Abstract
Purpose:
Cyclin D1 and cyclin-dependent kinases (CDK) are commonly activated in colorectal cancer. The activity of cyclin D1 can be blocked by CDK inhibitors, including p27 (CDKN1B) and p21 (CDKN1A, which is induced by p53). However, prognostic significance of tumoral cyclin D1 remains uncertain, and no previous study has considered potential confounding effect of p53, p21, p27, and related molecular events [microsatellite instability (MSI), CpG island methylator phenotype, and LINE-1 hypomethylation].
Experimental Design:
Among 602 colon cancer patients (stage I-IV) in two prospective cohort studies, cyclin D1 overexpression was detected in 330 (55%) tumors by immunohistochemistry. Cox proportional hazard models computed hazard ratios (HR) of colon cancer – specific and overall mortalities, adjusted for patient characteristics and tumoral molecular features, including p53, p21, p27, cyclooxygenase-2, fatty acid synthase, LINE-1 methylation, CpG island methylator phenotype, MSI, BMI, KRAS, and BRAF.
Results:
Cyclin D1 overexpression was associated with a low cancer-specific mortality in Kaplan-Meier analysis (P = 0.006), and in both univariate Cox regression [unadjusted HR, 0.64; 95% confidence interval (CI), 0.47-0.88; P = 0.0063] and multivariate analyses (adjusted HR, 0.57; 95% CI, 0.39-0.84; P = 0.0048). Similar findings were observed for an overall mortality (adjusted HR, 0.74; 95% CI, 0.57-0.98; P = 0.036). Notably, the effect of cyclin D1 on survival might differ by MSI status (Pinteraction = 0.008). Compared with tumors that were both cyclin D1–negative and MSI-low/microsatellite stable, the presence of either cyclin D1 or MSI-high or both seemed to confer better clinical outcome (adjusted HR point estimates, 0.10-0.65).
Conclusions:
Cyclin D1 overexpression is associated with longer survival in colon cancer.
Cyclin D1 (CCND1, the official gene symbol) plays a key role in cell cycle control, particularly in the transition from G1 to S phase, which is regulated by cyclin-dependent kinases (1). The ability of cyclin D1 to drive the cell cycle forward can be blocked by cyclin-dependent kinase (CDK) inhibitors, such as p27 (CDKN1B) and p21 (CDKN1A, which is induced by p53; ref. 1). Cyclin D1 overexpression occurs in one-third or more of colorectal cancers (2-20). Cyclin D1 activation by APC mutation/WNT signaling seems to contribute to colon neoplasia initiation (21, 22).
Despite a well-established role of cyclin D1 in cell cycle progression, previous data on cyclin D1 and clinical outcome in colon cancer have been conflicting (4-20). Although cyclin D1 expression has been associated with poor prognosis in two studies (4, 5), another study showed good prognosis associated with cyclin D1 expression (6), and most studies revealed no independent prognostic value of cyclin D1 (7-20). However, most previous studies had limited sample sizes, and only three studies (11, 15, 16) had sample sizes >170 (up to n = 363; ref. 16). In addition, cyclin D1 expression in colon cancer is related with microsatellite instability (MSI), the CpG island methylator phenotype (CIMP), and BRAF mutation (23). Although these molecular features have been associated with patient outcome (24-26), none of the previous studies (4-20) has considered confounding or modifying effect of MSI, CIMP, and BRAF.
Translational Relevance.
Cyclin D1 and cyclin-dependent kinase (CDK) activation has been shown to play an important role in carcinogenesis in various organ systems including colon. CDK inhibitors have been shown to be effective in cancer treatment. However, the relation between cyclin D1 expression in colon cancer and patient survival has been controversial. We have used the database of >600 colon cancer in two independent, prospective cohort studies, with available clinical information, adequate follow-up, and other important molecular events in colon cancers. To our knowledge, this is the first large study to show influence of cyclin D1 expression on clinical outcome independent of related molecular events including p53, p21, p27, KRAS, BRAF mutation, microsatellite instability, the CpG island methylator phenotype, and LINE-1 hypomethylation, all of which are potential confounders. Thus, our findings are relevant to practice in oncology.
In this study, using a large number (n = 602) of stage I to IV colon cancers in two independent cohort studies, we have examined the effect of cyclin D1 expression in colon cancer on patient survival. Because we concurrently assessed other related molecular variables including p53, p21, p27, KRAS, BRAF, MSI, CIMP, and LINE-1 hypomethylation, we could evaluate the independent effect of cyclin D1 after controlling for these potential confounders. In particular, it is important to control for the effect of MSI, CIMP, and LINE-1 hypomethylation because these molecular characteristics reflect genomic and epigenomic status of cancer cells, and have been related with patient survival in colon cancer (24-27).
