Abstract
While there is a correlation between hypertension and levels of IL-6, the exact role of this cytokine in myocardial remodeling is unknown. This is complicated by the variable tissue and circulating levels of IL-6 reported in numerous experimental models of hypertension. Accordingly, we explored the hypothesis that elevated levels of IL-6 mediate adverse myocardial remodeling. To this end, adult male Sprague Dawley rats were infused with IL-6 (2.5 μg·kg-1·hr-1, IP) for 7 days via osmotic minipump and compared to vehicle infused aged-matched controls. Left ventricular function was evaluated using a blood-perfused isolated heart preparation. In addition, myocardial interstitial collagen volume fraction and isolated cardiomyocyte size were also assessed. Isolated adult cardiac fibroblast experiments were performed to determine the importance of the soluble IL-6 receptor in mediating cardiac fibrosis. IL-6 infusions in vivo resulted in concentric left ventricular hypertrophy, increased ventricular stiffness, a marked increase in collagen volume fraction (6.2 vs. 1.7%; p < 0.001), and proportional increases in cardiomyocyte width and length; all independent of blood pressure. The soluble IL-6 receptor in combination with IL-6 was found to be essential in increasing collagen content regulated by isolated cardiac fibroblasts, and also played a role in mediating a phenotypic conversion to myofibroblasts. These novel observations demonstrate that IL-6 induces a myocardial phenotype almost identical to that of the hypertensive heart, identifying IL-6 as potentially important in this remodeling process.
Keywords: myocardial fibrosis, cardiomyocyte dimensions, ventricular compliance, ventricular function, cytokine
Introduction
Inflammation is a key component in the myocardial remodeling process that takes place in response to hypertension.1-5 However, the respective roles of specific cytokines in this process are not well defined. IL-6 has attracted attention in regard to myocardial dysfunction because increased levels correlate with the severity of heart failure and are strongly prognostic of one-year mortality.6, 7 There is a growing body of evidence that a similar association exists in hypertensive patients.8-10 Lee et al. 11 found that induction of IL-6 by angiotensin II contributes to elevations in blood pressure, however, the contribution of IL-6 to myocardial remodeling has not been firmly established. Hirota et al. demonstrated that concomitant overexpression of both IL-6 and the IL-6 receptor in mice induced concentric hypertrophy typical of that occurring in a hypertensive heart.12 While these observations suggest that IL-6 may directly mediate hypertrophic remodeling associated with hypertension, no studies have directly investigated the role of IL-6 in mediating cardiac fibrosis or diastolic dysfunction that are also characteristic features of the hypertensive heart. The effects of IL-6 on collagen synthesis by isolated cardiac fibroblasts have been inconsistent.13, 14 Siwik et al. found that IL-6 produced a modest decrease in collagen synthesis, together with increased matrix metalloproteinase activity in neonatal cardiac fibroblasts.14 However, these studies did not investigate the role of the soluble IL-6 receptor (sIL-6R). We hypothesized that elevations of IL-6 in vivo would produce cardiac fibrosis, in addition to inducing cardiac hypertrophy, and furthermore that the sIL-6R would be important in the regulation of collagen by adult cardiac fibroblasts.
Materials and Methods
Adult male Sprague-Dawley rats weighing between 250 and 300 g were housed under standard environmental condition and maintained on commercial rat chow and tap water ad libitum. This investigation conformed with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and the experimental protocol had Institutional Animal Care and Use Committee approval. Anesthesia for the non-terminal surgical procedure was achieved by inhalation of isoflurane (2%). Analgesia was achieved by administration of buprenorphine HCL (0.025 mg·kg-1, SQ) to the rats at the time of surgery. At the experimental endpoint the rats were anesthetized with pentobarbital (70 mg·kg-1, IP) and euthanized by removal of the heart.
Implantation of Osmotic Pumps and Experimental Protocol
IL-6 (Invitrogen, USA) was administered at a rate of 2.5 μg·kg-1·hr-1 for 7 days using osmotic minipumps (Alzet model 2001, Durect Corporation, Cupertino CA) implanted in the peritoneal cavity. The lyophilized IL-6 was dissolved in a vehicle solution containing 10% rat albumin and. At the experimental endpoint, blood pressure and body weights were measured and left ventricular (LV) function assessed.
