentress, 1984; Moore, 2004; Thelen, 1991), specialized neural infrastructures (e.g., central pattern generator) that are greatly influenced by maturation, development, and use (e.g., Barlow, Finan, Andreatta, 1997; Grillner, 1991), or balanced, bidirectional mapping among linguistic and vocalization systems (Smith, 2006) and between the auditory and articulatory systems (e.g., Callan, Kent, Guenther, & Vorperian, 2000; Guenther, 2006). These factors require that neural networks dynamically combine at different levels of the nervous system organizing mandibular muscle activity suited to perform a given oromandibular behavior, such as chewing (e.g., Green, et al., 1997), sucking (e.g., Finan & Barlow, 1998), or speech (e.g., Moore & Ruark, 1996). This process allows a significant degree of plasticity for mandibular coordination among these behaviors that differentially controls the jaw during development.
Physiological studies have supported the concept that neural networks organize distinctively across oromandibular behaviors. One empirical method for measuring task-related differences in neural control for coordination of the mandible is to compute the coherence function for EMG signals obtained from pairs of synergistic muscles, such as left and right masseter muscles (Smith & Denny, 1990). The coherence function computes the degree of correlation between two EMG signals as it varies across frequencies with changes in neural input correlating with changes in spectral content across EMG channels (Richardson & Mitchell, 1982). This analytic process used on EMG data from adult participants has provided evidence that neural input to muscle groups of the mandible is different across speech and nonspeech behaviors (Smith & Denny, 1990). Central pattern generation appears to be a dominant control mechanism influencing motor behaviors of the jaw for chewing but not for speech (Smith & Denny, 1990).
A different analytic approach for investigating whether neural control structures organize differently among various oromandibular behaviors is using EMG to measure timing and amplitude of muscle activation (coordination) across mandibular muscle groups. The hypothesis is that consistent differences in the coordinative organization for mandibular muscle groups among specified oromandibular behaviors reflects differences in organization for neural networks controlling jaw movement. For example, correlational analyses performed on the EMG signals of jaw elevator and depressor muscle groups in mature speakers reveal distinct patterns of activation for speech versus nonspeech behaviors (Moore, 1993; Moore, Smith, & Ringel, 1988). Mandibular antagonist muscle comparisons activate reciprocally during chewing, which is defined as alternating activation of jaw elevator with depressor muscle groups as observed in humans (e.g., Møller, 1966) and animals (e.g., Lund, 1991; Luschei & Goldberg, 1981). In contrast, mandibular antagonist muscle comparisons coactivate during speech, which is defined as synchronous activation of jaw elevator and depressor muscle groups (Moore, 1993; Moore, et al., 1988).
Correlational analysis performed on mandibular EMG of very-young children has revealed task-related differences for oromandibular coordination for infants, toddlers, and young children (12 to 48 months, Green, et al., 1997; 15 months, Moore & Ruark, 1996; 9 months, Steeve, et al., 2008). What is most remarkable about these investigations is that by 9-months of age, infants exhibit the essential organization of the mature model for task-related differences in mandibular coordination (Steeve, et al., 2008), and these identifiable patterns of motor organization are observed in infants whose anatomy and physiology is significantly different from the adult and is in a state of growth and development (Kent & Vorperian, 1995; Netsell, 1981). Specifically, the coordinative organization among mandibular muscle groups of 9-month-old infants for sucking, chewing, and babble resembles that observed for toddlers and older children (Steeve et al., 2008; Green et al., 1997; Moore & Ruark, 1996), and motor behaviors associated with chewing and babble in 9-month-olds are fundamental patterns of those observed for chewing and speech in adults (Moore, 1993; Moore et al., 1988).
1.2 Jaw kinematics: Task-dependent differences
The adult model for jaw kinematics is that distinct, task-dependent differences are observed among trajectories for chewing and speech (Gibbs & Masserman, 1972), which is an observation that is consistent with empirical measures for mandibular motor control (Moore, 1993; Moore et al., 1988; Smith & Denny, 1990). In comparison to chewing, the adult produces speech with reduced excursions (i.e., displacement) of the mandible while maintaining a more elevated vertical position of the mandible, and jaw trajectories are more complex and faster (Gibbs & Masserman, 1972). These earlier findings of task-related differences for adult jaw kinematics are easily replicated. Figure 1 includes comparative kinematic records of vertical changes in jaw height obtained while an adult subject chewed a soft, malleable piece of candy (Star Burst ®, left panel) and while the same subject spoke conversationally (right panel). The amplitude range for each kinematic waveform (vertical displacement) is equivalent across the tasks of chewing and speech (Figure 1). In Figure 1, average vertical position of the mandible is maintained at a higher elevation during speech with average total jaw displacement being less in comparison to those observed for chewing. The trajectory of jaw movement during chewing is highly repetitive across cycles, characterizing a sinusoidal waveform, whereas variability across cycles is much greater during speech characterizing a complex waveform (Figure 1). In comparison to speech, chewing exhibits less variability for measures of peak vertical jaw position, total jaw displacement, and for speed of jaw motion (Figure 1). Measures are defined in section 2.7, Measures of Jaw Kinematics, for (a) average vertical jaw position, (b) average total jaw displacement, and measures of variability for (c) peak vertical jaw position, (d) total jaw displacement, and (e) speed of jaw movement.
Figure 1.
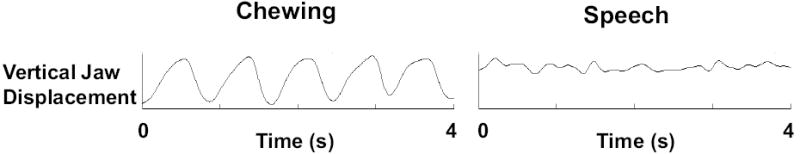
Vertical jaw displacement traces recorded from an adult during chewing (left) and conversational speech (right). The gain setting for these traces are equivalent.
A previous investigation we conducted provided evidence that task-dependent distinctions observed for coordinated activity among mandibular muscle groups is also apparent in associated jaw trajectories during development (8 – 22 months; Steeve & Moore, 2009). Spectrographic measures of jaw kinematic records (i.e., vertical jaw position over time) showed that cycles of movement for chewing are mainly represented by a single frequency below 2 Hz; while cycles for babble are represented by one or more frequencies, with the lowest frequency being above 2 Hz (Steeve & Moore, 2009). These results suggest that jaw trajectories are comparatively more complex and faster for babble in comparison to chewing (Steeve & Moore, 2009). However, this analysis only provided a gross measure of jaw movement. We could not determine, for example, whether the adult model illustrated in Figure 1 is representative of infant jaw kinematics. It is very possible that the adult-model for average vertical jaw position and average jaw displacement is not representative of infant babble and chewing. For instance, the jaw appears to function differently for vocalizations at 1 and 2-years in comparison with the adult. Jaw displacement, in comparison with that for the upper and lower lips, is primarily responsible for closing the oral aperture for bilabial vocalizations at 1 and 2-years, but the jaw apparently functions differently by adulthood because lip displacement contributes equally to that of the jaw for oral closure (Green, Moore, Higashikawa, & Steeve, 2000; Green, Moore, & Reilly, 2002). Therefore, the adult model for jaw kinematics during speech may not represent similar oromandibular behaviors observed in infant.
Non-overlapping task demands may also influence mandibular movement among various behaviors. Variability among peaks for vertical jaw position may be comparatively less for chewing because these values are most likely related to a stable point of occlusion for mastication of a food item (Kent & Vorperian, 1995), but for vocalizations, this measure is associated with modulation of aerodynamic energy. Non-overlapping task-demands may also differ among classifications of babble. For example, jaw kinematic measures may differ between specific categories of babble because of differences in phonetic complexity. Vowel babble is void of supra-laryngeal obstruent-like productions, whereas other babble classifications are composed of these sounds. Thus, vowel babble may exhibit less variability among kinematic measures in comparison to variegated babble. Also, average vertical jaw position or total jaw displacement may differ for vowel babble because of a lack of supraglottal articulation for these productions.
Other task-related distinctions for jaw kinematics may be due to differences in neural control. Mandibular control for chewing is presumed to be greatly influenced by pattern generation and a core characteristic of this control is the repetitive nature of the movement (e.g., Delcomyn, 1980; Marder & Bucher, 2001). Jaw kinematics for chewing may exhibit less variability for peak vertical jaw position, total jaw displacement, and speed of jaw displacement because masticatory CPGs are modeled as dominate control structures for chewing constraining jaw movements in both humans and animals. In contrast, neural networks for vocalizations are modeled as being more diffuse, located in various regions of the brain (e.g., Delcomyn, 1980; Jürgens, 2009; Jürgens, see open peer commentary in MacNeilage, 1998; Marder & Bucher, 2001; Smith & Denny, 1990). These diffuse neural regions allow greater variability for jaw movement during babble or speech.
