Abstract
Not all tumor vessels are equal. Tumor-associated vasculature includes immature vessels, regressing vessels, transport vessels undergoing arteriogenesis and peritumor vessels influenced by tumor growth factors. Current techniques for analyzing tumor blood flow do not discriminate between vessel subtypes and only measure average changes from a population of dissimilar vessels. We have developed methodologies for simultaneously quantifying blood flow (velocity, flux, hematocrit and shear rate) in extended networks at single capillary resolution in vivo. Our approach relies on deconvolution of signals produced by labeled red blood cells as they move relative to the scanning laser of a confocal or multiphoton microscope and provides fully-resolved three-dimensional flow profiles within vessel networks. Using this methodology, we show that blood velocity profiles are asymmetric near intussusceptive tissue structures in tumors in mice. Furthermore, we show that subpopulations of vessels, classified by functional parameters, exist in, around a tumor and in normal brain.
Introduction
Tumor blood vessels are heterogeneous in their structure and function, likely due to differential exposure to growth factors in the microenvironment and non-synchronous progression of angiogenesis and maturation 1–4. Because of technical limitations, most measurements of tumor vessel function are performed on single vessels or measured as bulk parameters over a large network. This limits our ability to detect responses to vascular-targeted drugs, which usually affect specific vessel subpopulations. To determine how individual vessels, or classes of vessels, are involved in tumor growth and response to treatment, tools for studying blood flow in extended vessel networks – at the spatial resolution of single capillaries – are urgently needed.
Many techniques have been used to analyze tumor hemodynamics. Single photon video-rate imaging has been the standard method for many years for measuring blood flow in normal and tumor vessels 1–3. Coupled with algorithms for automated detection and tracking of fluorescently-labeled red blood cell (RBC) 5 or two slit and 4 slit cross-correlation methods6 flow analysis in single vessels is possible. However, single photon techniques have relatively poor spatial resolution in the “z” direction (along the light path), and are less accurate when the vessels do not lie within the x–y plane.
Recently, a multiphoton laser scanning microscopy (MPLSM) based technique for single vessel blood flow analysis using line scanning was developed to quantify blood flow in individual cerebral cortical capillaries7 and tumor blood vessels8. This axial line scanning (ALS) quantifies flow velocity within selected blood vessels by scanning along the central axis of the blood vessel at high frequency, relying on contrast between an injected plasma fluorophore and the erythrocytes, which are dark. In an intensity plot of location along the line scan vs. time, streaks are produced with angles proportional to the RBC velocity (Fig. 1a). Line scanning along the central axis of a vessel allows quantification of centerline velocity, which can be used to estimate average flow velocity. ALS has good accuracy and sensitivity, especially when implemented with appropriate analysis algorithms 9. It can measure a wide range of velocities, but does not allow accurate quantification of erythrocyte flux (number of RBCs per second) or hematocrit (fraction of blood volume occupied by RBCs). Also, since there are generally more vessels than can be analyzed in a single session, this approach is subjective, as the operator must choose which vessels to measure.
