Abstract
An elevated level of homocysteine (Hcy), known as hyperhomocysteinmia (HHcy), was associated with neurovascular diseases. At physiological levels, hydrogen sulfide (H2S) protected the neurovascular system. Because Hcy was also a precursor of hydrogen sulfide (H2S), we sought to test whether the H2S protected the brain during HHcy. Cystathionine-β-synthase heterozygous (CBS+/−) and wild type (WT) mice were supplemented with or without NaHS (30 µM/L, H2S donor) in drinking water. Blood flow and cerebral microvascular permeability in pial vessels were measured by intravital microscopy in WT, WT+NaHS, CBS−/+ and CBS−/+ + NaHS treated mice. The brain tissues were analyzed for matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) by Western blot and RT-PCR. The mRNA levels of CBS and cystathionine gamma lyase (CSE, enzyme responsible for conversion of Hcy to H2S) genes were measured by RT-PCR. The results showed a significant increase in MMP-2, MMP-9, TIMP-3 protein and mRNA in CBS (−/+) mice, while H2S treatment mitigated this increase. Interstitial localization of MMPs was also apparent through Immunohistochemistry. A decrease in protein and mRNA expression of TIMP-4 was observed in CBS (−/+) mice. Microscopy data revealed increase in permeability in CBS (−/+) mice. These effects were ameliorated by H2S and suggested that physiological levels of H2S supplementation may have therapeutic potential against HHcy-induced microvascular permeability, in part, by normalizing the MMP/TIMP ratio in the brain.
Keywords: Blood Brain Barrier, Tissue inhibitors of matrix metalloproteinases, Cysthathionine-β-synthase (CBS) and Cysthathionine-γ-lyase (CSE)
1. INTRODUCTION
Homocysteine (Hcy) was a sulfur-containing, non-protein amino acid, and an intermediate of methionine metabolism (Selhub, 1999). Elevated levels of Hcy caused hyperhomocysteinemia (HHcy, Rabaneda et al, 2008). HHcy displayed various neurological abnormalities, such as mental retardation, seizures, and Alzheimer's disease (Robert et al, 2005; Sachdev et al, 2002; Santiard-Baron et al, 2005). Interestingly, in addition to cysteine, Hcy metabolites can also produce hydrogen sulfide (H2S) by cystathionine beta synthase (CBS), cystathionine gamma lyase (CSE) and mercapto sulfur transferase (MST, Abe and Kimura, 1996; Wang, 2002; Zhao et al, 2001). CBS is abundantly present in the brain, kidney and liver, whereas CSE is localized in smooth muscle and the heart (Ishii et al, 2004; Miles and Kraus, 2004). CBS and CSE have been identified to be the main enzyme responsible for the biosynthesis of H2S in the brain (Abe and Kimura, 1996; Hosoki et al, 1997). The exogenous supply of NaHS (donor of H2S), which generated H2S, produced physiological responses in many biological systems (Sun et al, 2008). H2S was reported to suspend metabolic activity in mice, thus can be used for better survival in severe injuries like myocardial Infarction and stroke (Blackstone et al, 2005). Physiological concentrations of H2S specifically potentiated the activity of the N-methyl-D-aspartate (NMDA) receptor and improved the induction of hippocampal long-term potentiation, which was associated with learning and memory (Abe and Kimura, 1996). H2S relaxed vascular smooth muscle and inhibited platelet aggregation (Zagli et al, 2007; Zhao et al, 2001). In the gastrointestinal system, H2S relaxed smooth muscle cells (Hosoki et al, 1997; Teague et al, 2002) increased colonic secretion and blood flow (Schicho et al, 2006). We recently reported that H2S attenuated Hcy-induced oxidative stress in brain endothelial cells (Tyagi et al, 2009). These multiple lines of evidence suggested that H2S function as a type of armor in the brain. However, the role of H2S in Hcy-induced brain damage was unclear. There was evidence that H2S acted as an endogenous scavenger for ROS (Whiteman et al, 2005). In addition, the present study was undertaken to determine the potential role of H2S in Hcy-mediated cerebrovascular remodeling and role of CBS and CSE. We demonstrated that elevated levels of homocysteine activated matrix metalloproteinases (MMPs) and inactivated tissue inhibitors of matrix metalloproteinases (TIMPs), which degraded the matrix, leading increase in cerebrovascular permeability. The pretreatment with H2S can prevent these alterations.