Assessment of Ventricular Size and Function
LV volume and function were evaluated ex vivo using a blood-perfused isolated heart preparation as previously described.15, 16 Briefly, the descending thoracic aorta was cannulated for continuous retrograde perfusion of the heart. The heart was then extirpated and attached to a pressurized reservoir (95-105 mm Hg) containing arterial blood from a support rat. The pulmonary artery was then transected to allow unrestricted drainage of coronary venous flow, which was collected in a reservoir and returned to the support rat, where it was re-oxygenated. The left atrium was removed and a compliant latex balloon was inserted through the mitral valve orifice into the LV in order to obtain ventricular pressure-volume relationships. Once the heart developed stable isovolumetric contractions, the balloon volume which produced an LV end diastolic pressure (EDP) of 0 mm Hg (V0) was determined. The balloon volume was then increased in 10 μl increments until an LV EDP of 25 mm Hg was attained. Following each increase in balloon volume, the end diastolic and peak isovolumetric pressures were recorded. After completion of these functional studies, the atria and great vessels were removed and the right ventricle (RV) and LV plus septum were separated and weighed. A mid-ventricular, transmural LV section was placed in 4% paraformaldehyde and the remaining tissue snap-frozen in liquid nitrogen and stored at -80°C for further analysis.
Morphologic and Histological Evaluation
LV tissue from vehicle (n=6) and IL-6 infused (n=8) rats was processed for routine histopathology and 5 μm-thick paraffin-embedded sections were stained with hematoxylin and eosin for the evaluation of myocardial morphology. A serial section was stained with picrosirius red and 20 randomly chosen fields per section were analyzed by light microscopy (400×) to determine the average collagen volume fraction (CVF) as previously described.1, 17 Perivascular areas were excluded from the CVF analysis.
Determination of Cardiomyocyte Size
Cardiomyocytes from the LV of vehicle (n=6) and IL-6 infused (n=8) rats were isolated using a modified KOH procedure initially described by Gerdes et al.18 to assess the extent of cellular remodeling. Briefly, formalin fixed tissue from a mid-level transmural section of the LV trimmed into 1 mm3 pieces was placed in a 0.1M KOH solution at room temperature for 24 hours. The tissue was then transferred to a 0.1 M PBS solution and continuously agitated for 10 minutes. Cardiac myocytes thus obtained were purified using a 10% Ficoll density gradient. Finally, the cells were resuspended in 10% buffered formalin and transferred to a glass microscope slide, using a cytospin apparatus, and stained with 1.5% gallocyanin and 5% chromium potassium sulfate dodecahydrate. The length and width of 50 rod-shaped cardiomyocytes were measured for each individual heart using ImagePro® Plus software (Media Cybernetics, LP, Silver Springs, MD).
Measurement of IL-6 and TNF-α
Myocardial levels of IL-6 and TNF-α were determined using commercially available kits (Quantikine by R&D Systems, Minneapolis, MN) in vehicle (n=6) and IL-6 infused (n=8) rats. Approximately 100 mg of LV tissue was homogenized for the assays. All samples were run in duplicate with the average of the two replicates reported.
Isolated Cardiac Fibroblast Studies
Primary cultures of adult cardiac fibroblasts were obtained from male Sprague Dawley rats (n=4). Briefly, hearts were homogenized and digested with Liberase 3 (Roche) with fibroblasts purified by selective attachment to the plastic culture ware. These cells were maintained in Dulbecco's Modified Eagle's Media (DMEM) containing 10% neonatal bovine serum and 5% fetal calf serum, with media replacement every other day and used prior to passage number 3. 19 One million fibroblasts were allowed to adhere in DMEM with 10% neonatal bovine serum and 5% fetal calf serum for 24 hours, before rinsing with Mosconas Salt Solution, and serum starvation for 24 hrs in DMEM-F12. Media was then replaced with DMEM (1.5 % fetal bovine serum) containing 10 or 50 ng/mL of IL-6 for 24 hours. Additional experiments were performed where IL-6 incubation occurred in the presence of varying concentrations of the sIL-6R (i.e., 0.5, 5 and 50 ng/mL). Cardiac fibroblasts were incubated in IL-6 (50 ng/mL 1.5% FBS DMEM) one hour before the sIL-6R treatment. The sIL-6R (Peprotech) was diluted in DMEM and added to the pretreated media to achieve final concentrations of 0.5, 5, and 50 ng/mL for 24 hours. The concentrations of IL-6 and sIL-6R were previously published by Yamaguchi et al.20 They reported that IL-6 signaling in the presence of the soluble form of IL-6 receptor leads to the phosphorylation of IL-6 signal transducer gp130 in human gingival fibroblasts. Though we used adult rat cardiac fibroblasts, we postulated that the same concentration of IL6 and IL-6 soluble receptor would produce the same signaling events. The concentrations of IL-6 used were previously shown to cause a response in skin and lung fibroblasts.21, 22 Collagen synthesis was determined by hydroxyproline analysis of collected media as described by Edwards et al.23
Western Blot Analysis
Western Blot Analysis was used to determine the relative amounts of α smooth muscle actin (SMA-α). Male adult cardiac fibroblasts were treated as previously stated. Following the 24 hour treatment, fibroblasts were rinsed with phosphate-buffer saline (PBS) and extracted in PBS containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The protein concentrations of the fibroblast were determined with a standardized colorimetric assay (Bio-Rad Protein Assay). Thirty micrograms of extracted protein was loaded onto a 10% SDS-polyacrylamide gels and separated at 150 Volts for an hour. The separated proteins were then transferred to 0.45 μm nitrocellulose membranes (ThermoScientific, Waltham, MA) at 30 V overnight. Following transfer, nitrocellulose membranes were stained with Ponceau solution (Sigma-Aldrich), to confirm the transference of proteins to the membrane. The membranes were then rinsed with Tris-Buffer Saline containing 0.15% Tween 20 (TBS-T). Membranes were blocked with TBS-T containing 5% powdered milk for 2 hours. Membranes were incubated with mouse monoclonal antibody against α-SMA (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:500 in 5% milk/TBS-T for 2 hours. Membranes were then rinsed several times with TBS-T and incubated with horseradish peroxidase conjugated goat anti-mouse antibody (1:2000 dilution, Santa Cruz) for 2 hours. Membranes were rinsed several times in TBS-T and developed using the ECL detection Kit (ThermoScientific).The luminescent signal was detected by exposure to x-ray film (Phenix Research Products, Chandler, NC) for 5 minutes. Band density was detected by using GS 800 Calibrated Densitometer and Quality One Software by Bio-Rad. The membrane was later reprobed for glyceraldehyde 3-phospate dehydrogenase (GAPDH) as an internal control. Results are presented as the ratio of α-SMA to GAPDH.
