Abstract
Although matrix metalloproteinase-9 (MMP-9) is involved in cardiomyocytes contractility dysfunction, tissue inhibitor of metalloproteinase-4 (TIMP-4) mitigates the effect of MMP-9, and proteinase-activated receptor-1 (PAR-1, a G-protein couple receptor, GPCR) is involved in the signaling cascade of MMP-9-mediated cardiac dysfunction, the mechanism(s) are unclear. To test the hypothesis that induction of dicer and differential expression of microRNAs (miRNAs) contribute, in part, to the down regulation of sarcoplasmic reticulum calcium ATPase isoform 2a (serca-2a) in MMP-9 and PAR-1-mediated myocytes dysfunction, ventricular cardiomyocytes were isolated from C57BL/6J mice and treated with 3 ng/ml of MMP-9, 12 ng/ml of TIMP-4, and 10 and 100 µM of PAR-1 antagonist with MMP-9. Specific role of MMP-9 was determined by using MMP-9 knock out (MMP-9KO) and their corresponding control (FVB) mice. Ion Optics video-edge detection system and Fura 2-AM loading were used for determining the contractility and calcium release from cardiomyocytes. Quantitative and semi-quantitative PCR were used to determine the expression of dicer, TIMP-4 and serca-2a. miRNA microarrays were used for assessing the expression of different miRNAs between MMP-9KO and FVB cardiomyocytes. The results suggest that MMP-9 treatment attenuates the voltage-induced contraction of primary cardiomyocytes while TIMP-4, an inhibitor of MMP-9, reverses the inhibition. MMP-9 treatment is also associated with reduced Ca2+ transients. This effect is blocked by a PAR-1 antagonist, suggesting that PAR-1 mediates this effect. The effect is not as great at high concentrations (100 µM) perhaps due to mild toxicity. The PAR-1 antagonist effect did not affect calcium transients unlike TIMP-4. Interestingly, we show that MMP-KO myocytes contract more rapidly and release more Ca2+ than FVB. The relevant RNA species serca-2a is induced and dicer is inhibited. There is selective inhibition of miR-376b and over-expression of miR-1, miR-26a, miR-30d, and miR-181c in MMP-9KO that are implicated in regulation of G-PCR and calcium handling.
Keywords: MMP, PAR-1, TIMP, Cardiac dysfunction, Dicer, Serca-2a, Calcium, Cardiomyocytes, Contractility
Introduction
Cardiovascular diseases are often associated with the structural and functional remodeling in cardiomyocytes and the surrounding matrix. Matrix synthesis and degradation is mainly regulated by the balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) axis [1]. In heart, MMP-9 and TIMP-4 are predominantly involved in cardiac remodeling. MMP-9 remains latent but activated during pathological condition and degrades matrix resulting into deposition of oxidized collagen leading to fibrosis [1, 2]. The MMP-9 signals are transduced through proteinase-activated receptor-1 (PAR-1)—a transmembrane G-protein-coupled receptor [3, 16]. The expression of PAR-1 has been established in cardiomyocytes as a high-affinity receptor for thrombin that can also be activated by the tethered-ligand domain sequence (SFLLRN) [4]. It promotes inositol trisphosphate accumulation, stimulates extracellular signal-regulated protein kinase and modulates contractile function [4]. In fibrosis, the increased matrix turn over [5, 6] and reduced elasticity impairs cardiomyocytes contractile function. To compensate the function, cardiomyocytes undergo structural remodeling that causes hypertrophy and culminates into heart failure. Although the fact that MMP-9 is involved in cardiomyocytes contractility dysfunction, TIMP-4 mitigates the effect of MMP-9 and PAR-1 is involved in the signaling cascade of MMP-9-mediated contractile dysfunction is documented, the mechanism is incompletely understood.
The contractility of cardiomyocytes is dependant on intracellular calcium influx [7]. Calcium is released from sarcoplasmic reticulum of the cell and the extracellular calcium enters into the cell through voltage-gated calcium ion channel. The sarcoplasmic reticulum calcium ATPase isoform 2a (serca-2a) is a calcium signaling related gene that modulates contractility in cardiomyocytes and is tightly associated with the intracellular calcium influx [8]. The transmembrane PAR-1 is reported to increase the intracellular calcium [9]. Calcium flux also depends on opening and closing of calcium ion channel, which is maintained by calcium channel beta-2 (CACNB2) gene in a precise manner.
MicroRNA (miRNA or miR) are ~22 nucleotide long, conserved, and non-coding RNAs that negatively modulates gene expression, and are recognized as key regulators of biological processes [10]. The selective inhibition of miRNAs leads to hypertrophy, fibrosis, and arrhythmia [10]. The ion channels required for proper functioning of heart is also regulated by miRNAs. For example, miR-30 regulates CACNB2—a calcium ion channel gene [11]. The biogenesis of miRNAs requires dicer, an RNase III endonuclease [10]. In addition to the miRNA maturation, dicer is also indispensable for survival [12]. The expression of dicer changes in pathological condition. It is found that dicer is induced during compensatory heart failure [12] suggesting its direct involvement in maintaining the homeostasis.