2. MATERIAL AND METHODS
2.1. Animals
Breeding pair of CBS−/+ mice were obtained from Jackson Laboratories (Bar Harbor, ME).The mice were grouped: (wild type, WT, WT+NaHS, CBS(−/+) and CBS (−/+) + NaHS) and housed in the animal care facility at University of Louisville. All animal procedures were carried out in accordance with the National Institute of Health Guidelines for animal research. The Institutional Animal Care and Use Committee of the University of Louisville, School of Medicine approved this protocol. All the mice were treated with 30 µMol/L NaHS for 8 weeks in drinking water. Every alternate day drinking waters was supplemented with NaHS.
2.2. Rationale for NaHS (H2S donor) dose
Human brain contained 50–160 µM H2S (Goodwin et al, 1989), which occurred naturally (~50–100 µM) in both human (Richardson et al, 2000) and rat (Zhao et al, 2001) serum. NaHS in the aqueous phase produced exact equal concentration of H2S gas. Therefore, we used 30 µmol/l NaHS in the drinking water to supplement animals with 30 µmol/l of H2S (Sen et al, 2009). Therefore, the pharmacological doses of H2S that were administered render the study to relate to the human condition.
2.3. Genotyping and measurement of homocystine
Mice were genotyped with a specific set of primer to confirm they were heterozygous of CBS (−/+). To determine the plasma levels of Hcy in experimental and control samples, HPLC was performed. Hcy from the samples identified according to the retention times and standards as described (Sen et al, 2007).
2.4. Measurement of permeability in brain microcirculation
Blood flow in brain microvasculature was measured in anesthetized mice as described (Kumar et al, 2008a). Mice were injected with FITC conjugated to bovine serum albumin (BSA-FITC-albumin, 70 KDa) through the tail vein. After 30 minutes, 14-mm hole was made in the skull using a high-speed micro drill (Fine scientific Tools). The exposed area was examined using in-vivo imaging fluorescent microscopy (Olympus, Japan) and the data was interpreted with software provided with the microscope.
2.5. RNA isolation and expression study
Total RNA from the mouse brain was isolated using Trizol Reagent (Gibco BRL) by following the instructions provided by the manufacturer. The cDNA was prepared using the Promega kit. Expression levels of MMP-2, MMP-9, TIMP-3, TIMP-4, CBS and CSE were measured using SYBR green assay kit on Mx3000p QPCR as described earlier (Kumar et al, 2008a). Optimal concentrations of primers used for the assay were between (750 nmol/L) to (1000nmol/L). PCR amplification was performed using a cyclic pattern: 94°C for 10 minutes followed by 35 cycles of 94°C for 1minute, 58°C for 1 minute, 72°C for 1 minute and final extension at 72°C for 5 minutes. For the determination of SYBR Green based melting peak (Tm) of the products, an additional cycle of 95°C for 1 minute, 55°C for 30 sec, 95°C for 30 sec was performed. CSE gene cloned in pIRES2-AcGFP1 vector system (Clontech) was used to normalize the mRNAs. A standard curve was generated by amplification of known amount of CSE gene in pIRES2-AcGFP1 vector system depending on Ct values (threshold cycle). The parameter Ct was defined as the fractional cycle number at which the fluorescence passes the fixed threshold. Data were expressed as copy number based on plasmid standard curve and normalized by dividing the copy number for each sample. Samples and control were run in duplicate for each gene. A standard curve was plotted using a CSE-cloned gene, and the amplification of each gene was measured against the standard plot. Each of the genes analyzed had different melting temperatures. Additionally, MMP-2 and MMP-9 mRNA levels were measured and normalized with internal control GAPDH.