Statistical Analysis
Statistical analysis was performed using SPSS 11.5 software (SPSS; Chicago, IL). Results are presented as mean ± standard deviation (SD) or standard error (SEM) as appropriate. Unpaired t-test was used to make group comparisons, except for the sIL-6R comparisons in Figure 4 where a one-way ANOVA with Bonferroni post-testwas applied. The end diastolic pressure-volume curves for each heart were fit to a third-order nonlinear regression (Graph Pad, La Jolla, CA) and the volumes corresponding to 2.5 mm Hg pressure increments were determined. The volumes for each pressure increment were then averaged to obtain the pressure-volume relationship for each group. Statistical significance was taken to be p < 0.05.
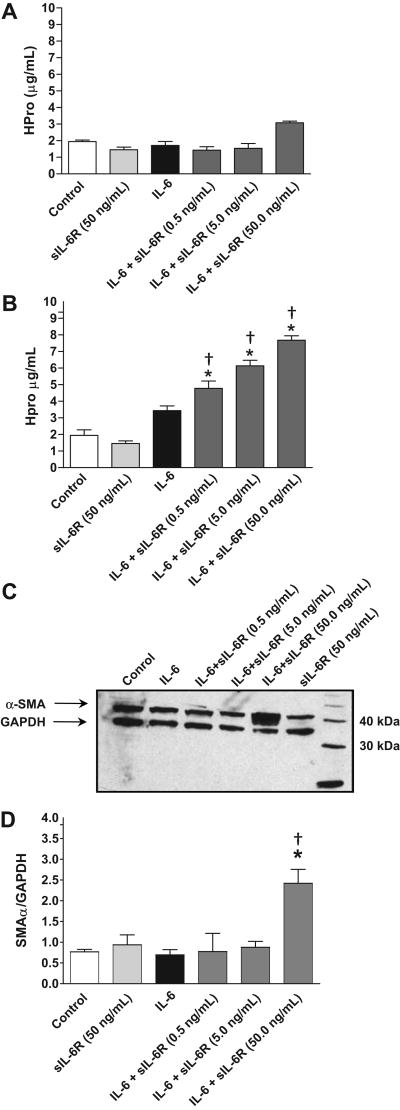
Figure 4.
Hydroxyproline synthesis by isolated adult cardiac fibroblasts following treatment with IL-6 (10 ng/mL, A; 50 ng/mL, B) in the presence of increasing concentrations of the sIL-6R. (C) Representative Western blot analysis and quantification (D) of α-SMA levels in isolated adult cardiac fibroblasts following treatment with IL-6 and sIL-6R. All values are mean ± SEM. *= p < 0.05 vs control; †=p < 0.05 vs IL-6.
Results
Morphometric Parameters
Average body, LV, RV and lung weights, as well as systolic (SBP) and diastolic blood pressures (DBP) for vehicle and IL-6 infused groups are presented in Table 1. LV weights of IL-6 infused hearts were significantly increased relative to vehicle infused hearts. No significant differences in RV and lung weights were observed between the groups, indicating that the animals were still in compensated hypertrophy. IL-6 infusion did not significantly alter systolic or diastolic blood pressures.
Table 1.
