Abstract
Migraine attacks with auras are sometimes associated with underlying hereditary or acquired cerebrovascular disorders. A unifying pathophysiological explanation linking migraine to these conditions has been diffcult to identify. On the basis of genetic and epidemiological evidence, we suggest that changes in blood vessels, hypoperfusion disorders, and microembolisation can cause neurovascular dysfunction and evoke cortical spreading depression, an event that is widely thought to underlie aura symptoms. In fact, recent experimental data have indicated that focal, mild, and transient ischaemia can trigger cortical spreading depression without an enduring tissue signature. Although migraine with aura has many causes (eg, neuronal network excitability), it seems that migraine and stroke might both be triggered by hypoperfusion and could therefore exist on a continuum of vascular complications in a subset of patients who have these hereditary or acquired comorbid vascular conditions.
Introduction
Migraine headache can occur as a comorbidity of ischaemic stroke, carotid or vertebral artery dissection, arteriovenous malformations, cerebral auto somal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL syndrome), or platelet disorders (eg, thrombocytosis) among other disorders (panel).1–5 Although diffcult to identify a unifying hypothesis linking these disorders to migraine pathogenesis, a disturbance within brain vessels might be common to a subset of patients who have migraine with aura. Migraine with aura has been identified as an independent risk factor for ischaemic stroke6 and possibly for white matter hyperintensities, suggesting common pathophysiological mechanisms that implicate the neurovascular unit.7,8 Although several possibilities can explain the comorbidity of migraine and vascular diseases (eg, shared mutations or a consequence of repeated migraine attacks), an emerging hypothesis, which we find persuasive, places stroke and migraine on a continuum of vascular complications caused by, for example, focal and transient hypoperfusion. Recent experimental data in mice indicate that cerebral microembolism triggers cortical spreading depression (CSD), a biological substrate for migraine aura, without causing requisite tissue injury.9 Although the brain’s vulnerability to injury is well recognised, it is only with the help of emerging data from experiments in animals that we are now able to examine the possibility that even focal, brief hypoxic-ischaemic episodes could trigger CSD without an obvious or enduring tissue signature. If this possibility is true in human beings, blood vessel and blood flow disorders would then be acknowledged as a migraine trigger, and vascular causes and risk factors for migraine with aura would be more aggressively sought.
In this Personal View, we first consider the evidence and possible mechanisms that link CSD to migraine aura and local hypoperfusion. We then examine what is known about blood vessel and microcirculatory disorders implicated in migraine with aura to identify the important subset of patients in whom these conditions might be linked. Because migraine is a disorder of brain function and CSD is a putative biological substrate for migraine aura, we begin with a brief description of the relevance and pathophysiological importance of CSD.
Migraine aura and CSD
CSD is a slowly propagating wave of neuronal and glial depolarisation that can be evoked in the cortex, cerebellum, basal ganglia, thalamus, and hippocampus. Although well documented and easily evoked in the lissencephalic brain, only recently has its existence been unequivocally shown in the human brain in the context of subarachnoid haemorrhage, malignant stroke, and head injury.10–12 CSD provides the most likely explanation for migraine visual aura, although the evidence from patients with migraine is still indirect. The earliest and strongest support for this view came from blood flow imaging studies by Olesen and colleagues,13 which showed that, during aura-like symptoms, slowly spreading oligaemia propagated anteriorly from the occipital pole. This finding was corroborated during a spontaneous migraine attack without aura seen during PET imaging,14 and also by use of magneto encephalography, which showed multiple cortical areas activated in spontaneous and visually induced migraine aura.15 The most technically advanced demonstration was provided by a functional imaging study during visual aura, which showed a slowly propagating perturbation of the magnetic resonance signal in the primary visual cortex that had at least eight characteristics of CSD, including transient hyperperfusion followed by sustained hypoperfusion.16
Not unlike migraine aura, CSD develops in response to several types of stress, including excitotoxic or hypoxic-ischaemic stress. Unsurprisingly then, CSD is noxious in some studies: it activates the trigeminovascular system in rodents17 and has been proposed as a trigger for headache in human beings.18,19 CSD susceptibility is modulated by genetic and environmental factors: increases in extracellular potassium ion and glutamate concentrations might initiate CSD during intense cortical excitation, perhaps in response to local depolarisation. Furthermore, migraine has a strong but complex genetic component that is susceptible to modulation by endogenous biological and environmental factors such as oestrogen withdrawal, sleep, and stress, and might also be an expression of neuronal network excitability (figure 1).
Figure 1. CSD and factors involved in the initiation of vascularly triggered migraine aura.
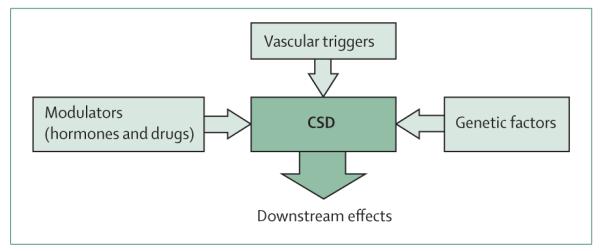
CSD has a fundamental role in the genesis of migraine aura. Susceptibility to CSD is conferred by genes and modulated by hormones (ovarian and testicular) as well as by drugs that suppress CSD and prevent migraine attacks. Recently identified vascular triggers initiate CSD by causing transient, mild, and focal hypoperfusion, as determined experimentally. CSD=cortical spreading depression.
In addition to intrinsic brain parenchymal mechanisms, as noted above, CSD is sometimes triggered by brief hypoxic-ischaemic episodes, including endothelin-1-induced vasospasm and microinfarcts or aneurysmal subarachnoid haemorrhage,10,20 as well as in vitro brief hypoxia and mitochondrial inhibition.21,22 CSD develops during exposure to carbon monoxide or before the development of terminal depolarisation in animals breathing a fraction of inspired oxygen (FiO2) of less than 0·08.23,24 Recurrent and slowly propagating peri-infarct depolarisations that resemble CSD are generated in focal cerebral ischaemia.12 In fact, perfusion pressure and oxygen levels have been reported to affect the electrophysiological recovery of repetitive CSD events, or of CSD developing in the context of ischaemia.25 Hence, CSD develops as a consequence of mild and transient hypoxic-ischaemic perturbations, which in turn affect recovery; severe episodes eventually lead to terminal anoxic depolarisation, as frequently found in prolonged ischaemia.