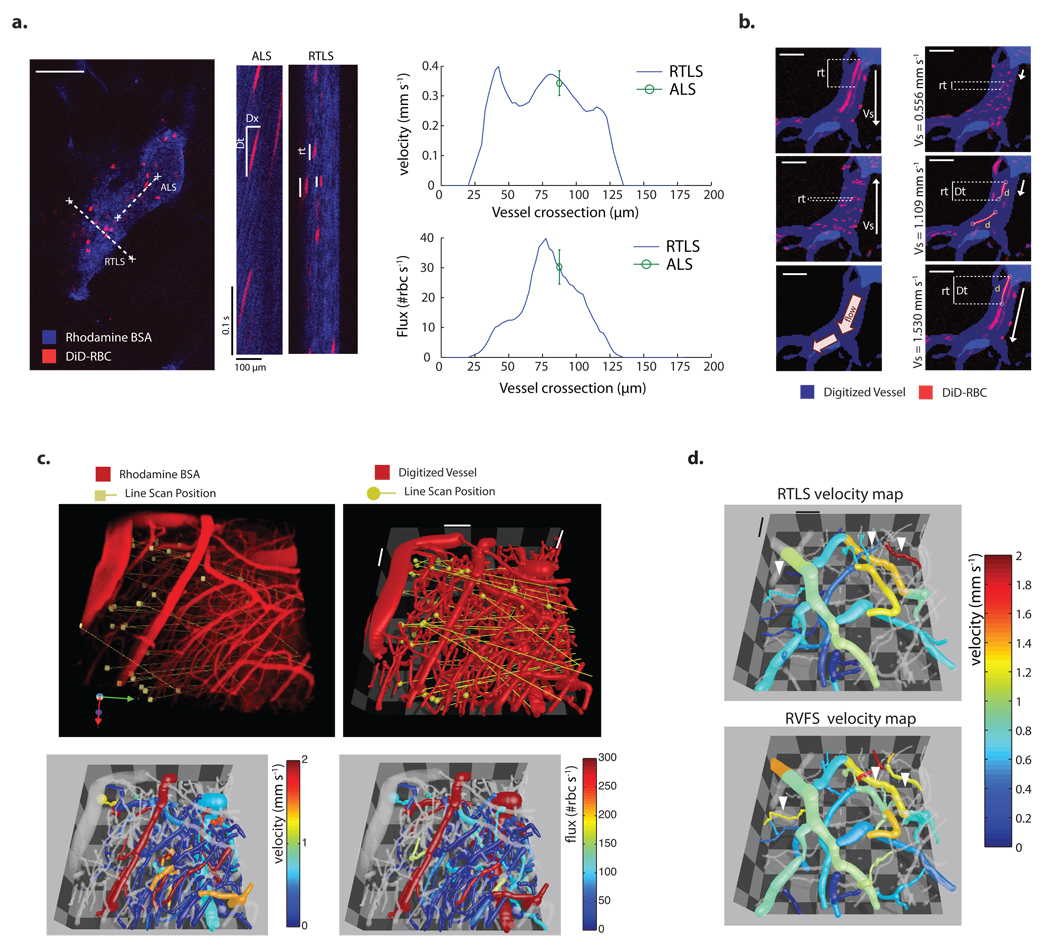
Figure 1. Description, validation and application of RTLS and RVFS.
(a) Representative MPLSM angiogram of a tumor blood vessel. x vs t plots were generated by scanning along the centerline of the vessel with ALS and perpendicular to the vessel with RTLS. Analysis of flow velocity is based on the slope of the RBC signal (Δx/Δt) for ALS and on residence time (rt) for RTLS. RBC velocity and flux measured along the vessel cross-section using RTLS are compared to ALS-based analysis of flow (mean ± s.e.m.). (b) A single vessel scanned with a range of scanning velocities in two opposing scanning directions (scanning velocity - Vs). Scanning from top to bottom with velocities from 1.1 to 1.5 mm s−1 caused "velocity-matched" red blood cells with higher residence times (rt) and a measurable traveled distance (d), which can be used to measure velocity. (DiD, 1,1-dioctadecyl-3,3,3,3-tetramethylindodicarbocyanine perchlorate) (c) 3D MPLSM angiogram of the brain of a tumor-bearing mouse illustrating the position of the line scans performed at several z planes. Comprehensive velocity and flux 3D maps were generated using RTLS (d) 3D Velocity map of a glioma network analyzed by RTLS and RVFS. Vessels in which RVFS velocity measurement differs from RTLS due to low RBC flux (closed arrowheads). Scale bars, 100 µm (a), 50 µm (b), 100 µm (c), 100 µm (d).