During the first 2-years of life, biologic growth and development or changes in task requirements may alter jaw movement among various oromandibular behaviors. For example, jaw kinematics associated with chewing may change during the first 2-years of life due to eruption of molars or growth and development of the oromandibular region (Kent & Vorperian, 1995), as well as changes in food consistency (Wilson & Green, 2009). The eruption of molars has been discussed as providing a stable point of occlusion during chewing (Kent & Vorperian, 1995), and speed of jaw closing for pureed food consistencies decreases significantly from 18 to 24 months of age (Wilson & Green, 2009). These factors associated with infant chewing may influence kinematic measures of variability for peak vertical jaw position (point of occlusion), average total jaw displacement, and speed of jaw movement. Changes may also be noted for vocalizations. These developmental changes may influence jaw kinematics differently across categories of babble.
1.3 Experimental Aims
Physiologic investigations support the idea that mandibular motor control organizes differently for speech and non-speech during a period when these oromandibular behaviors co-emerge, exhibiting task-related differences for jaw kinematics (Green, et al., 1997; Moore & Ruark, 1996; Steeve, et al., 2008). These kinematic measures remain the least understood given the investigator’s challenge to achieve experimental control and maintain accurate physiologic measures in behaviors produced by infants. The present investigation addressed these challenges by making frequent longitudinal measurements of jaw movement during chewing, and several types of early multisyllables in a single child during the first 2-years of life.
This investigation examined whether the adult model for jaw kinematics is representative of infant chewing and specific types of babble, and if so, whether significant task-related differences should be observed for each dependent measure of jaw kinematics within each age group (defined below). If the adult model for jaw kinematics for chewing and speech was not representative of infant kinematics for chewing and babble, then the following two research questions address infant chewing and babble comparisons. The first question for this study was whether measures of jaw kinematics exhibit task-specific differences among those oromandibular behaviors observed including chewing and specific types of babble. The second question was whether developmental changes for a given dependent measure of jaw kinematics would be observed within those oromandibular behaviors observed across age: chewing, babble (collapsed across categories), and non-variegated babble (defined below). The present investigation was designed to address these empirical gaps by measuring jaw movement during chewing and three distinct classifications of early multisyllabic babble (defined below, Stoel-Gammon, 1989). Dependent measures were derived from kinematic data of vertical changes in jaw height and include: (a) average vertical jaw position, (b) average total jaw displacement, and the coefficient of variation for (c) peak vertical jaw position, (d) total jaw displacement, and (e) a general index of speed for changes in vertical jaw motion.
2. METHOD
2.1 Subject
Longitudinal data were gathered from one normally developing male infant at 4-to-6 week intervals from 8 to 22 months (i.e., 8.2, 9.1, 9.3, 10.2, 12.1 13.2, 14.3 16.3, 17.1, 19.2, and 21.3 months). The child participated in this experiment as part of an ongoing longitudinal investigation of early development for emerging oromandibular behaviors. Complimentary EMG analyses and spectrographic measures of jaw trajectories have been reported previously from this child (Steeve & Moore, 2009). The parents were monolingual, American-English speakers, who indicated scheduling availability and a willingness to have their child participate over the course of the experiment, and the infant demonstrated the ability to participate in the experimental protocol. These factors are important because of the experimental and technological challenges in obtaining physiologic data from infants.
A certified speech-language pathologist conducted informal screenings at each session, and the parents provided information regarding communicative abilities using the MacArthur Communicative Development Inventory: Words and Gestures. The infant passed screenings for oral motor function and speech development. Speech development was evaluated on parent-report and on the basis of the participant’s observed production of age-appropriate phonemes and syllable structures, which have been reported in the literature (e.g. Smith, Brown-Sweeny, & Stoel-Gammon, 1989; Stoel-Gammon, 1985, 1998). The infant passed all screenings administered at 6, 12, 18, and 24 months by his pediatrician for cognitive and motor development, and there were no signs of middle ear infection or other health problems. He passed hearing screenings administered by an audiologist at birth, 12, and 24 months. There was no indication during the experimental sessions that hearing, communication skills, cognition, or motor abilities were abnormal.
2.2 Experimental Protocol
Initial recordings of jaw kinematics were obtained from this infant at 8 months when he began producing multisyllabic, vowel babble (defined below; Stoel-Gammon, 1989). Physiologic data were recorded from the participant while he was seated in an adjustable chair, eating and vocalizing as he wished. Each session lasted from 40 to 60 minutes. The parents and experimenter played / interacted with the infant to elicit vocalizations. Sometimes the adult remained silent while engaged in play activities with the infant and the participant would vocalize during this activity. Infant vocalizations also occurred while the adult was talking as a part of the play activity, such as vocalizing for a puppet. Careful review of the video recordings indicated that the infant’s productions were not replications of vocalizations from the adult speakers.
2.3 Acquisition of Kinematic Data
Vertical jaw position was recorded using an infrared-sensitive, monochrome camera (Burle, TC351A) connected to a video-recorder (Panasonic, AG-1980). The infant pictured in Figure 2, Panel A, is from a sample video clip. Three flat, circular, reflective markers (~3 mm in diameter) were placed in the midline on the tip of the nose, the nasion, and superior to the protuberance of the mandible. The reference markers on the tip of the nose and the nasion were used to correct for head movement, and the nose marker served as the origin for the jaw marker. Target behaviors were identified subsequently in reviewing the videotaped session, and these video clips were digitized using a commercially available video tracking system (Motus, version 6.0, Peak Performance). The kinematic system used pattern recognition and tracking algorithms to automatically extract position traces from digital video recordings. The sampling rate for these kinematic data was 60 Hz, and these data were subsequently low-pass filtered (flp = 8 Hz) using a digital, zero-phase shift forward and reverse digital filter (Butterworth, 8 pole, Matlab, version 6.5, The MathWorks, 2003). Static calibration of this kinematic system has indicated that jaw excursion in the ordinate plane (i.e., Y axis) is accurate to at least 1 mm, which was equivalent to the precision used during the calibration procedure (Green et al., 2000).
Figure 2.
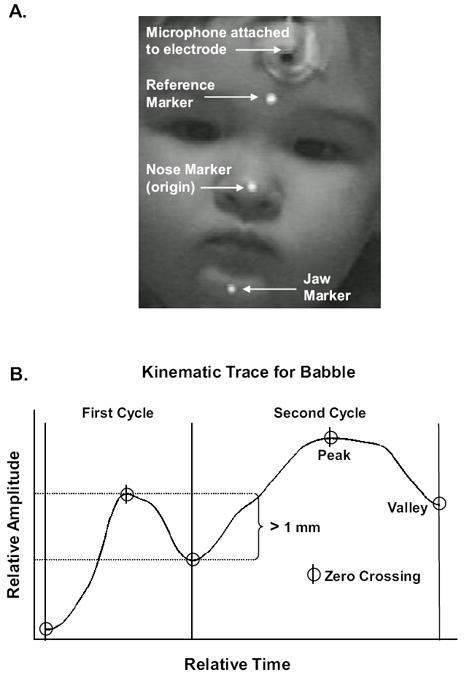
Panel A illustrates placement of kinematic markers and microphone on the participant. Panel B illustrates the location of zero crossings in the displacement trace and how these landmarks defined the peak(s) and valley(s) in the displacement trace. Panel B also illustrates that vertical changes in jaw height had to be greater than 1 mm.
2.4 Acquisition of Audio Data
A miniature, omni-directional, microphone (ECM-77B, Sony) was adhered to a disposable EMG electrode mounted on the forehead for the purpose of maintaining a constant mouth-to-microphone distance during the recording session (see Figure 2, Panel A). The microphone signal was high-pass filtered at 50 Hz and pre-amplified (1 pole, 6 dB / octave, Pro MPA, Applied Research Technology) with the microphone preamplifier coupled to both the video-recorder (Panasonic, AG-1980), which recorded the kinematic data, and to a computer. Audio data digitally recorded to the computer were anti-alias filtered (flp = 5000 Hz, 8-pole Butterworth, Alligator Technologies, Costa Mesa, CA) prior to digitization (sampling rate = 10,000 Hz; Dataq Instruments, Inc., Natick, MA). Audio recordings from the computer were used for perceptually classifying the prelinguistic vocalizations.