Here, we investigated the impact of MMP-9, TIMP-4, and PAR-1 on contractile function of cardiomyocytes extracted from C57BL/6J mice. The specific role of MMP-9 on contraction/relaxation cycle and calcium flux was studied by using MMP-9KO and their FVB control mice. Further, the discoordination in calcium handling and alteration in serca-2a expression was determined. Our focus was to understand the mechanism of MMP-9-mediated contractile dysfunction of cardiomyocytes through differential expression of miRNAs and dicer.
Materials and Methods
Animal Models
Three types of mice namely C57BL/6J, MMP-9KO, and FVB were procured from The Jackson Laboratory (Bar Harbor, ME), and were maintained in 12:12 h light–dark cycle with normal mouse chaw diet in the animal facility of the University of Louisville. When mice attained the age of 10 weeks, they were sacrificed following the protocol approved by the Institutional Animal care and Use Committee of the University of Louisville. Further, the animal care and use programs were carried out according to standard protocol and guidelines of National Institute of Health (NIH) and Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 86-23, revised 1985) and the regulation of the Animal Welfare Act.
Experimental Protocols
Freshly isolated cardiomyocytes from 10- to 11-week-old male C57BL/6J mice were separately incubated with MMP-9—3 ng/ml (Abcam catalogue# ab39308), TIMP-4 (Abcam catalogue #ab39319)—12 ng/ml, and PAR-1(Sigma-M2192)—10 and 100 µM for 20 min. To determine the effect of TIMP-4, cardiomyocytes were pre-incubated with TIMP-4 (12 ng/ml) for 20 min before MMP-9 treatment. The rate of systolic contraction (dL/dt) and diastolic relaxation (−dL/dt) was measured by video-based edge detection system (IonOptix, Milton, MA). Additionally, calcium transients were also measured. Age- and sex-matched MMP-9KO and FVB mice were used for comparing the rate of contraction and relaxation as well as calcium flux. To understand the mechanism of contractile dysfunction, the MMP-9KO and FVB cardiomyocytes were used for determining the expression of serca-2a, dicer, and microRNAs.
Protocol for Adult Cardiomyocytes Isolation from Ventricles
Cardiomyocytes from 10- to 11-week-old male mice were isolated by the enzymatic dissociation of Liberase Blendzyme 4 (Roche Diagnostics, IN, USA). The animal was injected with heparin (1000 units/kg i.p.) and anaesthetized with TBE (Avertin 100 mg/kg BW i.p.). The heart was rapidly removed and perfused (37°C) with oxygenated (5% CO2/95% O2) perfusion buffer (135 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM HEPES, 0.33 mM NaH2PO4, 10 mM glucose, 10 mM BDM) at a flow rate of 4.0 ml/min for 4 min. The time from heart extraction to perfusion was limited to 1 min. Subsequently, heart was perfused with digestion buffer (30 ml perfusion buffer and 1.0 ml of 10 mg/ml Liberase Blendzyme 4) for 7–8 min that made heart flaccid. After perfusion left ventricle was removed, minced, and resuspended in the same buffer at room temperature (RT). Extracellular calcium was added to digestion buffer in increments to 1.2 mM/l. Isolated myocytes were maintained at RT in the digestion buffer with calcium.
Measurement of Contraction and Relaxation of Cardiomyocytes
Mechanical properties of cardiomyocytes were determined by video-based edge detection system (IonOptix, Milton, MA) as described elsewhere [13]. In brief, contraction and relaxation of individual cardiomyocyte was scored by an IonOptix MyoCam camera after stimulation by a MyoPacer field stimulator at a frequency of 1.0 Hz, pulse duration of 4 ms, and amplitude of 10 V. After scoring 5 stimulated myocytes, it was replaced with a fresh batch of unstimulated myocytes to minimize the effect of prolong stimulation. For each data set, 20–25 cardiomyocytes with good pattern of amplitude and shortening and lengthening were recorded from a single animal. The number of mice for each experiment was 5–7. The rate of contraction and relaxation as well as calcium consumption was determined by Soft-Edge software.
Measurement of Intracellular Calcium of Cardiomyocytes
The Fura-2 AM was used to detect the intracellular calcium influx. A separate cohort of myocytes was loaded with Fura 2-AM (1.0 µmol/l) for 30 min, and fluorescence was recorded with a dual-excitation fluorescence photomultiplier system (IonOptix). Myocytes were stimulated at the frequency of 1.0 Hz. During contraction/relaxation cycle fluorescence was detected between 480 and 520 nm by a photomultiplier tube after first illuminating the cells at 360 nm for 0.5 s and then at 380 nm for the duration of the recording (333-Hz sampling rate). Intracellular Ca2+ transients were measured in terms of Fura 2 fluorescence intensity (ΔFFI) and determined by calculating the difference between systolic and diastolic Ca2+ levels (ΔFFI = peak FFI – baseline FFI). The time course of the fluorescence signal decay (Tau: the duration where Ca2+ transient decays 67% from the peak level) was calculated to assess intracellular Ca2+ clearance rate.
RNA Extraction and Quality Assessment
Freshly isolated cardiomyocytes were immediately processed for RNA extraction using the mirVana™ miRNA isolation kit (Ambion, Part number #AM1560) following the kit protocol. The quality of total RNA was determined by NanoDrop ND-1000 and only highly pure quality RNA (260/280—2.00 and 260/230—2.00) was used for RT-PCR, QPCR, and miRNA microarrays.
