Abstract
Top-down signals from frontal cortex (FC) are conjectured to play a critical role in cognitive control of sensory processing. To explore this interaction, we compared activity in ferret FC and primary auditory cortex (A1) during auditory and visual tasks requiring discrimination between classes of reference and target stimuli. FC responses were behaviorally-gated, selectively encoded the timing and invariant behavioral meaning of target stimuli, could be rapid in onset, and sometimes persisted for hours following behavior. This mirrors earlier findings in A1that attention triggered rapid, selective, persistent, task-related changes in spectrotemporal receptive fields. Simultaneously recorded local field potentials (LFPs) revealed behaviorally-gated changes in inter-areal coherence, selectively modulated between FC and focal regions of A1 responsive to target sounds. These results suggest that A1 and FC dynamically establish a functional connection during auditory behavior that shapes the flow of sensory information and maintains a persistent trace of recent task-relevant stimulus features.
Keywords: Frontal Cortex, Prefrontal Cortex, Attention, Top-down, Gating, Auditory, Ferret
INTRODUCTION
Studies of prefrontal cortex (PFC) have provided considerable evidence for its role in high-level executive functions, including stimulus categorization1-3, planning and decision-making4 and working memory5. A fundamental component of these functions is the cognitive control of the flow of sensory inputs through cortex via top-down feedback from PFC6,7. The top-down signals are thought to facilitate the processing of task-relevant information and their effects are manifested by attention-driven changes in spatial or feature stimulus selectivity that occur in sensory areas when an animal engages in goal-directed behavior8-11. Premotor cortex (PMC) shares some common response properties with PFC such as similar attentional modulation and representation in the same task conditions12,13.
If frontal cortex (FC) is a source of top-down command signals that modulate sensory representations for optimal processing of task-relevant information, one would predict that this modulation would be contingent on behavioral state and task-dependent stimulus meaning. Another prediction would be the existence of a strong correspondence between modulation of evoked sensory responses in FC and in task-relevant sensory areas. To explore and test this hypothesis, we have developed the ferret as a new animal model for studying FC control of auditory behavior. Recent anatomical studies have shown that the ferret FC includes areas in the orbital gyrus (OBG) and anterior sigmoid gyrus (ASG) that share common features of neuroanatomical structure and connectivity with PFC in primates, other carnivores and rodents14 (current studies by SRS) and thus are likely to play a similar role in brain function as PFC in other species.
We recorded the activity of single neurons and the LFP in two cortical areas of ferret FC (ASG and the dorsal aspect of OBG) during auditory discrimination behaviors (tone detection and two-tone discrimination) that we have previously shown8,9 to drive rapid receptive field plasticity in the primary auditory cortex (A1). For example, in A1, tasks that require the identification of a pure tone target in a sequence of broadband noise distractors cause an enhancement of responses to stimuli at the frequency of the target tone. We predicted activity in FC that would be consistent with an output signal that could control the frequency specific enhancement observed in A1 for attended tonal targets. In order to assess changes in LFP coherence between these two regions during behavior, we also recorded activity simultaneously in A1 and FC. Finally, in order to dissociate sensory effects from motor and motivational factors, we compared activity in FC during auditory and visual discrimination tasks with the same operant structure.
RESULTS
To study the representation of auditory stimuli during behavior we recorded the activity of 766 single units in frontal cortex (FC) of five ferrets, trained on a variety of auditory and visual discrimination tasks. Neural activity was recorded during behavior and during passive presentation of an identical sequence of task stimuli before and after behavior. These recordings (locations shown in Fig. 1) were made in the dorsal aspect of OBG, which is homologous to the primate dorsolateral prefrontal cortex (dlPFC), and the rostral ASG14 (unpublished data, SRS and JBF). Responses were similar in both frontal areas, and hence were grouped together in all the analyses presented in this study.
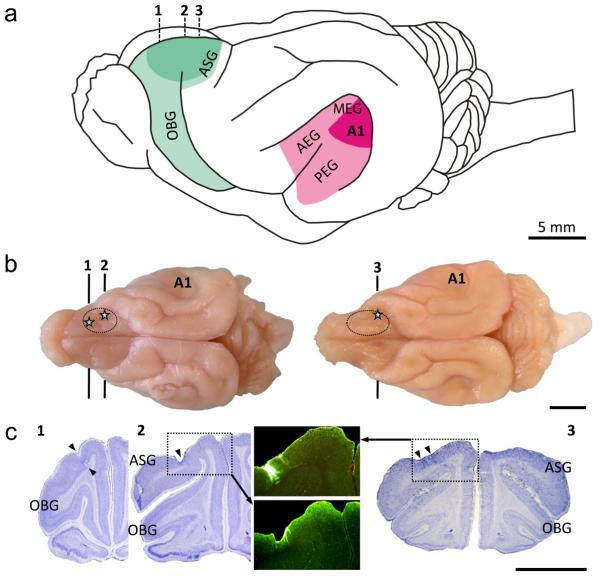
Figure 1.
Sites of physiological recordings. (a) Lateral view of the ferret brain indicating location of auditory (AC) and frontal (FC) cortices. The AC is located on the anterior, middle and posterior ectosylvian gyri (AEG, MEG, PEG), with primary auditory cortex (A1) situated in posterior MEG. Ferret PFC includes orbital gyrus (OBG) and the rostral portion of anterior sigmoid gyrus (ASG)14. We recorded from dorsal OBG and/or rostral ASG (dark green), and simultaneously in A1 (dark red). The numbers (1-3) indicate rostrocaudal position of neuroanatomically confirmed recording sites in PFC, shown in two representative brains in b and corresponding coronal sections in c. (b) Dorsal view of brains of two experimental ferrets. FC recording areas are encircled (recordings were made bilaterally, but for simplicity, are shown only in the right hemisphere). Numbered lines indicate rostrocaudal position in PFC of coronal sections shown in c. Stars indicate recording sites marked by lesions and fluorescent dye. (c) Coronal Nissl-stained sections from three rostrocaudal levels of FC (as indicated in b) showing recording sites. (1): section through OBG. Arrowheads indicate entrance and endpoint of penetrations in dorsal OBG marked by lesions. (2): section at rostrocaudal level of the transition between OBG and rostral ASG. The arrowhead points to cortical depression caused by numerous electrode penetrations superficially marked by fluorescent dye (green beads) shown in lower inset. (3): section through rostral ASG. Arrowheads point to two recording sites labelled with fluorescent dye (green beads), shown in greater detail in upper inset.
All tasks shared the same basic structure, in which animals learned by conditioned avoidance8 to lick water from a spout during the presentation of a class of “reference” stimuli, and to cease licking after the presentation of the class of “target” stimuli in order to avoid a mild shock (Fig. 2a, Supplementary Fig. 1). The spectro-temporal features of reference and target classes varied between tasks. Although the ferrets learned multiple tasks, the majority of data was recorded during performance of the pure tone detection task (Fig. 2a, top row). The effects of behavior on neural activity were similar for other tasks, and a detailed breakdown for different tasks appears in Table 1.
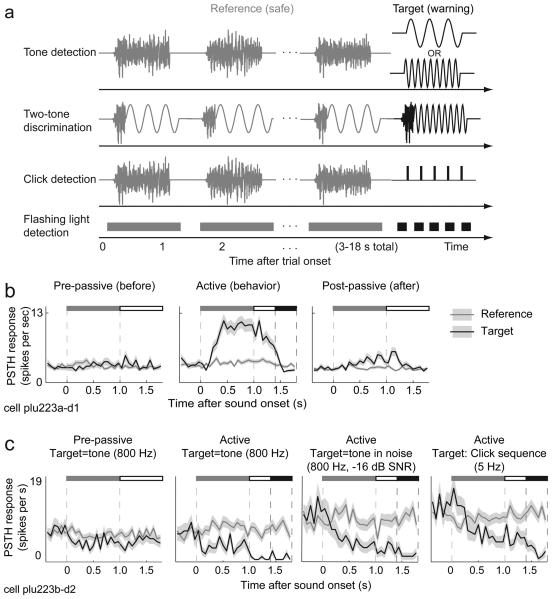
Figure 2.