Materials and Methods
Study population
We used the databases of two independent prospective cohort studies; the Nurses' Health Study (N = 121,700 women followed since 1976) and the Health Professionals Follow-up Study (N = 51,500 men followed since 1986; ref. 28). Every 2 years, participants have been sent follow-up questionnaires to update information on potential risk factors and to identify newly diagnosed cancer and other diseases in themselves and their first-degree relatives. We calculated body mass index (BMI, kg/m2), using self-reported height from the baseline questionnaire and weight from the biennial questionnaire that immediately preceded the diagnosis of colon cancer. In validation studies in both cohorts, self-reported anthropometric measures were well-correlated with measurements by trained technicians (r > 0.96). On each biennial follow-up questionnaire, participants were asked whether they had a diagnosis of colon cancer during the previous 2 years. When a participant (or next of kin for decedents) reported colon cancer, we sought permission to obtain medical records. Study physicians, while blinded to exposure data, reviewed all records related to colon cancer, and recorded American Joint Committee on Cancer tumor stage and tumor location. For nonresponders, we searched the National Death Index to discover deaths and ascertain any diagnosis of colon cancer that contributed to death or was a secondary diagnosis. Approximately 96% of all incident colon cancer cases were identified through these methods. We collected paraffin-embedded tissue blocks from hospitals where colon cancer patients underwent tumor resections (28). Tissue sections from all colon cancer cases were reviewed and confirmed by a pathologist (S.O.). Tumor grade was categorized as high (≤50% glandular area) or low (>50% glandular area). Based on availability of tissue samples, we included a total of 602 stage I to IV colon cancer cases diagnosed up to 2002. There were only ~2.5% of Asians, Hispanics, and African Americans and the remaining 97.5% were non-Hispanic Caucasians. Written informed consent was obtained from all study subjects. This study was approved by the Human Subjects Committees at Brigham and Women's Hospital and the Harvard School of Public Health.
Measurement of mortality
Patients were observed until death or June 2006, whichever came first. Ascertainment of deaths included reporting by the family or postal authorities. In addition, the names of persistent nonresponders were searched in the National Death Index. The cause of death was assigned by physicians blinded to information on life-style exposures and molecular changes in colon cancer. In rare patients who died as a result of colon cancer not previously reported, we obtained medical records with permission from next of kin. More than 98% of deaths in the cohorts were identified by these methods.
DNA extraction, pyrosequencing of KRAS and BRAF, and MSI analysis
Genomic DNA from paraffin-embedded tissue was extracted, and whole genome amplification was done (29). PCR and Pyrosequencing targeted for KRAS codons 12 and 13 (29), and BRAF codon 600, were done (30). MSI status was determined using 10 microsatellite markers (D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67, and D18S487; ref. 31). MSI-high was defined as the presence of instability in ≥30% of the markers, MSI-low as the presence of instability in <30% of the markers, and microsatellite stability (MSS) as no unstable marker.
Real-time PCR (MethyLight) to determine CIMP status
Sodium bisulfite treatment on tumor DNA and subsequent real-time PCR (MethyLight) assays were validated and done as previously described (32). We quantified promoter methylation in 8 CIMP-specific genes (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1; refs. 33-35). CIMP-high was defined as ≥6/8 methylated promoters using the 8-marker CIMP panel, CIMP-low/0 as 0 to 5 methylated promoters, according to the previously established criteria (34).
Pyrosequencing to measure LINE-1 methylation
In order to accurately quantify relatively high LINE-1 methylation levels, we used Pyrosequencing as previously described (27).
Immunohistochemistry for cyclin D1, p53, p21, p27, cyclooxygenase-2 and fatty acid synthase
Tissue microarrays were constructed as previously described (37). Two 0.6-mm tissue cores each from tumor and normal colonic mucosa were placed in each tissue microarrays block. Methods of immunohistochemical procedures and interpretation were previously described as follows: cyclin D1 (23), cyclooxygenase (COX)-2 and fatty acid synthase (FASN; ref. 28, 31), p21 and p27 (38), and p53 (39). For cyclin D1, antigen retrieval was done, and deparaffinized tissue sections in Target Retrieval Solution (Dako) were treated with microwave in a pressure cooker for 15 min. Tissue sections were incubated with 3% H2O2 (10 min) to block endogenous peroxidase, and with 10% normal goat serum (Vector Laboratories) in PBS (10 min). Primary antibody against cyclin D1 [rabbit monoclonal (SP4) to cyclin D1, 1:100 dilution; Abcam] was applied, and the slides were maintained overnight at room temperature. This antibody has been shown to be a robust antibody to detect cyclin D1 in paraffin tissue (40), and its specificity has been confirmed by Western blot (41). In addition, CCND1 gene amplification has been correlated with protein expression level assessed by this antibody (42). Next, we applied an anti-rabbit IgG antibody (Vector Laboratories) for 30 min, followed by an avidin-biotin complex conjugate (Vector Laboratories) for 30 min. The immunochemical reaction was revealed by diaminobenzidine (5 min) and methyl-green counterstain. Nuclear cyclin D1 expression was recorded as no expression, weak expression, or moderate/strong expression, and the proportion of positive tumor cells was recorded. Moderate or strong staining for cyclin D1 in any fraction of tumor cells was interpreted as positive. Appropriate positive and negative controls were included in each run of immunohistochemistry. All immunohistochemically-stained slides for cyclin D1 were interpreted by one of the investigators (K.N.) unaware of other data. A random sample of 160 tumors were reexamined by a second observer (K.S.) unaware of other data. The concordance between the two observers was 0.83 (κ = 0.64; P < 0.0001), indicating substantial agreement. For the other markers, a random selection of 108 to 246 cases was reexamined for each marker by a second pathologist (p53 and FASN by K.N.; p21 and p27 by K.S.; COX-2 by R. Dehari, Kanagawa Cancer Center, Japan) unaware of other data, and concordance rates and κ coefficients between the two pathologists were as follows: 0.87 (κ = 0.75; n = 118) for p53; 0.93 (κ = 0.57; n = 246) for FASN; 0.83 (κ = 0.62; n = 179) for p21; 0.94 (κ = 0.60; n = 114) for p27; 0.92 (κ = 0.62; n = 108) for COX-2, indicating good to substantial agreement.