Semi-Quantitative Reverse Transcription-PCR (RT-PCR)
The total RNA (200–500 ng) was reverse transcribed by two-step process using Promega RT-PCR kit. The program and methodology has been described elsewhere [14].
Quantitative Real-Time PCR (QPCR)
The Syber-green dye and Stratagene Mx3000p cycler was used for Real-time quantitative PCR as described elsewhere [14]. The primers for dicer, TIMP-4, serca-2a, and GAPDH were designed using primer 3 input (primer3_www.cgiv02) and their sequences are listed in the Table 1.
Table 1.
Primers used for real-time PCR and RT-PCR of cardiomyocytes from MMP-9KO and FVB mice
| Primers | Forward | Reverse |
|---|---|---|
| GAPDH | ATG GGA AGC TGG TCA TCA AC | TGT GAG GGA GAT GCT CAG TG |
| Dicer | TGG AGC GAA TTC TCA GGA GT | CAC AGG GCG TGT ATT TTC CT |
| Serca-2a | TCT TCA TAA CAC ACG CCA ATT | CCC TTT GCT GCC AAT TAA CTA |
| TIMP-4 | CTT ATC TGC CTT GCC CTC AG | TGT TCA GCT CCC ACT GTG TC |
miRNA Microarrays Analyses
The RNA extracted from MMP-9KO and FVB cardiomyocytes by mirVana method was used for miRNA microarrays analyses. Taqman Rodent MicroRNA array A (Applied Biosystems, Part No: 4398979) was used for determining differential expression of miRNAs in MMP-9KO and FVB cardiomyocytes following the protocol of company. Only good quality amplifications were used for data analyses using SDS RQ Manager Software by Applied Biosystems.
Statistics
The contractility and calcium measurement was performed on 20–25 cardiomyocytes from single animal and 5–7 animals from each group. The values were represented as average ±SD (standard deviation). The significance level of 95% was thoroughly used and P < 0.05 was represented as star (*). Student t-test was applied for determining statistically significant difference.
Results
MMP-9 Attenuated Myocytes Contraction/Relaxation and TIMP-4 Mitigated the Myocytes Dysfunction
The contraction/relaxation cycle of C57BL/6J untreated cardiomyocytes was used as control. The treatment of MMP-9 significantly decreased the cell shortening, rate of contraction (dL/dt), and relaxation (−dL/dt) of cardiomyocytes (Fig. 1a–d). However, pre-incubation with TIMP-4 reduced the effect of MMP-9 (Fig. 1a–d). Nevertheless, TIMP-4 itself did not affect the contractile function (Fig. 1a–d).
Fig. 1.
Percent (%) myocyte contraction and relaxation: a Adult cardiomyocytes were isolated from C57BL/6J mouse heart (control). Myocytes were treated with MMP-9 (3 ng/ml), prior to stimulation (MMP-9). To inhibit MMP-9, myocytes were pre-treated with TIMP-4 (12 ng/ml) prior to adding MMP-9 (TIMP-4 + MMP-9) and control myocytes treated with TIMP-4 (TIMP-4). Myocytes were stimulated at 1 Hz. b % Myocyte cell shortening, c Rate of systolic contraction; d Rate of diastolic relaxation. Each bar represents average ± SD from 5 to 7 sets of myocytes preparation. * P < 0.05 compared with untreated control
MMP-9 Attenuated the Calcium Contraction and TIMP-4 Mitigated the MMP-9-Mediated Calcium Mishandling in Cardiomyocytes
The untreated C57BL/6J cardiomyocytes were used as control for calcium consumption. The ratio of intracellular calcium released by sarcoplasmic reticulum was significantly reduced in MMP-9 treated myocytes (Fig. 2a, b). Although TIMP-4 did not have independent effect on the calcium ratio, it improves the calcium release by attenuating the effect of MMP-9 (Fig. 2a, b).
Fig. 2.
Calcium (Ca2+) transients. a The ratio of FURA-2 fluorescence binding to calcium was recorded. Myocytes were treated with MMP-9 (3 ng/ml) or TIMP-4 (12 ng/ml), prior to stimulation (MMP-9 or TIMP-4). To inhibit MMP-9, myocytes were pre-treated with TIMP-4 (12 ng/ml) prior to adding MMP-9 (TIMP-4 + MMP-9). Myocytes were stimulated at 1 Hz. b Histographic presentation of Ca2+ transients. Each bar represents average ± SD from 5 to 7 sets of myocytes preparation. * P < 0.05 compared with untreated control
PAR-1 Antagonist Mitigated MMP-9-Mediated Myocytes Dysfunction
To determine the role of the transmembrane G-coupled PAR-1 in MMP-9-mediated contractile dysfunction, cardiomyocytes were pre-incubated with different doses (10 and 100 µM) of PAR-1 antagonist before MMP-9 treatment. Both lower and higher doses of PAR-1 antagonist attenuated the effect of MMP-9. It ameliorated the rate of contraction and relaxation and improved cell shortening of MMP-9 treated cardiomyocytes (Fig. 3a–d) pointing to involvement of PAR-1 in the signaling cascade of MMP-9-mediated contractile dysfunction.