Behavioral paradigm and examples of typical FC neuron responses. (a) Conditioned avoidance task structure. In each trial, the animal was presented with a random number (1-7) of reference sounds (gray), to be discriminated from a target sound (black) that differed along a feature dimension that defined the task. Animals licked water from a spout throughout the reference stimuli, and learned to refrain from licking upon hearing the target sound in order to avoid receiving a mild shock during the “shock window” 400-800 ms after target offset. All auditory stimuli were of equal duration (either 1 s or 1.5 s in a given behavioral block) and sound level (70 dB). In most tasks, reference sounds consisted of spectro-temporally modulated broadband noise48. In detection tasks, the target stimulus could vary substantially. The animals were trained to respond to a range of acoustic targets such as a pure tone (fixed or variable frequency), tone-in-noise, or click-train. In the two-tone discrimination task, both reference and target stimuli were hybrid sounds, consisting of modulated noise stimuli with a tone attached at the end: the two end-tones for reference and target differed in frequency9. In the visual task, reference stimuli consisted of a series of steady lights followed by a brighter, rapidly flashing target light. (b)Unit showing enhanced responses during a tone-detection task. The peri-stimulus time histogram (PSTH) response to the target tone (black) and the reference noise (gray) were aligned to the onset of 1-s long stimuli (indicated by gray bar at top; white bar indicates silent “reaction interval” period – extending from target offset to 400 ms post-offset; black bar indicates 400 ms shock window between 400-800 ms following target offset). No responses were observed during passive presentation of the acoustic stimuli prior to the task. During the task, only the target tone elicited strong responses that declined gradually after the end of the tone. Post-task, the response to the target persisted weakly. (c)PSTH response of a suppressed cell in a detection task sequence. Panels display responses of the same FC neuron before behavior, and during a series of detection tasks (for three types of target – tone, tone-in-noise and click train). Prior to behavior, activity was weakly suppressed by target sounds, perhaps because of persistent effects from prior behavioral sessions earlier in the same recording day. During the behavior, suppression was stronger and built up over time, becoming substantially stronger well past the offset of the target tone, through the 400 ms reaction window and into the 400 ms shock window.
Table 1.
Each entry shows the fraction of units whose spiking activity was significantly modulated by sensory (target or reference onset/offset), motor (licking) or either task parameter (sensory or motor) during the specified task and behavior condition (pre-, during-, or post-behavior). Modulation is significant (p<0.05) according to the stepwise regression described in the Methods. Because data were not collected from all behavior conditions for all neurons (because cells could not always be held for the required hour to obtain all responses), the first column reports all cells with data collected during behavior and the second column reports only cells tested under all behavior conditions. The columns at right show the fraction of the cells modulated during behavior that were also modulated during passive stimulation either before (column 3) or after (column 4) behavior. The bottom row summarizes modulation for all cells observed during any task, after correcting for multiple comparisons when data were collected from multiple tasks.
| Task / response type | All units | Complete data sets |
Subset of units modulated during behavior |
||
|---|---|---|---|---|---|
| During | During | Pre | Post | ||
| 1. | Tone detect – any modulation | 541/727 (74%) |
423/536 (79%) |
-- | -- |
| 2. | Tone detect – sensory | 268/727 (37%) |
208/536 (38%) |
66/208 (32%) | 99/208 (48%) |
| 3. | Tone detect – target | 243/727 (33%) |
188/536 (35%) |
55/208 (26%) | 84/208 (40%) |
| 4. | Tone detect – reference | 65/727 (9%) | 53/536 (10%) | 14/208 (7%) | 23/208 (11%) |
| 5. | Tone detect – motor | 175/727 (24%) |
132/536 (25%) |
-- | -- |
| 6. | Tone detect – motor and sensory |
13/727 (2%) | 11/536 (2%) | -- | -- |
| 7. | Tone discrimination – sensory | 33/125 (26%) | 20/74 (27%) | 3/20 (15%) | 5/20 (20%) |
| 8. | Detect & discrimination – sensory |
26/115 (23%) | 12/55 (22%) | 3/12 (25%) | 5/12 (42%) |
| 9. | Click detect – sensory | 28/105 (27%) | 19/74 (26%) | 4/19 (21%) | 5/19 (26%) |
| 10. | Tone in noise detect – sensory | 22/73 (30%) | 15/47 (32%) | 1/15 (7%) | 7/15 (47%) |
| 11. | Visual flash detect – sensory | 56/169 (33%) | 40/100 (40%) | 15/40 (38%) | 19/40 (48%) |
| 12. | Visual & auditory – sensory | 27/157 (17%) | 17/78 (22%) | 3/17 (18%) | 9/17 (53%) |
| 13. | Total – sensory | 294/766 (38%) |
230/596 (39%) |
65/230 (28%) | 100/230 (43%) |
FC responses are gated by behavior
During tone detection behavior, the spiking activity of a majority of FC neurons (541/727, 74%) was significantly modulated by task events (p<0.05, jackknifed t-test), while it was not modulated or only weakly modulated by passive presentation of stimuli before behavior. In some cells, activity clearly correlated with sensory stimuli (268/727, 39% of neurons, designated as “sensory”, Supplementary Fig. 2), whereas in others, activity was correlated with task-related licking (243/727, 33%, “motor”). In a few cases, the source of modulation could not be determined unambiguously (43/727, 6%). For most sensory neurons, activity reflected selective responses to the target (243/268, 91% of modulated neurons) rather than to reference sounds (65/268, 24%). Even when significant, reference responses were much weaker than target responses (Fig. 3b). In general, sensory responses were gated by behavior, and a much smaller proportion of neurons responded to stimuli in the pre-behavioral passive state (55/536, 10% of neurons with data collected passively before and after behavior, see Table 1, Fig. 5, Supplementary Fig. 3). When present, these responses were likely the result of persistent effects from earlier behavioral sessions on the same day. Thus, many FC neurons did not respond to sensory stimuli unless they were behaviorally salient targets in either an ongoing (or very recent) task, and in this sense, the target responses were behaviorally gated.
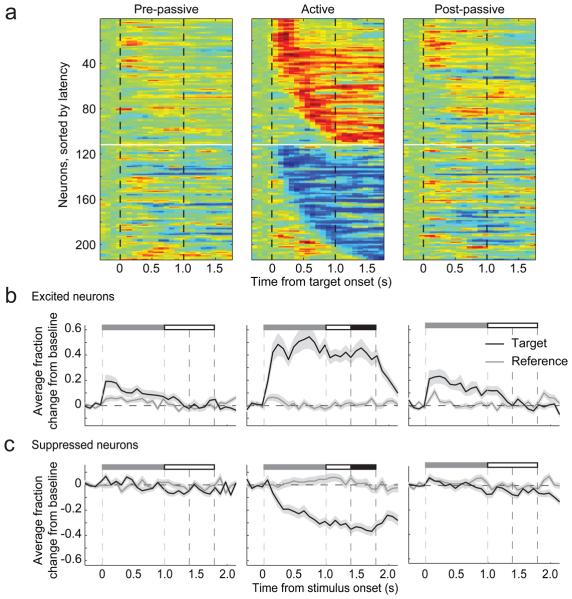
Figure 3.
FC population target responses during tone-detection behavior. (a) Three heat maps show population responses (n=208) to target sounds during presentation of tone-detection stimuli in pre-behavior passive condition (left panel), during active behavior (middle panel), and in post-behavior passive condition (right panel). Each horizontal line illustrates modulation in a single neuron for 400 ms before target onset, 1 sec during the stimulus and 800 ms after offset. Neurons were normalized to have the same peak modulation, grouped by sign (enhanced versus suppressed) and ordered by onset latency of modulation during behavior. Red indicates activity enhanced from baseline and blue indicates suppressed activity. After behavior, there were some faint, persistent post-behavioral passive effects (right panel). In contrast, the little modulation present in the left panel pre-behavioral passive heat map is likely due to the imprint of previous behavioral sessions on the same recording day. (b) Each panel displays the population average target and reference PSTH for neurons with enhanced target responses, during behavior in a. Stimulus and shock windows are labeled as in Figure 2. During behavior, population activity increased rapidly after the target onset (black line) and was sustained through the shock window before returning rapidly to baseline. Very little modulation to reference stimuli was observed (gray line). In addition to being weaker, persistent modulation after behavior returned to baseline soon after target offset. (c) Average target and reference modulation for target-suppressed cells in a. During behavior, response latency was slightly slower than for enhanced neurons, but otherwise showed similar persistence.