Statistical analysis
We used stage-matched, conditional Cox proportional hazard models to calculate hazard ratios (HR) of death according to tumoral cyclin D1 status, adjusted for age, sex, year of diagnosis, BMI, family history of colorectal cancer in any first-degree relative, tumor location, stage, grade, and status of MSI, CIMP, LINE-1, KRAS, BRAF, p53, p21, p27, COX-2, and FASN. In addition, we also did unconditional Cox regression analysis to assess the unadjusted, main effect of cyclin D1 expression on mortality. For analyses of colon cancer–specific mortality, death as a result of colon cancer was the primary end point and deaths as a result of other causes were censored. To adjust for potential confounding, age, year of diagnosis, and LINE-1 methylation were used as continuous variables, and all of the other covariates were used as categorical variables. We dichotomized family history (present versus absent), BMI (<30 kg/m2 versus ≥30 kg/m2), tumor location (proximal versus distal), tumor grade (high versus low), CIMP (high versus low/0), MSI (high versus low/MSS), p53, p21, p27, COX-2, FASN, KRAS, and BRAF. For cases with missing tumor location (1.5% missing) or p27 (4.5% missing), we assigned a separate (“missing”) indicator variable and included those cases in the multivariate analysis models. For missing information in other covariates [including BMI (3.8% missing), tumor grade (0.3% missing), MSI (1.0% missing), KRAS (0.7% missing), BRAF (3.2% missing), p53 (0.7% missing), p21 (2.8% missing), COX-2 (0.3% missing), and FASN (1.7% missing)], we included those cases in a majority category in the particular missing variable, to minimize the number of “missing” indicator variables and maximize the efficiency of multivariate Cox regression analyses. We confirmed that excluding cases with missing information in any of the covariates did not substantially alter results (data not shown). An interaction was assessed by including the cross product of the cyclin D1 variable and another variable of interest in a multivariate Cox model, and the likelihood ratio test was done. Considering multiple hypotheses testing, P values for interactions were conservatively interpreted and higher level of significance (i.e., P < 0.01) was considered to be statistically significant. To assess an interaction of cyclin D1 and stage, we treated stage as a binary variable (I-II versus III-IV) as well as an ordinal categorical variable (I-IV). To confirm the relation between cyclin D1 expression and survival, we computed HR according to the proportion of tumor cells positive for cyclin D1 (as a continuous variable), nonparametrically with restricted cubic splines (43), which was independent of predetermined categorization of cyclin D1 status.
The Kaplan-Meier method was used to describe the distribution of colon cancer-specific and overall survival time, and the log-rank test was done. The χ2 test was used to examine an association of cyclin D1 with any of the categorical variables. The t test assuming unequal variances was done to compare mean age and mean LINE-1 methylation level. All analyses used SAS version 9.1 (SAS Institute) and all P values were two-sided.
Results
Cyclin D1 expression in colon cancer and patient survival
Among 602 patients with stage I to IV colon cancer, cyclin D1 overexpression was observed in 330 (55%) tumors by immunohistochemistry (Supplementary Figure). We assessed clinical and molecular characteristics of colon cancers, according to tumoral cyclin D1 status (Table 1). Compared with cyclin D1 – negative tumors, cyclin D1 – positive tumors were more likely to show MSI (MSI-high, P < 0.0001), CIMP (CIMP-high, P = 0.0003), BRAF mutation (P = 0.006), p21 expression (P < 0.0001), p27 nuclear expression (P = 0.023), and FASN expression (P = 0.022).
Table 1.
Clinical and molecular characteristics according to cyclin D1 status in colon cancer
| Clinical or molecular feature | All cases | Cyclin D1 |
P | |
|---|---|---|---|---|
| Negative | Positive | |||