Migraine and cerebral microembolism
The association between migraine, stroke, and patent foramen ovale (PFO) remains controversial and incomplete. Although the aggregate of the clinical evidence is more convincing than individual reports, initial reports linking PFO with migraine aura had severe selection bias and the quality of the evidence has therefore been judged as only moderate to low. Furthermore, a recent population-based study in the elderly did not confirm this association.26 However, data from most studies indicate that if there is an association, it is between PFO and migraine with aura, not migraine without aura. In a meta-analysis of 1517 patients, increased prevalence of PFO was found in migraineurs with aura compared with people without migraine, and a higher prevalence of migraine and migraine with aura was found in individuals with PFO than in individuals without PFO.27 The estimated odds ratio was 5·1 for an association between PFO and migraine and 3·2 for an association between PFO and migraine with aura. The prevalence of PFO in 665 patients with migraine with aura ranged from 41% to 72%.27 Calculations based on the available data suggest that PFO could be a source of migraine attacks in a subset of patients, but we and others26 believe that PFO probably does not account for all migraine attacks in these susceptible individuals.
Panel: Some comorbidities of migraine with aura.
Cardiac and pulmonary
Patent foramen ovale (associated with large openings, atrial septal aneursyms, and right-to-left shunting)
Mitral valve prolapse
Pulmonary arteriovenous malformations
Vascular
Stroke
Carotid or vertebral artery dissection
Carotid artery puncture
Brain arteriovenous malformations
Hereditary disorders (CADASIL, Col4A1 mutations, AD-RVCL, hereditary vascular retinopathy)
Inflammatory
Raynaud’s phenomenon
Sjögren’s syndrome
Antiphospholipid antibodies
Coagulopathy
Thrombocytosis
Polycythemia vera
AD-RVCL=autosomal dominant retinal vasculopathy and cerebral leukodystrophy. CADASIL=cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.
Disorders that might make PFO a putative risk factor for stroke, such as right-to-left shunt and an associated atrial septal aneurysm, have been documented; the same association is beginning to emerge for migraine with aura.28 The presence of spontaneous right-to-left cardiac shunt at rest has been implicated in the pathogenesis of stroke. The risk of systemic embolisation increases when right atrial pressure exceeds that on the left, such as during the Valsalva manoeuver or in disorders in which pulmonary artery pressure can be increased. Patients with postulated paradoxical embolism can have larger PFOs than individuals without paradoxical embolism, and MRI findings have implicated cerebral emboli as a potential mechanism in patients with stroke and a large PFO.29.30 Thrombi have been observed to traverse a PFO in some patients,31 thereby raising the probability that small blood clots and microaggregates escape from being trapped within the lungs.
Although the association between migraine and ischaemic stroke is primarily seen in patients with a low vascular risk profile,32 other comorbid disorders that increase the risk of stroke might also increase the risk of migraine with aura. Right-to-left shunts within the heart and also within the pulmonary circulation facilitate the transit of fibrin-rich, soft, red-type venous thrombi.33 Venous emboli might be more susceptible to fibrinolysis and disintegration by the intrinsic thrombolytic system than platelet-rich, white, often calcified emboli originating from the wall of atherosclerotic arteries.34,35 Accordingly, these fragmented, small emboli might have a higher probability of transiently occluding the microcirculation, causing small foci of hypoxia/ischaemia. Moreover, these emboli might create a surface for coagulation and platelet activation as well as release of vasoactive chemicals and pro-inflammatory factors from the occluded microvessel wall and entrapped blood cells that contribute to ischaemia and hypoxia.36 Thrombosis and micro embolisation could thereby create a transient hypoxic-ischaemic focus to induce CSD followed by a migraine attack, whereas more prolonged occlusion of larger vessels might cause transient ischaemic attack or stroke.37
Migraine with aura also develops as a complication of injury to large vessels—for example, after acute vertebral or carotid artery dissections.38 A clot can form within a narrow and irregular arterial lumen in the dissected segment and trigger distal embolism before it is completely thrombosed.39 Similarly, puncturing the carotid artery can cause embolism and trigger the onset of migraine aura attacks.40 These pathophysiological considerations take on greater importance in light of recent experimental studies indicating that microembolism can serve as a trigger for CSD. Indeed, recent experimental data show that small brain foci of ischaemia trigger CSD in mice.9 Small microemboli (microspheres of 10 μm) provide the trigger, with minimum or no histological damage in some animals but microscopic infarcts in less than half of the other animals. The small infarcts were found within the injected carotid artery territory and not within the brainstem. Conversely, air microbubbles were particularly effective as CSD triggers but did not cause any tissue damage. CSD occurrence was associated with the depth and duration of blood flow deficit (figure 2) but did not correlate with the presence of microscopic lesions regardless of whether air (0·8 μL), microspheres (10 μm), or cholesterol crystals (<70 μm) were injected.
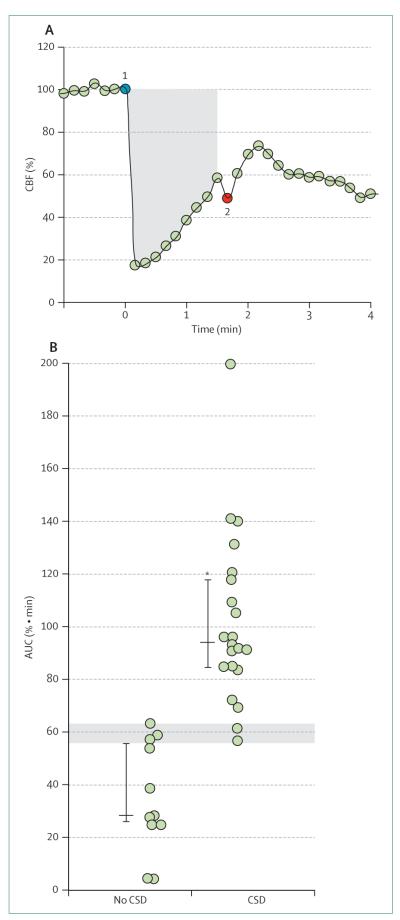
Figure 2. Association between CSD and CBF.