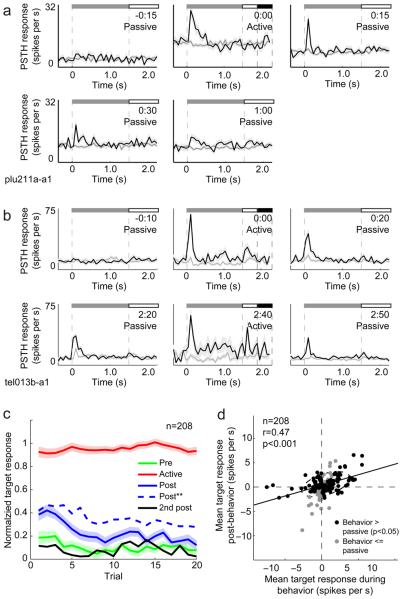
Figure 5.
Persistence and extinction of FC responses following behavior. (a) Neuron showing persistent target modulation following tone detection behavior. Behavior condition (passive or active) and time relative to the beginning of behavior (hours:minutes) is indicated in the upper right of each panel, with PSTHs plotted as in Fig. 2. The target response post-behavior was more phasic than during behavior and faded within one hour after the task performance. (b) Neuron showing exceptionally persistent modulation, plotted as in a. Target responses persisted over two hours after the task was completed and returned rapidly to high levels during a second behavior session. (c) Average normalized response magnitude for the population of 208 behaviorally activated neurons, as a function of trial number since the beginning of each epoch (i.e., before (green), during (red), after (blue), and long after (black) behavior). The dashed blue line indicates responses of the subset of 99 “persistent” neurons that were significantly modulated after the end of the behavioral session. (d) Scatter plot comparing target modulation during behavior (horizontal axis) versus post-behavior for all neurons showing sensory modulation during behavior (n=208). Black dots indicate units for which target responses were either significantly weaker or not significantly modulated during passive listening (p<0.05, t-test, 144/208, 69%). Responses post-behavior (vertical axis) were similar to responses during behavior (horizontal axis), although weaker (r=0.55, p<0.001, slope=0.31).
Examples illustrating two general patterns of behavioral gating in FC neurons are shown in the peri-stimulus time histograms (PSTHs) in Fig. 2b,c. In the first example (Fig. 2b), the unit gave a strong sustained response to the target tone during a tonedetection task (middle panel). In contrast, there was no response elicited by the same sounds in the identical sequence presented passively prior to the behavior (left panel). This unit had a persistent, but much weaker response after behavior (right panel). In 54% (112/208) of unit responsive to sensory stimuli during behavior, the firing rate was enhanced by the target. In the remaining 46%, the firing rate was suppressed by the target during behavior, as illustrated by the second unit (Fig. 2c). We did not observe spatial segregation of FC neurons with these two response polarities. Neighboring frontal neurons with opposite signs of modulation could be found in the same penetration, or even at the same recording site. For example, the second unit (showing suppression, Fig. 2c) was recorded deeper in the same electrode penetration, 150 μ away from the first unit (showing enhancement, Fig. 2b). During the recording from the second unit, the animal performed a series of detection tasks in successive behavioral blocks, each with different target stimuli. In each case, neural activity was suppressed by the target, regardless of its acoustic structure (tone, tone-in-noise or click train). The overall time-course and pattern of responses was similar for all three target conditions (although it is possible that small differences between PSTHs could reflect specific features of the stimuli). Trial-by-trial rasters of these neurons' activity are shown in Supplementary Fig. 4.
The pattern of FC responses varied considerably from unit to unit. Fig. 3a shows heat maps that summarize the target responses of the 208 units that gave significant target responses during the tone detection task and for which data was also collected during passive presentation of task stimuli pre- and post-behavior. Neurons were grouped by whether their activity was enhanced or suppressed during behavior and then ordered by response latency. The same neuronal grouping and ordering was also used for both passive epochs. A broad view of the FC responses in the heat maps illustrates several important properties: (1) Excitatory and inhibitory responses to the target tones during behavior were found in roughly equal numbers of neurons; (2) Latencies ranged from as short as 20-30 ms to more than 1 s, and were distributed nearly uniformly (see Supplementary Fig. 5). Although the functional significance of these variable latencies is unknown, they could potentially provide a precise temporal representation of both target and decision periods; (3) Responses to target tones in the passive state prior to behavior were generally weak or absent. If present, responses tended to have the same polarity as those observed for the same cell during behavior (e.g., Fig. 2c) and these small pre-passive responses may reflect an attenuated persistence from previous behavioral blocks on the same day (see Supplementary Fig. 3); (4) Response profiles could be phasic, sustained, built up or ramped down during the time course of the target stimulus (more examples below).
The average population responses of FC neurons to target tones are shown grouped according to their polarity in Fig. 3b,c. As the average PSTHs (middle panels) indicate, both enhanced and suppressed populations maintained activity from the onset of target “recognition” ~25-250 ms after onset of the target stimulus window until the end of the shock window 1.8 s later. The enhanced cells tended to respond with slightly shorter latency and return to baseline more rapidly after the end of the shock window.
There was a striking asymmetry in FC responses during behavior to the classes of target and reference sounds, with a high selectivity for target responses, signaling a clear distinction between the two stimulus classes. Interestingly, when present, reference responses exhibited an opposite polarity to the target responses (compare average target and reference responses in middle panels, Fig. 3b,c). Thus, even when reference responses occurred, the difference in response polarity enhanced discriminability between the stimulus classes.
These effects were largely independent of behavioral performance and recording location in FC. We observed a weak correlation between target response strength and task performance (Supplementary Fig. 6), but this effect was not significant, perhaps because data was collected only after animals were completely trained on the task. There was also a small but significant tendency for neurons from the same recording site and/or from the same penetration to show either sensory or motor modulation during behavior, suggesting some local topography of these effects in FC (see Supplementary Fig. 7). However, we observed no large-scale, global effects, and, as mentioned above, neighboring neurons could exhibit opposite polarity of modulation (Fig. 2b,c).
FC responses encode behavioral meaning of stimuli
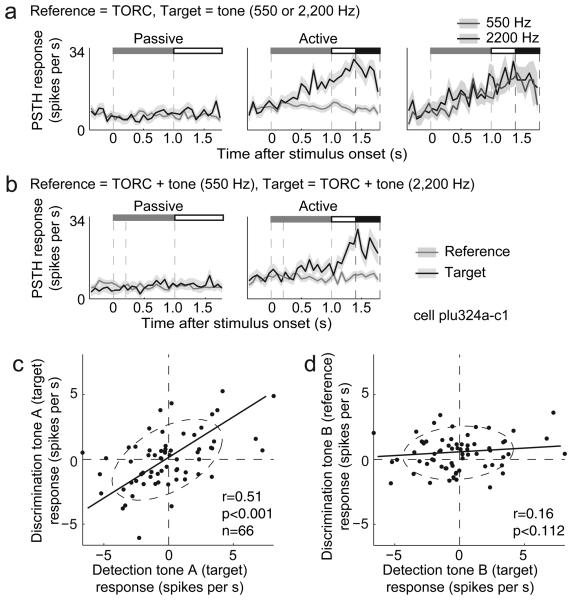
As suggested by Fig. 2c, FC activity encoded the task-related meaning of the stimulus as a “target” during behavior, rather than the physical nature of task stimuli. A striking example of FC responses encoding functional meaning independent of physical properties is shown in Fig. 4. Data from this unit were recorded during both tone detection and tone discrimination tasks. In the initial tone-detection task (Fig. 4a), the target varied between two randomly alternating tones (550 or 2200 Hz), while references were a class of thirty different broadband noises. During this task, both target tones evoked equally strong responses, whereas references evoked no response. In the second, two tone-discrimination task (Fig. 4b), the target was the 2200 Hz tone, while the 550 Hz tone acted as a reference. In this second task, the unit ceased responding to the 550 Hz tone, as its meaning had been changed to reference, and responded only to the 2200 Hz tone, which had retained its original meaning as target in the new task.