| Total n | 602 | 272 | 330 | |
| Sex | 0.19 | |||
| Male (HPFS) | 261 (43%) | 110 (40%) | 151 (46%) | |
| Female (NHS) | 341 (57%) | 162 (60%) | 179 (54%) | |
| Mean age ± SD | 66.5 ± 8.3 | 66.4 ± 8.4 | 66.6 ± 8.3 | 0.76 |
| BMI | 0.68 | |||
| <30 kg/m2 | 482 (83%) | 217 (82%) | 265 (84%) | |
| ≥30 kg/m2 | 97 (17%) | 48 (18%) | 49 (16%) | |
| Family history of colorectal cancer in any 1st degree relative | 0.35 | |||
| Absent | 454 (75%) | 210 (77%) | 244 (74%) | |
| Present | 148 (25%) | 62 (23%) | 86 (26%) | |
| Year of diagnosis | 0.25 | |||
| Before 1990 | 91 (15%) | 45 (17%) | 46 (14%) | |
| 1990-1999 | 440 (73%) | 190 (70%) | 250 (76%) | |
| 2000-2002 | 71 (12%) | 37 (14%) | 34 (10%) | |
| Tumor location | 0.080 | |||
| Proximal (cecum to transverse) | 344 (58%) | 145 (54%) | 199 (61%) | |
| Distal (splenic flexure to sigmoid) | 249 (42%) | 123 (46%) | 126 (39%) | |
| AJCC tumor stage | 0.054 | |||
| I | 123 (20%) | 48 (18%) | 75 (23%) | |
| IIA | 187 (31%) | 78 (29%) | 109 (33%) | |
| IIB | 18 (3.0%) | 11 (4.0%) | 7 (2.1%) | |
| IIIA | 21 (3.5%) | 8 (2.9%) | 13 (3.9%) | |
| IIIB | 80 (13%) | 39 (14%) | 41 (12%) | |
| IIIC | 54 (9.0%) | 31 (11%) | 23 (7.0%) | |
| IV | 78 (13%) | 43 (16%) | 35 (11%) | |
| Unknown | 41 (6.8%) | 14 (5.1%) | 27 (8.2%) | |
| Tumor grade | 0.40 | |||
| Low | 534 (89%) | 238 (88%) | 296 (90%) | |
| High | 66 (11%) | 33 (12%) | 33 (10%) | |
| Mean LINE-1 methylation (%) ± SD | 60.9 ± 9.6 | 59.9 ± 9.5 | 61.8 ± 9.6 | 0.014 |
| MSI | <0.0001 | |||
| MSI-low/MSS | 487 (82%) | 242 (89%) | 245 (75%) | |
| MSI-high | 109 (18%) | 29 (11%) | 80 (25%) | |
| CIMP | 0.0003 | |||
| CIMP-low/0 | 488 (81%) | 238 (88%) | 250 (76%) | |
| CIMP-high | 114 (19%) | 34 (12%) | 80 (24%) | |
| BRAF mutation | 0.006 | |||
| (−) | 489 (84%) | 234 (88%) | 255 (80%) | |
| (+) | 95 (16%) | 31 (12%) | 64 (20%) | |
| KRAS mutation | 0.98 | |||
| (−) | 377 (63%) | 171 (63%) | 206 (63%) | |
| (+) | 221 (37%) | 100 (37%) | 121 (37%) | |
| p53 expression | 0.38 | |||
| (−) | 366 (61%) | 160 (59%) | 206 (63%) | |
| (+) | 232 (39%) | 110 (41%) | 122 (37%) | |
| p21 | <0.0001 | |||
| Expressed | 125 (21%) | 32 (12%) | 93 (29%) | |
| Lost | 460 (79%) | 229 (88%) | 231 (71%) | |
| p27 | 0.023 | |||
| Nuclear expression | 117 (20%) | 42 (16%) | 75 (24%) | |
| Cytoplasmic expression or loss of nuclear expression | 458 (80%) | 218 (84%) | 240 (76%) | |
| COX-2 | 0.80 | |||
| (−) | 100 (17%) | 44 (16%) | 56 (17%) | |
| (+) | 500 (83%) | 227 (84%) | 273 (83%) | |
| Fatty acid synthase (FASN) | 0.022 | |||
| (−) | 511 (86%) | 240 (90%) | 271 (83%) | |
| (+) | 81 (14%) | 27 (10%) | 54 (17%) | |
NOTE: % indicates the proportion of tumors with a specific clinical or molecular feature in cyclin D1− (or cyclin D1+) tumors. Abbreviations: AJCC, American Joint Commission on Cancer; LINE-1, long interspersed nucleotide element-1.
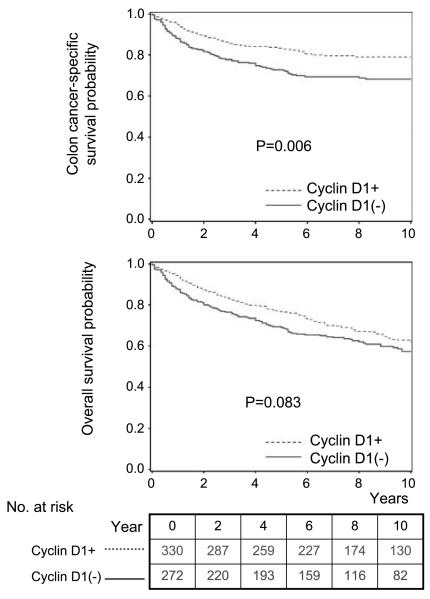
During follow-up, there were 259 deaths, including 153 colon cancer–specific deaths. We assessed the influence of cyclin D1 on patient survival. In Kaplan-Meier analysis, 5-year colon cancer–specific survival probability among patients with cyclin D1–positive tumors was higher than patients with cyclin D1–negative tumors (83% versus 73%, long rank P = 0.006; Fig. 1). In univariate Cox regression analysis, compared with patients with cyclin D1–negative tumors, those with cyclin D1–positive tumors experienced a significantly lower cancer-specific mortality [HR, 0.64; 95% confidence interval (CI), 0.47-0.88; P = 0.0063; Table 2]. In the multivariate Cox model adjusting for potential predictors of patient outcome, cyclin D1 was associated with a significantly lower colon cancer–specific mortality (adjusted HR, 0.57; 95% CI, 0.39-0.84; P = 0.0048) and overall mortality (adjusted HR, 0.74; 95% CI, 0.57-0.98; 0.036). No major confounder was present.
Fig. 1.
Kaplan-Meier curves for colon cancer – specific survival (top) and overall survival (bottom) according to cyclin D1 status in colon cancer.
Table 2.