2.5 Event Related Classification of Data
As illustrated in Figure 2, Panel B, zero-crossings were computed from the first-order derivative of the vertical displacement trace of the jaw (i.e., velocity). Multisyllabic babble and chewing samples were included in the corpus if the sample contained at least two cycles of jaw movement defined as changes in vertical jaw depression or elevation between subsequent zero-crossings (e.g., peak to valley) that were greater than 1 mm (see Figure 2, Panel B). The requirement for two cycles of jaw movement was chosen because scientists commonly suggest that central pattern generation dominates neural control of the mandible for chewing, and a core characteristic of pattern generation is the repetitive nature of this behavior (e.g., Delcomyn, 1980; Marder & Bucher, 2001). A main objective in this investigation was addressing how jaw movement differs across multisyllabic babble and chewing. Furthermore, changes in jaw elevation and depression had to be greater than 1mm as a conservative means to ensure that these kinematic measures were above the noise floor of the video tracking system. Less than 1% of the total kinematic samples contained a change in jaw displacement that was less than 1mm.
2.5.1 Chewing data
Chewing samples only included active patterns of mastication (Luschei & Goldberg, 1981); the initial bite, bolus positioning, and the final swallow movements were excluded. Food consistencies that the child ate from 8 to 13-months tended to be pureed and semi-solids food consistencies (e.g., cooked carrots) with more solid foods being presented to the child in small quantities per bite (e.g., a single Cheerios ®, a small piece of banana, teaspoon of pureed food), and from 14 to 22-months, the child was eating both semi-solid and solid food consistencies with greater amounts per bite (e.g., 5 to 10 Cheerios ®, 2 to 3 Goldfish ® crackers, a full bite of banana or apple, cookies, meat). Two individuals were trained to use the above criteria and independently identified the chewing samples using the video-recording from the experimental session (discussed below) and by examining the kinematic traces. These individuals had to reach 100% agreement before a sample was included in the study for further analysis. Table 1 shows the distribution of samples across age for chewing.
Table 1.
Distribution of multisyllabic vocalization and chewing samples across age.
| Age (mos.) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 1 | Age 2 | |||||||||||
| Behavior | 8.2 | 9.1 | 9.3 | 10.2 | 12.0 | 13.2 | 14.3 | 16.3 | 17.1 | 19.2 | 21.3 | Total |
| Babble | ||||||||||||
| Vowel | 9 | 28 | 15 | 7 | 0 | 7 | 1 | 3 | 2 | 2 | 2 | 76 |
| Non-variegated | 15 | 38 | 3 | 38 | 0 | 4 | 9 | 32 | 24 | 41 | 55 | 259 |
| Variegated | 1 | 3 | 1 | 2 | 0 | 1 | 3 | 22 | 17 | 24 | 16 | 90 |
| Total | 25 | 69 | 19 | 47 | 0 | 12 | 13 | 59 | 43 | 67 | 73 | 425 |
| Nonspeech | ||||||||||||
| Chewing | 24 | 19 | 67 | 30 | 38 | 164 | 51 | 69 | 21 | 38 | 20 | 541 |
2.5.2 Speech data
A multisyllabic vocalization was included in the corpus if it: (a) did not occur in concert with chewing food, (b) included at least two syllables, (c) was produced with expiratory breath support, and (d) was speech-like (e.g., no crying, screaming, or coughing). Each utterance was isolated from other events by at least 200 milliseconds of silence. Speech data were categorized according to phonetic and syllabic complexity (linguistic complexity, Stoel-Gammon, 1989), which has been suggested to best distinguish stages of speech motor development (e.g., Kent, 1992). The categories proposed by Stoel-Gammon (1989) are labeled as level 1, level 2, and level 3. The definition for level 1 vocalizations is consistent with ‘vowel babble’, level 2 vocalizations is consistent with non-variegated babble, and level 3 vocalizations is consistent with variegated babble. Canonical babble is defined as containing obstruent consonants and sounding speech-like, and these productions are consistent with non-variegated and variegated babble (Stoel-Gammon, 1989).
The number of syllable-like structures was perceptually judged in each vocalization sample. Multisyllabic vocalizations were composed of more than one consonant-vowel-like (CV) or vowel-consonant-like (VC) sequence (vocant and closant; Kent & Hodge, 1990; Stoel-Gammon, 1989). Vowel babble was composed of syllable sequences that included a consonant-vowel (CV) or vowel-consonant (VC), with the consonant being a glide or glottal (i.e., not involving supralaryngeal constriction). Non-variegated babble was defined as multisyllables composed of CV or VC sequences where at least one consonant was produced with supralaryngeal constriction (i.e., excluding glide and glottal). If a sequence of at least two CV or VC syllables were composed of two consonants (i.e., excluding glide and glottal) that had the same placement and manner (voicing could change), the syllables were considered reduplicative, even if a syllable with a glide or glottal proceeded or followed this reduplicative sequence. Variegated babble was composed of multisyllables that contained at least two consonants produced with supralaryngeal constriction (i.e., excluding glide and glottal) that differed in place and manner of articulation (i.e., variegated syllables). Although the infant produced a number of vocalizations that appeared to contain a referent (true words), especially for the later age groups, the current protocol was not designed for reliably identifying and classifying these vocalizations; instead, vocalizations were classified according to linguistic complexity.
Two transcribers were trained to use the above procedures for classifying these samples of prelinguistic vocalizations according to whether it was a multisyllabic production and according to level of linguistic complexity: 1) vowel babble, 2) non-variegated babble, or 3) variegated babble. Once trained, these transcribers independently reviewed the video clips of each vocal production. A total of 757 samples from these longitudinal observations were categorized. The Kappa level for category agreement between the two transcribers was .86 for number of syllables and .88 for level of linguistic complexity (N = 757). Only those vocalizations that both transcribers agreed were multisyllabic were considered for this investigation. These samples of multisyllabic vocalizations had to be unambiguously assigned to a specific babble category; therefore the two transcribers and a third judge classified those few tokens where there was disagreement and a consensus had to be reached. If one of the three perceptual judges did not agree with either classification, or judged that the token could not be reliably classified, then the token remained unclassified and was not included in the study. Table 1 shows the distribution of 425 multisyllabic vocalizations that were perceptually classified with 100% agreement. Furthermore, judges 1 and 2 respectively perceived 70% and 75% of the non-variegated babble for age 1 as reduplicative babble, and within age 2, 90% and 91% of these productions were perceived as reduplicative babble.
2.6 Post-Processing of Event Related Data
2.6.1 Synchronization
A custom syncing routine was written for Matlab, a commercially available signal-processing package, to synchronize the audio and kinematic data (version 6.5, The MathWorks, 2003). Once these data sets were synchronized, the jaw kinematic data, sampled at 60 samples/s, were up-sampled to match the sampling rate of the audio, which were sampled at 10,000 samples/s. The kinematic and audio data were then represented in a single, time-aligned data set. Oversampling of the kinematic channel was necessitated by the frequency bandwidth requirements of the audio signal.
2.6.2 Parsing event related data
The kinematic and audio data were simultaneously parsed from a continuous data set using boundaries operationally defined using the jaw kinematic trace. The first-order derivative of this displacement trace (i.e., velocity) was used to identify movement boundaries; parsing for the onset and offset boundaries were determined using zero-crossings identified in this velocity signal. Speech-related events were bounded by the zero-crossings immediately before and after the audio signal, and chewing-related events were bounded by the zero-crossings closest to the given event.
2.6.3 Inclusion criteria for kinematic data
Each video recording from which kinematic data were measured was reviewed to ensure that the reflective markers were within the measurement space, and that the infant was facing the camera such as illustrated in Figure 2, Panel A. Furthermore, samples of kinematic data were free of movement artifact, which was defined as abrupt (high frequency) changes in the waveform not associated with mandibular excursion. The sample had to contain at least two cycles of jaw movement defined as changes in vertical jaw excursion between subsequent zero-crossings (velocity trace; e.g., valley to peak) that were greater than 1 mm (see Figure 2, Panel B). Any sample not reaching the above criteria was removed from the kinematic data set.
2.7 Measures of Jaw Kinematics
These analyses measured a set of parameters for vertical jaw movement during categories of multisyllabic babble and chewing. Based on observations from adult participants (e.g., Gibbs & Masserman, 1972; Smith, 2006) it was hypothesized that (a) average vertical jaw position, (b) average total jaw displacement, and the coefficient of variation for (c) peak vertical jaw position, (d) total jaw displacement, and (e) a general index of speed for changes in jaw height would differentiate and describe mandibular kinematics associated with infant babble and chewing. These measures were derived from a defined landmark on each kinematic trace. The adult model indicates that jaw kinematics for chewing is typically produced with greater total jaw displacement than those associated with speech (Gibbs & Masserman, 1972), so calculations of the average and standard deviation values for total jaw displacement would routinely be larger for chewing than speech. Addressing this issue meant that a normalized measure, the coefficient of variation, was required for directly comparing variability across infant oromandibular behaviors that could typically differ in terms of total jaw displacement. The coefficient of variation calculated the percentage of variability associated with a given mean value, and thus normalized a score across kinematic samples for indicating the degree of dispersion around the mean. The coefficient of variation is defined as the ratio of the standard deviation to the mean, multiplied by 100, and expressed as a percentage. These measures were computed using a custom Matlab algorithm (version 7.5, The MathWorks, 2007).