Morphometric Parameters.
| Group | n | LV Weight (mg) |
RV Weight (mg) |
Lung Weight (mg) |
SBP (mmHg) |
DBP (mmHg) |
|---|---|---|---|---|---|---|
| Vehicle Infused | 12 | 660 ± 19 | 186 ± 19 | 1273 ± 117 | 114 ± 11 | 83 ± 3 |
| IL-6 Infused | 8 | 775 ± 92* | 198 ± 31 | 1275 ± 84 | 125 ± 18 | 95 ± 13 |
Values are means ± SD; LV= Left ventricle; RV= right ventricle; SBP= systolic blood pressure; DBP= diastolic blood pressure.
p < 0.01 vs. vehicle infused
Histological Analysis
The effect of IL-6 infusion on LV collagen volume fraction is presented in Figure 1. A greater than 3-fold increase in interstitial myocardial collagen was observed in the IL-6 infused group compared to control (6.2 ± 1.4% vs. 1.7 ± 0.5 %, respectively; p<0.001). No evidence of inflammatory cell infiltration or necrosis was observed.
Figure 1.
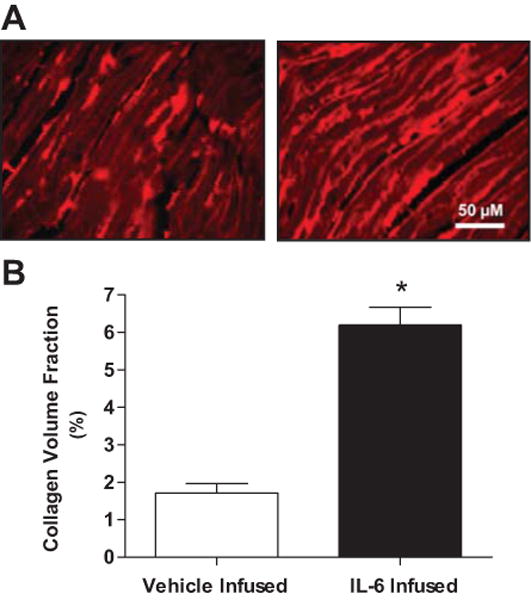
(A) Representative photomicrographs of picrosirus red stained myocardium depicting the marked induction of interstitial collagen in hearts infused with IL-6 (right) relative to that in hearts infused with vehicle (left). (B) Graphic representation of left ventricular collagen volume fraction from vehicle and IL-6 infused groups. All values are mean ± SEM. * p < 0.001 vs. vehicle infused group.
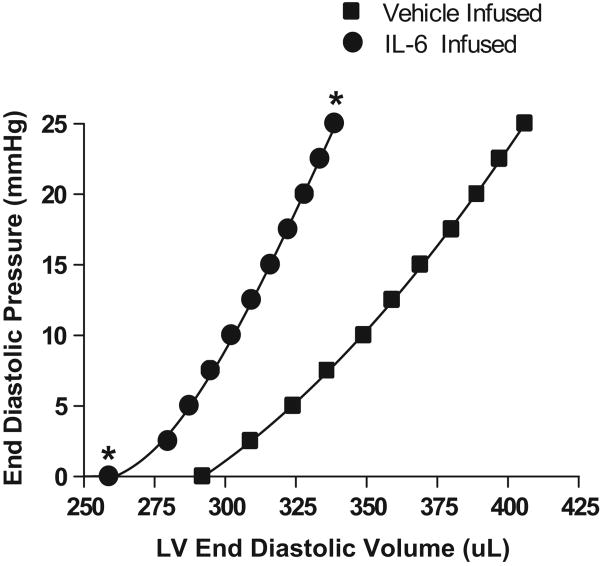
LV Diastolic Function
As can be seen in Figure 2, IL-6 infusion produced a leftward shift of the EDP-EDV relationship together with an increased slope, indicative of a smaller chamber and stiffer ventricle. These changes were quantified as the difference in unstressed LV volume (V0) and the volume required to increase EDP from 0 to 25 mmHg (ΔV0-25). As detailed in Table 2, V0 and ΔV0-25 were significantly decreased in the IL-6 infused group when compared to control.
Figure 2.
Left ventricular end-diastolic pressure-volume (P-V) relationships for IL-6 and vehicle infused groups. *p<0.001 vs vehicle infused group at the corresponding pressure.
Table 2.
Isolated Heart: Left Ventricular Volume and Chamber Stiffness.
Values are means ± SEM; V0 = left ventricular volume (LVEDV) at a LV end diastolic pressure (EDP) of 0 mm Hg; ΔV 0-25 = LVEDV at LVEDP of 25 mmHg - V0.
p < 0.001 vs. vehicle infused.
LV Cardiomyocyte Size
Average LV cardiomyocyte length and width for IL-6 and vehicle infused groups are presented in Table 3. Both length and width were significantly increased after 7 days of IL-6 infusion compared to cardiomyocytes from control hearts. However, the average cardiomyocyte length/width ratio remained similar for both groups.
Table 3.