After microemboli were injected into a rodent carotid artery, CSD occurrence was associated with the extent of CBF perturbation. (A) The time course of transient oligaemia after microembolisation (shown at point 1), followed by a gradual increase until CSD occurrence (shown at point 2). The shaded area (AUC) shows the ischaemic burden, a measure of depth and duration of oligaemia, from point 1 to point 2 or until CBF returned to baseline. (B) AUC values for individual animals with or without CSD show that the ischaemic burden (AUC) was greater in animals with CSD than in those without CSD. Evidence of cell or tissue damage was present in only a few animals with CSD. The shaded area indicates the threshold above which CSD is likely to occur. Bars indicate median (25–75% range). *Significant difference between animals with and without CSD. Modified from Nozari and colleagues,9 with permission from the American Neurological Association. AUC=area under the curve. CBF=cerebral blood flow. CSD=cortical spreading depression.
We believe that these findings are relevant to human beings because the terminal vascular beds of the mouse and human brain are not too dissimilar. We also believe that these findings are of relevance to migraine auras and headaches in patients that have PFO and right-to-left cardiac shunts, wherein the filtering capacity of the lungs is bypassed. Lending support to this view, a recent study in patients with migraine with aura as well as PFO showed that injection of air bubbles into a peripheral vein induced multiple focal or bilateral temporo-occipital electroencephalographic (EEG) disturbances.41 Bubble injection induced an attack of migraine with aura in one of the seven patients, as is occasionally reported during the microbubble studies used to detect PFO.42,43 Interestingly, the EEG abnormality induced by bubble injection was not observed in patients without migraine history but in patients with large PFOs and right-to-left shunts. Although the observed EEG changes are not equivalent to CSD, they do indicate the susceptibility of the brain in patients with migraine with aura to perturbations within the microcirculation.
The risk of developing a migraine attack during microembolisation most probably depends not only on the location, size, and duration of transient vascular micro-occlusion, but also on the susceptibility of the brain to developing CSD. Therefore, the fact that PFO closure reduces attack frequency but does not eliminate migraine attacks is not surprising, although published case series of PFO closure were not properly controlled. In fact, the results of the only randomised trial were mostly negative, indicating the need for caution about PFO closure as a routine treatment option.44 Nevertheless, a substantial reduction in frequency and severity of migraine recurrence might be achieved by PFO closure in migraineurs with a large PFO and subclinical brain MRI lesions,45 suggesting that only carefully selected patients with migraine with aura who have PFO could benefit from this intervention; this hypothesis would be best tested in the context of a new prospective randomised clinical trial.
Although there is little experimental evidence at present, alternative mechanisms for PFO-induced migraine have been proposed, including hypoxia-induced migraine caused by brief episodes of oxygen desaturation or migraine caused by high concentrations of serotonin or other vasoactive substances in the arterial circulation. These explanations are not mutually exclusive. A shared genetic predisposition for the atrial defect and tendency for migraine with aura has also been suggested.
Susceptibility to migraine aura of vascular origin
CADASIL syndrome, an autosomal dominant disorder, is a prototypical disorder that emphasises a strong link between blood vessels and migraine aura.4 20–40% of patients with CADASIL have migraine with aura, often as the first symptom of the disease. Caused by mutations in NOTCH3, which encodes a transmembrane receptor expressed by vascular smooth muscle cells, this disorder affects both systemic and cerebral blood vessels. More than 95% of point mutations have been identified in the extracellular domain of the affected protein.
The precise pathophysiological explanation for attacks of migraine with aura in patients with CADASIL is not known. After the onset of migraine aura in the second or third decade of life, patients develop transient ischaemic attacks and strokes in their fourth or fifth decades, accompanied by early cognitive decline.46 The constellation of complications is consistent with a spectrum disorder in which migraine is an identifying feature of a subset of patients with mild vascular dysfunction. On the basis of the clear association between transient vascular occlusion and CSD, we suspect that transient occlusion within the microcirculation that causes local hypoperfusion is the most likely trigger of CSD in susceptible individuals. In studies in patients with CADASIL, reduced blood flow and low mean regional cerebral metabolic rate for glucose were reported for the young adult patients.47 Hence, fluctuations in the mean arterial blood pressure, normally compensated for by autoregulatory mechanisms at the microcirculation level, might cause focal hypoperfusion and episodes of compromised blood flow in the presence of reduced vascular compliance. Strokes are the identifying feature for the severe end of the ischaemic continuum, whereas transient ischaemic attacks and perhaps silent small infarcts indicate an intermediary stage.
Lending support to the idea that vascular dysfunction might underlie diverse clinical phenotypes, patients with CADASIL have reduced cerebral vasoreactivity to carbon dioxide inhalation,48 as well as impaired postocclusive hyperaemia in the skin.49 Similarly, mice that express human mutations in Notch3 (Arg90Cys) have abnormal myogenic responses, including constriction and dilation of cerebral and systemic vessels,50 and have significantly larger brain infarcts after middle cerebral artery occlusion compared with mice without these mutations,51 suggesting a deficit in compensatory mechanisms such as collateral blood flow.
As noted above, the mechanism(s) linking mutations in vascular smooth muscle cells and CSD is poorly understood. In addition to a transient perfusion abnormality similar to the complications of microembolisation, migraine aura or CSD could indicate dysfunction of the neurovascular unit, a concept that emphasises the importance of cell-cell signalling between component cells of the blood vessel wall, astrocytes, neurons, and the brain matrix.8 Astrocytic foot processes surround small brain vessels and can affect vascular smooth muscle and regulate blood flow. These foot processes also provide spatial buffering of potassium and promote glutamate uptake—two key players in the generation of CSD. Precisely how the neurovascular unit is affected by NOTCH3 mutations in vascular smooth muscle or other vascular disorders implicated in migraine aura pathogenesis needs further study. Of interest, genetically engineered mice that overexpress the Arg90Cys mutation have a lower threshold for evoking CSD than wild-type mice, consistent with a migraine aura phenotype.52
Migraine is also associated with less frequent vascular syndromes, including the rare angiopathy due to mutations in collagen type IV alpha-1 chain (Col4A1) and the three prime repair exonuclease 1 (TREX1). Col4A1 is an essential component of the basal membrane. TREX1 is a 3′→5′ DNA exonuclease and mutations in this protein might cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy (ADRVCL).53 Not unlike patients with CADASIL, patients with hereditary vascular retinopathy can also have migraine, cerebral infarcts, vascular dementia, and Raynaud’s phenomenon as shown in a Dutch pedigree.54 Together, these genetic disorders strongly implicate neurovascular dysfunction in migraine pathophysiology, particularly in the early events that precede headache onset.