Figure 4.
FC class-specific responses to task-relevant sounds. (a) A single unit's response to two randomly alternating target tones in a tone detection task, plotted as in Fig. 2. This cell responded equally well to either of the two target tones (550 or 2200 Hz) but not to any of the 30 different reference noise stimuli. (b) During two-tone discrimination the 550 Hz (low) tone became a reference sound, and the unit now stopped responding to this tone, while maintaining its response to the 2200 Hz (high) tone, which remained a target. (c) Each point indicates the response of a neuron (n=66/115 responsive neurons) to the same target tone during tone detection (vertical axis) and discrimination (horizontal axis). Responses to the target tone were strongly correlated between behavior conditions (r=0.51, p<0.001). The dashed ellipse indicates the first (major axis) and second (minor axis) principal components of the covariance matrix of target responses under the two task conditions. The regression line (black) has a slope of 0.9, indicating that response magnitude was similar in both conditions. (d) Responses of the same neurons to the tone whose task-related ‘stimulus class’ or ‘meaning’ switched from target during tone detection to reference during the two-tone discrimination task, plotted as in c. Responses were much weaker when the tone acted as a reference in the two-tone discrimination task, and the responses were not correlated between behavior conditions (r=0.16, p>0.2).
This flexible and adaptive response to changing stimulus meaning was observed across a large number of FC cells (Fig. 4c). For the set of 66 units for which data were collected during both behaviors and which gave significant sensory responses during at least one behavior, we contrasted the average response to the target tone in both tasks (left panel) against the responses to the tone that switched meaning from target to reference (right panel). Across the population, responses to the tone that remained a target in both tasks were significantly correlated (r=0.51, p<0.001), while responses to the tone that switched meaning between tasks were not (r=0.16, p>0.2).
While FC responses primarily encoded stimulus meaning and were highly abstracted from physical stimulus properties, they also preserved detailed information about temporal stimulus features. For example, responses were commonly time-locked to stimulus onset and offset (Fig. 3), and they sometimes were phase-locked to the rate of a train of stimuli such as a series of clicks or light flashes (Supplementary Fig. 8).
Persistence and extinction of post-behavioral responses
The behaviorally-gated modulation of many FC neurons was accompanied by a lack of responsiveness to passively presented stimuli. Many units (109/208, 52%) had a “tabula rasa” character, responding vigorously to target stimuli only during the task and losing responsiveness to identical stimuli presented passively immediately afterwards. However, the remaining behaviorally-modulated neurons (99/208, 48%) manifested persistent post-behavioral responses to target stimuli. The responses of these neurons smoothly decreased in amplitude, and finally extinguished, over a variable time course of minutes to an hour or more (e.g., Fig. 5a). Thus the “behavioral gate” for these neurons opened rapidly after task onset but then closed slowly with a variable half-life after task offset. For a small number of FC neurons, post-behavior persistent responses were exceptionally long-lasting. The neuron shown in Fig. 5b continued responding to the target sound more than two hours after behavior was completed. The slightly diminished response (after two hours) was potentiated and restored to a much stronger response when the behavior was repeated. We note that these effects cannot be attributed to persistent motor behavior since the ferrets showed virtually no licking during passive epochs.
An overall view of the time course of persistent responses in FC can be discerned by examining the average target response magnitude as a function of trial number following the onset of the recording epochs over the entire population of cells that responded to task stimuli during behavior and with data available before and after behavior (n=208, Fig. 5c). Relative to the pre-behavioral passive epoch (green line), there was clear enhancement of responsiveness during the first post-passive epoch (blue line) following task performance (red line). After the task was concluded, population responses gradually weakened over about 20 trials (~200 s), although individual neurons could maintain an elevated response for extended durations (as in the examples above). More prolonged enhancement was observed in the subset of 99 units that showed significant modulation after behavior (dashed blue line). Fig. 5d provides another global view of this post-task enhancement on a cell-by-cell basis through a scatter plot of the amplitude of modulation by target stimuli during behavior versus during post-behavioral passive stimulus presentation. The two measures are well correlated (r=0.55, p<0.001), indicating that persistent responses, while gradually weakening, tended to resemble responses during the task.
Given the long-term persistence of responses after behavior, some of the modulation observed during passive stimulation before behavior may reflect persistence from earlier behaviors. Pre-behavior modulation was generally weaker if only the first behavioral session of the day was considered (Supplementary Fig. 5), suggesting that effects of the most recent task target persisted for hours, rather than days.
Category-specific changes in LFP within and across FC and A1
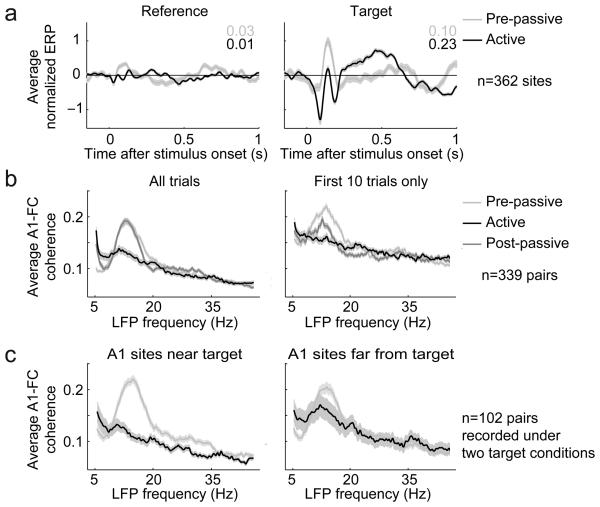
In order to better understand the mechanisms giving rise to the behavioral gating of spiking responses in FC, we extracted the LFP by low pass filtering the recordings made during auditory tasks. Fig. 6a shows the event-related potential (ERP) following the onset of reference (left panel) and target sounds (right panel) under active and pre-passive conditions (n=362 FC recording sites). Surprisingly, despite the absence of substantial spiking responses during pre-passive stimulus presentation (Figs. 2-3), we did observe significant ERPs for both reference and target stimuli. Then, during behavior, the response evoked by reference sounds was attenuated, while the response evoked by the target tone showed an overall amplification, including an increased early hyperpolarization (~100 ms), later depolarization (~450-650 ms) and late hyperpolarization (>700 ms). This pattern of ERPs occurred regardless of whether there were spiking responses to references or targets at the recording site (Supplementary Fig. 9). These responses are consistent with a network in which auditory signals arrive at the FC in the passive state, but a gating mechanism prevents spiking responses to either reference or target stimuli. Subsequently, during behavior, the input to FC is selectively attenuated for reference responses while the input for targets is selectively enhanced. With the gate now open, the target inputs elicit spiking in FC during behavior.
Figure 6.
Selective top-down modulation of LFP in A1 and FC. (a) Average event related potential (ERPs) in FC (n=362 sites) evoked by reference (left panel) and target stimuli (right panel) during pre-behavior passive presentation (gray line) of tone detection stimuli and during behavior (black line). Variance of responses is reported for passive (gray) and behavior (black) in the upper right corner of each panel. ERPs occurred for both references and targets during passive presentation, despite the absence of spiking responses. During behavior, the magnitude of ref-ERP was reduced, while the tar-ERP showed two peaks of stronger, early hyperpolarization (100-200 ms) and stronger, late depolarization (400-600 ms) and later hyperpolarization (> 600 ms). (b) Average coherence of LFP recorded simultaneously in A1 and FC during reference phase of tone detection behavior (left panel, n=339 A1-FC site pairs). During passive presentation of reference sounds (both pre-behavior, light gray curve, and post-behavior, dark gray curve), there was strong coherence in the alpha-beta frequency range (10-20 Hz). This coherence was greatly diminished during behavior (black). Average coherence measured during the first 10 trials of each condition (~40 reference stimuli) reveals that the passive post-behavior condition was only partially restored to the original pre-behavior baseline, reflecting the persistent change in coherence and the gradual return of intra-areal communications to the passive state. (c) In some recordings (n=102 site pairs), there were two successive tone detection behavioral blocks with different target frequencies. Data were divided into two groups, pairs where the target frequency was near the best frequency (BF) of the A1 site (“near,” left panel) or far from the BF (“far,” right panel). The behaviorally-induced decrease in 10-20 Hz modulation occurred for near A1 sites. The decrease in FC-A1 coherence was dramatically diminished for far A1 sites.