Figure 3, Panels A and B, are composed of a kinematic trace recording vertical jaw displacement while an infant chewed. As illustrated in Figure 3, Panel A, average vertical jaw position was computed across all data points that composed a given sample, so the waveform in Figure 3, Panel A, is composed of 17,300 data samples (10,000 samples/s) that were included for calculating the average vertical jaw position of -5.14 cm. Figure 3, Panel B, illustrates measures of total jaw displacement and peak vertical jaw position values. For each sample, total jaw displacement was measured between zero crossings, or peaks and valleys, in the displacement trace. Average total jaw displacement was the average of these values. In Figure 3, Panel B, for example, four total jaw displacement values were measured (i.e., 1.30 cm, 0.85 cm, 1.06 cm, 0.96 cm) and the averaged displacement was calculated (i.e., 0.98 cm). In Figure 3, Panel C, the calculation for the coefficient of variation for total jaw displacement is demonstrated; the ratio of the standard deviation (i.e., .09 cm) to the mean (i.e., 0.98 cm) is calculated and multiplied by 100 expressing the percentage of dispersion around the mean (i.e., 9.18%). The calculation for the coefficient of variation across peak vertical jaw position values is illustrated in Figure 3, Panels B and D. Figure 3, Panel B, illustrates the measure of two peak values (i.e., -4.89 cm, -4.68 cm), and Figure 3, Panel D, shows the calculation of the mean (i.e., -4.79 cm) and standard deviation (i.e., 0.15 cm) that were used to determine the coefficient of variation (i.e., 3.13%). A coefficient of variation was also calculated for a general index of speed for mandibular movement. This measure is a general index of speed because the kinematic tracking system only measured in two-dimensional space, but jaw movement occurred in three-dimensional space. Furthermore, mandibular movement is estimated from a chin surface target that is subject to error due to surface movements of this tissue. The first-order derivative of the vertical displacement trace of the jaw (i.e., velocity) was calculated and the sign was removed (i.e., absolute value; speed) prior to calculating the mean and standard deviation for these data; the coefficient of variation was then determined.
Figure 3.
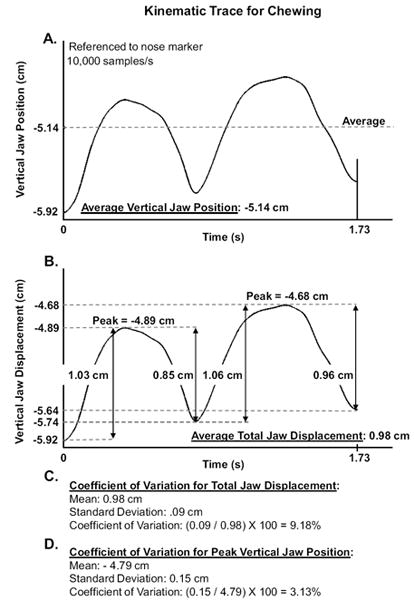
Panel A illustrates the measure for average jaw height that was computed across this kinematic waveform of jaw displacement recorded during chewing. Panel B illustrates the peak-to-valley measures for jaw excursion that were used to compute average jaw displacement. Panel C illustrates the calculation of the coefficient of variation for jaw displacement. Panel B also illustrates the peak amplitude measures for jaw displacement and how these values were included in Panel D to calculate the coefficient of variation for peak amplitude values.
2.8 Statistical Treatment: Planned comparisons
Non-parametric statistical analysis across sets of planned comparisons was necessary because the distributions of these data did not meet the required assumptions of normality and/or equal variance. Statistical analyses further stipulated that each cell in a comparison contained at least ten samples. Table 1 illustrates the distribution of multisyllabic babble and chewing samples. The top of the table indicates the age of the child when these data were acquired (e.g., 8.2 months means 8 months, 2 weeks). When the child was 12.0, 13.2, and 14.3 months few examples of multisyllabic babble were recorded, and since this investigation is comparing chewing with multisyllabic babble, these age groups were not included in the comparisons. Multisyllabic vocalization and chewing samples were reduced into two main age groups. At 8.2, 9.1, 9.3, and 10.2 months the vocalization data contained significantly more vowel babble and fewer variegated babble, and at 16.3, 17.1, 19.2, and 21.3 months the vocalization data contained significantly more variegated syllables and fewer vowel utterances (see Table 1). These observations were measured by a Spearman rank order test that indicated a significant, negative correlation (r = - .85, p < .001) for the proportion of vowel babble distributed across age, and a positive correlation (r = .80, p = .003) for variegated babble distributed across age. Age 1 was composed of data points observed at 8.2, 9.1, 9.3, and 10.2 months, and these observations included the behavior of chewing and vowel and non-variegated babble. Age 2 was composed of data points observed at 16.3, 17.1, 19.2, and 21.3 months, and these observations included the behaviors of chewing and non-variegated and variegated babble (see Table 1).
The research question for this investigation was that if the adult model for jaw kinematics was representative of infant oromandibular behaviors then significant differences between early vocalizations and chewing would be observed for each dependent measure within each age group (i.e., age 1 and age 2). If the adult model was not representative of infant jaw trajectories, then the first question was whether task-related differences for measures of jaw kinematics would be significant among specific types of oromandibular behaviors. A Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks was used to test for a main effect across the three oromandibular behaviors that were observed at age 1 (i.e., vowel babble, non-variegated babble, and chewing) and at age 2 (i.e., non-variegated babble, variegated babble, and chewing) and multiple pairwise testing was conducted using Dunn’s Method. A second question addressed whether developmental changes would be observed for jaw kinematics associated with chewing, non-variegated babble, or babble (collapsed across categories). Non-variegated babble was the only category observed across age (see Table 1). A Mann-Whitney rank sum test was used to compare each dependent measure across age. Alpha level for all statistical testing was .05 to be considered significant.
3. RESULTS
3.1 Measure of Duration
The duration of each sample for a given behavior was measured. The average duration for samples of chewing was 1.55 seconds (SD = .47), vowel babble was 1.41 seconds (SD = .75), non-variegated babble was 1.58 seconds (SD = .73), and variegated babble was 1.66 seconds (SD = .63). These measures of duration were statistically compared for behavior across age group (e.g., chewing X age) and compared across behaviors (e.g., chewing X vowel babble) within an age group. A Kruskal-Wallis ANOVA on ranks did not reveal significant differences for behaviors across age group comparisons nor was a main effect obtained for comparisons across behaviors within a given age group.
3.2 Jaw Kinematic Measures
3.2.1 Average vertical jaw position
Figure 4 shows the median and 25th and 75th percentiles computed for average vertical jaw position by oromandibular behavior and age. A Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect for behavioral differences of average jaw position for age 1 (p = .005), with average elevation being greater for vowel babble than chewing (Q = 3.217) as tested by Dunn’s Method (p < .05; see Figure 4a). At age 2, a Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect for behavioral differences of average jaw position (p < .001), with average position being higher for chewing than non-variegated babble (Q = 3.974) as tested by Dunn’s Method (p < .05; see Figure 4b).
Figure 4.
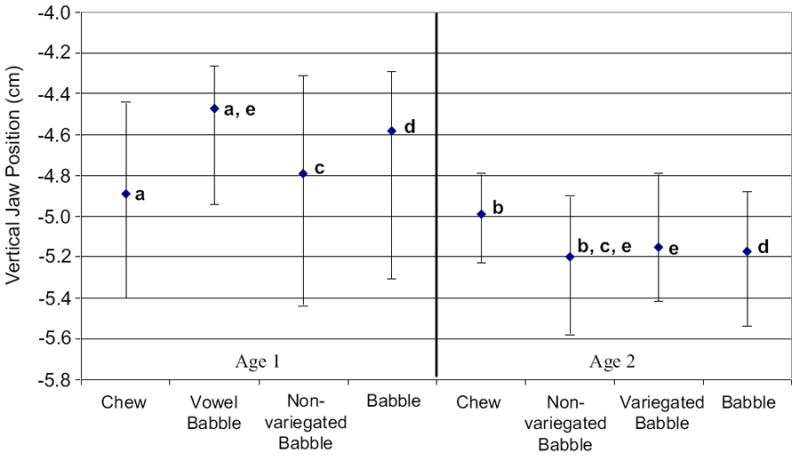
The median and 25th and 75th percentiles are depicted for average vertical jaw position plotted by oromandibular behavior and age, with letters depicting comparisons that were significant and are discussed in the text.