A strong association between migraine and some acquired vascular disorders is suggested by epidemiological data. For example, migraine is diagnosed in 46% of patients with primary Sjögren’s syndrome.55 An increased prevalence of migraine was found in patients with Raynaud’s phenomenon (odds ratio 5·4; 95% CI 2·8–10·3).56 Chest pain was more common in patients with Raynaud’s phenomenon who had coexisting migraine than in those without coexisting migraine. Interestingly, migraine and Raynaud’s phenomenon are both reportedly associated with endothelial cell dysfunction. In Japanese patients, the prevalence of Raynaud’s phenomenon with vasospastic angina was higher than that reported from North America, although the prevalence of migraine was the same, suggesting a genetic component in the underlying vasculopathy.57 A history of migraine is reported to be associated with vascular damage in the organs of patients with systemic lupus erythematosus, lending support to the idea of an inherent vascular dysfunction in migraineurs.58 Active migraine was associated with more severe disease activity, higher concentrations of antiphospholipid antibodies, and worsening of Raynaud’s phenomenon. Similarly, in a community-based prospective cohort of 15792 participants, Rose and colleagues59 reported an increased incidence of retinopathy signs in individuals with migraine and other headaches, lending support to the hypothesis that neurovascular dysfunction might underlie some types of vascular headaches. These authors also reported an association between migraine and exertional angina, although a direct correlation with coronary artery disease was not identified.60 Consistent with these observations, an increased incidence of migraine was reported in several vascular disorders (eg, moyamoya disease,61 livedo reticularis,62 and preeclampsia63). An important role for immune factors and inflammation is also suggested in some diseases noted above by links between migraine and Sjögren’s syndrome, systemic lupus erythematosus, Raynaud’s phenomenon, and evidence of endothelium dysfunction and its effect on the brain microcirculation. Taken together, these findings lend support to the idea that migraine might share a common pathophysiological mechanism with these vascular disorders, although whether there is an association with migraine with aura requires further clarification.
In view of these findings, it might be possible someday to classify migraine vascular triggers according to whether blood vessel dysfunction is caused by release of vasoactive substances, disturbances in myogenic responses, or endothelial-dependent relaxation, by hypercoagulable states, inflammation, platelet and white blood cellendothelial interactions, or by a combination of the above.
Platelets, coagulation disorders, endothelial dysfunction, inflammation, and migraine
Although insuffcient, some evidence from small, unvalidated clinical studies lends some support to a possible association between platelet and coagulation disorders and migraine, as well as between migraine and endothelial dysfunction.64 However, most of these early studies were done on small samples with mostly unreliable and questionable methods that are now not considered appropriate. In a recent study, patients with migraine had significantly more platelet-leukocyte aggregates in their serum compared with controls.5 Thrombocytosis might have been implicated in attacks of migraine aura in patients with polycythemia vera.65
Endothelial dysfunction might predispose individuals to ischaemic stroke and coronary disease, possibly by impairing vascular reactivity or by promoting platelet activation and enhancing the inflammatory process. We believe that the evidence for migraine is incomplete at present. Antiphospholipid antibodies might increase risk of clotting or might serve as a marker of endothelial perturbation, but are probably not causally associated with migraine.66,67 Impaired endothelium-dependent relaxation of the cerebral and systemic arteries has nevertheless been reported in migraineurs.68 Concentrations of von Willebrand factor antigen activity levels, accepted biomarkers of endothelial dysfunction, are reportedly increased between attacks in patients with migraine and are particularly high in migraineurs with a history of livedo reticularis, a cutaneous marker of endothelial damage.69 Concentrations of von Willebrand factor are also reportedly increased during a migraine attack.70 Additionally, migraineurs have decreased concentrations of circulating endothelial progenitor cells, a non-specific marker of vascular function that is inversely associated with the risk for cardiovascular disease.71 Lending support to the possibility of endothelial dysfunction in migraineurs, forearm blood flow responses were impaired in response to the cerebral vasodilators acetylcholine and sodium nitroprusside, indicating dysfunction at the vascular smooth muscle level.72 Genetic susceptibility to endothelial dysfunction, such as a deletion poly morphism in the angiotensin-converting enzyme gene (ACE-DD genotype) or the methylenetetrahydrofolate reductase C677-TT polymorphism, have been associated with migraine in some, but not all, studies73 and these mutations might increase the risk of ischaemic stroke in patients with migraine aura. Finally, Dreier and colleagues20 have implicated endothelin, the endothelium-derived peptide that causes powerful vasoconstriction, in a proposed model of migraine by showing that local peptide infusion causes CSD as well as brain microinfarcts.
Migraine as a risk factor for ischaemic cerebrovascular disorders
Migraine with aura has been consistently identified as an independent risk factor for ischaemic stroke.6,74–78 Several potential mechanisms have been hypothesised, including alterations in vasoreactivity and cerebral blood flow due to vessel wall dysfunction, release of vasoactive substances such as prostaglandins and endothelins, and, as discussed above, platelet hyperactivity and paradoxical embolism through a cardiac or extracardiac shunt. We presume that focal areas of hypoperfusion trigger CSD as an underlying mechanism for some of the attacks in patients with migraine aura (figure 3). It is likely that the brains of patients with migraine with aura are particularly prone to generating CSD after such perturbations, and the experimental model that has been proposed by Dreier and colleagues supports this possibility.20 Possibly related is the observation that posterior circulation silent microinfarcts occur with greater frequency in young patients,79 suggesting a cardiac source for embolism.80 Kruit and colleagues81 reported MRI evidence of posterior circulation territory microinfarcts in patients with migraine with aura (13 of 161; 8·1%), whereas the occurrence was significantly smaller in patients with migraine without aura (three of 134; 2·2%) or controls (one of 140; 0·7%). A high number of migraine attacks predisposed individuals to ischaemic lesions, as patients who had more than one attack per month reportedly had the highest risk for posterior circulation territory infarcts (odds ratio 15·8). Nevertheless, cardiovascular risk factors were not more prevalent in migraineurs, although PFO was not specifically studied in this cohort of patients.