In order to study interaction and functional connectivity between auditory and frontal areas, we recorded LFP simultaneously from A1 and FC during the tone detection task. We used coherence to measure synchronous activity between these areas15 during the presentation of reference sounds and observed a strong depression in the beta-band range of 10-20 Hz during behavior, compared to pre-behavior passive presentation of the same stimuli (Fig. 6b, left panel). This decrease in inter-areal coherence was strongly specific to the regions of A1 “near” the target frequency (i.e., tonotopic regions where cells responded preferentially to target sounds, Fig. 6c), and the change was much smaller when measured only for A1 sites “far” from the attended target frequency. Thus the behaviorally-driven change in LFP coherence was selective for the region of the A1 tonotopic map that encoded the target tone, suggesting an attentional spotlight directed toward the task-relevant frequency domain in A1.
The behaviorally driven decrease in coherence did not reverse immediately after behavior was complete. When we analyzed only the first ten passive trials after behavior (~100 sec), we observed only partial return to baseline coherence (Fig. 6b, right panel). This persistent post-task change in LFP coherence mirrors the post-task persistence of FC spiking responses (Fig. 5c) and of behaviorally-triggered tuning changes8 in A1.
Overlapping and non-overlapping FC responses to auditory and visual target stimuli
Two ferrets were also trained on a visual task that followed the same behavioral paradigm as the auditory tasks (Fig. 2a). In the visual task, references were a sustained light, whereas targets were a flashing light. Auditory and/or visual stimuli evoked vigorous responses in many FC neurons during task performance (76/157, 48%). Activity during behavior in both modalities was qualitatively similar, in that it was gated by behavior and responses were consistently larger to the target stimulus.
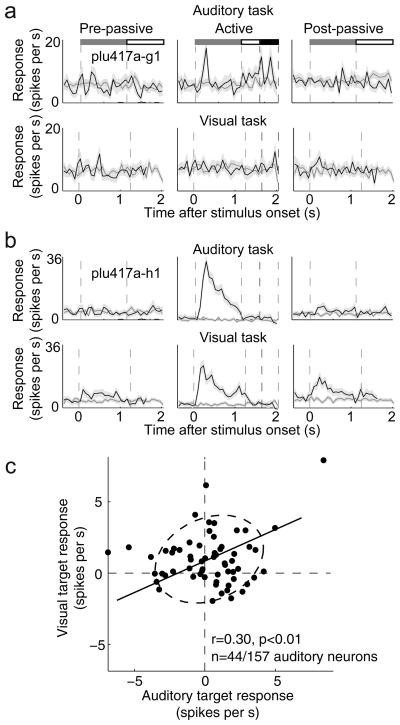
Fig. 7a,b illustrates simultaneous recordings from 2 FC neurons during a sequence of two tasks—auditory (tone detection) and visual (flashing-light detection). An auditory “tabula rasa” neuron (top row) responded significantly to target only during the auditory task, and displayed no response to the target in the visual task. By contrast, a bimodal (i.e., auditory and visual) unit (second row) responded to both auditory and visual targets (although post-behavioral persistence was stronger after the visual task). When neurons responded to stimuli of both modalities, the responses were often similar (Fig. 7c, r=0.30, p<0.01). Of the FC neurons studied during both visual and auditory tasks, about one third (27/76, 36%) of the responsive cells were bimodal, showing a common representation of target stimuli irrespective of sensory modality (Supplementary Fig. 10). The remaining modulated units were divided between unimodal auditory (17/76, 22%) and visual responses (32/76, 42%). This distribution and the amount of overlap is in the range of what would be expected for two equal and randomly overlapping populations of visual and auditory inputs. Analysis of auditory and visual modulation within and across electrode penetrations also showed no clear anatomical segregation of auditory and visual modulation (Supplementary Fig. 7b).
Figure 7.
Bimodal and unimodal sensory responses in FC. (a) Example of single unit responses to successive auditory and visual tasks, plotted as in Fig. 2. This cell showed modulation during behavior only to the auditory target. (b) A second neuron showed target modulation by both the visual and the auditory stimuli. This cell also responded persistently after behavior, particularly to the visual target. (c) Scatter plot of auditory (horizontal axis) and visual (vertical axis) target responses shows that cells responded similarly if they were bimodal (i.e., responded to both auditory and visual stimuli, n=93, r=0.30, p<0.01).
The blend of sensory modality-independent and modality-dependent target coding provides further evidence for the sensory origin of the modulation observed in many FC neurons (268/541, 50%, Supplementary Fig. 2). In both the auditory and visual tasks, animals performed the same motor behavior, namely to lick during reference stimuli and cease licking after target presentation, and they showed comparable behavioral performance in both tasks. If FC neurons encoded strictly motor-related decisions or commands (i.e., inhibition of licking), their responses should have been identical in both auditory and visual tasks, but this was not true of the unimodal cells.
DISCUSSION
The ferret offers a new animal model to explore the neural basis of auditory attention and top-down control of auditory processing during goal-directed behavior16. The results of our previous work on task-related receptive field plasticity8,9,17 and other studies18 in A1 have suggested the hypothesis that top-down signals trigger changes in the receptive field properties of A1 neurons that optimize signal processing for task-salient features of target sounds. We initiated recordings in ferret FC since it is a likely source of top-down control in primates2, and anatomical evidence suggests a homology between ferret FC and primate PFC, and specifically a homology between dorsal OBG and macaque dlPFC14 (unpublished data SRS). The present study is the first to explore neuronal responses in FC of the behaving ferret, and based upon simultaneous recordings from the two regions, offers new insights into interactions between FC and A1.
Behavioral gating of FC responses
The majority of responses in FC were behaviorally gated and highly selective for target stimuli, with roughly equal numbers of neurons showing enhanced or suppressed modulation during behavior. These responses likely reflect target recognition or a cognitive decision process rather than other possible origins, including general arousal, purely sensory or motor effects, or motor planning. The activity of FC neurons cannot simply be attributed to arousal, because we observed strong responses only to the class of target sounds, nor is it simply a sensory response because FC activity is absent or weak during passive stimulus presentation. We also performed a thorough stepwise correlation analysis (see Methods) to exclude all activity that could be explained by correlated licking events (the relevant motor activity during behavior) before testing for correlation between neural activity and sensory events. Admittedly, neural activity involved in decision-making and motor planning is difficult to disambiguate from activity underlying target recognition, since the decision to behave is often closely coupled to perception. However, two important observations argue against a pre-motor or motor planning interpretation of our data: (1) the presence of persistent, target-specific responses lasting 5-100 minutes in the complete absence of motor responses, and (2) the fact that the majority of cells tested on both auditory and visual tasks showed modulation for only the auditory or the visual target, but not both, despite similar motor behavior in both behaviors. This last result suggests that some FC responses are sensory modality-specific and hence cannot simply reflect motor planning or performance. Additional evidence that the observed FC activity is related to target recognition rather than motor planning will require a different experimental design in which the appropriate motor response is cued only after the process of stimulus recognition has been completed, thus permitting temporal dissociation of perceptual, decision-making and motor-planning processes. It is also possible that populations of attention and pre-motor neurons are intermingled in ferret FC, as has been shown in monkey frontal eye fields19.
Our finding of behavioral gating in the ferret FC derives from a contrast between neural activity during the pre-passive epoch (which provides a critical “low-attention” baseline to measure the effects of behavioral modulation) and during goal-directed behavior. These results are in agreement with previous reports of gating in monkey PFC (Soc. Neurosci. Abstr. D. Freedman et al., 160.12, 2002; C. Hussar & T. Pasternak, 460.15, 2008). Thus, in addition to the valuable insights gained from a comparison between FC responses under different task conditions, our findings also emphasize the value of comparing passive and behavioral states as a window of insight into mechanisms of encoding salient events and stimuli in FC.
Finally, unlike single-unit responses, LFP measurements in the passive condition exhibited significant ERPs to both reference and target stimuli. During behavior, however, ERPs became sharply differentiated, selectively suppressed for reference stimuli and enhanced for target stimuli, suggesting a form of selective gating at the synaptic input level as well as at the neuronal level.