Developmental changes for average vertical jaw position was observed for non-variegated babble and babble (collapsed across categories) but not for chewing. A Mann-Whitney rank sum test indicated that average jaw position for non-variegated babble decreased in elevation (lowered) from age 1 to age 2 (T = 11655, p < .001; see Figure 4c), and this trend was also observed for babble in general (T = 29078, p < .001; see Figure 4d).
Post-hoc analyses were conducted to test differences for average vertical jaw position between vowel babble and non-variegated babble at age 2, and vowel babble and variegated babble at age 2. This testing was performed to better understand how jaw kinematics may change due to fewer instances of vowel babble and more productions of non-variegated babble together with the emergence of variegated babble from age 1 to age 2. Mann-Whitney rank sum tests indicated a significant difference across babble types for measures of average vertical jaw position; jaw position for vowel babble was more elevated than that measured for non-variegated babble (T = 7857, p < .001) and variegated babble at age 2 (T = 3735, p < .001; see Figure 4e).
3.2.2 Average total jaw displacement
Figure 5 shows the median and 25th and 75th percentiles computed for average total jaw displacement by oromandibular behavior and age. A Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect for behavioral differences for average jaw displacement at age 1 (p < .001), with average jaw displacement being greater for chewing than vowel babble (Q = 7.426) and non-variegated babble (Q = 5.369; see Figure 5a), and average displacement was greater for non-variegated babble than vowel babble (Q = 2.635; see Figure 5b) as tested by Dunn’s Method (p < .05). At age 2, a Kruskal-Wallis one-way ANOVA on ranks did not reach significance for comparisons among chewing, non-variegated babble, and variegated babble.
Figure 5.
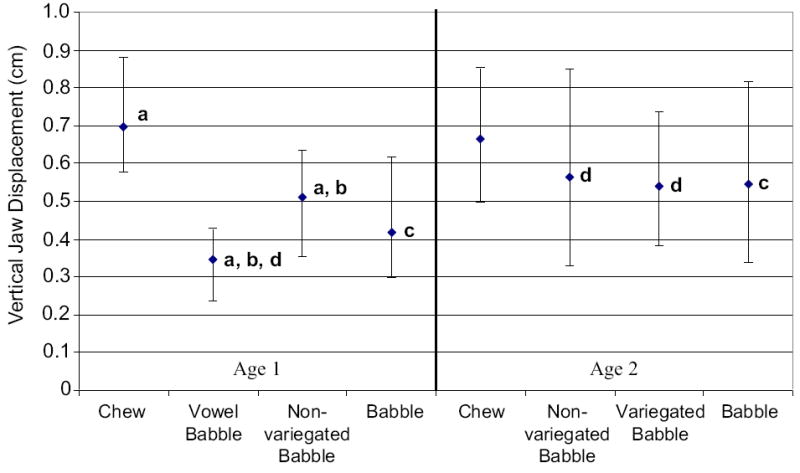
The median and 25th and 75th percentiles are depicted for average vertical jaw displacement plotted by oromandibular behavior and age, with letters depicting comparisons that were significant and are discussed in the text.
Developmental changes for average total jaw displacement were only significant for babble (collapsed across categories) but not for non-variegated babble and chewing. A Mann-Whitney rank sum test indicated a significant main effect for babble with jaw displacement increasing from age 1 to age 2 (T = 20320, p < .001; see Figure 5c).
Post-hoc analyses were conducted to test differences for average total jaw displacement between vowel babble and non-variegated babble at age 2, and vowel babble and variegated babble at age 2. This testing was conducted to better understand how jaw kinematics may change due to the transition of decreased instances for vowel babble and increased instances for non-variegated and variegated babble at age 2. Mann-Whitney rank sum tests indicated a significant difference across babble types for measures of average jaw displacement; jaw displacement for vowel babble was less than that measured for non-variegated babble (T=3784, p < .001) and variegated babble at age 2 (T = 2202, p < .001; see Figure 5d).
3.2.3 Coefficient of variation: Peak vertical jaw position values
Figure 6 shows the median and 25th and 75th percentiles computed for variation among peak vertical jaw position values by oromandibular behavior and age. A Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect for behavioral differences of variation for peak jaw position values at age 1 (p < .001), with variability being less for chewing than vowel babble (Q = 4.176) and non-variegated babble (Q = 4.218) as tested by Dunn’s method (p < .05; see Figure 6a). At age 2, a Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect of variation for peak jaw position values (p < .001), with variability being less for chewing than non-variegated babble (Q = 7.626) and variegated babble (Q = 6.645) as tested by Dunn’s Method (p < .05; see Figure 6b).
Figure 6.
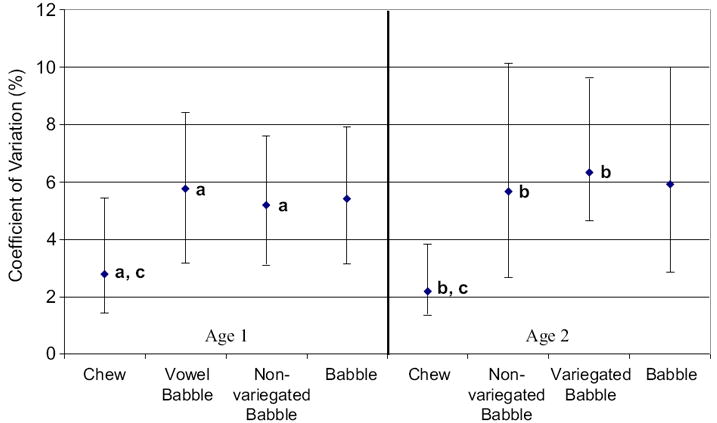
The median and 25th and 75th percentiles are depicted for the coefficient of variation computed among peak vertical jaw position values plotted by oromandibular behavior and age, with letters depicting comparisons that were significant and are discussed in the text.
Developmental changes for variation among peak vertical jaw position values were observed for chewing but not for non-variegated babble or babble (collapsed across categories). A Mann-Whitney rank sum test indicated that variability of peak jaw position values for chewing significantly decreased from age 1 to age 2 (T = 19248, p < .015; see Figure 6c).
3.2.4 Coefficient of variation: Total jaw displacement values
Figure 7 shows the median and 25th and 75th percentiles computed for variation among total jaw displacement values by oromandibular behavior and age. A Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect for behavioral differences of variation among total jaw displacement values at age 1 (p < .001), with less variability for chewing than vowel babble (Q = 8.101) and non-variegated babble (Q = 7.192) as tested using Dunn’s Method (p < .05; see Figure 7a). At age 2, a Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect for behavioral differences of variation in jaw displacement (p < .001), with less variability for chewing than non-variegated babble (Q = 3.826) and variegated babble (Q = 4.527) as tested by Dunn’s Method (p < .05; see Figure 7b).
Figure 7.
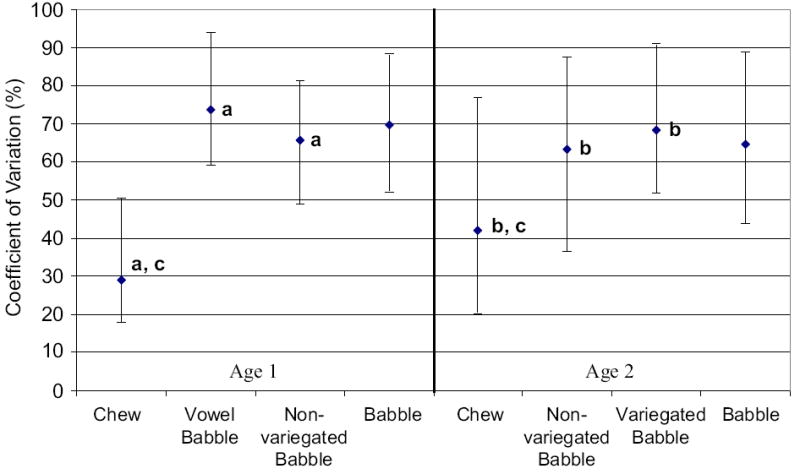
The median and 25th and 75th percentiles are depicted for the coefficient of variation computed among jaw displacement values plotted by oromandibular behavior and age, with letters depicting comparisons that were significant and are discussed in the text.
Developmental changes for variation among total jaw displacement values were observed for chewing but not for non-variegated babble or babble (collapsed across categories). A Mann-Whitney rank sum test indicated that variability for jaw displacement for chewing significantly increased from age 1 to age 2 (T = 15533, p < .001; see Figure 7c).