To overcome these limitations we developed two MPLSM-based methods for blood flow analysis in tumor and normal vascular networks. The first method, Residence Time Line Scanning (RTLS), allows direct analysis of flow velocity by scanning a line at an arbitrary angle to the vessel (Fig. 1a). The second method, relative velocity field scanning (RVFS), is a full-field method allowing simultaneous analysis of most of the vessels within a field of view by deconvolving the image distortion produced by the moving cells relative to the moving laser scans (Fig. 1b). Here, we describe the operational principles of RTLS and RVFS and then demonstrate the power of the methodologies by first measuring fully-resolved lumenal profiles of velocity, flux, hematocrit and shear rate in tumor vessels undergoing intussusceptive angiogenesis, and second by performing cluster analyses to identify “signatures” of vessels based on location and function.
Results
Residence time line scan operational principles
To perform either RTLS or RVFS, we label RBCs ex-vivo with a far-red lipophilic fluorescent dye (1,1-dioctadecyl-3,3,3,3-tetramethylindodicarbocyanine perchlorate (DID)) allowing observation deep within the tissue via MPLSM. The labeled RBCs are mixed with the endogenous mouse blood via systemic injection at a ratio of 3–5 labeled RBCs per 100; the labeled RBCs are ideal for long-term studies, with a half-life of approximately 10–14 days in the circulation, comparable to erythrocyte half-life in mice 10. RTLS is performed by scanning along a single line that intersects the vessel (Fig. 1a and Supplementary Fig. 1a). Repeated scanning along this line generates fluorescence intensity data along the line over time (x-t) in which “images” of the fluorescent RBCs are compressed or elongated depending on the residence time of the cells within the scan. The length of each cell signal in the x-t domain along the time axis depends on the number of times the laser scans the fluorescent cell as it passes. Thus, we can extract velocities from the line scan frequency, scanning angle and the length of the x-t cell images.
Since RBCs are biconcave flat discs (~1 µm thick, 7µm in diameter) that tumble in the blood stream, they can have various orientations when crossing the scan line; this could affect their x-t cell image independently of their velocity. The most rigorous and direct test of the potential error due to orientation is shown in Supplementary Fig. 2a and Supplementary Fig. 2b, where we directly compare RTLS with ALS, a method that is insensitive to RBC orientation. We find good correspondence between the two techniques, with no apparent biases at the low or high velocity ranges.
A major advantage of RTLS is that, compared with ALS, it is relatively insensitive to the orientation of the scan line with respect to the vessel. This means that RTLS scans can be randomly placed, or systematically arranged (for example in a grid pattern), removing operator dependence. Furthermore, each scan can intersect several vessels, allowing simultaneous measurement of multiple velocity profiles (Fig. 1c and Supplementary Fig. 1a). The technique is sufficiently robust to map velocities and fluxes in complex glioma vasculatures with its abundance of small vessels (Fig. 1c).
Although RTLS has advantages over ALS, it has certain limitations. Blood velocity can vary dramatically along a given tumor vessel due to abrupt changes in diameter or the presence of bifurcations, and RTLS still only analyzes a subset of these vessel cross sections. Another approach, therefore, might be to perform full-field imaging at sufficiently high frame rates and use cell tracking algorithms or correlation analyses to calculate blood flow throughout a network. Unfortunately, full-field acquisition rates for standard confocal and multiphoton microscopes are too slow to be used in this way.
Relative velocity field scanning operational principles
To circumvent the slow acquisition rates, of around 1 frame per second, we developed RVFS, which uses conventional laser scanning systems (MPLSM or confocal) to perform full field analysis of flow. Instead of requiring high frame rates, the method takes advantage of the relatively low speed of the laser field scans, which results in distortion of RBC images. RVFS is based on an analysis of the length of time a given cell spends in the scan line, that is a RBC’s residence time (RT) and traveled distance, extracted from the distortion of the images of RBCs as they move during scanning. While ALS and RTLS use stationary lines, RVFS uses a moving line scan. Because the line takes a finite amount of time to scan across the field, the resulting x–y image contains signals from cells that are distorted, and the distortion depends on the velocity of the cells relative to the moving scan line. RVFS analysis is then based on the relationship between RBC distortion and laser scanning velocity. The RT is maximum when the scan velocity approximates that of the moving RBCs. To fully deconvolve the velocities and generate reliable data for all vessels in the field, we need to scan in multiple directions and speeds, and collect accurate morphological information of the vessel network (for calculating relative scan angles). Blood velocity can be resolved by comparing the RTs and the scanning velocities for scans in the same direction as the flow and fitting the data to the appropriate function (Fig. 1b and Supplementary Fig. 3 – Equation derived from the Doppler effect). Furthermore, flux in a given vessel can be calculated from the number of RBCs and the scanning velocities.