Cyclin D1 expression in colon cancer and patient mortality
| Total n | Colon cancer–specific mortality |
Overall mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths/ person- years |
Univariate HR (95% CI) |
Stage- matched HR (95% CI) |
Multivariate HR (95% CI) |
Deaths/ person- years |
Univariate HR (95% CI) |
Stage- matched HR (95% CI) |
Multivariate HR (95% CI) |
||
| Cyclin D1 (−) | 272 (45%) |
83/2080 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 124/2080 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Cyclin D1 (+) | 330 (55%) |
70/2855 | 0.64 (0.47-0.88) |
0.66 (0.46-0.93) |
0.57 (0.39-0.84) |
135/2855 | 0.81 (0.63-1.03) |
0.80 (0.61-1.04) |
0.74 (0.57-0.98) |
| P | 0.0063 | 0.018 | 0.0048 | 0.084 | 0.090 | 0.036 | |||
NOTE: The multivariate, stage-matched (stratified) conditional Cox regression model included age, year of diagnosis, sex, family history of colorectal cancer, BMI, tumor location, grade, KRAS, BRAF, p53, p21, p27, COX-2, FASN, LINE-1 methylation, MSI, and CIMP.
To confirm the relation between cyclin D1 expression and survival, we also visually estimated the proportion of tumor cells positive for cyclin D1 (although not primarily used for categorization of cyclin D1 status). Then, we computed HR according to the proportion of positive cells as a continuous variable, nonparametrically with restricted cubic splines (Fig. 2; ref. 43). This method allowed us to assess the relationship between cyclin D1 expression and survival, besides any predetermined categorization of cyclin D1 status. It was evident that, as the proportion of positive cells increased, the HR for colon cancer-specific and overall mortality decreased.
Fig. 2.
Smoothing spline plot of unadjusted HRs for colon cancer – specific (left) and overall mortality (right) according to the proportion of tumor cells positive for cyclin D1 (with 0% as a reference). Hatched lines, 95% CI.
Effect of MSI on the relation between cyclin D1 and mortality
We found a modifying effect of MSI on the relation between cyclin D1 and patient mortality (Pinteraction = 0.061 for colon cancer-specific mortality and Pinteraction = 0.008 for overall mortality). This was not unexpected, considering a potential pathogenetic link between cyclin D1 expression and MSI in colon cancer (23). Thus, we stratified patients into four categories based on MSI and cyclin D1 status (Table 3). Compared with tumors that were both MSS/MSI-low and cyclin D1-negative, tumors that were either MSI-high or cyclin D1-positive seemed to be associated with lower cancer-specific mortalities (adjusted HR point estimates, 0.10-0.52) and overall moralities (adjusted HR point estimates, 0.29-0.65).
Table 3.
Combined MSI/cyclin D1 category and patient mortality in colon cancer
| Combined MSI/cyclin D1 category |
Total n | Colon cancer–specific mortality |
Overall mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deaths/ person- years |
Univariate HR (95% CI) |
Stage- matched HR (95% CI) |
Multivariate HR (95% CI) |
Deaths/ person- years |
Univariate HR (95% CI) |
Stage- matched HR (95% CI) |
Multivariate HR (95% CI) |
|||
| MSS/MSI-low | ||||||||||
|
|
Cyclin D1 (−) | 242 | 82/1769 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 118/1769 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Cyclin D1 (+) | 245 | 57/2199 | 0.61 (0.43-0.85) |
0.60 (0.42-0.87) |
0.52 (0.35-0.78) |
101/2199 | 0.71 (0.54-0.92) |
0.69 (0.52-0.91) |
0.65 (0.48-0.87) |
|
| MSI-high | ||||||||||
|
|
Cyclin D1 (−) | 29 | 1/303 | 0.080 (0.011-0.58) |
0.14 (0.02-1.04) |
0.10 (0.01-0.78) |
6/303 | 0.31 (0.13-0.70) |
0.42 (0.18-0.97) |
0.29 (0.12-0.70) |
| Cyclin D1 (+) | 80 | 13/591 | 0.45 (0.25-0.80) |
0.67 (0.36-1.25) |
0.40 (0.18-0.86) |
34/591 | 0.86 (0.58-1.26) |
1.09 (0.73-1.63) |
0.65 (0.39-1.10) |
|
NOTE: The multivariate, stage-matched (stratified) Cox model included the combined MSI/cyclin D1 category, age, year of diagnosis, sex, family history of colorectal cancer, BMI, tumor location, grade, KRAS, BRAF, p53, p21, p27, COX-2, FASN, LINE-1 methylation, and CIMP. Pinteraction (cyclin D1 and MSI) = 0.061 for colon–cancer specific mortality; Pinteraction (cyclin D1 and MSI) = 0.008 for overall mortality.
Stratified analysis of cyclin D1 and mortality
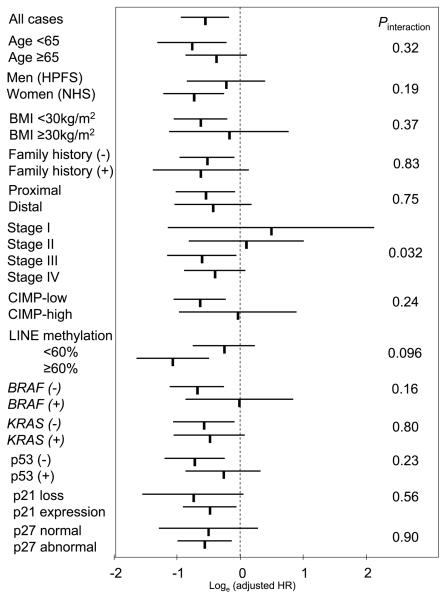
We further examined the influence of cyclin D1 overexpression on colon cancer–specific mortality across strata of other potential predictors of patient survival (Fig. 3). Considering multiple hypotheses testing, there was no evidence for significant effect modification by any of the variables (all Pinteraction ≥ 0.03). Notably, the effect of cyclin D1 did not significantly differ between the two independent cohort studies (Pinteraction = 0.19). The effect of cyclin D1 might differ according to stage (I-II versus III-IV;Pinteraction = 0.030; ordinal scale from I-IV;Pinteraction = 0.032); however, considering multiple hypothesis testing, this could be a chance event.