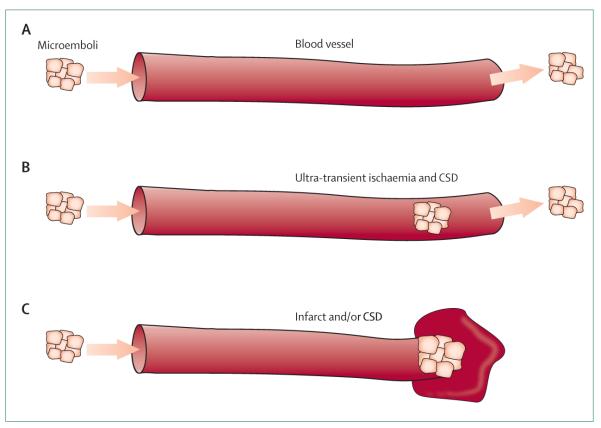
Figure 3. The risk of developing CSD after microembolisation partly depends on the location, size, and duration of vascular occlusion.
Although some microemboli can traverse the brain microcirculation without pathophysiological consequence (A), other microemboli can transiently occlude the circulation to a critical volume of tissue to initiate CSD (B) followed by recovery; more prolonged occlusion (C) will cause tissue microinfarction. CSD=cortical spreading depression.
The small size and territorial distribution of these microinfarcts provide information about their potential source and pathophysiology. In a systematic analysis of the topographical details of these parenchymal defects, Kruit and colleagues82 found that more than 90% of the infarct-like lesions were located in the deep arterial border zone areas of the cerebellum. If implicated, CSD might directly decrease the cerebral perfusion pressure and blood flow and increase the ischaemic burden by slowing the clearance of occluding particles. Additional mechanisms noted above might also contribute, including the release of procoagulant factors and enhanced susceptibility to platelet aggregation,70,83 decreased endothelium-dependent relaxation,84 and increased oxidative stress and inflammation of the vessel wall.5
Consistent with these observations, a recent publication by Scher and colleagues85 documented an increased risk of infarct-like lesions in women who had reported migraine with aura earlier during their life (odds ratio 1·4). Similarly, Kurth and colleagues86 reported a significantly increased risk for ischaemic stroke in women with active migraine aura. Specifically, in a large prospective cohort of women aged more than 45 years, these investigators found a greater than fourfold increase in the risk for ischaemic stroke in individuals with active migraine with aura if the frequency of migraine attacks exceeded once per week. Interestingly, the authors also reported an association between migraine and major cardiovascular events, although the strength and direction of this association seemed to vary with migraine frequency.
Search strategy and selection criteria.
References for this Personal View were identified through searches of PubMed with the search terms “migraine”, “cortical spreading depression”, “cerebrovascular”, “cardiovascular”, “patent foramen ovale”, and “pathophysiology” in combination with “stroke”, “ischemia”, “coagulation”, “hypoxia”, “CADASIL”, “white matter lesions”, “carotid puncture”, “cholesterol”, “atherosclerosis”, “dissection”, “Raynaud”, “Sjögren’s syndrome”, “SLE”, and “antiphospholipid antibody”. Preference was given to papers published between January, 2000, and November, 2009. Only papers published in English were reviewed in detail. The final reference list was selected on the basis of relevance to the topics covered in this paper.
Conclusions
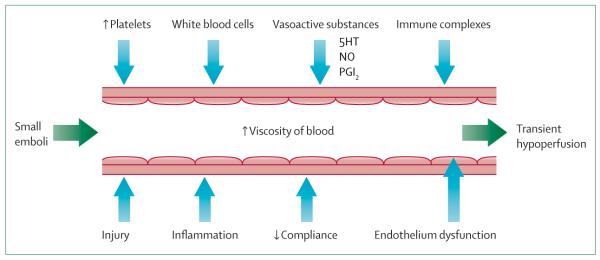
Although an increasing amount of evidence lends support to an association between migraine with aura and ischaemic cerebrovascular disorders, a causative relationship is diffcult to prove and a coherent vascular aetiology is unlikely to account for the triggering of all types of migraine with aura. Nevertheless, the multitude of clinical observations, as well as recent experimental data, suggest a common pathophysiology for these disorders, and indicate that, at least in a subset of patients of underdetermined size, migraine aura exists on a continuum of hypoperfusion disorders that includes transient ischaemic attacks and cerebral infarcts. According to this view, brief periods of hypoperfusion, a final common event for CSD initiation, develop as a consequence of local endothelial or smooth muscle dysfunction together with changes in circulating blood elements, or develop as a downstream complication of events that originate within larger blood vessels (figure 4). As we begin to acquire new knowledge about the regulation of the microcirculation during health and disease, and begin to understand the full effect of how perturbations in microvasculature and neurovascular units modulate brain function in human beings, we will better understand one important trigger for migraine aura.
Figure 4. Cerebral blood vessels are important for the triggering of cortical spreading depression and the pathophysiology of migraine aura.
We propose that in patients with patent foramen ovale, brief periods of local and mild hypoperfusion develop as a consequence of microemboli arising from the venous circulation, or might develop in other conditions in response to injury to the vessel wall, local release of vasoactive substances, increased blood viscosity, circulating immune complexes, endothelial dysfunction, enhanced platelet-endothelial interaction, or platelet-leucocyte interaction among other mechanisms. The potentially important astrocytes and other components of the neurovascular unit are not depicted.
Acknowledgments
Some of the studies cited in this paper were supported by a National Institute of Neurological Disorders and Stroke grant NS35611 (MAM).
Footnotes
Conflicts of interest We have no conflicts of interest.