Adaptive and selective representation of target stimuli
The ability to change behavioral and neural responses to identical sensory stimuli, depending upon current task and context, is an essential component of flexible, goal-directed behavior1,2. Neurons in FC are likely to contribute to this adaptive ability because of their extraordinary flexibility, responding differently to identical stimuli depending upon the task requirements and behavioral contexts1-3,13,20. Our results are consistent with these findings, as demonstrated by the rapid, adaptive change in coding between tone detection and discrimination tasks. When task conditions changed, the strong responses to the acoustic target stimulus during tone detection disappeared when the same sound became a reference stimulus during two-tone discrimination. Another demonstration of rule encoding in FC neurons is provided by similar responses of bimodal neurons to auditory and visual stimuli, suggesting that some FC responses form multisensory or supramodal representations similar to those reported in the primate PFC21,22.
Categorical representation of visual and auditory stimuli has been demonstrated in primate PFC23,24. Similar to our results, neurons in vlPFC24 generally encoded the category to which a percept belonged, rather than the acoustical features of the stimulus. However, unlike these studies, ferret FC responses were highly specific to the class of “target” rather than “reference” sounds. This striking response asymmetry may reflect differences in task structure between the conditioned avoidance paradigm used here8 and the 2AFC positive-reinforcement paradigms used in other studies23-25, highlighting an important direction for future research on FC representation of stimulus meaning in the context of varying task structure and valence.
Array of response latencies and event timing in FC
The time at which cortical neurons respond to sensory stimuli can provide insight into their functional role and the level the neurons occupy in the bi-directional sensory to decision-making hierarchy. In FC neurons, target modulation latencies ranged from early to late (20 ms to over 1500 ms). Although the functional significance of this array of variable latencies is uncertain, it could serve as a continuous neural representation of time and behavioral state during working memory or other cognitive tasks26,27. Our findings are compatible with recent studies of dynamic population coding of category information in PFC28 and supplementary motor cortex29,30.
The lasting imprint of attention in A1 and FC
FC neurons exhibited adaptive responses that were strikingly similar in their rapid onset with behavior, and variable persistence following behavior, to changes in spectro-temporal receptive field shape8,9 for salient acoustic features in A1. As in A1, FC recordings post-behavior revealed responses that could persist for minutes to hours following task performance. We suggest that these persistent responses, triggered by attention, correspond to changes in stimulus meaning that last long after the behavioral epoch. FC persistence reported here closely resembled findings in monkey dlPFC31 and predictions from models of learning32. Specifically, (1) neuronal responses to the target had latencies as short as 20-60 ms; (2) an approximately equal percentage of frontal cells were either activated or suppressed during behavior; and (3) a population profile of post-behavioral diminution (“forgetting” or “extinction”) on multiple timescales.
The potential functional significance of this persistence is multifaceted and reflects the variety of functional roles that have been ascribed to FC in behavioral control of attention and executive function33 and/or in information storage for working memory34. For instance, FC persistence can be viewed as the imprint of attention – an early indicator of physiological learning mechanisms that “tag” and maintain responsiveness to behaviorally relevant, salient signals such as our target sounds35-36. On a shorter timescale, persistence may also represent the temporary storage of information necessary to provide the bridge between the stimulus cue and its contingent response34.
LFP gating and feature-selective changes in inter-areal coherence
Neuropsychological studies suggest that prefrontal damage disrupts normal inhibitory modulation37,38 of inputs to A1. We speculate that FC may play a similar inhibitory role in our conditioned avoidance paradigm, in which target recognition is critical for guiding behavior. Our simultaneous measurements of LFP in A1 and FC revealed coherence that changed significantly depending on behavioral state and the behavioral meaning of incoming stimuli. The clear decrease in coherence was selective for A1 sites that responded to the target frequency (“near” cells), whereas only a small change was observed when target frequency was distant from the best frequency of the A1 site (“far” cells). Previous studies have suggested that beta band activity may be related to maintenance of the current cognitive set39. In this light, the decrease in coherence at target-frequency locations may reflect the selective removal of inhibition specific to that frequency or, equivalently, enhancement of processing for the salient target frequency during behavior8.
We also observed that the change in LFP coherence persisted in the post-behavioral epoch, mirroring post-task persistence of FC spiking responses and of behaviorally-triggered receptive field changes8,9,17 in A1. The interactions reflected by coherence could be mediated by a direct influence of the FC on A1 or by multiple, polysynaptic routes. Our observations are consistent with the hypothesis that FC can shape the responses of specific sensory areas during behavior by directing selective attention11 and by changing cortical sensory filter properties through activation of neuromodulator systems40-42.
Summary
Our physiological study of FC in the behaving ferret provides a new animal model for studying the role of the FC in attention. In ferret FC, as in monkey dlPFC3, neurons selectively and adaptively encode task-relevant information, show enhanced responses to target stimuli, are modulated by selective attention and contribute to the top-down control of sensory processing. Notwithstanding neuroanatomical differences in cytoarchitecture, topology and connectivity across species43, the overall functional homology of responses is remarkable, and may be understood as shared neuronal solutions to the common problem confronting many animals of representing currently salient stimuli relevant to shifting behavioral goals in an ever-changing environment.
The central hypothesis that motivated this work, that the FC exerts dynamic and selective control over sensory filters in A1 during auditory behavior, is in accord with prevailing views of PFC as the source of top-down modulatory influence on other brain areas, particularly sensory cortices, in the service of behavioral goals1,2. In support of this hypothesis, we observed a rich tapestry of response patterns in FC that shared key properties of task-related receptive field plasticity8 in A1, specifically behavioral contingency with a rapid time course of change and a persistence that closely mirrored the time-course of A1 plasticity. Moreover, FC responses sharply distinguished task-salient stimuli, and exhibited LFPs that were consistent with it being a source of frequency-specific top-down signals to A1. These findings highlight the challenge for future work in integrating the interplay of attention, reward44,45 and the role of FC in top-down signaling that triggers adaptive, task-related plasticity in auditory cortex.
METHODS
All experimental procedures were approved by the University of Maryland Animal Care and Use Committee and were in accord with National Institutes of Health Guidelines.
Behavior
All five adult female ferrets in the study were trained on a variety of auditory tasks, and two of the ferrets were also trained on the visual task. These task variants were based on the conditioned avoidance behavioral paradigm8. Fig. 2 illustrates the basic structure of trials in four task variants (three auditory and one visual task) and the acoustic and visual stimuli used (Fig. 2a). Each behavioral trial consisted of a sequence of reference stimuli (randomly ranging from 1-6) followed by a target (except on catch trials in which 7 reference stimuli were presented with no target). Ferrets licked water from a spout during presentation of the reference stimuli and learned to stop licking upon presentation of a target stimulus to avoid a mild shock (shock window, 400-800 ms after target offset). All stimulus presentation and behavior control were performed by custom software (Matlab).
Ferrets were trained once a day (for 50-200 trials to satiation) in a sound-attenuated test box until they reached criterion, defined as consistent performance for two sessions with >80% hit rate accuracy and >80% safe rate for a discrimination rate of >0.65. Initial training to criterion on the simplest task (i.e. tone detection – detecting a tone in the presence of noise) in the free-running test box took ~3-6 weeks for each ferret. Ferrets were subsequently trained on tauditory and visual task variants and could readily switch from one to another (responses of a frontal neuron when the animal engaged sequentially in three different task variants are shown in Fig. 2c).
After the initial training was completed, surgery was conducted to implant a stainless steel headpost for head restraint during physiological recording (see below). After recovery from surgery, ferrets were retrained on the task, while restrained in a lucite holder, with their head fixed to enable stable recordings. Animals were used in behavioral physiology experiments no more than 1-2 times per week, for 6-8 hours per experiment. During experiments, each task condition block contained ~40 trials, and animals worked for reward in 1-6 task blocks on each recording day. Overall performance of the ferrets on two of the auditory task variants, single tone detection and two-tone discrimination tasks8,9 is shown in Supplementary Fig. 1b,c. Comparable performance was achieved for other task variants.