Fig. 3.
Stratified analysis of cyclin D1 status and colon cancer mortality. Loge(adjusted HRs) with 95% CI for colon cancer – specific mortality in cyclin D1+ tumors (versus cyclin D1–negative tumors) in various strata are shown. A Pinteraction value indicates statistical significance of an interaction between cyclin D1 and a given variable in a multivariate Cox model. *,“p27 abnormal” is defined as cytoplasmic localization or loss of nuclear expression. HPFS, Health Professionals Follow-up Study; NHS, Nurses' Health Study.
Discussion
We conducted this study to examine the relation between cyclin D1 expression and patient survival in stage I to IV colon cancer. Cyclin D1 (CCND1, the official gene symbol) activation has been implicated in colon carcinogenesis (1, 21, 22), among many other molecular changes. We found that cyclin D1 overexpression was associated with longer survival, independent of patient characteristics and other related molecular variables including p53, p21, p27, KRAS, BRAF, LINE-1 methylation, MSI, and the CIMP. All of these characteristics are potential confounders in analysis of tumoral cyclin D1 status and patient survival. Our results indicate that cyclin D1 expression in colon cancer is associated with superior prognosis.
It is very common to hypothesize that oncogene activation (or tumor suppressor inactivation) is associated with aggressive tumor behavior. However, this hypothesis does not always hold true. This is well exemplified by the association between MSI and good prognosis (24). MSI is known to cause inactivation of a number of tumor suppressors (including TGFBR2, BAX, and many others); yet MSI is associated with better patient outcome. This probably reflects a fundamental molecular difference between MSI-high tumors and non–MSI-high tumors. Our current findings also imply a molecular difference between cyclin D1–positive and cyclin D1–negative tumors. It is well known that colon cancers develop through accumulation of multiple genetic and epigenetic events. Some tumors activate cyclin D1, whereas others do not. In order to acquire malignant characteristics, cyclin D1–negative cancers might have bypassed the necessity of cyclin D1 activation, which might cause more aggressive behavior than cyclin D1–activated cancers. Thus, an aberration of a given oncoprotein such as cyclin D1 or tumor suppressor can be associated with indolent behavior.
Examining molecular changes and prognostic factors is important in colon cancer research (44-51). Previous studies have examined the relationship between tumoral cyclin D1 expression and clinical outcome in colon cancer (4-20). However, those studies have yielded inconsistent results. Although two studies (4, 5) have shown that cyclin D1 expression has been associated with poor prognosis, most studies have shown no independent prognostic value of cyclin D1 (7-20), and one study has shown good prognosis associated with cyclin D1 expression (6). A study has reported associations of cyclin D1 expression with MSI, CIMP, and BRAF mutation (23), and CIMP, MSI, and BRAF mutation in colon cancer have been related with clinical outcome (25, 26, 44). Thus, CIMP, MSI, and BRAF are potential confounders in analysis of cyclin D1 and clinical outcome. However, none of the previous studies (4-20) have examined confounding or modifying effect of these tumoral molecular events. Moreover, most previous studies were limited by small sample sizes (n < 170), and only 3 studies (11, 15, 16) examined >170 cases (up to n = 363; ref. 16). In the current study, we concurrently examined a number of molecular events, which have been related with both tumoral cyclin D1 expression and patient prognosis. In addition, our study had adequate statistical power with a large number (n = 602) of stage I to IV colon cancers, and our results have been consistent across the two independent cohort studies.
In addition, we found a potential modifying effect of MSI on the relation between cyclin D1 and clinical outcome in colon cancer. Specifically, compared with tumors that were both MSI-low/MSS (microsatellite stable) and cyclin D1–negative, tumors with either cyclin D1–positive or MSI-high seemed to be associated with lower mortalities. We have previously shown that cyclin D1 expression is associated with MSI, independent of CIMP status (23). Although the mechanistic link between MSI and cyclin D1 has not been elucidated, it is conceivable that cyclin D1 may exert different effects on tumor behavior according to tumoral MSI status.
There are advantages in using the database of the two independent prospective cohort studies, the Nurses' Health Study and Health Professionals Follow-up Study. Exposure and other clinical information were prospectively collected, and entered into the database blinded to patient and tumoral features, and outcome. Data were updated every 2 years. Cohort participants who developed colon cancer were treated at hospitals throughout the United States. Tumor specimen procurement rate has been ~60%, and there were no significant demographic difference between cases with tumor tissue analyzed and those without tumor tissue analyzed (28). However, a limitation of this study is that data on cancer treatment were limited. Nonetheless, it is unlikely that chemotherapy use differed according to tumoral cyclin D1 status because such data were not available to patients or treating physicians. In addition, beyond cause of mortality, data on cancer recurrences were not available in these cohorts. Nonetheless, given the median survival for metastatic colon cancer was ~10 to 12 months during much of the time period of this study (27), colon cancer–specific survival should be a reasonable surrogate for cancer-specific outcomes.
There are currently no standardized methods to assess cyclin D1 in colon cancer. Nonuniformity in methods to evaluate tumoral cyclin D1 expression may contribute to the inconsistent results in the previous studies. Nonetheless, our method yielded highly significant associations between cyclin D1 expression and other related molecular variables (including MSI, CIMP, BRAF, and p21; see Table 1). Moreover, any random misclassification of tumors in terms of cyclin D1 expression would drive our results on patient outcome toward the null hypothesis.