Cardiomyocyte Size.
| Group | n | Cell Width (μM) |
Cell Length (μM) |
CL/CW ratio |
|---|---|---|---|---|
| Vehicle Infused | 6 | 46.5 ± 2.4 | 108.9 ± 7.8 | 2.3 ± 0.2 |
| IL-6 Infused | 8 | 61.9 ± 2.5* | 138.2 ± 15* | 2.2 ± 0.2 |
Values are means ± SEM. CL = Cell Length; CW = Cell Width;
p < 0.001 vs. vehicle infused.
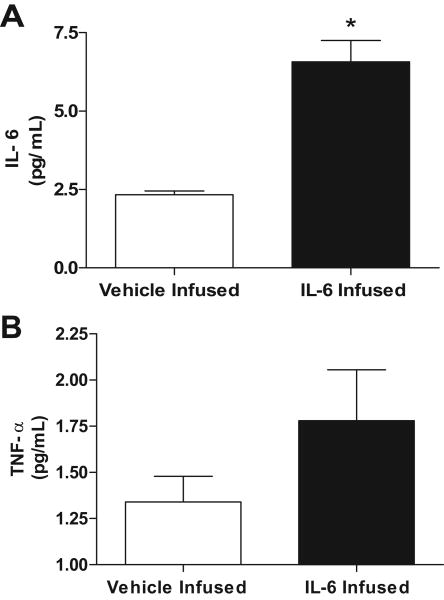
LV levels of IL-6 and TNF-α
Levels of myocardial IL-6 and TNF-α from IL-6 and vehicle infused rats are shown in figures 3A and B respectively. IL-6 levels were significantly increased in the IL-6 infused group compared to controls (6.5 ± 1.6 vs. 2.3 ± 0.3 pg/mL, respectively; p < 0.001). TNF-α was not significantly increased in the IL-6 infused group compared to controls (1.7 ± 0.7 vs. 1.3 ± 0.3 pg/mL).
Figure 3.
Myocardial levels of IL-6 (A) and TNF-α (B) following vehicle and IL-6 infusions. All values are mean ± SEM. * p < 0.001 vs. vehicle infused group.
Cardiac Fibroblast Collagen Synthesis and Myofibroblast Formation
As can be seen in Figure 4A, incubation of isolated adult cardiac fibroblasts with IL-6 at a concentration of 10 ng/mL had no effect on collagen content either alone or in the presence of varying concentrations of the sIL-6 receptor. However, IL-6 at a concentration of 50 ng/mL produced a significant concentration dependent increase in collagen content for all three of the concentrations of the sIL-6R tested (Figure 4B). IL-6 alone had no effect on fibroblast phenotype as assessed by α-smooth muscle actin (SMA) (Figure 4C). However, IL-6 administered in conjunction with sIL-6R at 50 ng/mL resulted in a dramatic increase in α-SMA in fibroblasts indicative of a conversion of phenotype to that of myofibroblasts.
Discussion
Both experimental studies and epidemiology point to an emerging recognition of inflammatory cytokines as biomarkers of cardiovascular disease.24 However, the relative importance of specific cytokines in the regulation of arterial pressure and in the pathogenesis of hypertension has yet to be fully elucidated. Increased levels of circulating IL-6 in patients correlate with the severity of heart failure and are predictive of mortality. 6, 7 A similar relationship has also been identified between IL-6 and hypertension,8, 9 with increases in mean arterial pressure in normal healthy men being significantly associated with elevated levels of circulating IL-6.10 However, the exact contribution of IL-6 to the pathogenesis of hypertension is unclear due to the variable levels of IL-6 reported in experimental models of hypertension.1, 25-27 Nevertheless, the data presented herein clearly demonstrate the ability of IL-6 to induce a pattern of myocardial remodeling consistent with that occurring in the hypertensive heart, including concentric hypertrophy, fibrosis and diastolic dysfunction. Further, we have identified a critical role for the sIL-6R in the collagen synthesis induced by IL-6.
Our findings are the first to establish that pathologic elevations in circulating IL-6 result in extensive cardiac fibrosis. The IL-6 family of cytokines has the capacity to regulate cell function through a cell surface receptor composed of two trans-membrane proteins,28 a ligand-binding subunit designated as the IL-6 receptor and a signal transducing glycoprotein (gp130). 29, 30 The presence of the IL-6 receptor has previously been demonstrated in adult cardiac fibroblasts, where it was reported to be essential for fibroblast growth.31. Furthermore, Siwik et al.14 found that incubation of adult rat cardiac fibroblasts with IL-6 reduced collagen content in the media by 11%. In addition, they also reported that matrix metalloproteinase activity was significantly increased after incubation of neonatal cardiac fibroblasts with IL-6. Somewhat consistent with their observation, we found no effect of IL-6 alone on collagen content in the media of isolated adult cardiac fibroblasts. However, addition of the sIL-6R, which was not evaluated in the studies by Siwik et al., elicited concentration dependent increases in collagen content. Incubation of fibroblasts with IL-6 in the presence of the sIL-6R produced a 4-fold increase in collagen content at the highest concentration of the sIL-6R. Moreover, this combination also induced a conversion in fibroblast phenotype to that of a myofibroblast, indicating the ability of the sIL-6R to regulate fibroblast function by several mechanisms. The sIL-6R is naturally occurring in the body and is the result of proteolysis of the membrane receptor or alternative mRNA splicing. 32, 33 While most soluble receptors act as antagonists in the sense that they compete with the corresponding membrane-bound receptor for the specific ligand, this is not the case with the sIL-6R, which instead acts as an agonist to activate signal transduction on cells that are not stimulated by IL-6 alone. 32 The IL-6/sIL-6 R does this by interacting with membrane-bound gp130, 33 which in turn leads to phosphorylation of downstream second messengers such as JAK and STAT and stimulation of various cellular events.