Fig. 3.
Percent (%) myocyte contraction and relaxation: a Adult cardiomyocytes were isolated from C57BL/6J mouse heart (control). Myocytes were treated with MMP-9 (3 ng/ml) or TIMP-4 (12 ng/ml), prior to stimulation (MMP-9 or TIMP-4). To block PAR-1, myocytes were pre-treated with PAR-1 antagonist (10 and 100 µM), prior to adding MMP-9 (10 µM PAR + MMP-9) and (100 µM PAR + MMP-9). Myocytes were stimulated at 1 Hz. b % Myocyte cell shortening, c Rate of systolic contraction, d Rate of diastolic relaxation. Each bar represents average ± SD from 5 to 7 sets of myocytes preparation. * P < 0.05 compared with untreated control
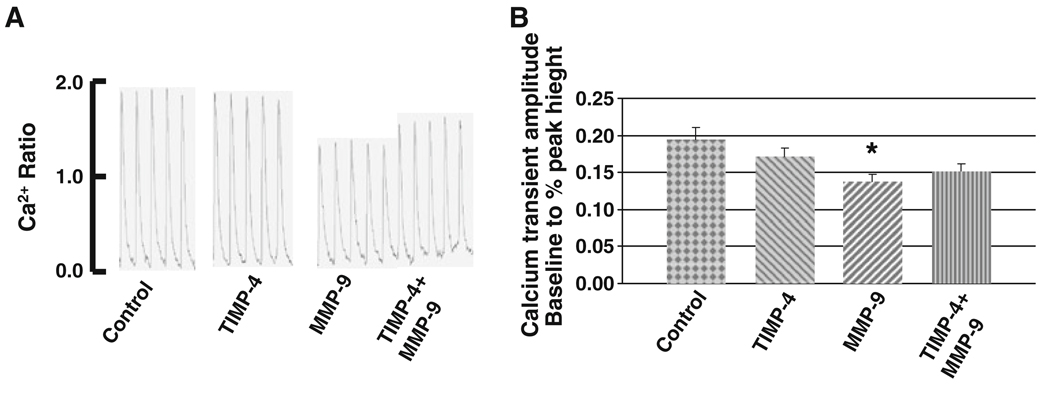
Effect of MMP-9 and PAR-1 Antagonist on Calcium Transients in Cardiomyocytes
Although the improvement of contractile function after treatment with PAR-1 antagonist was apparent, the calcium handling show different trend. The baseline to % peak height (calcium transient amplitude) decreased significantly after treatment with lower dose (10µM) of PAR-1 antagonist. However, the higher dose (100 µM) of PAR-1 antagonist increased the calcium ratio (Fig. 4a, b). On the other hand, calcium ratio for time to attain 90% baseline was lower in low dose of PAR-1 antagonist but significantly decreased in higher dose (Fig. 4c). It might be caused due to mild toxic effect of PAR-1 antagonist in higher dose.
Fig. 4.
Calcium (Ca2+) transients. a The fluorescence ratio of FURA-2 and binding to calcium was recorded (control). Myocytes were treated with MMP-9 (3 ng/ml) prior to stimulation (MMP-9). To block PAR-1, myocytes were pre-incubated with PAR-1 antagonist (10 and 100 µM), prior to adding MMP-9 (10 µM PAR + MMP-9) and (100 µM PAR + MMP-9). Myocytes were stimulated at 1 Hz. b Histographic presentation of Ca2+ transients during systolic contraction. c Histographic presentation of Ca2+ transients during diasystolic relaxation. Each bar represents average ± SD from 5 to 7 sets of myocytes preparation. * P < 0.05 compared with untreated control
Cardiomyocytes Contractions in MMP-9 Knock Out and Their Corresponding Control (FVB) Mice
Although baseline to % peak height was same, MMP-9KO required less time for contraction than FVB cardiomyocytes (Fig. 5a–c). The departure and relaxation velocity of MMP-9KO was higher than that of FVB cardiomyocytes (Fig. 5d, e).
Fig. 5.
Myocyte lengthening (micron, µM). a Myocytes from MMP-9KO and FVB mice were isolated. The cell lengthening and duration of lengthening were measured. Myocytes were stimulated at 1 Hz. b Time to contraction, c Time to relaxation, d Rate of systolic contraction, and e Rate of diastolic relaxation. Each bar represents average ± SD from 5 to 7 sets of myocytes preparation. * P < 0.05 compared with FVB myocytes
The increased contractile function of MMP-9KO was corroborated by the fact that it released more intracellular calcium in a particular time span (Fig. 6a). The rate of transient calcium decay (Tau) was significantly lower (that means decay was faster) in MMP-9KO cardiomyocytes (Fig. 6b). Further, the calcium released during baseline to peak height was higher in MMP-9KO cardiomyocytes (Fig. 6c).
Fig. 6.