2.6. Western Blots
The brain tissue homogenates were prepared using extraction buffer (0.01 M cacodylic acid pH 5.0, 0.15 M NaCl, 1 µM ZnCl2, 0.02 m CaCl2, 0.0015 M NaN3 and 0.01% v/v Triton X-100) and kept for overnight digestion in a cold room. The extracted proteins were collected by centrifugation at 10000×g for 10 minutes and concentration was estimated by Bradford method. Equal amounts of proteins were analyzed on 10% or 12% SDS-PAGE as described earlier (Tyagi et al, 2009). The developed X-ray images were analyzed using UMAX PowerLockII (Taiwan, R.O.C.).
2.7. Gelatin Gel Zymography
MMP-9 activities were measured using gelatin gel zymography as described previously (Tyagi et al, 2009). Samples were electrophoretically resolved on 7.5% SDS-PAGE containing 1.5% gelatin as a substrate. At the end, the gel was incubated in re-naturation buffer (2.5% Triton X-100) for 30 min to remove SDS, rinsed in distilled water, and then incubated for 24 hours at 37°C in water bath in an activation buffer (50 mM Tris.HCl, pH 7.4, and 5 mM CaCl2). The gels were stained in Coomassie blue R-250 for 1hr and then distained with destining buffer (10% acetic acid, 10%methnol and 80% distilled water). The clear digested regions representing MMP-9 activity, as accessed by running pre-stained molecular weight markers, were quantified densitometrically using Un-Scan-It software (Silk Scientific Inc., Orem, UT).
2.8. Immunohistochemistry
Mice were deeply anaesthetized with high doses of sodium pentobarbital. Mice were trans-cardialy perfused with 20 ml (PBS), followed by 20ml (4% Paraformaldehyde). The skull was opened and brain was kept in fixative for 2–3hrs, and incubated in 30% sucrose for overnight. The tissue was frozen in OCT compound and transverse sections of the brain were taken on a cryostat. 10µm sections were mounted on poly-lysine-pretreated microscope slides. For immuno-staining, the slides were brought to room temperature followed by two rinses in 0.1M PBS 5 min each, followed by antigen retrieval in 10mM Sodium Citrate buffer (pH 6.0). The endogenous peroxidase reaction was blocked by incubation in 3% hydrogen peroxide in PBS buffer. The non-specific sites were blocked by incubation in blocking solution (0.2M PBS pH 7.4 containing 1% BSA, 0.3% Triton X100, and 1% normal animal serum) in closed hydrated chamber for 1h. Slides were incubated for 1h at room temperature in humid chamber with primary antibodies (1:250). The sections were washed and incubated in secondary antibodies (1:100) for 30 min. After incubation sections were washed followed by incubation in liquid DAB (3, 3’ Diaminobenzidine) substrate chromogen system (DAKO) for 7–10 min and counter stained with hematoxylin. After rinsing in distilled water, the sections were dehydrated and mounted in Permount. The immunostained sections were then visualized on Olympus microscope at 40× magnification.
2.9. Statistical analysis
The means and standard errors of mean (SEM) in the four experimental groups of mice (WT, WT treated with H2S, CBS (−/+) and CBS (−/+) treated with H2S) were determined. For each of response, we checked normality assumptions, using Shapiro-Wilk test (Shapiro and Wilk, 1965) and found data to be valid. ANOVA procedure was used to analyze the data. The procedure was used to compare the different groups. The declared results were significant at alpha level of 0.05, (p<0.05).