3.2.5 Coefficient of variation: A general index of speed for jaw movement
Figure 8 shows the median and 25th and 75th percentiles computed for variation of a general index of speed for vertical jaw displacement by oromandibular behavior and age. A Kruskal-Wallis one-way ANOVA on ranks indicated a significant main effect for behavioral differences of variation for speed of jaw movement at age 1 (p < .001), with chewing exhibiting less variability than vowel babble (Q = 4.321) and non-variegated babble (Q = 4.501) as tested by Dunn’s Method (p < .05; see Figure 8a). At age 2, a Kruskal-Wallis one-way ANOVA on ranks indicated a significant behavioral difference for variation of speed of movement (p < .008), with chewing exhibiting less variability than non-variegated babble (Q = 2.772) and variegated babble (Q = 2.407) as tested by Dunn’s Method (p < .05; see Figure 8b).
Figure 8.
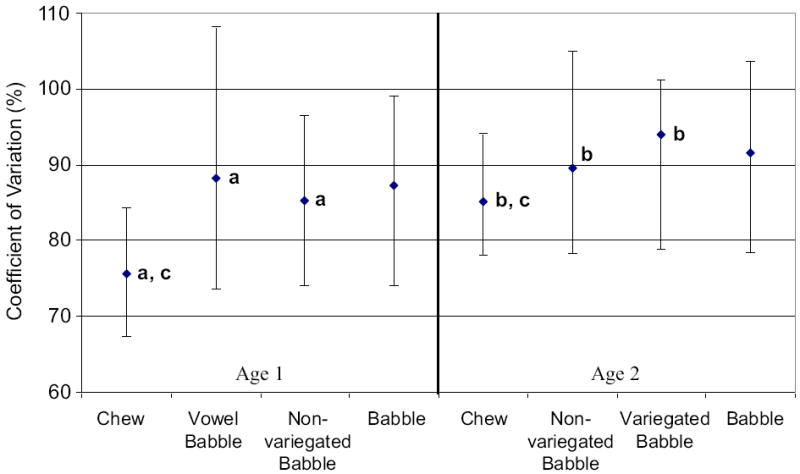
The median and 25th and 75th percentiles are depicted for the coefficient of variation computed among values for speed of jaw movement plotted by oromandibular behavior and age, with letters depicting comparisons that were significant and are discussed in the text.
Developmental changes for variation of speed for vertical jaw displacement were observed for chewing but not for non-variegated babble or babble (collapsed across categories). A Mann-Whitney rank sum test indicated that variability for this general index of speed changed significantly for chewing, with variability increasing from age 1 to age 2 (T = 13980, p < .001; see Figure 8c).
4. DISCUSSION
These longitudinal data provide insight into the developmental differences for infant jaw kinematics among oromandibular behaviors. However, an expanded study with more participants is required for increasing statistical power. The statistical comparisons in this investigation were quite robust; however, other details regarding infant jaw kinematics may surface with more participants.
Perceptual observations using phonetic complexity to categorize multisyllabic productions of babble appeared to reflect distinct stages of speech development (Kent, 1992; Stoel-Gammon, 1989; 1998). The distribution for specific categories of babble changed significantly across age groups. Vowel babble frequently occurred at age 1, but this vocalization type was seldom produced by age 2 (Table 1). Non-variegated babble was observed within both age groups, but proportionally, more samples of reduplicative babble were produced for the later age group. Variegated babble productions were virtually absent at age 1, but these vocalizations were frequently produced at age 2. Overall, vocal development for this infant replicates previous findings (Smith, et al., 1989; Stoel-Gammon, 1985, 1998).
4.1 Mandibular kinematics for infant
This investigation does not support the conclusion that the adult model for mandibular kinematics for chewing and speech is fully representative of infant trajectories for chewing and babble. Mainly, measures of average vertical jaw position were not greater for chewing than other babble categories, except for vowel babble (see Figure 4). At age 1, measures of average jaw displacement were significantly less for vowel babble and non-variegated babble than chewing, which represents the adult model, however, by age 2, these differences were no longer apparent among chewing and babble comparisons (see Figure 5). In contrast, adult maintain an elevated jaw position with smaller jaw excursions for speech in comparison to chewing (e.g., see Figure 1, Gibbs & Masserman, 1972). Although the adult model may not be completely represented in these kinematic data, jaw kinematics for this infant do exhibit task-specific organization among oromandibular behaviors. Specifically, kinematic differences are observed between chewing and babble, and among categories of babble.
4.2 Task-specific differences among chewing and babble
The measures of variability significantly distinguished jaw movement between infant chewing and babble, and these measures of jaw kinematics are consistent with previous observations for adults (Gibbs & Masserman, 1972). Jaw trajectories for infant chewing exhibited less variability for peak vertical jaw position (see Figure 6), total jaw displacement (see Figure 7), and speed of jaw movement (see Figure 8) than those measured for all other categories of multisyllabic babble at age 1 and age 2. These task-specific results for jaw kinematics are consistent with prior physiologic investigations supporting the idea that motor control of the jaw organizes distinctively for chewing and speech (or babble) in adults, children, and infants (Green, et al., 1997; Moore, 1993; Moore & Ruark, 1996; Moore, et al., 1988; Steeve, et al., 2008). A factor possibly influencing these kinematic differences for measures of variability is that mechanisms of mandibular motor control are reportedly different for chewing and vocalization (e.g., Jürgens, 2002, 2009; Moore, et al., 1988). For example, a core characterization for kinematics associated with specialized neural control mechanisms of pattern generation is the repetitive nature of the movement pattern (Delcomyn, 1980; Marder & Bucher, 2001), which may likely reflect a dominate role of central pattern generation controlling the mandible during chewing, as reported for adults (Smith & Denny, 1990).
Additionally, non-overlapping task-demands for chewing and babble may also influence jaw movement as observed in the measure of variability for peak vertical jaw position (see Figure 6). Peak vertical jaw position for chewing is related to a point of occlusion on a food item; whereas, peak jaw position for babble is not, and is related to modulation of aerodynamic energy. In a general sense, differences in coordination among mandibular muscle groups are required for generating appropriate forces and jaw trajectories across oromandibular behaviors to achieve task-dependent goals. Chewing, for example, requires a greater degree of occlusal force for mastication that is generated by reciprocal activation among muscle groups that elevate (e.g., masseter and temporalis muscles) and depress the jaw (e.g., anterior belly of digastric; Humphrey & Reed, 1983; Møller, 1966). Speech does not require these same forces. For example, coactivation among antagonist muscle groups of the mandible (e.g., Moore, et al., 1988) has been suggested to enhance the intrinsic biomechanics of the mandible for positional accuracy during vocalization (Bizzi, Polit, & Morasso, 1976; Perrier, Ostry, & Laboissière, 1996). This coordinative organization observed among mandibular muscle groups for adults (e.g., Moore, et al., 1988) is also observed for infants during babble and chewing (e.g., Moore & Ruark, 1996). The interaction among coordinated muscle groups and biomechanical properties of orofacial regions and other developmental processes will differentially influence jaw kinematics for different oromandibular behaviors. Furthermore, because there are anatomical and physiologic dissimilarities between infants and adults (Kent & Vorperian, 1995), the adult model for jaw kinematics among speech and nonspeech behaviors (e.g., Gibbs & Masserman, 1972) may not entirely represent what is observed in infants. In fact, this investigation supports the idea that task-specificity for jaw kinematics will present differently for the infant and for the adult.
In contrast to this perspective, commonly proposed phylogenetic / ontogenetic models of speech development do not consider a number of developmental processes, such as task-demands (e.g., Thelen, 1991; Fentress, 1984) or sensory experience (e.g., hearing, Callan, et al., 2000), as influencing control for mandibular movement (Davis & MacNeilage, 1995; MacNeilage, 1998; Meier, et al., 1997; von Hapsburg, et al., 2008). Instead, these models predict that early jaw trajectories are the same among early vocalizations and nonspeech oromandibular behaviors because development of motor control for speech is modeled as emerging from the serendipitous consequence of jaw motion in concert with vocalization. However, observations from this investigation do not support this assumption.