The RVFS imaging protocol depends on the size of the region or volume of interest. Since the sampling rate of RVFS per vessel is lower than that of ALS or RTLS, physiological fluctuations of blood flow induced by heart rate or vasomotion can distort the relationship of RT to scanning speed potentially generating multiple peaks. At maximum RT, since scan velocity approximates that of the moving RBCs, "velocity -matched" RBC streaks are generated. If the resulting stretched signals are significantly longer than the size of an RBC (> 4 × 7µm) they can be used to directly analyze traveled distance and calculate velocity (Fig. 1b and Supplementary Fig. 3). Measurement of flow based on velocity matched RBCs allows averaging of velocity over the imaging time and minimizes the effect of temporal fluctuations. For vessels where no velocity-matched RBCs are generated and RT vs scanning speeds do not follow the doppler equation, it is necessary to increase the range by scanning either faster or slower through the field.
Analysis of cross-sectional flow profiles within tumor vessels
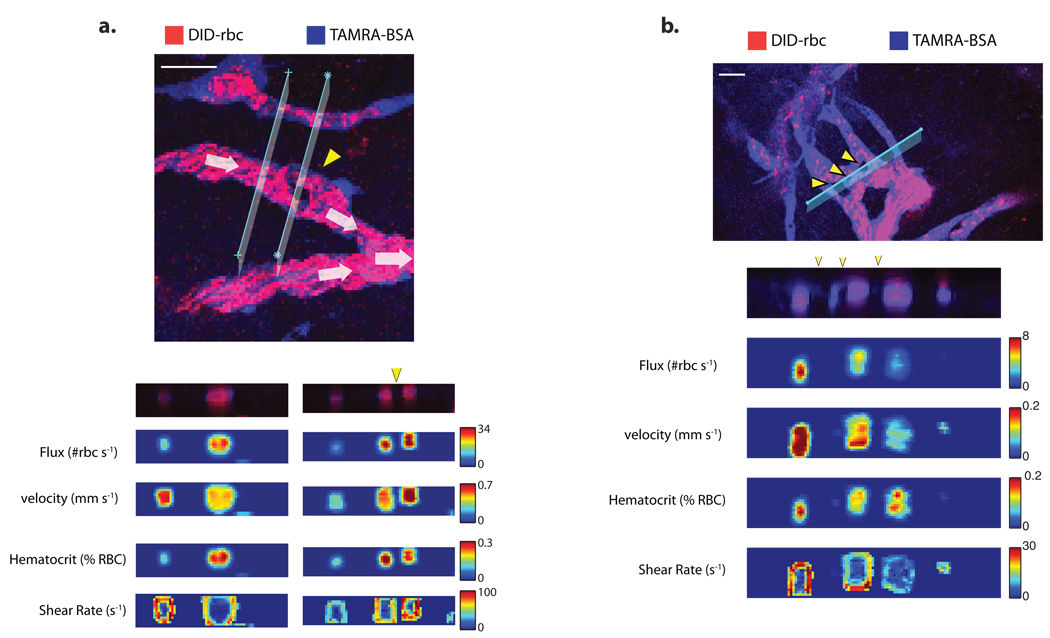
RVFS and RTLS yield similar results when applied to the same vessel segments (Fig. 1d and Supplementary Fig. 2c). Differences in velocities measured by RVFS and RTLS appear more frequently in small vessels with relatively low RBC flux, where RVFS is less accurate (Fig. 1d). By using RTLS to scan through segments of vasculature undergoing intussusceptive angiogenesis11, we were able record the first shear rate profiles around intussusceptive tissue structures (Fig. 2c,d). Shear rate is calculated as the difference in velocity between adjacent voxels along a vector perpendicular to the vessel wall. As shown in Fig. 2c and Fig. 2d, there are differences in flow and shear rates between the segments separated by the interstitial tissue structures, and the shear rates vary around the perimeter of each of the segments; this contrasts with vessels not undergoing intussusception, in which the shear rate is uniform around the perimeter (Supplementary Fig. 4). It is possible that these shear rate gradients help guide the insertion and growth of intussusceptive tissue structures.