In addition to cardiac fibrosis, we also found that IL-6 induced significant concentric LV hypertrophy. This finding is consistent with the previous reports by Hirota et al.12 who described a pattern of concentric hypertrophy in the hearts of mice over-expressing both IL-6 and the IL-6 receptor. However, our study extends these findings to demonstrate that individual cardiomyocytes underwent both elongation and thickening in response to IL-6 infusion. The fact that the ratio of cell length to width was similar to normal cardiomyocytes indicates this hypertrophy consisted of proportional growth. This is in agreement with the previous report by Korecky and Rakusan,34 who determined that cell length and width increase proportionally in concentric cardiac hypertrophy. Several studies have implicated cardiotrophin-1/gp130 induced phosphorylation of STAT3 as mediating myocardial hypertrophy, 35-37 and Wollert et al.,38 reported that cardiotrophin-1 stimulation in isolated neonatal cardiomyocytes induces hypertrophy consisting of in-series addition of sarcomeric units. While this may reflect intrinsic differences between adult and neonatal cardiomyocytes, or a differential response of cells to cardiotrophin-1 and IL-6, it seems likely that differential regulation of hypertrophy is more complex, involving induction of other in vivo pathways like the renin-angiotensin system. This is reflected in the findings of López et al.,37 who recently reported that in-series sarcomeric addition was induced by cardiotrophin-1 in adult cardiomyocytes isolated from normotensive Wistar rats, while a concentric hypertrophic response was observed in cardiomyocytes obtained from hypertensive SHR. Interestingly, cardiac fibroblasts stimulated with angiotensin II have been shown to secrete members of the IL-6 family, including IL-6 itself, which induced cardiomyocyte hypertrophy via activation of the gp130 receptor.39 In view of the possibility that increased LV stiffness could lead to higher left atrial and pulmonary pressures, it is interesting to note that RV hypertrophy was absent following IL-6 infusion. However, although LV stiffness was increased in the IL-6 infused animals, we do not know the actual LV pressures. Given that this was a one week infusion of IL-6 it may be that the pressures were not elevated long enough to result in RV hypertrophy in that time period. Furthermore, there is evidence from exercise studies in heart failure patients that pulmonary wedge pressure (a marker of LV pressure) is not coupled to right atrial pressure until a point of pericardial constraint is reached. 40 Thus, in the IL-6 infused animals, a large enough degree of LV hypertrophy and/or ventricular stiffness may not have been attained to influence the RV workload.
The IL-6 induced changes in myocardial structure were manifested functionally as a stiffer (decreased ΔV0-25), smaller ventricle (decreased V0). The increased stiffness was most likely the result of the marked myocardial fibrosis, since it has been shown that myocyte hypertrophy does not intrinsically alter LV stiffness.41 Together these events replicate the concentric cardiac remodeling typical of pressure overload, where there is an increase in LV mass concomitant with decreased LV chamber size and significant fibrosis. Consequently, these findings provide additional evidence suggesting IL-6 may contribute to the development of diastolic dysfunction in hypertensive patients, resulting in the eventual transition to heart failure.
The increased levels of myocardial IL-6 confirm the effectiveness of the IL-6 infusion. However, IL-6 did not induce an increase in blood pressure, nor was there an inflammatory cell response in the myocardium, indicating that: 1) IL-6 release likely occurs downstream of inflammatory cell infiltration; and 2) fibrosis and hypertrophy can be independent of blood pressure. While increases in TNF-α are known to induce subsequent increases in IL-6,42 the sustained increase in IL-6 did not induce a corresponding induction of myocardial TNF-α levels.
Perspectives
This is the first report demonstrating the ability of IL-6 to induce a pattern of myocardial remodeling that includes cardiac fibrosis and concentric hypertrophy. Overall, the remodeling induced by infusion of IL-6 was remarkably similar to that seen in hypertension and suggests that IL-6 may be responsible for inducing adverse remodeling in the hypertensive heart, thereby contributing to LV diastolic dysfunction. Furthermore, this study establishes the necessity of the sIL-6R for IL-6 to increase collagen content by cardiac fibroblasts. Accordingly, future investigation is warranted to evaluate IL-6 as a potential therapeutic target.