4.3 Jaw kinematics for chewing
Jaw kinematics for chewing underwent significant developmental changes as observed from measures of variability. Growth and development of the oromandibular system, and task-related factors may underlie these observations. Variability decreased for peak vertical jaw position from age 1 to age 2 (see Figure 6); whereas, variability increased for total jaw displacement (see Figure 7) and speed of jaw movement from age 1 to age 2 (see Figure 8). Variability for peak vertical jaw position is a measure related to a point of occlusion during chewing. Growth and development occurring within the oromandibular / facial region during the first 2-years of life includes the eruption of molars that is suggested to provide an occlusion contact area allowing the jaw to close to the same position (Kent & Vorperian, 1995). For this infant, molars were only present for kinematic measures obtained within age 2, and not age 1. Growth and development, with the eruption of molars, may have provided a more stable target for occlusion during chewing, decreasing variability across measures for peak vertical jaw position at age 2.
Task-related factors associated with food consistency and bolus size may provide a partial explanation for developmental changes observed for chewing. Variability increased for total jaw displacement (see Figure 7) and speed of jaw movement (see Figure 8) from age 1 to age 2. These measures of variability include the active segments of jaw movement; which includes variability among values for jaw displacement and speed of jaw movement. Task-related factors associated with food consistency and bolus size changed across age groups. Food consistencies within age 1 were pureed and semi-solid food items, bolus size for pureed items was a teaspoon and semi-solids were presented as single items (e.g., single Cheerios ®, a small slice of banana), and by age 2, the child was eating both semi-solid and solid food items with greater amounts per bite (e.g., 5 to 10 Cheerios ®, a large bite of banana or apple, meat, 2 to 3 Goldfish® crackers). Possibly, these changes for food consistency and bolus size across age for chewing increased variability for the active measures of jaw movement.
Growth and development of the mandible may have also contributed to increased variability observed for jaw displacement and speed of movement across age for chewing (see Figures 7 & 8). The mandible changes in size but not shape with the eruption of teeth (alveolar margin) and the elongation of ramus (Kent & Vorperian, 1995). Increased size of the mandible would influence the angular movement of the jaw and possibly make movements more variable for chewing at age 2. Additionally, changes to the soft tissues of the buccae (cheeks), such as decreased prominence of the buccal fat pad (Pad of Bichat), may also have contributed to greater variability. However, it is important to recall that average vertical jaw position (see Figure 4) and average jaw displacement (see Figure 5) did not significantly change across age for chewing; the difference in the median values across age is quite small. Furthermore, jaw movement for chewing exhibited less variability across all kinematic measures in comparison to all categories of babble (see Figures 6, 7, & 8), which includes vocalizations at age 1 that were produced with less jaw displacement than chewing (see Figure 5). Jaw kinematics is most likely influenced by the interaction among various developmental processes, which includes biomechanical properties of the mandible and task-related factors.
4.4 Task-specific differences among vowel babble and other behaviors
Jaw kinematics for vowel babble was distinctively different from other categories of babble and chewing. Overall, this infant produced vowel babble with reduced jaw displacement (see Figure 5) while maintaining a higher elevated position of the jaw (see Figure 4) in comparison to other oromandibular behaviors measured in both age groups. Developmentally, vowel babble is different for other types of babble. Vowel babble is a unique vocalization type in that it is produced by both normal hearing and profound hearing impaired infants; whereas, only normal hearing infants begin producing babble categories composed of greater phonetic complexity (Oller & Eilers, 1988; Stoel-Gammon & Otomo, 1986). Hearing does not influence the emergence of vowel babble, but hearing is very important for the emergence of non-variegated and variegated babble (i.e., canonical babble). For hearing infants, instances of vowel babble productions decrease over the course of development, such as observed for the infant in this study (Stoel-Gammon, 1985, 1989, 1998). Given that the emergence of vowel babble is not dependent on auditory-articulatory linkages (e.g., Callan, et al., 2000) and that jaw kinematics are different for these vocalizations; motor control for vowel babble may emerge from neural networks unlike those for non-variegated and variegated babble. Animal models suggest that neural control differs across a continuum of innate and learned vocalizations, with control for learned, complex vocalizations being associated with networks that include cortical and brainstem regions (Jürgens, 2002, 2009). Perhaps control for non-variegated and variegated babble (i.e., canonical babble) emerges from linkages with language centers in the brain (Smith, 2006).
4.5 Jaw kinematics and phonetic complexity
There are a variety of developmental factors that may influence jaw kinematics across oromandibular behaviors. Phonetic complexity may partially explain the kinematic differences observed among different types of babble. Adult speakers, for example, exhibit greater jaw displacement for speech tasks composed mainly of stop consonants (Tasko & McClean, 2004), and a universal regularity in phonemic acquisition for emerging speech is the production of stop consonants (Locke, 1983). Jaw kinematic measures for vowel babble probably differ from non-variegated and variegated types (see Figures 4 & 5) because vowel babble is basically composed of glottals and glides, and lacks supralaryngeal obstruent productions. Furthermore, if early canonical babble is composed of mainly stop consonants, this factor may influence why jaw kinematic comparisons between infant babble and chewing were unlike those observed for speech and chewing in adults (Gibb & Masserman, 1972). Average vertical jaw position was not significantly greater for canonical babble than chewing (see Figure 4), and average jaw displacement was not significantly less for canonical babble than chewing, except for non-variegated babble at age 1 (see Figure 5).
Phonetic complexity may also partially explain why jaw kinematics for this infant changed across age groups for non-variegated babble. Average vertical jaw position significantly lowered for non-variegated babble from age 1 to 2 (see Figure 4); while, average jaw displacement increased for babble (collapsed across babble categories) across age groups (see Figure 5). These changes for jaw kinematics may have occurred because the ratio of reduplicative syllable productions increased from age 1 to age 2. Additionally, both non-variegated and variegated babble productions were more frequently produced at age 2 and observations of jaw displacement were greater for these productions than for vowel babble at age 1 (see Figure 5). These factors most likely influenced the measure of jaw displacement across age for the ‘babble’ comparison (see Figure 5). More frequent productions of reduplicative and variegated syllables at age 2 may have altered jaw kinematics for canonical babble.
Growth and development of the mandible may have been partially responsible for across age changes observed for average vertical jaw position and jaw displacement that were associated with babble productions (see Figures 4 & 5). Increased size (length) of the mandible could account for a lower average jaw position with increased jaw displacement. However, it is important to observe that average vertical jaw position and average jaw displacement did not significantly change across age for chewing (see Figures 4 & 5). Furthermore, average jaw displacement did not change across age for non-variegated babble; instead, this measure mainly changed due to the decline of vowel babble and emergence of variegated babble across age, which influenced the ‘babble’ comparison (i.e., babble categories collapsed, see Figure 5). Again, it is the interaction among different developmental processes that influenced these within and across age comparisons.
5. Conclusion
Mandibular trajectories observed for chewing and speech in adults are not fully representative of infant jaw kinematics that naturally occur for chewing and babble during the first 2-years of life. Task-dependent distinctions for mandibular kinematics are observed among categories of early babble and chewing. These important distinctions among oromandibular behaviors suggests that an understanding of development for mandibular trajectories associated with early infant vocalizations cannot be predicted from jaw kinematics associated with non-speech behaviors or from jaw trajectories produced by adult speakers.