3. RESULTS
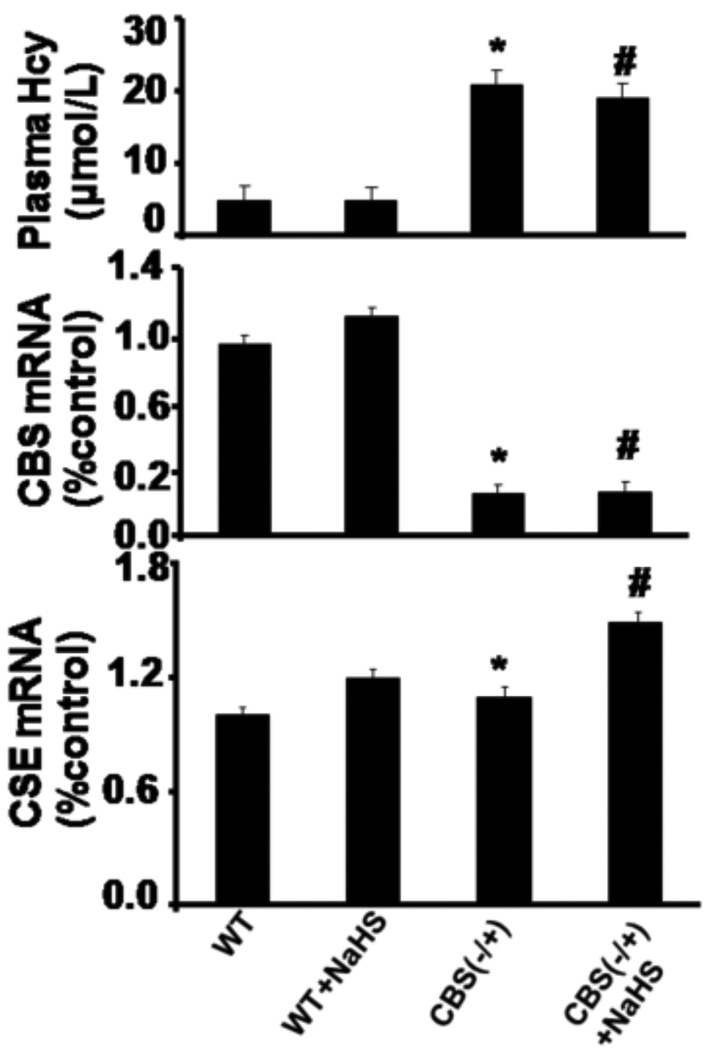
In CBS−/+ mice, the plasma Hcy level was found to be significantly higher as compared to WT mice. There was no change in the plasma Hcy levels in the group supplemented with NaHS (Figure 1).These results suggested that H2S supplementation did not have any role in the plasma Hcy levels. Since CBS and CSE produced H2S, we measured mRNA expression of CBS and CSE enzyme. As shown in Figure 1, CBS gene expression increased in WT+NaHS as compared to WT mice. However, CBS gene expression was low in CBS (−/+) and CBS (−/+)+NaHS mice. CSE gene expression significantly increased in WT+NaHS and CBS−/+ + NaHS mice compared to WT and CBS−/+ mice (Figure 1). These results suggested that both the enzymatic conversion and trans-sulfuration pathways of Hcy clearance were impaired in the CBS−/+ mice and supplementation of NaHS mitigated the decrease in Hcy metabolism.
Figure 1.
Effect of hydrogen sulfide on plasma Hcy levels (Top panel). Total Hcy was extracted from plasma and analyzed by HPLC, presented as µMol/L mean+SEM from n=5 in each group. Effect of hydrogen sulfide on mRNA expression of CBS (Middle panel) and CSE (Bottom panel): Real-time PCR analysis of CBS and CSE in brain tissue of WT, WT+NaHS, CBS(−/+) and CBS(−/+) mice treated with NaHS was performed. The bar graphs represent densitometric data normalized with beta actin and mean ± SEM from n=5 in each group were presented. *p<0.05 compared to WT. #p<0.05 compared to CBS (−/+).