Figure 2. Analysis of cross-sectional flow profiles within tumor vessels undergoing intussusceptive angiogenesis.
Cross-sectional velocity, flux, hematocrit shear rate profiles and raw data maps upstream and at the level of a single (a) or multiple (b) intussusceptions (yellow arrow heads Scale bars, 50 µm (a), 50 µm (b). Bigger images are scanned areas of the plane where the cross-sectional line scanning was done.
Multiparametric phenotypic vessel clustering to characterize glioma vessels
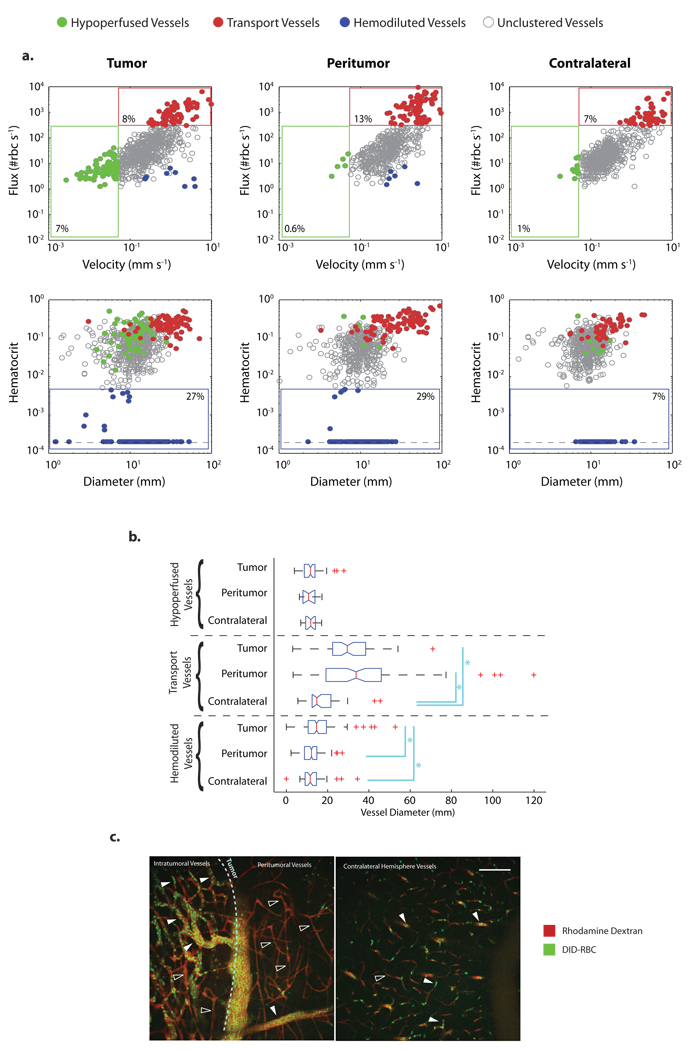
Current techniques for analyzing tumor blood vessels mainly rely on vessel structure or morphology to discriminate between vessel subtypes. To demonstrate the ability of our methodology to distinguish and analyze vessel populations, we used RTLS to measure velocities, fluxes and hematocrits of 2351 vessels from six glioma-bearing animals (Fig. 3). Using green fluorescence protein (GFP) -expressing glioma cells, we were able to record the location of each vessel relative to the tumor mass and classify each as tumor or peritumor; contralateral brain hemisphere vessels were also analyzed (Supplementary Fig. 5a). Considering only the average values, peritumor vessels had higher velocity, flux and wall shear rate compared to tumor or contralateral brain vessels (Supplementary Fig. 5b). Interestingly, average tumor vessel perfusion parameters (velocity and flux) were not different than the contralateral brain (Supplementary Fig. 5b). By clustering the vessels into specific phenotypes: Hypoperfused vessels – velocity < 0.05 mm s−1, Transport vessels - flux>300 RBC s−1, and. Hemodiluted vessels - hematocrit < 0.005, we could analyze the relationship between vessel location and function (Fig. 3a). For example, there is a population of vessels more prevalent in the tumor, characterized by low velocity and flux, high hematocrit and variable diameter (Fig. 3a). Transport vessels, defined as vessels with high flux, were present in all areas but with the highest fraction in the peritumor area, consistent with peritumoral arteriogenesis (Fig. 3a). Transport vessels had significantly larger diameters in the tumor and the peritumor compared to the contralateral brain, suggesting extensive dilation of feeding and draining vessels in these regions4 (Fig. 3a,b). Hemodiluted vessels, defined by their low hematocrit, were present in all