Surgery
In order to secure stability for electrophysiological recording, a stainless steel headpost was surgically implanted on the skull. Ferrets were initially anesthetized with ketamine/xylazine, then intubated and maintained in deep anesthesia with isoflurane throughout the surgery. Using sterile procedure, overlying tissue was removed to expose the dorsal and lateral skull surface. The headpost was mounted on the midline with Durelon bone cement, and secured to a headcap fastened to the skull with titanuium screws and Zimmer bone cement, leaving clear access to A1 and FC in both hemispheres. Antibiotics and analgesics were administered as needed before, during and after surgery.
Neurophysiological recording
Experiments were conducted in a double-walled sound attenuation chamber (IAC). Small craniotomies (1-2 mm in diameter) were made over primary auditory cortex (A1) and/or FC prior to 6-8 hour recording sessions. We recorded simultaneously from both loci using multiple independently moveable electrode drives (Alpha-Omega) to independently direct up to four electrodes in each cortical area. The electrodes were configured in a square pattern with ~800 u between electrodes. Responses were recorded with tungsten microelectrodes (3-10 MΩ;, FHC) and then stored for analysis off-line. A typical recording yielded 1-2 isolated single units from each electrode.
The A1 and FC regions were initially located with approximate stereotaxic coordinates, and then further identified physiologically. Physiological indicators of ferret A1 have been widely used in the past (Fig. 1c). They include a tonotopic map, short latency (~20 ms), vigorous sustained or transient responses to tones and noise, and spectrotemporal receptive fields (STRFs) with clearly defined excitatory and inhibitory response areas along both the spectral and temporal axes46. We also recorded from the FC of the ferret (in the dorsal orbital gyrus (OBG) and rostral ASG). To clarify neuroanatomical nomenclature, we note that these two cortical areas are both classified as PFC according to the criterion of strong reciprocal connections with the mediodorsal nucleus of the thalamus14. Based upon our further studies of cytoarchitecture and connectivity we prefer to refer to these areas as part of FC, although we agree that the dorsal portion of the ferret OBG, (likely to be homologous to the canine proreal gyrus) is probably homologous with primate dlPFC14 and that the rostral portion of the ASG corresponds to premotor cortex. However, further neuroanatomical work will be necessary to settle these issues.
Physiological localization of the different parts of the FC was particularly difficult because of the absence of a stereotaxic atlas for the ferret brain and was made even more challenging for a variety of reasons, including low neuronal firing rates, and poorly defined external landmarks. Although we were guided by a few reports on the anatomical and physiological properties of the ferret FC 14,47 another major challenge from an experimental point of view was finding responsive neurons in FC, given the commonly weak or absent response in the passive (non-behaving) animal (as shown in Results). Consequently, it was often necessary to engage the animal in a preliminary behavioral task in order to evoke responses on one or more of the electrodes in the FC and identify neurons suitable for recording.
Neuroanatomical localization of FC recording sites
Following extensive recording in FC, two of the ferrets in the study were injected with tracer (at each injection site 1 μl of green retrobeads – Lumafluor, Inc.) with a Hamilton syringe in order to mark the recording area, and explore its neuroanatomical connections. Two weeks following the injections, the ferrets were euthanized with an overdose of sodium pentobarbital (65 mg/kg ip) and transcardially perfused with saline followed by cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for subsequent histological study. The brains were removed, post-fixed overnight in 4% paraformaldehyde, followed by a series of 15% and 30% sucrose until sunk. The brain was cut in 50 μ coronal sections in a freezing microtome and collected in 0.1 M phosphate buffer. Some sections (1:4) were stained with cresyl violet for Nissl in order to view cytoarchitectural structure.
Stimuli
During physiological recording, digital stimuli were converted to analog signals, amplified, and delivered through inserted earphones (Etymotic) that were calibrated in situ at the beginning of each experiment to ensure uniform, equalized gain across a 5-octave frequency range from 500 Hz to 16 kHz. Visual stimuli were delivered through two LEDs positioned just to the left and right of the midline, 15 cm in front of the animal.
All auditory stimuli were presented at ~70 dB SPL, and references and targets were consistently of the same duration (either 1 or 1.5 sec) for a given behavioral block in a daily experimental session. For the tone detection task, references consisted of 30 different broad-band (500-16000 Hz) temporally orthogonal ripple combinations48 (TORCs) and targets consisted of pure tones. Although the target tone frequency in tone detection tasks varied randomly during training and over the course of behavioral physiology experiments, in any single block of recordings usually only one or at most two tone frequencies were used. In other detection tasks with different targets (such as detecting a click sequence target, or a tone embedded in noise), the reference sounds also consisted of TORCs, and the target, while fixed for a given experimental block, could vary in successive blocks. In two-tone discrimination tasks the reference stimuli were brief TORCs (0.5 sec) with attached tones of different frequencies (Fig. 1b). In visual discrimination experiments, a steady light (1 sec duration) served as a reference stimulus, whereas a spatially separate brighter flashing light served as the target.
All passive task measurements used precisely the same set of reference and target stimuli, in the identical order as the trial sequences in behavioral blocks, but without any behavioral contingencies. The animal was cued to the passive condition by the absence of water flow from the spout. No licking was observed during passive stimulus presentation. Conversely, the ferret was cued to the active condition by the presence of water flow from the spout, and drank avidly until the task was completed or the ferret reached satiation.
Analysis of single unit activity
To measure single unit spiking activity, the continuous electrophysiological signal was digitized and bandpass filtered between 300 and 6000 Hz. Single units were then classified using principal components analysis and k-means clustering49. Only clusters with 80% or greater isolation across all data files (i.e., 80% or more spikes in the cluster were likely to have been produced by a single neuron) were used for analysis. Varying the isolation threshold from 80% to 99% did not change any of the population-level effects observed in this study. The tail shock for incorrect responses introduced a strong electrical artifact, and signals recorded during this period were discarded before processing.
Significant neural modulation by auditory and visual stimuli was determined by stepwise linear regression of time-varying spike activity (binned at 50 ms) against stimulus (target and reference) and motor (licking) events. The complete regression modeled spiking activity as a function of reference, target and lick events,
| (1) |
The stimulus functions, sr(t) and st(t), are zero, except at times, t, of reference or target onset, respectively, when they have a value of one. Similarly, the motor function, m(t) has a value of zero except at times when lick events occur. The regression functions, hr(τ), ht(τ), and hm(τ), then indicate the average firing rate before and after each corresponding event. The regression functions were fit by normalized reverse correlation50, which discounted spurious effects that might arise due to correlations between stimulus events and changes in motor activity.
Neurons were classified as significantly modulated by sensory inputs if the occurrence of a stimulus predicted a change in firing rate that could not be explained by a simpler model based on motor activity alone,
| (2) |
Thus a neuron was considered to be modulated by sensory inputs only if the full model predicted spiking activity significantly better than the model based only on licking activity (p<0.05, jackknifed t-test). Examples of neurons with activity significantly correlated with sensory or motor events appear in Supplementary Fig. 2.
To compare responses across the neural population under different behavior conditions (passive versus active, tone detection versus discrimination, etc.), the average firing rate was measured during the 1-1.5 sec duration of stimulus and the subsequent 800 ms silent period/shock window. Similarity of population responses was then measured by the correlation coefficient between the average responses under the different conditions. Significance was determined by a randomized paired t-test (a bootstrapping procedure by which the probability of the measured correlation coefficient was computed directly from a distribution of correlation coefficients measured for randomly shuffled behavior conditions).
Analysis of local field potential
The local field potential (LFP) was extracted by low-pass filtering the same continuous recordings below 200 Hz. A hardware notch filter removed artifacts at 60 Hz, and activity between 55 and 65 Hz was discarded to avoid artifacts.