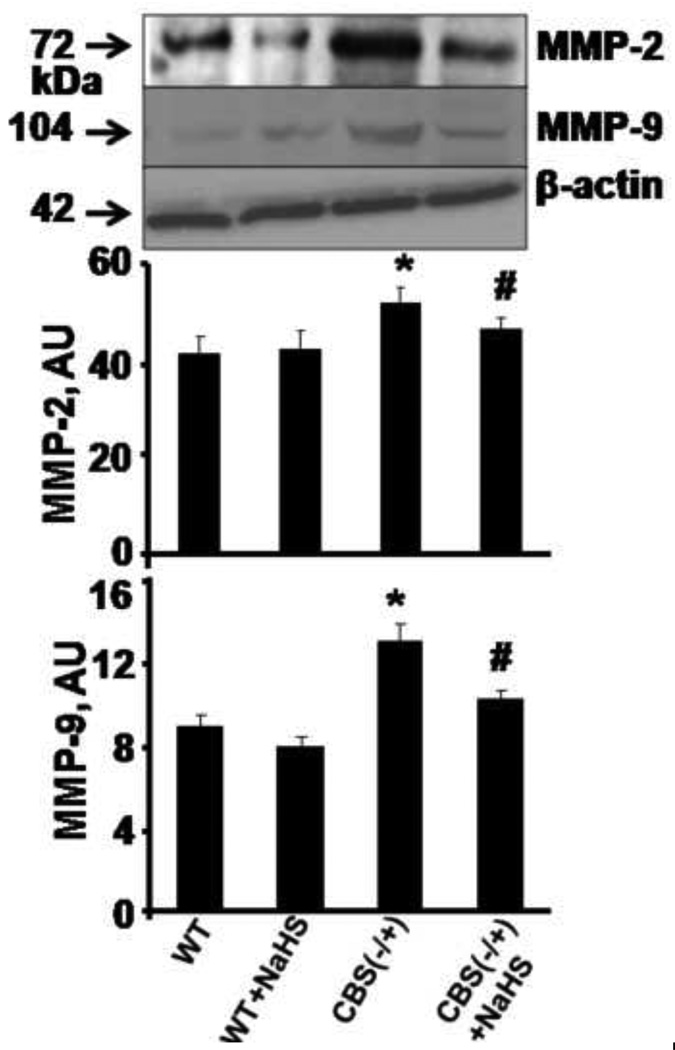
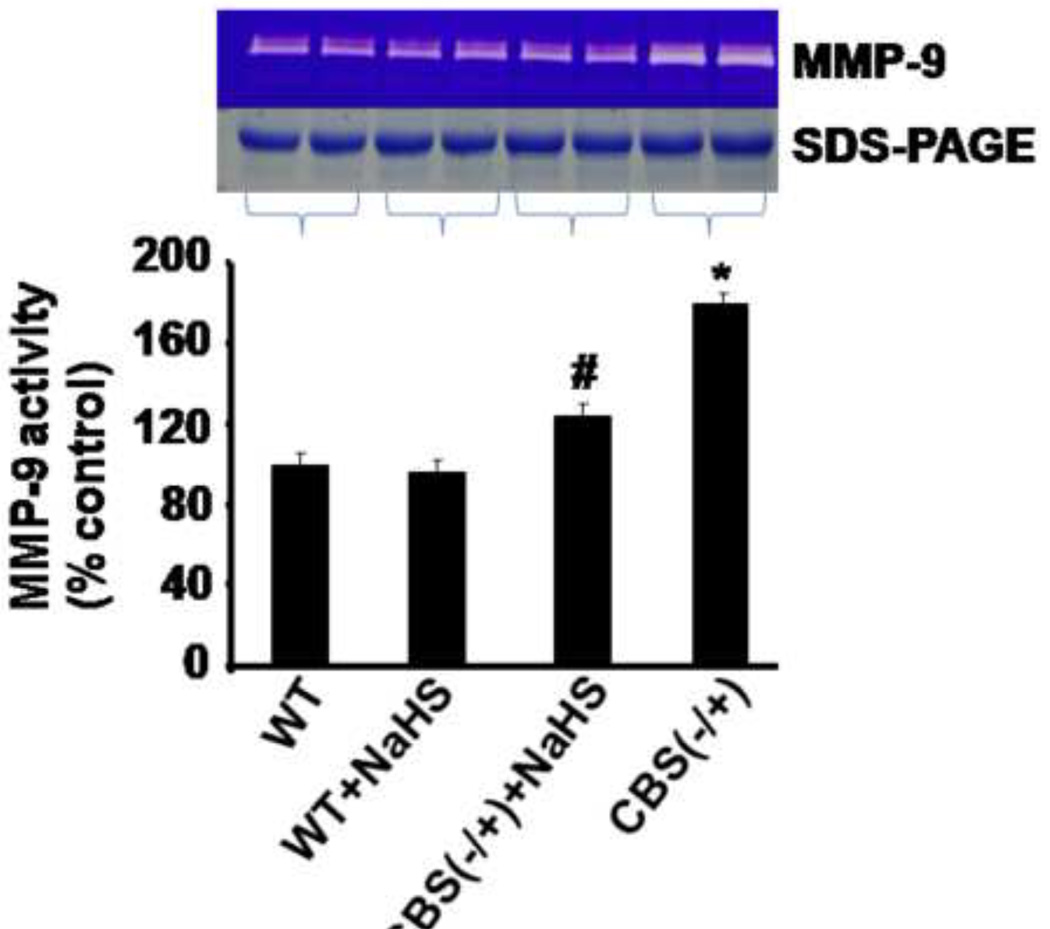
MMP-2 and 9 were critically involved in the brain matrix remodeling. Therefore, we examined the MMP-2, -9 protein levels and activity of MMP-9. As shown in Figure 2, protein levels of MMP-2 and MMP-9 were significant higher in CBS−/+ as compared to WT mice. Interestingly, CBS−/+ mice supplemented with NaHS showed a significant decrease in MMP-2 and -9 protein levels. In addition, we observed an increase in MMP-9 activity in CBS−/+ as compared to WT mice (Figure 3). Importantly, H2S supplementation almost normalized MMP-9 activity in CBS−/+ + NaHS mice as compared to CBS−/+ mice. These results suggested that both MMP-2 and -9 play an important role in HHcy associated neuropathy. The expression and activity of these proteinases was modulated by H2S, which suggested a possible trigger of both MMPs during HHcy causing permeability.
Figure 2.
Effect of hydrogen sulfide on MMPs expression. Western blot analysis of MMP-2 and MMP-9 protein levels in brain tissue of WT, WT treated with NaHS, CBS(−/+) and CBS(−/+) mice treated with NaHS and β-actin was used as control (gel panels). The bar graph represented mean ± SEM normalized with β-actin. n=5 in each group. *p<0.05 compared to WT. ≠p<0.05 compared to CBS (−/+) mice.