compartments but with higher fraction in the tumor and the peritumor areas (Fig. 3b). Low hematocrit vessels within the peritumor area were morphologically normal with diameters similar to normal brain and high RBC velocity. The variation in vessel hematocrit seen in the tumor and peritumor regions is likely due to loss of plasma by leaky vessels, which increases hematocrit. The extravasated plasma, which also increases tumor IFP 12, is eventually reabsorbed by other vessels, diluting their RBCs (Fig. 3c). Thus, RTLS and RVFS provides a framework for distinguishing and classifying vessel populations, providing more sensitive analyses of vascular physiology in and around tumors. This approach should enable focused studies of how vessel subpopulations shift during tumor growth and in response to therapies.
Figure 3. Multiparametric phenotipic vessel clustering to compare vessels within tumor, peritumor and contralateral brain regions in a glioma model.
(a) All the vessels analyzed in U87-bearing animals (n = 6) are plotted. Scatter plots of velocity vs flux and diameter vs hematocrit showing the gating applied to analyze three different vessel sub-types (hypoperfused, transport, and hemodiluted).(b) Box plots for vessel diameter from clustered vessels. (* P < 0.05). (c). U87 MPLSM micrographs. Rhodamine BSA was used for the angiographic contrast and fluorescent RBCs (green) were injected 1 day prior to imaging. Glioma cells expressed GFP which allowed accurate localization of the tumor and determination of the tumor edge (light blue dashed line). Tumor vessels are heterogeneous with high (closed arrow head) and low (open arrow head) hematocrit vessels. Peritumor vessels are morphologically normal and have low (open arrow head) hematocrit when compared to contralateral brain. Scale bars, 100 µm (c).
Discussion
Each of these techniques has advantages and disadvantages. RTLS has high sensitivity and allows cross-sectional analysis of flow within vessels, but flow analysis is limited to the location of intersection between the line and the vessel. RVFS allows analysis of entire vessel networks, integrating flow analysis at multiple positions along vessels. However, it is slower due to the need for multiple scan angles and speeds, and its accuracy can be affected by low RBC flux. A comparison of ALS, RTLS and RVFS is presented in Table 1.
Table 1.