Calcium (Ca2+) transients. a The fluorescence ratio of FURA-2 and binding to calcium was recorded. Myocytes from MMP-9KO and FVB mice were isolated. Calcium ratio at cell lengthening and the duration of lengthening was measured. Myocytes were stimulated at 1 Hz. b Rate of decay in calcium transients. c Histographic presentation of Ca2+ transients. Each bar represents average ± SD from 5 to 7 sets of myocytes preparation. * P < 0.05 compared with FVB myocytes
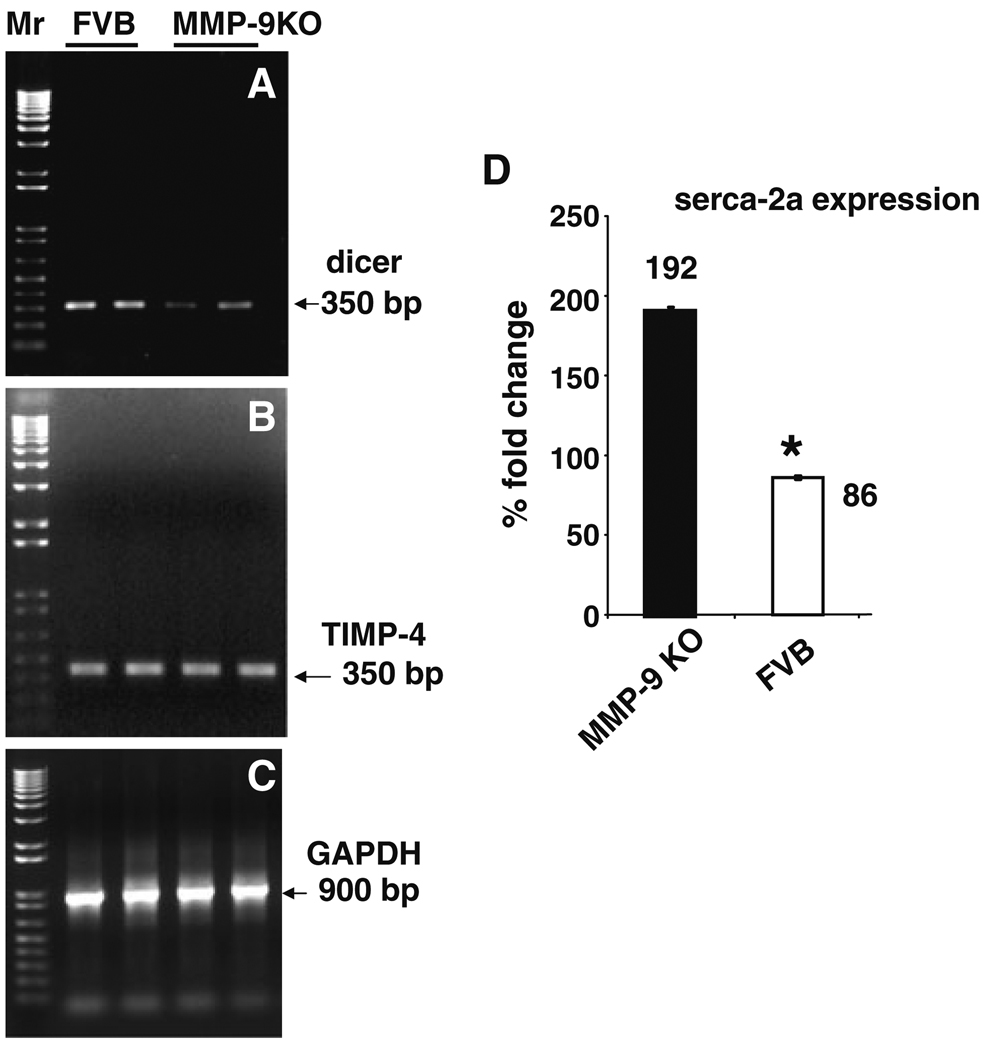
The molecular mechanism underlying the physiological changes in contraction/relaxation cycle of MMP-9 and FVB was determined by measuring the levels of dicer, TIMP-4 and serca-2a. The RT-PCR result showed attenuation of dicer in MMP-9KO mice (Fig. 7a). On the other hand, there was no change in TIMP-4 level in MMP-9KO and FVB cardiomyocytes (Fig. 7b). The quantitative PCR showed up regulation of calcium handling genes, serca-2a in MMP-9KO cardiomyocytes (Fig. 7d).
Fig. 7.
Semi-quantitative RT-PCR analysis of dicer, TIMP-4, and serca-2a. The mRNA from FVB and MMP-9KO cardiomyocytes was isolated and amplified for dicer (a) and TIMP-4 (b) regions. GAPDH (c) was used as loading control. d The bar graph represents real-time PCR amplification of serca-2a in FVB and MMP-9KO cardiomyocytes. GAPDH was used as endogenous control. Each bar represents average ± SD from 5 to 7sets of myocytes preparation. * P < 0.05 compared with FVB myocytes
The microRNA microarrays data revealed differential expression of several miRNAs in MMP-9KO cardiomyocytes (Table 2). Fifteen miRNAs showed robust expression while seventeen remain equal or mild up regulation. However, six miRNAs were down-regulated in MMP-9KO cardiomyocytes (Table 2).
Table 2.