Figure 3.
Effect of hydrogen sulfide on MMP-9 activity. Zymographic MMP-9 activity and SDS-PAGE control protein levels in brain tissue of WT, WT treated with NaHS, CBS (−/+) and CBS (−/+) mice treated with NaHS (gel panel). Bar graph presented MMP-9 activity as mean ± SEM, normalized with loading control (same volume of each sample was run on SDS-PAGE and stained by Coomassie blue). n=5 in each group. *p<0.05 compared to WT. ≠p<0.05 compared to CBS (−/+) mice.
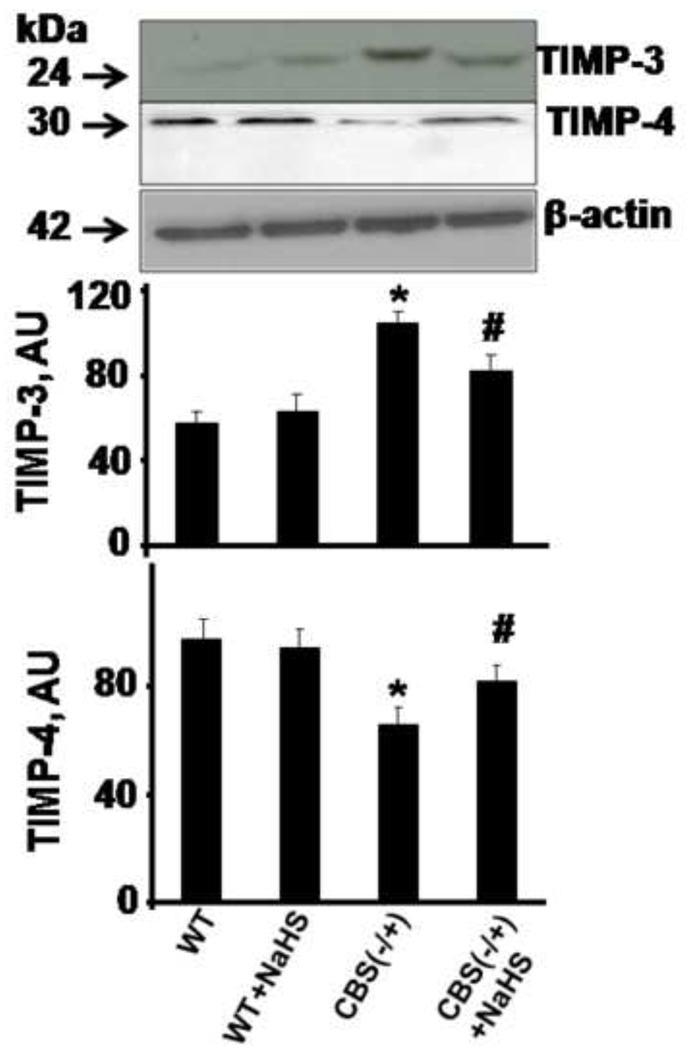
The activities of MMPs were controlled by TIMPs; therefore, we measured the protein level of TIMPs. As shown in Figure 4, protein expression of TIMP-3 significantly increased in CBS−/+ compared to WT mice. However, supplementation of NaHS significantly decreased TIMP-3 protein expression in CBS−/+ mice as compared to CBS−/+ mice without NaHS. TIMP-4 protein levels significantly decreased in CBS−/+ compared to the WT mice. Interestingly, CBS−/+ mice treated with NaHS showed a significant increase in TIMP-4 protein. This suggested that expression of TIMPs was differentially altered by H2S, which supported H2S as being a therapeutic agent to HHcy induced-permeability.