In summary, our large cohort study suggests that cyclin D1 expression is independently associated with good prognosis in colon cancer. Our findings may have considerable clinical implications. Future studies are needed to confirm this association as well as to elucidate exact mechanisms by which cyclin D1 affects tumor behavior.
Supplementary Material
Acknowledgments
We thank the Nurses' Health Study and Health Professionals Follow-up Study cohort participants who have generously agreed to provide us with biological specimens and information through responses to questionnaires; hospitals and pathology departments throughout the United States for providing us with tumor tissue materials; Frank Speizer, Walter Willett, Susan Hankinson, Graham Colditz, Meir Stampfer, and many other staff members who implemented and have maintained the cohort studies.
Grant support: U.S. NIH P01 CA87969 (S. Hankinson), P01 CA55075 (W. Willett), P50 CA127003 (C.S. Fuchs), K07 CA97992 (J.A. Meyerhardt), and K07 CA122826 (S. Ogino); the Bennett Family Fund; and the Entertainment Industry Foundation National Colorectal Cancer Research Alliance. K. Nosho was supported by a fellowship grant from the Japan Society for Promotion of Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Cancer Institute or NIH. Funding agencies did not have any role in the design of the study; the collection, analysis, or interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Alao J. The regulation of cyclin D1degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–74. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 3.Palmqvist R, Stenling R, Oberg A, Landberg G. Expression of cyclin D1 and retinoblastoma protein in colorectal cancer. Eur J Cancer. 1998;34:1575–81. doi: 10.1016/s0959-8049(98)00162-2. [DOI] [PubMed] [Google Scholar]
- 4.Maeda K, Chung YS, Kang SM, et al. Overexpression of cyclin D1 and p53 associated with disease recurrence in colorectal adenocarcinoma. Int J Cancer. 1997;74:310–5. doi: 10.1002/(sici)1097-0215(19970620)74:3<310::aid-ijc13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Bahnassy AA, Zekri AR, El-Houssini S, et al. Cyclin A and cyclin D1 as significant prognostic markers in colorectal cancer patients. BMC Gastroenterol. 2004;4:22. doi: 10.1186/1471-230X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland TA, Elder J, McCloud JM, et al. Subcellular localisation of cyclin D1 protein in colorectal tumours is associated with p21(WAF1/CIP1) expression and correlates with patient survival. Int J Cancer. 2001;95:302–6. doi: 10.1002/1097-0215(20010920)95:5<302::aid-ijc1052>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Handa K, Yamakawa M, Takeda H, Kimura S, Takahashi T. Expression of cell cycle markers in colorectal carcinoma: superiority of cyclin A as an indicator of poor prognosis. Int J Cancer. 1999;84:225–33. doi: 10.1002/(sici)1097-0215(19990621)84:3<225::aid-ijc5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Bukholm IK, Nesland JM. Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch. 2000;436:224–8. doi: 10.1007/s004280050034. [DOI] [PubMed] [Google Scholar]
- 9.Bhatavdekar JM, Patel DD, Chikhlikar PR, et al. Molecular markers are predictors of recurrence and survival in patients with Dukes B, Dukes C. colorectal adenocarcinoma. Dis Colon Rectum. 2001;44:523–33. doi: 10.1007/BF02234324. [DOI] [PubMed] [Google Scholar]
- 10.Pasz-Walczak G, Kordek R, Faflik M. P21 (WAF1) expression in colorectal cancer: correlation with P53 and cyclin D1 expression, clinicopathological parameters and prognosis. Pathol Res Pract. 2001;197:683–9. doi: 10.1078/0344-0338-00146. [DOI] [PubMed] [Google Scholar]
- 11.McKay JA, Douglas JJ, Ross VG, et al. Analysis of key cell-cycle checkpoint proteins in colorectal tumours. J Pathol. 2002;196:386–93. doi: 10.1002/path.1053. [DOI] [PubMed] [Google Scholar]
- 12.Cheah PY, Choo PH, Yao J, Eu KW, Seow-Choen F. A survival-stratification model of human colorectal carcinomas with β-catenin and p27kip1. Cancer. 2002;95:2479–86. doi: 10.1002/cncr.10986. [DOI] [PubMed] [Google Scholar]
- 13.Bondi J, Bukholm G, Nesland JM, Bukholm IR. Expression of non-membranous β-catenin and γ-catenin, c-Myc and cyclin D1 in relation to patient outcome in human colon adenocarcinomas. APMIS. 2004;112:49–56. doi: 10.1111/j.1600-0463.2004.apm1120109.x. [DOI] [PubMed] [Google Scholar]
- 14.Bondi J, Husdal A, Bukholm G, Nesland JM, Bakka A, Bukholm IR. Expression and gene amplification of primary (A, B1, D1, D3, and E) and secondary (Cand H) cyclins in colon adenocarcinomas and correlation with patient outcome. J Clin Pathol. 2005;58:509–14. doi: 10.1136/jcp.2004.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knosel T, Emde A, Schluns K, et al. Immunoprofiles of 11 biomarkers using tissue microarrays identify prognostic subgroups in colorectal cancer. Neoplasia. 2005;7:741–7. doi: 10.1593/neo.05178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilska M, Collan YU, VJ OL, et al. The significance of tumor markers for proliferation and apoptosis in predicting survival in colorectal cancer. Dis Colon Rectum. 2005;48:2197–208. doi: 10.1007/s10350-005-0202-x. [DOI] [PubMed] [Google Scholar]