Differential expression of selective miRNAs in MMP-9KO cardiomyocytes
| Down-regulated miRNAs |
Unchanged or mild up-regulated miRNAs |
Robust up-regulated miRNAs |
|---|---|---|
| mmu-miR-192 | mmu-let-7b,c,d,e,i | mmu-miR-1 |
| mmu-miR-197 | mmu-miR-19b | mmu-let-7 g |
| mmu-miR-188-5p | mmu-miR-27a | mmu-miR-24 |
| mmu-miR-376b | mmu-miR-30d | mmu-miR-26a |
| mmu-miR-494 | mmu-miR-92a | mmu-miR-29a |
| mmu-miR-574-3p | mmu-miR-106b | mmu-miR-30b, c, d |
| mmu-miR-152 | mmu-miR-98 | |
| mmu-miR-193b | mmu-miR-126-3p, -5p | |
| mmu-miR-218 | mmu-miR-133a | |
| mmu-miR-328 | mmu-miR-135a | |
| mmu-miR-342-3p | mmu-miR-145 | |
| mmu-miR-499 | mmu-miR-222 | |
| mmu-miR-744 | mmu-miR-223 | |
| mmu-miR-150 | ||
| mmu-miR-181c |
Discussion
The matrix turn over depends on activation of MMP-9 and affects contractile function of cardiomyocytes. Here, we used MMP-9, TIMP-4 and PAR-1 antagonist to substantiate the fact that MMP-9 compromises contractile function of cardiomyocytes through PAR-1 signaling cascade and TIMP-4 mitigates the effect of extracellular MMP-9 (Figs. 1, 2, 3, and 4). Extracellular MMP-9 treatment decreases the rate of contraction (dL/dt) and relaxation (−dL/dt) of C57BL/6J cardiomyocytes (Fig. 1a, c, d). The mitigation of effect of MMP-9 by pre-incubation with TIMP-4 (inhibitor of MMP-9) suggests that contractile dysfunction is mediated by MMP-9 (Fig. 1a, c, d). We measured intracellular calcium ions released from sarcoplasmic reticulum by using Fura-2. The calcium flux results showed that contraction/relaxation cycle is a calcium-dependant process and is influenced by MMP-9 (Fig. 2a, b). Blocking of MMP-9 by PAR-1 mitigated the effect of MMP-9 on contractility (Figs. 3, 4) suggesting that PAR-1 mediates the signaling cascade of MMP-9 [15].
The specific role of MMP-9 (intracellular as well as extracellular) was determined by contractility and calcium flux studies on MMP-9KO and FVB mice. It was found that MMP-9KO required significantly less time for contraction but had higher rate of contraction and relaxation than the corresponding control (FVB) cardiomyocytes (Fig. 5a–e). The intracellular calcium transients were measured as changes in Fura 2 fluorescence intensity (FFi). The change in intensity (ΔFFi) was determined by the difference between the levels of calcium in the systolic and diastolic conditions (ΔFFi = peak FFi − baseline FFi). The time course of the fluorescence decay (the duration where calcium transient decays 67% from the peak level) was used to calculate the intracellular calcium clearing rate. The amplitude and peak of Fura-2 fluorescence determines the release of intracellular calcium. The contractility and calcium flux study was performed on the same batch of cardiomyocytes for each group of animals. The rate of transient calcium decay (Tau)—an indicator of capability of cell to restore calcium [16], was low in MMP-9KO suggesting that it consumed relatively less calcium during contraction (Fig. 6a, b). The amplitude of contraction was higher in MMP-9KO (Fig. 6a, c) that points to better contraction and relaxation capacity. These findings extend support to the fact that inhibition of MMP-9 protects contractile dysfunction of myocytes [17].
The contraction/relaxation pattern and calcium cycling suggests that systolic and diastolic movement is a calcium dependant process and is rapid in MMP-9KO cardiomyocytes (Figs. 5, 6). The calcium flux is regulated by serca-2a [8, 18, 19]. As expected, it was up-regulated in MMP-9KO cardiomyocytes (Fig. 7d). The basal level of TIMP-4 was maintained in MMP-9KO and FVB (Fig. 7b) that can be explained by the reduced or normal activity of MMP-9 in both MMP-9KO and FVB mice.
Dicer is induced in compensatory stage of failing heart [12] and pathological conditions such as hyperhomocysteinemia [14]. Dicer mutant also have dysregulation of contractile proteins and sarcomere disarrays [12]. In MMP-9KO dicer was down-regulated (Fig. 7a) suggesting that pathological conditions can be ameliorated by inhibiting MMP-9. The selective inhibition of miRNAs also induces cardiovascular diseases [10]. The differential expression of miRNAs in MMP-9KO and FVB mice revealed several putative candidates (Table 2). Fifteen miRNAs showed robust up regulation in MMP-9KO cardiomyocytes while 17 miRNAs remained unchanged or showed mild up regulation. In contrast, six miRNAs were down-regulated in MMP-9KO cardiomyocytes (Table 2). Interestingly, miR-1 that target “Sri” proteins, which is implicated regulation of sarcoplasmic reticulum calcium release (http://mirdb.org/miRDB/) was robust in MMP-9KO. Similarly, miR-26a that regulates L-type voltage-gated calcium channel was over expressed in MMP-9KO (Table 2). Other miRNAs involved in regulation of calcium channel were miR-30d and miR-181c (Table 2). There were several miRNAs involved in regulation of G-protein coupled receptors and differentially expressed in MMP-9KO cardiomyocytes. These were miR-376b (down-regulated), miR-92a, miR-152, miR-218 (unchanged or mild up-regulated) and miR-181c (robust expression) (Table 2). Interestingly, miR-494, which is down-regulated in MMP-9KO cardiomyocytes targets MMP-13 (or MMP-1) that is implicated in mechanical adaptation (http://mirdb.org/miRDB/).