Figure 4.
Effect of hydrogen sulfide on TIMPs expression. Western blot analysis of TIMP-3 and TIMP-4 protein levels in brain tissue of WT, WT treated with NaHS, CBS (−/+) and CBS (−/+) mice treated with NaHS and β-actin was used as control (Gel panels). Scanned intensity of TIMP-3 and TIMP-4, normalized with control beta actin represented as mean ± SEM. n=5 in each group. *p<0.05 compared to WT. ≠p<0.05 compared to CBS (−/+) mice.
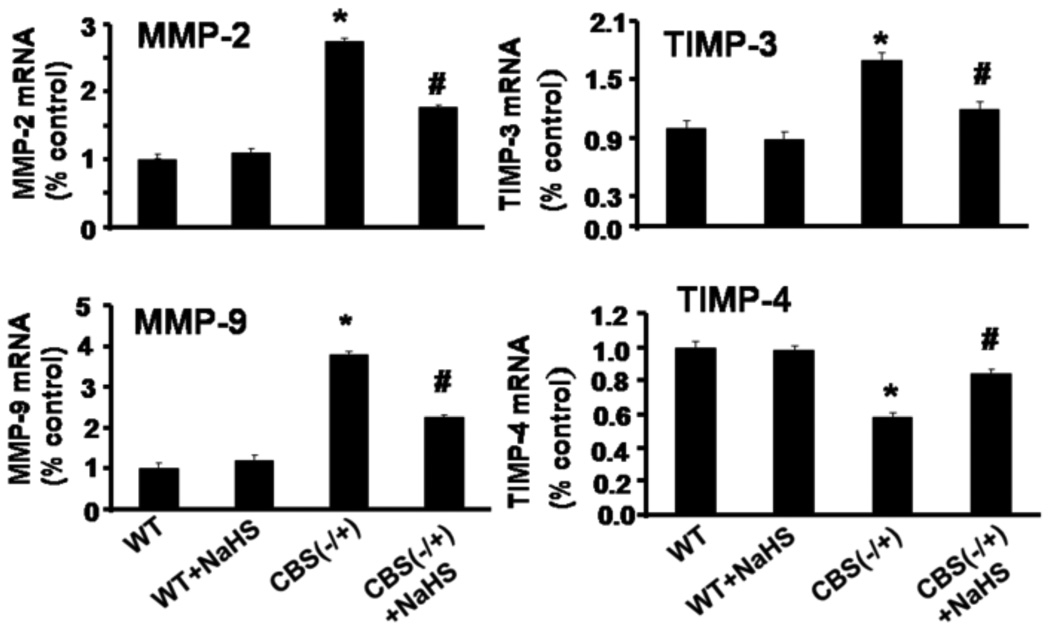
As show in Figure 5, mRNA levels of MMP-2, -9 were significantly increased in CBS (−/+) compared to WT mice (p<0.05. In CBS (−/+) mice supplementation with NaHS prevented the increased in MMP-2 and-9 (Figure 5). The TIMP-3 mRNA was significantly increased in CBS (−/+) compared with WT and NaHS-treated groups p<0.05 (Figure 5). Whereas, TIMP-4 mRNA was significantly decreased in CBS (−/+) compared with WT mice. However, NaHS treatment mitigated the increase in TIMP4 mRNA in CBS−/+ mice (Figure 5).These result suggested that expression of protein levels (Figures 3 and 4) were due to transcriptional regulation of the MMP-2,-9, TIMP-3 and -4 genes (Figure 5). Therefore, these evidences supported H2S as a regulator of matrix genes.