References
- 1.Kurth T. Associations between migraine and cardiovascular disease. Expert Rev Neurother. 2007;7:1097–104. doi: 10.1586/14737175.7.9.1097. [DOI] [PubMed] [Google Scholar]
- 2.Stang PE, Carson AP, Rose KM, et al. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2005;64:1573–77. doi: 10.1212/01.WNL.0000158326.31368.04. [DOI] [PubMed] [Google Scholar]
- 3.Post MC, Letteboer TG, Mager JJ, Plokker TH, Kelder JC, Westermann CJ. A pulmonary right-to-left shunt in patients with hereditary hemorrhagic telangiectasia is associated with an increased prevalence of migraine. Chest. 2005;128:2485–89. doi: 10.1378/chest.128.4.2485. [DOI] [PubMed] [Google Scholar]
- 4.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser M. CADASIL. Lancet Neurol. 2009;8:643–53. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 5.Zeller JA, Frahm K, Baron R, Stingele R, Deuschl G. Platelet-leukocyte interaction and platelet activation in migraine: a link to ischemic stroke? J Neurol Neurosurg Psychiatry. 2004;75:984–87. doi: 10.1136/jnnp.2003.019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330:63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Brain stem and cerebellar hyperintense lesions in migraine. Stroke. 2006;37:1109–12. doi: 10.1161/01.STR.0000206446.26702.e9. [DOI] [PubMed] [Google Scholar]
- 8.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 9.Nozari A, Dilekoz E, Sukhotinsky I, et al. Microemboli may link spreading depression, migraine aura and patent foramen ovale. Ann Neurol. 2009 doi: 10.1002/ana.21871. published online Sept 14. DOI:10.1002/ana.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–37. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 11.Strong AJ, Fabricius M, Boutelle MG, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–43. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- 12.Fabricius M, Fuhr S, Bhatia R, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–90. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- 13.Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981;9:344–52. doi: 10.1002/ana.410090406. [DOI] [PubMed] [Google Scholar]
- 14.Woods RP, Iacoboni M, Mazziotta JC. Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med. 1994;331:1689–92. doi: 10.1056/NEJM199412223312505. [DOI] [PubMed] [Google Scholar]
- 15.Bowyer SM, Aurora KS, Moran JE, Tepley N, Welch KM. Magnetoencephalographic fields from patients with spontaneous and induced migraine aura. Ann Neurol. 2001;50:582–87. doi: 10.1002/ana.1293. [DOI] [PubMed] [Google Scholar]
- 16.Hadjikhani N, Del Rio M Sanchez, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Nat Acad Sci USA. 2001;98:4687–92. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–42. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–68. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz MA, Bolay H, Dalkara T. Deciphering migraine mechanisms: clues from familial hemiplegic migraine genotypes. Ann Neurol. 2004;55:276–80. doi: 10.1002/ana.20035. [DOI] [PubMed] [Google Scholar]
- 20.Dreier JP, Kleeberg J, Petzold G, et al. Endothelin-1 potently induces Leao’s cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura? Brain. 2002;125:102–12. doi: 10.1093/brain/awf007. [DOI] [PubMed] [Google Scholar]
- 21.Gerich FJ, Hepp S, Probst I, Muller M. Mitochondrial inhibition prior to oxygen-withdrawal facilitates the occurrence of hypoxia-induced spreading depression in rat hippocampal slices. J Neurophysiol. 2006;96:492–504. doi: 10.1152/jn.01015.2005. [DOI] [PubMed] [Google Scholar]
- 22.Somjen GG, Aitken PG, Czeh GL, Herreras O, Jing J, Young JN. Mechanism of spreading depression: a review of recent findings and a hypothesis. Can J Physiol Pharmacol. 1992;70(suppl):S248–54. doi: 10.1139/y92-268. [DOI] [PubMed] [Google Scholar]
- 23.Kunimatsu T, Asai S, Kanematsu K, et al. Transient in vivo membrane depolarization and glutamate release before anoxic depolarization in rat striatum. Brain Res. 1999;831:273–82. doi: 10.1016/s0006-8993(99)01481-x. [DOI] [PubMed] [Google Scholar]
- 24.Mayevsky A, Meilin S, Rogatsky GG, Zarchin N, Thom SR. Multiparametric monitoring of the awake brain exposed to carbon monoxide. J Appl Physiol. 1995;78:1188–96. doi: 10.1152/jappl.1995.78.3.1188. [DOI] [PubMed] [Google Scholar]
- 25.Sukhotinsky I, Dilekoz E, Moskowitz MA, Ayata C. Hypoxia and hypotension transform the blood flow response to cortical spreading depression from hyperemia into hypoperfusion in the rat. J Cereb Blood Flow Metab. 2008;28:1369–76. doi: 10.1038/jcbfm.2008.35. [DOI] [PubMed] [Google Scholar]
- 26.Rundek T, Elkind MS, Di Tullio MR, et al. Patent foramen ovale and migraine: a cross-sectional study from the Northern Manhattan Study (NOMAS) Circulation. 2008;118:1419–24. doi: 10.1161/CIRCULATIONAHA.108.771303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia. 2008;28:531–40. doi: 10.1111/j.1468-2982.2008.01554.x. [DOI] [PubMed] [Google Scholar]
- 28.Rigatelli G. Migraine and patent foramen ovale: connecting flight or one-way ticket? Expert Rev Neurother. 2008;8:1331–37. doi: 10.1586/14737175.8.9.1331. [DOI] [PubMed] [Google Scholar]
- 29.Desai AJ, Fuller CJ, Jesurum JT, Reisman M. Patent foramen ovale and cerebrovascular diseases. Nat Clin Practice Cardiovasc Med. 2006;3:446–55. doi: 10.1038/ncpcardio0597. [DOI] [PubMed] [Google Scholar]
- 30.Steiner MM, Di Tullio MR, Rundek T, et al. Patent foramen ovale size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;29:944–48. doi: 10.1161/01.str.29.5.944. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava TN, Payment MF. Images in clinical medicine. Paradoxical embolism—thrombus in transit through a patent foramen ovale. N Engl J Med. 1997;337:681. doi: 10.1056/NEJM199709043371005. [DOI] [PubMed] [Google Scholar]