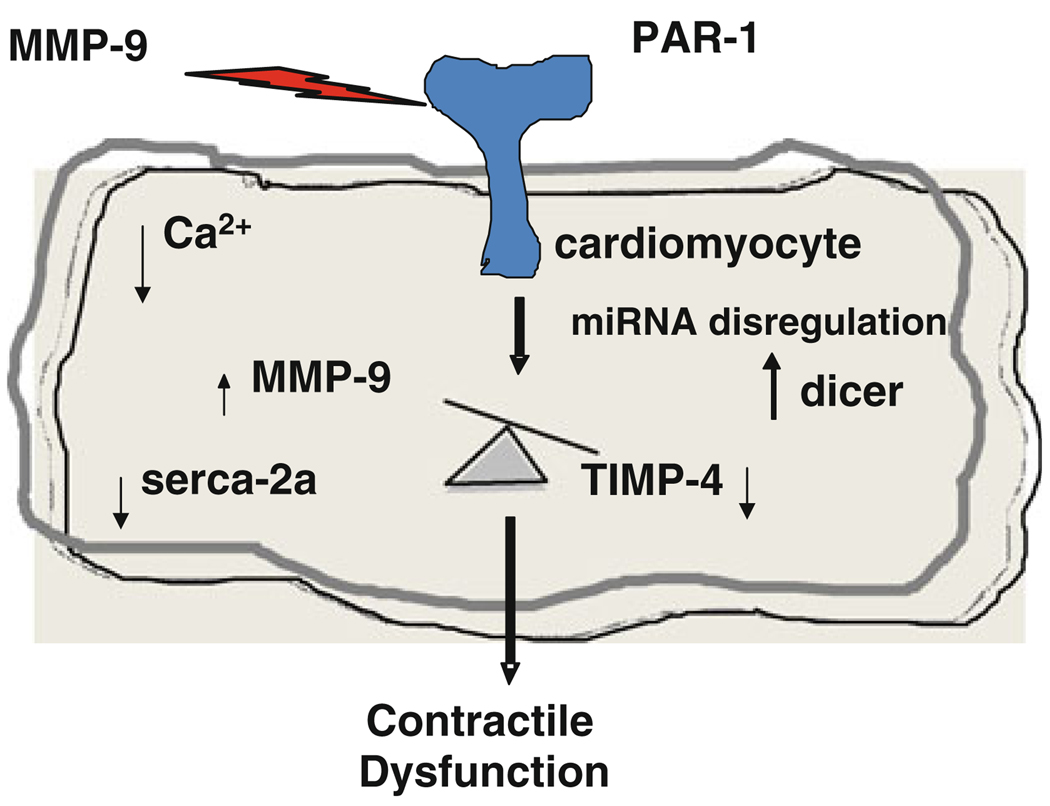
In summary, the treatment of C57BL/6J cardiomyocytes with MMP-9 decreases rate of contraction and relaxation (±dL/dt) and TIMP-4 ameliorates it (Fig. 1). Additionally, PAR-1 antagonist also improves contraction and relaxation (Fig. 3). The MMP-9KO cardiomyocytes show increased contraction and relaxation than the corresponding control (FVB) reinforcing the fact that endogenous MMP-9 also influenced contractility of cardiomyocytes. The corresponding increase in serca-2a expression in MMP-9KO mice suggests that dysregulation of sercoplasmic calcium flux plays important role in the contractile dysfunction. The decrease in dicer expression in MMP-9KO and differential expression of miRNAs points to plausible role of miRNAs in MMP-9-mediated contractile function of cardiomyocytes. Based on these findings, we hypothesize that active MMP-9 induce signaling cascade into cytoplasm through PAR-1 receptor and disturbs the intracellular balance between MMP-9/TIMP-4 axis. The high cytoplasmic MMP-9 causes contractile dysfunction in cardiomyocytes by inducing dicer and inhibiting serca-2a. The differential expression of selective miRNAs plays important role in contractile function (Fig. 8b).
Fig. 8.
Model showing effect of MMP-9 on contractile dysfunction: The active MMP-9 passes signal into cytoplasm through PAR-1 receptor and disturbs the intracellular balance of MMP-9/TIMP-4 axis. The high cytoplasmic MMP-9 causes contractile dysfunction in cardiomyocytes through altering the expression of dicer and serca-2a. Differential expression of selective miRNAs plays important role in contractility dysfunction
Perspective
The inhibition of MMP-9 by either TIMP-4 or PAR-1 antagonist or by knocking down the gene itself improved cardiac function. It indicates that MMP-9, TIMP-4, and PAR-1 are promising therapeutic candidates for cardiovascular diseases. Additionally, considering the emerging role of miRNAs as a biomarker and therapeutic target, the present findings provides putative candidate miRNAs implicated in MMP-9-mediated contractility dysfunction of cardiomyocytes.