Acknowledgments
Sources of Funding: This work was supported in part by American Heart Association Mid-Atlantic Affiliate Postdoctoral Fellowship (grant number 0825510E to SPL) and National Institutes of Health (grant numbers HL62228, HL073990 to JSJ).
Footnotes
Conflict of Interest: None.
Reference List
- 1.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 2.Hinglais N, Huedes D, Nicoletti A, Manset C, Laurent M, Bariety J, Michel JB. Colocalization of myocardial fibrosis and inflammatory cells in rats. Lab Invest. 1994;70:286–294. [PubMed] [Google Scholar]
- 3.Tomita H, Egashira K, Kubo-Inoue M, Usui M, Koyanagi M, Shimokawa H, Takeya M, Yoshimura T, Takeshita A. Inhibition of NO synthesis induces inflammatory changes and monocyte chemoattractant protein-1 expression in rat hearts and vessels. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1456–1464. doi: 10.1161/01.atv.18.9.1456. [DOI] [PubMed] [Google Scholar]
- 4.Koyanagi M, Egashira K, Kitamoto S, Ni W, Shimokawa H, Takeya M, Yoshimura T, Takeshita A. Role of monocyte chemoattractant protein-1 in cardiovascular remodeling induced by chronic blockade of nitric oxide synthesis. Circulation. 2000;102:2243–2248. doi: 10.1161/01.cir.102.18.2243. [DOI] [PubMed] [Google Scholar]
- 5.Kagitani S, Ueno H, Hirade S, Takahashi T, Takata M, Inoue H. Tranilast attenuates myocardial fibrosis in association with suppression of monocyte/macrophage infiltration in DOCA/salt hypertensive rats. J Hypertens. 2004;22:1007–1015. doi: 10.1097/00004872-200405000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Haugen E, Gan LM, Isic A, Skommevik T, Fu M. Increased interleukin-6 but not tumour necrosis factor-alpha predicts mortality in the population of elderly heart failure patients. Exp Clin Cardiol. 2008;13:19–24. [PMC free article] [PubMed] [Google Scholar]
- 7.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 8.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez-Oliva G, Fernandez-Real JM, Zamora A, Vilaseca M, Badimon L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Hum Hypertens. 2005;19:457–462. doi: 10.1038/sj.jhh.1001845. [DOI] [PubMed] [Google Scholar]
- 10.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 11.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci U S A. 1995;92:4862–4866. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar S, Vellaichamy E, Young D, Sen S. Influence of cytokines and growth factors in ANG II-mediated collagen upregulation by fibroblasts in rats: role of myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H107–H117. doi: 10.1152/ajpheart.00763.2003. [DOI] [PubMed] [Google Scholar]
- 14.Siwik DA, Chang DLF, Colucci WS. Interleukin-1β and Tumor Necrosis Factor-α Decrease Collagen Synthesis and Increase Matrix Metalloproteinase Activity in Cardiac Fibroblasts In Vitro. Circ Res. 2000;86:1259–1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 15.Brower GL, Levick SP, Janicki JS. Inhibition of matrix metalloproteinase activity by ACE inhibitors prevents left ventricular remodeling in a rat model of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H3057–H3064. doi: 10.1152/ajpheart.00447.2006. [DOI] [PubMed] [Google Scholar]
- 16.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Cardiac Fail. 2005;11:548–556. doi: 10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. Journal of Molecular and Cellular Cardiology. 2008;45:56–61. doi: 10.1016/j.yjmcc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerdes AM, Onodera T, Tamura T, Said S, Bohlmeyer TJ, Abraham WT, Bristow MR. New method to evaluate myocyte remodeling from formalin-fixed biopsy and autopsy material. J Card Fail. 1998;4:343–348. doi: 10.1016/s1071-9164(98)90240-8. [DOI] [PubMed] [Google Scholar]
- 19.Burgess ML, Terracio L, Hirozane T, Borg TK. Differential integrin expression by cardiac fibroblasts from hypertensive and exercise-trained rat hearts. Cardiovascular Pathology. 2002;11:78–87. doi: 10.1016/s1054-8807(01)00104-1. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Naruishi K, Arai H, Nishimura F, Takashiba S. IL-6/sIL-6R enhances cathepsin B and L production via caveolin-1-mediated JNK-AP-1 pathway in human gingival fibroblasts. J Cell Physiol. 2008;217:423–432. doi: 10.1002/jcp.21517. [DOI] [PubMed] [Google Scholar]
- 21.Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, Mutsaers SE, Thompson PJ, Knight DA. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am J Pathol. 2003;163:345–354. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luckett LR, Gallucci RM. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol. 2007;156:1163–1171. doi: 10.1111/j.1365-2133.2007.07867.x. [DOI] [PubMed] [Google Scholar]