- 17.Kouraklis G, Theocharis S, Vamvakas P, et al. Cyclin D1 and Rb protein expression and their correlation with prognosis in patients with colon cancer. World J Surg Oncol. 2006;4:5. doi: 10.1186/1477-7819-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyall MS, Dundas SR, Curran S, Murray GI. Profiling markers of prognosis in colorectal cancer. Clin Cancer Res. 2006;12:1184–91. doi: 10.1158/1078-0432.CCR-05-1864. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz KJ, Wohlschlaeger J, Alakus H, et al. Activation of extracellular regulated kinases (ERK1/2) but not AKT predicts poor prognosis in colorectal carcinoma and is associated with k-ras mutations. Virchows Arch. 2007;450:151–9. doi: 10.1007/s00428-006-0342-y. [DOI] [PubMed] [Google Scholar]
- 20.Theocharis S, Giaginis C, Parasi A, et al. Expression of peroxisome proliferator-activated receptor-γ in colon cancer: correlation with histopathological parameters, cell cycle-related molecules, and patients' survival. Dig Dis Sci. 2007;52:2305–11. doi: 10.1007/s10620-007-9794-4. [DOI] [PubMed] [Google Scholar]
- 21.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 23.Nosho K, Kawasaki T, Chan AT, et al. Cyclin D1 is frequently overexpressed in microsatellite unstable colorectal cancer, independent of CpG island methylator phenotype. Histopathology. 2008;53:588–98. doi: 10.1111/j.1365-2559.2008.03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popat S, Hubner R, Houlston RS. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J Clin Oncol. 2005;23:609–18. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 25.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–9. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 26.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–6. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogino S, Nosho K, Kirkner GJ, et al. A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer. J Natl Cancer Inst. 2008;100:1734–8. doi: 10.1093/jnci/djn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 29.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–21. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–8. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 32.Ogino S, Kawasaki T, Brahmandam M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–17. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogino S, Cantor M, Kawasaki T, et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55:1000–6. doi: 10.1136/gut.2005.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–14. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 36.Ogino S, Kawasaki T, Nosho K, et al. LINE-I hypomethylation is inversely associated with microsatellite instability and CPG island methylator phenotype in colorectal cancer. Int J Cancer. 2008;122:2767–73. doi: 10.1002/ijc.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogino S, Brahmandam M, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia. 2006;8:458–64. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogino S, Meyerhardt JA, Cantor M, et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin Cancer Res. 2005;11:6650–6. doi: 10.1158/1078-0432.CCR-05-0738. [DOI] [PubMed] [Google Scholar]
- 39.Ogino S, Kawasaki T, Kirkner GJ, Yamaji T, Loda M, Fuchs CS. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Mod Pathol. 2007;20:15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 40.Cheuk W, Wong KO, Wong CS, Chan JK. Consistent immunostaining for cyclin D1 can be achieved on a routine basis using a newly available rabbit monoclonal antibody. Am J Surg Pathol. 2004;28:801–7. doi: 10.1097/01.pas.0000126054.95798.94. [DOI] [PubMed] [Google Scholar]
- 41.Fleming IN, Hogben M, Frame S, McClue SJ, Green SR. Synergistic inhibition of ErbB signaling by combined treatment with seliciclib and ErbB-targeting agents. Clin Cancer Res. 2008;14:4326–35. doi: 10.1158/1078-0432.CCR-07-4633. [DOI] [PubMed] [Google Scholar]
- 42.Gibcus JH, Mastik MF, Menkema L, et al. Cortactin expression predicts poor survival in laryngeal carcinoma. Br J Cancer. 2008;98:950–5. doi: 10.1038/sj.bjc.6604246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 44.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zlobec I, Terracciano LM, Lugli A. Local recurrence in mismatch repair-proficient colon cancer predicted by an infiltrative tumor border and lack of CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res. 2008;14:3792–7. doi: 10.1158/1078-0432.CCR-08-0048. [DOI] [PubMed] [Google Scholar]
- 46.Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:1413–7. doi: 10.1158/1078-0432.CCR-07-1994. [DOI] [PubMed] [Google Scholar]
- 47.Zlobec I, Baker K, Terracciano LM, Lugli A. RHAMM, p21 combined phenotype identifies microsatellite instability-high colorectal cancers with a highly adverse prognosis. Clin Cancer Res. 2008;14:3798–806. doi: 10.1158/1078-0432.CCR-07-5103. [DOI] [PubMed] [Google Scholar]
- 48.Suehiro Y, Wong CW, Chirieac LR, et al. Epigenetic-genetic interactions in the APC/WNT, RAS/RAF, P53 pathways in colorectal carcinoma. Clin Cancer Res. 2008;14:2560–9. doi: 10.1158/1078-0432.CCR-07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.French AJ, Sargent DJ, Burgart LJ, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res. 2008;14:3408–15. doi: 10.1158/1078-0432.CCR-07-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng YW, Pincas H, Bacolod MD, et al. CpG island methylator phenotype associates with low-degree chromosomal abnormalities in colorectal cancer. Clin Cancer Res. 2008;14:6005–13. doi: 10.1158/1078-0432.CCR-08-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weichert W, Roske A, Niesporek S, et al. Class I his-tone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14:1669–77. doi: 10.1158/1078-0432.CCR-07-0990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.