| Axial Line Scan (ALS) |
Residence Time Line Scan (RTLS) |
Relative Velocity Field Scan (RVFS) |
||
|---|---|---|---|---|
| Description | Protocol | Repeatedly scan along a line parallel to the vessel wall - Fig. 1a - |
Repeatedly scan along a line intersecting one or multiple vessels - Fig. 1a and Supplementary Fig. 1 - |
Progressively scan a line over an area. Repeat at various scan speeds and angles - Fig. 1b and Supplementary Fig. 3 - |
| Velocity Analysis |
Velocity is given by the angle of the signals in the x-t image |
Velocity is extracted from the residence times of individual RBCs as they pass the scan line |
Velocity is deconvolved from the stretched RBC signal, which is determined by the relative velocity between the field scan and the RBC |
|
| Yield | Single “lane” within the analyzed vessel; resolution determined by resolution of laser scan |
Cross-sectional velocity profile of all intersected vessels |
Full velocity field within all vessels (within the range of sensitivity) |
|
| Sensitivity | Depends on line scan rate and line length |
Depends on line scan rate | Depends on Sampling (number of scan rate and angle variations) |
|
| Application | Monitor velocity with high time resolution in a single vessel with moderate or fast flow. |
Monitor velocity and shear rate with high time resolution simultaneously in multiple vessels with moderate or slow flow. |
Measure average flow velocity in all the vessels of a region or volume of interest (within a predefined range of sensitivity). |
|
| Limitations | High velocity vessels Arteries |
Line scan rates of standard systemsa limit sensitivity. Solution: Increasing line length increases sensitivity |
Line scan rates of standard systemsa limit sensitivity. Solution: Analyze residence time at a single pixel instead of a line |
Field scan speed and angle specifications have to be optimized specifically to analyze high velocity vessels |
| Low velocity vessels |
No limitations | No limitations | Field scan speed and angle specifications have to be optimized specifically to analyze low velocity vessels |
|
| 3D Vessels | Analyzes flow in the scanned plane. Velocity components perpendicular to the imaged plane are not resolved |
No limitations | Analyzes flow in the scanned plane. Velocity components perpendicular to the imaged plane are not resolved |
|
| Yield | Limited to a specific location in the chosen vessel. Biased to operator-selected vessels. |
Generally limited to a single cross- section of each intersected vessel. |
Limited to those vessels that have RBC velocity similar to the specified field scan velocity (speed and direction) |
|
| Temporal Resolution |
Limited by laser scan frequency (typically 500–1000 scans per second) |
Limited by laser scan frequency (typically 500 – 1000 scans per second) |
Typical acquisition rates: for X-Y area, 12 secondsb; for X–Y–Z volume: 8 minutesc |
|
| Potential Error | Accuracy of RBC d–t image angle is critical. |
Accuracy of RBC residence time is critical. RBC preferential orientation can contribute error to the residence time analysis. |
Accuracy of measurement of the distance travelled by the RBC is critical. |
|
| Advantages | Higher sensitivity than RTLS for high velocity vessels. |
Measures velocity profile and shear rates. |
Measures all vessels in the area of interest. | |
| No need to label tracer RBCs. | Analyzes flow in penetrating and diving vessels. |
|||
| Can analyze multiple vessels simultaneously. |
||||
Olympus confocal or multiphoton laser scanning microscope. Olympus FV300 Scanning unit: 256×256 pixels (660 × 660 µm) scanned in 0.45 seconds.
assumes 660×660 µm field sampled at 6 angles with 2 speeds at each angle.
assumes 660×660×100 µm field sampled at 6 angles with 2 speeds at each angle (40 z-slices).
An important feature of RTLS is its ability to resolve velocities and fluxes spatially within individual vessels. The technique generates profiles of RBC velocity and flux (and therefore hematocrit) across each sampled blood vessel cross section (Fig. 1a and Supplementary Fig. 1). Furthermore, when combined with measurements of vessel cross-section assessed using a second fluorophore restricted to the plasma, accurate estimations of wall shear rates and stresses can be extracted.
Such spatially-resolved measurements of wall shear rates have the potential to answer long-standing questions of how blood shear forces exerted on endothelial cells contribute to processes such as atherogenesis and angiogenesis. For example, it is well known that endothelial cells sense shear stress gradients and respond by activating genes related to cell migration, vasoregulation and proliferation 13. But since there were previously no methods for measuring shear stress gradients in vivo, it is not known to what extent they affect the organization or migration of endothelial cells during angiogenesis. Systematic application of RTLS during the process of angiogensis may resolve these issues. Although demonstrated here for tumor vessels, the techniques can be used to analyze any vasculature accessible by confocal or multiphoton microscopy.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Padera for his editorial comments and S. Roberge for her outstanding technical assistance. This work was supported in part by National Institutes of Health Grants R01HL064240 (LLM), R01CA149285 (LLM) and P01CA80124 (RKJ), and by postdoctoral fellowship from the Susan G. Komen Foundation (WSK).