- 32.Kurth T, Schurks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ. 2008;337:a636. doi: 10.1136/bmj.a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilmshurst P, Pearson M, Nightingale S. Re-evaluation of the relationship between migraine and persistent foramen ovale and other right-to-left shunts. Clin Sci. 2005;108:365–67. doi: 10.1042/CS20040338. [DOI] [PubMed] [Google Scholar]
- 34.Jang IK, Gold HK, Ziskind AA, et al. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989;79:920–28. doi: 10.1161/01.cir.79.4.920. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs IB, Gorog DA, Yamamoto J. Enhanced spontaneous thrombolysis: a new therapeutic challenge. J Thromb Thrombolysis. 2006;21:221–27. doi: 10.1007/s11239-006-6579-0. [DOI] [PubMed] [Google Scholar]
- 36.Wilmshurst PT, Nightingale S, Walsh KP, Morrison WL. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or stroke or for haemodynamic reasons. Lancet. 2000;356:1648–51. doi: 10.1016/s0140-6736(00)03160-3. [DOI] [PubMed] [Google Scholar]
- 37.Heckmann JG, Lang CJ, Dietrich W, Neidhardt B, Neundorfer B. Symptomatic migraine linked to stroke due to paradoxical embolism and elevated thrombosis risk. Cephalalgia. 2002;22:154–56. doi: 10.1046/j.1468-2982.2002.00338.x. [DOI] [PubMed] [Google Scholar]
- 38.Tzourio C, Benslamia L, Guillon B, et al. Migraine and the risk of cervical artery dissection: a case-control study. Neurology. 2002;59:435–37. doi: 10.1212/wnl.59.3.435. [DOI] [PubMed] [Google Scholar]
- 39.Olesen J, Friberg L, Olsen TS, et al. Ischaemia-induced (symptomatic) migraine attacks may be more frequent than migraine-induced ischaemic insults. Brain. 1993;116:187–202. doi: 10.1093/brain/116.1.187. [DOI] [PubMed] [Google Scholar]
- 40.Lauritzen M, Skyhoj Olsen T, Lassen NA, Paulson OB. Changes in regional cerebral blood flow during the course of classic migraine attacks. Ann Neurol. 1983;13:633–41. doi: 10.1002/ana.410130609. [DOI] [PubMed] [Google Scholar]
- 41.Sevgi E. PhD thesis. Hacettepe University; Ankara, Turkey: 2008. A study on the effect of air micro embolism on cerebral bioelectrical activity with spectral EEG in migraine patients with aura and patent foramen ovale. [Google Scholar]
- 42.Dinia L, Roccatagliata L, Bonzano L, Finocchi C, Del Sette M. Diffusion MRI during migraine with aura attack associated with diagnostic microbubbles injection in subjects with large PFO. Headache. 2007;47:1455–56. doi: 10.1111/j.1526-4610.2007.00948.x. [DOI] [PubMed] [Google Scholar]
- 43.Zaletel M, Zvan B, Kozelj M, et al. Migraine with aura induced by artificial microbubbles. Cephalalgia. 2008;29:480–83. doi: 10.1111/j.1468-2982.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- 44.Dowson A, Mullen MJ, Peatfield R, et al. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 2008;117:1397–404. doi: 10.1161/CIRCULATIONAHA.107.727271. [DOI] [PubMed] [Google Scholar]
- 45.Vigna C, Marchese N, Inchingolo V, et al. Improvement of migraine after patent foramen ovale percutaneous closure in patients with subclinical brain lesions: a case-control study. J Am Coll Cardiol Intv. 2009;2:107–13. doi: 10.1016/j.jcin.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Desmond DW, Moroney JT, Lynch T, Chan S, Chin SS, Mohr JP. The natural history of CADASIL: a pooled analysis of previously published cases. Stroke. 1999;30:1230–33. doi: 10.1161/01.str.30.6.1230. [DOI] [PubMed] [Google Scholar]
- 47.Tuominen S, Miao Q, Kurki T, et al. Positron emission tomography examination of cerebral blood flow and glucose metabolism in young CADASIL patients. Stroke. 2004;35:1063–67. doi: 10.1161/01.STR.0000124124.69842.2d. [DOI] [PubMed] [Google Scholar]
- 48.Pfefferkorn T, von Stuckrad-Barre S, Herzog J, Gasser T, Hamann GF, Dichgans M. Reduced cerebrovascular CO(2) reactivity in CADASIL: a transcranial Doppler sonography study. Stroke. 2001;32:17–21. doi: 10.1161/01.str.32.1.17. [DOI] [PubMed] [Google Scholar]
- 49.Gobron C, Vahedi K, Vicaut E, et al. Characteristic features of in vivo skin microvascular reactivity in CADASIL. J Cereb Blood Flow Metab. 2007;27:250–57. doi: 10.1038/sj.jcbfm.9600356. [DOI] [PubMed] [Google Scholar]
- 50.Lacombe P, Oligo C, Domenga V, Tournier-Lasserve E, Joutel A. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke. 2005;36:1053–58. doi: 10.1161/01.STR.0000163080.82766.eb. [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, Eikermann-Haerter K, Joutel A, Moskowitz MA, Ayata C. Enlarged infarcts in mice expressing the archetypal NOTCH3 R90C CADASIL mutation. J Cereb Blood Flow Metab. 2009;29:S253–54. [Google Scholar]
- 52.Eikermann-Haerter K, Wang Y, Dilekoz E, et al. Increased susceptibility to cortical spreading depression in CADASIL mutant mice. J Cereb Blood Flow Metab. 2009;29:S53–S58. [Google Scholar]
- 53.Kavanagh D, Spitzer D, Kothari PH, et al. New roles for the major human 3′-5′ exonuclease TREX1 in human disease. Cell Cycle. 2008;7:1718–25. doi: 10.4161/cc.7.12.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hottenga JJ, Vanmolkot KR, Kors EE, et al. The 3p21.1-p21.3 hereditary vascular retinopathy locus increases the risk for Raynaud’s phenomenon and migraine. Cephalalgia. 2005;25:1168–72. doi: 10.1111/j.1468-2982.2005.00994.x. [DOI] [PubMed] [Google Scholar]
- 55.Pal B, Gibson C, Passmore J, Griffths ID, Dick WC. A study of headaches and migraine in Sjogren’s syndrome and other rheumatic disorders. Ann Rheum Dis. 1989;48:312–16. doi: 10.1136/ard.48.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Keeffe ST, Tsapatsaris NP, Beetham WP., Jr Increased prevalence of migraine and chest pain in patients with primary Raynaud disease. Ann Intern Med. 1992;116:985–89. doi: 10.7326/0003-4819-116-12-985. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura Y, Shinozaki N, Hirasawa M, et al. Prevalence of migraine and Raynaud’s phenomenon in Japanese patients with vasospastic angina. Jpn Circ J. 2000;4:239–42. doi: 10.1253/jcj.64.239. [DOI] [PubMed] [Google Scholar]