Acknowledgments
This work was supported in part by the National Institutes of Health Idea Network of Biomedical Research Excellence Program (INBRE), National Center for Research Resources Grant P20 RR16474, and the Experimental Program to Stimulate Competitive Research (EPSCoR), National Science Foundation Grant EPS 0447681, as well as by the National Institute on Deafness and Other Communication Disorders Grants R01 DC00822, T32 DC00033, and F31 DC00295, and the University of Wyoming in Laramie, and the University of Washington in Seattle. The author would like to acknowledge Christie Samuelson and Jolene Samuelson for their assistance with this investigation, and to Dr. David Jones for commentary on earlier versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barlow SM, Finan DS, Andreatta R. Neuronal group selection and emergent orofacial motor control: Towards a unifying theory of speech development. In: Hulstijn W, Peters HFM, van Lieshout PHHM, editors. Speech production: Motor control, brain research, and fluency disorders. Elsevier; Amsterdam: 1997. pp. 529–546. [Google Scholar]
- Bizzi E, Polit A, Morasso P. Mechanisms underlying achievement of final head position. Journal of Neurophysiology. 1976;39:435–444. doi: 10.1152/jn.1976.39.2.435. [DOI] [PubMed] [Google Scholar]
- Callan DE, Kent RD, Guenther FH, Vorperian HK. An auditoryfeedback- based neural network model of speech production that is robust to developmental changes in the size and shape of the articulatory system. Journal of Speech, Language, and Hearing Research. 2000;43:721–736. doi: 10.1044/jslhr.4303.721. [DOI] [PubMed] [Google Scholar]
- Davis BL, MacNeilage PF. The articulatory basis of babbling. Journal of Speech, Language, and Hearing Research. 1995;38:1199–1211. doi: 10.1044/jshr.3806.1199. [DOI] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Fentress JC. The development of coordination. Journal of Motor Behavior. 1984;16:99–134. doi: 10.1080/00222895.1984.10735315. [DOI] [PubMed] [Google Scholar]
- Finan DS, Barlow SM. Intrinsic dynamics and mechanosensory modulation of non-nutritive sucking in human infants. Early Human Development. 1998;52:181–197. doi: 10.1016/s0378-3782(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Gibbs CH, Masserman T. Jaw motion during speech. ASHA Reports, Report 7. 1972:R104–R112. [Google Scholar]
- Green JR, Moore CA, Higashikawa M, Steeve RW. The physiologic development of speech motor control: lip and jaw coordination. Journal of Speech, Language, and Hearing Research. 2000;43:239–55. doi: 10.1044/jslhr.4301.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Moore CA, Reilly KJ. The sequential development of jaw and lip control for speech. Journal of Speech, Language, and Hearing Research. 2002;45(1):66–79. doi: 10.1044/1092-4388(2002/005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Moore CA, Ruark JL, Rodda PR, Morvée WT, VanWitzenburg MJ. Development of chewing in children from 12 to 48 months: Longitudinal study of EMG patterns. Journal of Neurophysiology. 1997;77:2704–2727. doi: 10.1152/jn.1997.77.5.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Recombination of motor pattern generators. Simple neuronal networks combine to produce complex versatile motor patterns. Current Biology. 1991;1(4):231–233. doi: 10.1016/0960-9822(91)90066-6. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. Journal of Communication Disorders. 2006;39:350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Humphrey DR, Reed DJ. Separate cortical systems for control of joint movement and joint stiffness: Reciprocal activation and coactivation of antagonist muscles. In: Desmedt JE, editor. Motor control mechanisms in health and disease. New York: Raven Press; 1983. pp. 347–372. [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neuroscience and Biobehavioral Reviews. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The neural control of vocalization in mammals: a review. Journal of Voice. 2009;23(1):1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Kent RD. The biology of phonologic development. In: Ferguson CA, Menn L, Stoel-Gammon C, editors. Phonological development: Models, research, and implications. Timonium, MD: York Press; 1992. pp. 65–90. [Google Scholar]
- Kent RD, Hodge MM. The biogenesis of speech: Continuity and process of early speech and language development. In: Miller JF, editor. Progress in research on child language disorders. Austin, Texas: Pro-Ed; 1990. pp. 25–53. [Google Scholar]
- Kent RD, Vorperian MA. Development of the craniofacial-oral-laryngeal anatomy: A review. Journal of Medical Speech-Language Pathology. 1995;3(3):145–90. [Google Scholar]
- Lund JP. Mastication and its control by the brain stem. Critical Reviews in Oral Biology and Medicine. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goldberg LJ. Neural mechanisms of mandibular control: Mastication and voluntary biting. In: Brooks VB, editor. Handbook of physiology – section I: The nervous system, volume II, motor control. Bethesda, MD: American Physiological Society; 1981. pp. 1237–74. [Google Scholar]
- MacNeilage PF. The frame / content theory of evolution of speech production. Behavioral and Brain Sciences. 1998;21:499–546. doi: 10.1017/s0140525x98001265. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. The American Physiological Society. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Current Biology. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Matlab [version 6.5, Computer software] Natick, MA: The MathWorks; 2003. [Google Scholar]
- Matlab [version 7.5, Computer software] Natick, MA: The MathWorks; 2007. [Google Scholar]
- Meier RP, McGarvin L, Zakia RAE, Willerman R. Silent mandibular oscillations in vocal babbling. Phonetica. 1997;54:153–71. doi: 10.1159/000262219. [DOI] [PubMed] [Google Scholar]
- Møller E. The chewing apparatus. An electromyographic study of the action of muscles of mastication and its correlation to facial morphology. Acta Physiologica Scandinavica. 1966;69(Supplement 280) [PubMed] [Google Scholar]
- Moore CA. Symmetry of mandibular muscle activity as an index of coordinative strategy. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA. Physiologic development of speech production. In: Maassen B, Hulstijn W, Peters HFM, Lieshout PHHM, editors. Speech motor control in normal and disordered speech. Oxford University Press; Oxford: 2004. [Google Scholar]
- Moore CA, Ruark JL. Does speech emerge from earlier appearing motor behaviors? Journal of Speech and Hearing Research. 1996;39:1034–47. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel RL. Task-specific organization of activity in human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–80. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Motus [version 6.0, Computer software] Englewood, CO: Peak Performance Technologies; 2000. [Google Scholar]
- Netsell R. The acquisition of speech motor control: A perspective with direction for research. In: Stark RE, editor. Language behavior in infancy and early childhood. New York: Elsevier/North Holland; 1981. pp. 127–56. [Google Scholar]
- Locke JL. Phonological acquisition and change. San Diego, CA: Academic Press; 1983. [Google Scholar]
- Oller DK, Eilers RE. The role of audition in infant babbling. Child Development. 1988;59(2):441–49. [PubMed] [Google Scholar]
- Perrier P, Ostry DJ, Laboissière R. The equilibrium point hypothesis and its application to speech motor control. Journal of Speech and Hearing Research. 1996;39:365–378. doi: 10.1044/jshr.3902.365. [DOI] [PubMed] [Google Scholar]
- Richardson CA, Mitchell RA. Power spectral analysis of inspiratory nerve activity in the decerebrate cat. Brain Research. 1982;233:317–336. doi: 10.1016/0006-8993(82)91205-7. [DOI] [PubMed] [Google Scholar]
- Smith A. Speech motor development: Integrating muscles, movements, and linguistic units. Journal of Communication Disorders. 2006;39:331–349. doi: 10.1016/j.jcomdis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Smith A, Denny M. High-frequency oscillations as indicators of neural control mechanisms in human respiration, mastication, and speech. Journal of Neurophysiology. 1990;63(4):745–758. doi: 10.1152/jn.1990.63.4.745. [DOI] [PubMed] [Google Scholar]
- Smith BL, Brown-Sweeny S, Stoel-Gammon C. A quantitative analysis of reduplicative and variegated babbling. First Language. 1989;9:175–90. [Google Scholar]
- Steeve RW, Moore CA. Mandibular motor control during early development of speech and nonspeech behaviors. Journal of Speech, Language, and Hearing Research. 2009;52:1530–54. doi: 10.1044/1092-4388(2009/08-0020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeve RW, Moore CA, Green JR, Reilly KJ, Ruark McMurtrey JL. Babbling, chewing, and sucking: Oromandibular coordination at 9-months. Journal of Speech, Language, and Hearing Research. 2008;51(6):1390–1404. doi: 10.1044/1092-4388(2008/07-0046). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoel-Gammon C. Phonetic inventories, 15-24 months: Longitudinal study. Journal of Speech and Hearing Research. 1985;28:505–512. doi: 10.1044/jshr.2804.505. [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon C. Prespeech and early speech development of two late talkers. First Language. 1989;9:207–24. [Google Scholar]
- Stoel-Gammon C. The role of babbling and phonology in early linguistic development. In: Wetherby AM, Warren SF, Reichle J, editors. Transitions in prelinguistic communication. Baltimore: Paul H. Brookes Publishing Co; 1998. pp. 87–110. [Google Scholar]
- Stoel-Gammon C, Otomo K. Babbling development of hearing-impaired and normally hearing subjects. Journal of Speech and Hearing Disorders. 1986;51:33–41. doi: 10.1044/jshd.5101.33. [DOI] [PubMed] [Google Scholar]
- Tasko SM, McClean MD. Variations in articulatory movement with changes in speech tasks. Journal of Speech, Language, Hearing Research. 2004;47:85–100. doi: 10.1044/1092-4388(2004/008). [DOI] [PubMed] [Google Scholar]
- Thelen E. Motor aspects of emergent speech: A dynamic approach. In: Krasnegor NA, Rumbaugh DM, Schiefelbusch RL, Studdert-Kennedy Michael, editors. Biological and behavioral determinants of language development. Hillsdale, NJ: Lawrence Erlbaum Associates Publishers; 1991. pp. 339–62. [Google Scholar]
- von Hapsburg D, Davis BL, MacNeilage PF. Frame Dominance in Infants with Hearing Loss. Journal of Speech, Language, Hearing Research. 2008;51:306–20. doi: 10.1044/1092-4388(2008/023). [DOI] [PubMed] [Google Scholar]
- Wilson EM, Green JR. The development of jaw motion for mastication. Early Human Development. 2009;85(5):303–311. doi: 10.1016/j.earlhumdev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windaq Acquisition [version 2.54, Computer software] Akron, OH: Dataq Instruments; 2001. [Google Scholar]