Footnotes
Author Contributions
W.S.K., Conception and design, technique validation, implementation and data collection, manuscript writing and final approval of manuscript; S.S.C, implementation and data collection; D.A.L, implementation and data collection; J.A.T., conception and design and technique validation; M.M., technique validation; M. A. G., implementation and data collection; D.F., conception and design and administrative support; R.K.J, administrative support, financial support and final approval of manuscript; L.L.M, Conception and design, administrative support, financial support, manuscript writing and final approval of manuscript.
Authors’ Disclosures of Potential Conflicts of Interest
Rakesh K. Jain has an advisory role in the following companies: SynDevRx, AstraZeneca, Dyax, Millenium and Honoraria role in AstraZeneca
AOP
Multiphoton laser scanning microscopy paired either with stationary line scans across a vessel or moving line scans across a network of vessels allows the profiling of key parameters that describe red blood cells.
Issue
Multiphoton laser scanning microscopy paired either with stationary line scans across a vessel or moving line scans across a network of vessels allows the profiling of key parameters that describe red blood cells.
References
- 1.Fukumura D, Yuan F, Monsky WL, Chen Y, Jain RK. Effect of host microenvironment on the microcirculation of human colon adenocarcinoma. Am J Pathol. 1997;151:679–688. [PMC free article] [PubMed] [Google Scholar]
- 2.Brizel DM, et al. A comparison of tumor and normal tissue microvascular hematocrits and red cell fluxes in a rat window chamber model. Int J Radiat Oncol Biol Phys. 1993;25:269–276. doi: 10.1016/0360-3016(93)90348-y. [DOI] [PubMed] [Google Scholar]
- 3.Endrich B, Reinhold HS, Gross JF, Intaglietta M. Tissue perfusion inhomogeneity during early tumor growth in rats. J Natl Cancer Inst. 1979;62:387–395. [PubMed] [Google Scholar]
- 4.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100:865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamoun WS, Schmugge SJ, Kraftchick JP, Clemens MG, Shin MC. Liver microcirculation analysis by red blood cell motion modeling in intravital microscopy images. IEEE Trans Biomed Eng. 2008;55:162–170. doi: 10.1109/TBME.2007.910670. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum WI, El-Sabban F. Measurement of red cell velocity with a two-slit technique and cross-correlation: use of reflected light, and either regulated dc or unregulated ac power supplies. Microvasc Res. 1981;22:225–227. doi: 10.1016/0026-2862(81)90092-3. [DOI] [PubMed] [Google Scholar]
- 7.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A. 1998;95:15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown EB, et al. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- 9.Drew PJ, Blinder P, Cauwenberghs G, Shih AY, Kleinfeld D. Rapid determination of particle velocity from space-time images using the Radon transform. J Comput Neurosci. 2009 doi: 10.1007/s10827-009-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsey ES, Donaldson GW, Woodruff MF. Erythrocyte survival in normal mice and in mice with autoimmune haemolytic anaemia. Clin Exp Immunol. 1966;1:85–98. [PMC free article] [PubMed] [Google Scholar]
- 11.Patan S, et al. Vascular morphogenesis and remodeling in a human tumor xenograft: blood vessel formation and growth after ovariectomy and tumor implantation. Circ Res. 2001;89:732–739. doi: 10.1161/hh2001.097872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- 14.Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer. 2002;2:266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 15.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Tyrrell JA, et al. Robust 3-D modeling of vasculature imagery using superellipsoids. IEEE Trans Med Imaging. 2007;26:223–237. doi: 10.1109/TMI.2006.889722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.