Coherence of simultaneously recorded LFP was computed using a multi-taper method15. Because coherence has a fixed lower bound of zero, noise biases coherence toward positive values. Thus, when comparing coherence between behavior conditions, the number of trials included in each condition was fixed so as to keep the noise bias the same in each condition. Significant differences in coherence between behavior conditions were determined by a jackknifed t-test measured across different recording sites.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Alvaro Duque, Jenny Bizley, Vicky Bajo, Fernando Nodal and Andy King for discussion and for generously sharing their unpublished neuroanatomical data on the ferret FC and its connectivity with AC, Nima Mesgarani for assistance with customized MATLAB software development, Mounya Elhilali for discussion and for analysis of memory effects in A1, Steve Gotts for discussion of LFP coherence, Catherine Carr for cheerfully sharing lab resources for neuroanatomical studies, and Alexandra Israelson, Sasha Rubin, Kevin Donaldson and David Levitt for assistance in training the ferrets in this study. This research was funded by grants from the National Institutes of Health, R03DC005938 (JBF), R01DC005779 (SAS, JBF), and F32DC008453 (SVD).
Footnotes
AUTHOR CONTRIBUTIONS
JBF designed and conducted all behavioral physiological experiments; SVD analyzed data; JBF, SVD, SAS evaluated results; JBF and PBY made neuroanatomical tracer injections; SRS processed all histological tissue, analyzed neuroanatomical results and made one figure; SVD made all other figures; JBF, SAS and SVD wrote paper.
BIBLIOGRAPHY
- 1.Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nature Reviews Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- 2.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann. Review Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 3.Everling S, Tinsley CJ, Gaffan D, Duncan J. Selective representation of task-relevant objects and locations in the monkey prefrontal cortex. Europ. J. Neurosci. 2006;23:2197–2214. doi: 10.1111/j.1460-9568.2006.04736.x. [DOI] [PubMed] [Google Scholar]
- 4.Gold JI, Shadlen MN. The neural basis of decision making. Ann. Review Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 5.Funahashi S. Prefrontal cortex and working memory processes. Neurosci. 2006;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 7.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz JB, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat. Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- 9.Fritz JB, Elhilali M, Shamma SA. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J. Neurosci. 2005;25:6723–7635. doi: 10.1523/JNEUROSCI.1318-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anton-Erxleben K, Stephan VM, Treue S. Attention reshapes center-surround receptive field structure in macaque cortical area MT. Cer. Cortex. 2009;19:2466–2478. doi: 10.1093/cercor/bhp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat. Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- 12.Di Pellegrino G, Wise SP. Effects of attention on visuomotor activity in the premotor and prefrontal cortex of a primate. Somatosensory & Motor Res. 1993;10:245–262. doi: 10.3109/08990229309028835. [DOI] [PubMed] [Google Scholar]
- 13.Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J. Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- 14.Duque A, McCormick DA. Circuit based localization of ferret prefrontal cortex. Cer. Cortex. 2010;20:1020–1036. doi: 10.1093/cercor/bhp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra P, Bokil H. Observed Brain Dynamics. Oxford Univ. Press; New York: 2008. [Google Scholar]
- 16.Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention – focusing the searchlight on sound. Current Opinion in Neurobiology. 2007;17:437–455. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Fritz JB, Elhilali M, Shamma SA. Adaptive changes in cortical receptive fields induced by attention to complex sounds. J. Neurophysiol. 2007;98:2337–2346. doi: 10.1152/jn.00552.2007. [DOI] [PubMed] [Google Scholar]
- 18.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J. Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy A, Thompson KB, Schall JD. Dynamic dissociation of visual selection from saccade programming in frontal eye field. J. Neurophysiol. 2001;86:2634–2637. doi: 10.1152/jn.2001.86.5.2634. [DOI] [PubMed] [Google Scholar]
- 20.Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- 21.Romanski LM. Domain specificity in the primate prefrontal cortex. Cognitive and Affective Behavioral Neuroscience. 2004;4:421–429. doi: 10.3758/cabn.4.4.421. [DOI] [PubMed] [Google Scholar]
- 22.Sugihara T, Diltz MD, Averbeck BB, Romanski LM. Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J. Neurosci. 2006;26:11138–11147. doi: 10.1523/JNEUROSCI.3550-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 24.Russ BE, Lee Y-S, Cohen YE. Neural and behavioral correlates of auditory categorization. Hear. Res. 2007;229:204–212. doi: 10.1016/j.heares.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Lemus L, Hernandez A, Romo R. Neural encoding of auditory discrimination in ventral premotor cortex. Proc. Nat. Acad. Sciences. 2009;106:14640–14645. doi: 10.1073/pnas.0907505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman Memory without feedback in a neural network. Neuron. 2009;61:621–634. doi: 10.1016/j.neuron.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat. Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyers EM, Freedman DJ, Kreiman G, Miller EK, Poggio T. Dynamic population coding of category information in inferior temporal and prefrontal cortex. J. Neurophysiol. 2008;100:1407–1419. doi: 10.1152/jn.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shima K, Tanji J. Neuronal activity in the supplementary and pre-supplementary motor areas for temporal organization of multiple movements. J. Neurophysiol. 2000;84:2148–2160. doi: 10.1152/jn.2000.84.4.2148. [DOI] [PubMed] [Google Scholar]
- 30.Salinas E. Rank-order-selective neurons form a temporal basis set for the generation of motor sequences. J. Neurosci. 2009;29:4369–4380. doi: 10.1523/JNEUROSCI.0164-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7:1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- 32.Fusi S, Asaad WF, Miller EK, Wang XJ. A neural circuit model of flexible sensorimotor mapping: learning and forgetting on multiple timescales. Neuron. 2007;54:319–333. doi: 10.1016/j.neuron.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung MW, Baeg EH, Kim MJ, Kim YB, Kim JJ. Plasticity and memory in the prefrontal cortex. Reviews in Neuroscience. 2008;19:29–46. doi: 10.1515/revneuro.2008.19.1.29. [DOI] [PubMed] [Google Scholar]
- 34.Baddeley AD. Working memory. Clarendon/Oxford Univ. Press; Oxford: 1986. [Google Scholar]
- 35.Morris RGM. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Europ. J. Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- 36.Frey S, Frey JU. ‘Synaptic tagging’ and ‘cross-tagging’ and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog. Brain Res. 2008;169:117–143. doi: 10.1016/S0079-6123(07)00007-6. [DOI] [PubMed] [Google Scholar]
- 37.Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 38.Ehlis AC, Ringel TM, Plichta MM, Richter MM, Herrmann MJ, Fallgatter AJ. Cognitive correlates of auditory sensory gating: A simultaneous near infrared spectroscopy event-related potential study. Neuroscience. 2009;159:1032–1043. doi: 10.1016/j.neuroscience.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Engel AK, Fries P. Beta-band oscillations – signaling the status quo? Current Opinion in Neurobiol. 2010;20:1–10. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 41.Rasmusson DD, Smith SA, Semba K. Inactivation of prefrontal cortex abolishes cortical acetylcholine release evoked by sensory or sensory pathway stimulation in the rat. Neuroscience. 2007;149:232–241. doi: 10.1016/j.neuroscience.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 42.Roelfsema PR, van Ooyen A, Watanabe T. Perceptual learning rules based on reinforcers and attention. Trends Cog. Sci. 2010;14:64–71. doi: 10.1016/j.tics.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodman GF, Luck SJ, Schall JD. The role of working memory representations in the control of attention. Cer. Cortex. 2007;17:118–124. doi: 10.1093/cercor/bhm065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messinger A, Lebedev MA, Kralik JD, Wise SP. Multitasking of attention and memory functions in the primate prefrontal cortex. J. Neurosci. 2009;29:5640–5653. doi: 10.1523/JNEUROSCI.3857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bizley J, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cer. Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
- 47.Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated though a dynamic balance of excitation and inhibition. J. Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein DJ, Depireux DA, Simon JZ, Shamma SA. Robust spectrotemporal reverse correlation for the auditory system: optimizing stimulus design. J. Computational Neurosci. 2000;9:85–111. doi: 10.1023/a:1008990412183. [DOI] [PubMed] [Google Scholar]
- 49.David SV, Mesgarani N, Fritz JB, Shamma SA. Rapid synaptic depression explains nonlinear modulation of spectro-temporal tuning in primary auditory cortex by natural stimuli. J. Neurosci. 2009;29:3374–3386. doi: 10.1523/JNEUROSCI.5249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theunissen FE, David SV, Singh NC, Hsu A, Vinje WE, Gallant JL. Estimating spatial temporal receptive fields of auditory and visual neurons from their responses to natural stimuli. Network: Comp. in Neural Systems. 2001;12:289–316. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.