- 58.Appenzeller S, Costallat LT. Clinical implications of migraine in systemic lupus erythematosus: relation to cumulative organ damage. Cephalalgia. 2004;24:1024–30. doi: 10.1111/j.1468-2982.2004.00785.x. [DOI] [PubMed] [Google Scholar]
- 59.Rose KM, Wong TY, Carson AP, Couper DJ, Klein R, Sharrett AR. Migraine and retinal microvascular abnormalities: the Atherosclerosis Risk in Communities Study. Neurology. 2007;68:1694–700. doi: 10.1212/01.wnl.0000261916.42871.05. [DOI] [PubMed] [Google Scholar]
- 60.Rose KM, Carson AP, Sanford CP, et al. Migraine and other headaches: associations with Rose angina and coronary heart disease. Neurology. 2004;63:2233–39. doi: 10.1212/01.wnl.0000147289.50605.dc. [DOI] [PubMed] [Google Scholar]
- 61.Park-Matsumoto YC, Tazawa T, Shimizu J. Migraine with aura-like headache associated with moyamoya disease. Acta Neurol Scand. 1999;100:119–21. doi: 10.1111/j.1600-0404.1999.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 62.Tietjen GE, Al-Qasmi MM, Shukairy MS. Livedo reticularis and migraine: a marker for stroke risk? Headache. 2002;42:352–55. doi: 10.1046/j.1526-4610.2002.02106.x. [DOI] [PubMed] [Google Scholar]
- 63.Facchinetti F, Allais G, Nappi RE, et al. Migraine is a risk factor for hypertensive disorders in pregnancy: a prospective cohort study. Cephalalgia. 2009;29:286–92. doi: 10.1111/j.1468-2982.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 64.Tietjen EG. Migraine and ischaemic heart disease and stroke: potential mechanisms and treatment implications. Cephalalgia. 2007;27:981–87. doi: 10.1111/j.1468-2982.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 65.Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets. 2006;17:528–44. doi: 10.1080/09537100600758677. [DOI] [PubMed] [Google Scholar]
- 66.Williams FM, Cherkas LF, Bertolaccini ML, et al. Migraine and antiphospholipid antibodies: no association found in migraine-discordant monozygotic twins. Cephalalgia. 2008;28:1048–52. doi: 10.1111/j.1468-2982.2008.01646.x. [DOI] [PubMed] [Google Scholar]
- 67.Tietjen GE, Day M, Norris L, et al. Role of anticardiolipin antibodies in young persons with migraine and transient focal neurologic events: a prospective study. Neurology. 1998;50:1433–40. doi: 10.1212/wnl.50.5.1433. [DOI] [PubMed] [Google Scholar]
- 68.de Hoon JN, Willigers JM, Troost J, Struijker-Boudier HA, van Bortel LM. Cranial and peripheral interictal vascular changes in migraine patients. Cephalalgia. 2003;23:96–104. doi: 10.1046/j.1468-2982.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 69.Tietjen GE, Al-Qasmi MM, Athanas K, Dafer RM, Khuder SA. Increased von Willebrand factor in migraine. Neurology. 2001;57:334–36. doi: 10.1212/wnl.57.2.334. [DOI] [PubMed] [Google Scholar]
- 70.Cesar JM, Garcia-Avello A, Vecino AM, Sastre JL, Alvarez-Cermeno JC. Increased levels of plasma von Willebrand factor in migraine crisis. Acta Neurol Scand. 1995;91:41–13. doi: 10.1111/j.1600-0404.1995.tb07030.x. [DOI] [PubMed] [Google Scholar]
- 71.Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008;70:1510–17. doi: 10.1212/01.wnl.0000294329.93565.94. [DOI] [PubMed] [Google Scholar]
- 72.Napoli R, Guardasole V, Zarra E, et al. Vascular smooth muscle cell dysfunction in patients with migraine. Neurology. 2009;72:2111–14. doi: 10.1212/WNL.0b013e3181aa53ce. [DOI] [PubMed] [Google Scholar]
- 73.Lea RA, Ovcaric M, Sundholm J, MacMillan J, Griffths LR. The methylenetetrahydrofolate reductase gene variant C677T influences susceptibility to migraine with aura. BMC Med. 2004;2:3. doi: 10.1186/1741-7015-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henrich JB, Horwitz RI. A controlled study of ischemic stroke risk in migraine patients. J Clin Epidemiol. 1989;42:773–80. doi: 10.1016/0895-4356(89)90075-9. [DOI] [PubMed] [Google Scholar]
- 75.MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438–45. doi: 10.1161/STROKEAHA.107.488395. [DOI] [PubMed] [Google Scholar]
- 76.Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64:1020–26. doi: 10.1212/01.WNL.0000154528.21485.3A. [DOI] [PubMed] [Google Scholar]
- 77.Tzourio C, Iglesias S, Hubert JB, et al. Migraine and risk of ischaemic stroke: a case-control study. BMJ. 1993;307:289–92. doi: 10.1136/bmj.307.6899.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donaghy M, Chang CL, Poulter N. European Collaborators of The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone C. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry. 2002;73:747–50. doi: 10.1136/jnnp.73.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milhaud D, Bogousslavsky J, van Melle G, Liot P. Ischemic stroke and active migraine. Neurology. 2001;57:1805–11. doi: 10.1212/wnl.57.10.1805. [DOI] [PubMed] [Google Scholar]
- 80.Hafeez F, Levine R, Dulli D, Razzaq M. Differing mechanisms between posterior and middle cerebral artery infarctions. J Stroke Cerebrovasc Dis. 1998;7:250–54. doi: 10.1016/s1052-3057(98)80034-7. [DOI] [PubMed] [Google Scholar]
- 81.Kruit MC, van Buchem MA, Hofman PAM, et al. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–34. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- 82.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain. 2005;128:2068–77. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 83.Couch JR, Hassanein RS. Platelet aggregability in migraine. Neurology. 1977;27:843–48. doi: 10.1212/wnl.27.9.843. [DOI] [PubMed] [Google Scholar]
- 84.Olesen J, Thomsen LL, Iversen H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol Sci. 1994;15:149–53. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 85.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–70. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurth T, Schurks M, Logroscino G, Buring JE. Migraine frequency and risk of cardiovascular disease in women. Neurology. 2009;73:581–88. doi: 10.1212/WNL.0b013e3181ab2c20. [DOI] [PMC free article] [PubMed] [Google Scholar]