Limitation
The present findings showed inhibition of dicer in MMP-9KO. However, it can be just coincidental, or a consequence or prerequisite of MMP-induced cardiomyocytes malfunction. To confirm the role of dicer in MMP-9-mediated contractile dysfunction, two approaches can be used: (i) targeted deletion of dicer in heart and measuring the level of MMP-9 and (ii) over expressing dicer in MMP-9KO heart and determining the contractile function of cardiomyocytes. Although the present findings suggest the role of MMP-9 in contractile dysfunction and involvement of dicer, miRNAs and serca-2a in the mechanism of contractility dysfunction, further investigations will be required to establish the findings. The differential expression of miRNAs provides only potential candidates. To confirm the exact role of each miRNAs, functional studies for contractility dysfunction are required after knocking down (robust miRNAs) or over-expressing (down-regulated miRNAs) these putative miRNAs in mice.
Acknowledgment
A part of this study was supported by NIH grants: HL-74185 and HL-88012.
Contributor Information
Paras Kumar Mishra, Email: pkmish01@louisville.edu.
Suresh C. Tyagi, Email: suresh.tyagi@louisville.edu.
References
- 1.Tyagi SC, Hoit BD. Metalloproteinase in myocardial adaptation and maladaptation. Journal of Cardiovascular Pharmacology and Therapeutics. 2002;7:241–246. doi: 10.1177/107424840200700407. [DOI] [PubMed] [Google Scholar]
- 2.Ali MA, Schulz R. Activation of MMP-2 as a key event in oxidative stress injury to the heart. Frontiers in Bioscience. 2009;14:699–716. doi: 10.2741/3274. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacological Reviews. 2001;53:245–282. [PubMed] [Google Scholar]
- 4.Sabri A, Muske G, Zhang H, Pak E, Darrow A, Andrade-Gordon P, et al. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circulation Research. 2000;86:1054–1061. doi: 10.1161/01.res.86.10.1054. [DOI] [PubMed] [Google Scholar]
- 5.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham HK, Horn M, Trafford AW. Extracellular matrix profiles in the progression to heart failure. European Young Physiologists Symposium Keynote Lecture-Bratislava 2007. Acta Physiol (Oxf) 2008;194:3–21. doi: 10.1111/j.1748-1716.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Kain V, Sitasawad SL. Cardiotoxicity of calmidazolium chloride is attributed to calcium aggravation, oxidative and nitrosative stress, and apoptosis. Free Radical Biology and Medicine. 2009;47:699–709. doi: 10.1016/j.freeradbiomed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Khan H, Metra M, Blair JE, Vogel M, Harinstein ME, Filippatos GS, et al. Istaroxime, a first in class new chemical entity exhibiting SERCA-2 activation and Na-K-ATPase inhibition: A new promising treatment for acute heart failure syndromes? Heart Failure Reviews. 2009;14:277–287. doi: 10.1007/s10741-009-9136-z. [DOI] [PubMed] [Google Scholar]
- 9.Ide J, Aoki T, Ishivata S, Glusa E, Strukova SM. Proteinase-activated receptor agonists stimulate the increase in intracellular Ca2+ in cardiomyocytes and proliferation of cardiac fibroblasts from chick embryos. Bulletin of Experimental Biology and Medicine. 2007;144:760–763. doi: 10.1007/s10517-007-0425-z. [DOI] [PubMed] [Google Scholar]
- 10.Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. Journal of Cellular and Molecular Medicine. 2009;13:778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latronico MV, Catalucci D, Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circulation Research. 2007;101:1225–1236. doi: 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- 12.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, et al. Targeted deletion of dicer in the heart leads to dilated cardiomyopathy and heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, et al. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 14.Mishra PK, Tyagi N, Kundu S, Tyagi SC. MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochemistry and Biophysics. 2009;55:153–162. doi: 10.1007/s12013-009-9063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moshal KS, Tyagi N, Henderson B, Ovechkin AV, Tyagi SC. Protease-activated receptor and endothelial-myocyte uncoupling in chronic heart failure. American Journal of Physiology. Heart and Circulatory Physiology. 2005;288:H2770–H2777. doi: 10.1152/ajpheart.01146.2004. [DOI] [PubMed] [Google Scholar]
- 16.Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. American Journal of Physiology. Heart and Circulatory Physiology. 2008;295:H890–H897. doi: 10.1152/ajpheart.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leon H, Baczko I, Sawicki G, Light PE, Schulz R. Inhibition of matrix metalloproteinases prevents peroxynitrite-induced contractile dysfunction in the isolated cardiac myocyte. British Journal of Pharmacology. 2008;153:676–683. doi: 10.1038/sj.bjp.0707621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz JJ, Glascock BJ, Witt SA, Nieman ML, Nattamai KJ, Liu LH, et al. Accelerated onset of heart failure in mice during pressure overload with chronically decreased SERCA2 calcium pump activity. American Journal of Physiology. Heart and Circulatory Physiology. 2004;286:H1146–H1153. doi: 10.1152/ajpheart.00720.2003. [DOI] [PubMed] [Google Scholar]
- 19.Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, et al. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovascular Research. 2001;50:495–508. doi: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]