- 23.Edwards CA, O'Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clinica Chimica Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 24.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- 25.Andrzejczak D, Gorska D, Czarnecka E. Influence of amlodipine and atenolol on lipopolysaccharide (LPS)-induced serum concentrations of TNF-alpha, IL-1, IL-6 in spontaneously hypertensive rats (SHR) Pharmacol Rep. 2006;58:711–719. [PubMed] [Google Scholar]
- 26.Shiota N, Rysa J, Kovanen PT, Ruskoaha H, Kokkonen JO, Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21:1823–1825. doi: 10.1097/00004872-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Ishimaru K, Ueno H, Kagitani S, Takabayashi D, Takata M, Inoue H. Fasudil attenuates myocardial fibrosis in association with inhibition of monocyte/macrophage infiltration in the heart of DOCA/salt hypertensive rats. J Cardiovasc Pharmacol. 2007;50:187–194. doi: 10.1097/FJC.0b013e318064f150. [DOI] [PubMed] [Google Scholar]
- 28.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 29.Wollert KC, Chien KR. Cardiotrophin-1 and the role of gp130-dependent signaling pathways in cardiac growth and development. J Mol Med. 1997;75:492–501. doi: 10.1007/s001090050134. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi-Takihara K, Kishimoto T. Cytokines and their receptors in cardiovascular diseases--role of gp130 signalling pathway in cardiac myocyte growth and maintenance. Int J Exp Pathol. 2000;81:1–16. doi: 10.1046/j.1365-2613.2000.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuruda T, Jougasaki M, Boerrigter G, Huntley BK, Chen HH, D'Assoro AB, Lee SC, Larsen AM, Cataliotti A, Burnett JC., Jr Cardiotrophin-1 stimulation of cardiac fibroblast growth: roles for glycoprotein 130/leukemia inhibitory factor receptor and the endothelin type A receptor. Circ Res. 2002;90:128–134. doi: 10.1161/hh0202.103613. [DOI] [PubMed] [Google Scholar]
- 32.Rose-John S. Interleukin-6 biology is coordinated by membrane bound and soluble receptors. Acta Biochim Pol. 2003;50:603–611. [PubMed] [Google Scholar]
- 33.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 34.Korecky B, Rakusan K. Normal and hypertrophic growth of the rat heart: changes in cell dimensions and number. Am J Physiol. 1978;234:H123–H128. doi: 10.1152/ajpheart.1978.234.2.H123. [DOI] [PubMed] [Google Scholar]
- 35.Ancey C, Menet E, Corbi P, Fredj S, Garcia M, Rucker-Martin C, Bescond J, Morel F, Wijdenes J, Lecron JC, Potreau D. Human cardiomyocyte hypertrophy induced in vitro by gp130 stimulation. Cardiovasc Res. 2003;59:78–85. doi: 10.1016/s0008-6363(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 36.Fukuzawa J, Booz GW, Hunt RA, Shimizu N, Karoor V, Baker KM, Dostal DE. Cardiotrophin-1 increases angiotensinogen mRNA in rat cardiac myocytes through STAT3 : an autocrine loop for hypertrophy. Hypertension. 2000;35:1191–1196. doi: 10.1161/01.hyp.35.6.1191. [DOI] [PubMed] [Google Scholar]
- 37.Lopez N, Diez J, Fortuno MA. Differential hypertrophic effects of cardiotrophin-1 on adult cardiomyocytes from normotensive and spontaneously hypertensive rats. J Mol Cell Cardiol. 2006;41:902–913. doi: 10.1016/j.yjmcc.2006.03.433. [DOI] [PubMed] [Google Scholar]
- 38.Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, Vernallis AB, Heath JK, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996;271:9535–9545. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- 39.Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, Takahashi T, Makino S, Kato T, Ogawa S. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275:29717–29723. doi: 10.1074/jbc.M003128200. [DOI] [PubMed] [Google Scholar]
- 40.Janicki JS. Influence of the pericardium and ventricular interdependence on left ventricular diastolic and systolic function in patients with heart failure. Circulation. 1990;81(2 Suppl):III15–III20. [PubMed] [Google Scholar]
- 41.Janicki JS, Matsubara BB. Myocardial collagen and left ventricular diastolic dysfunction. In: Gaasch WH, LeWinter MM, editors. Left Ventricular Diastolic Dysfunction. Philadelphia: Lea & Febiger; 1994. pp. 125–140. [Google Scholar]
- 42.Turner NA, Mughal RS, Warburton P, O'Regan DJ, Ball SG, Porter KE. Mechanism of TNFα-induced IL-1α, IL-1β and IL-6 expression in human cardiac fibroblasts: Effects of statins and thiazolidinediones. Cardiovascular Research. 2007;76:81–90. doi: 10.1016/j.cardiores.2007.06.003. [DOI] [PubMed] [Google Scholar]