Figure 5.
Effect of hydrogen sulfide on mRNA expression of MMP-2 and -9 and TIMP-3 and -4. Real-time PCR analysis of MMP-2, MMP-9, TIMP-3, and TIMP-4 in brain tissue of WT, WT mice treated with NaHS, CBS(−/+) and CBS(−/+) mice treated with NaHS and GAPDH was used as control. The bar graphs represent mean ± SEM, normalized with GAPDH. n=5 in each group. *p<0.05 compared to WT. ≠p<0.05 compared to CBS (−/+) mice.
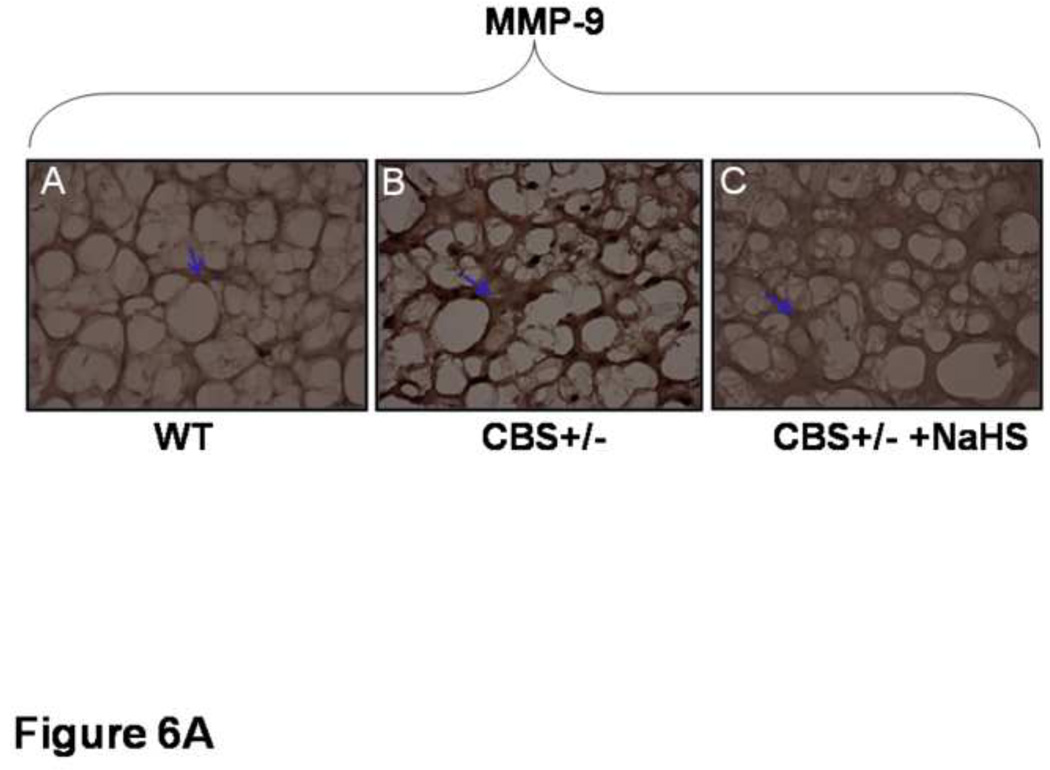
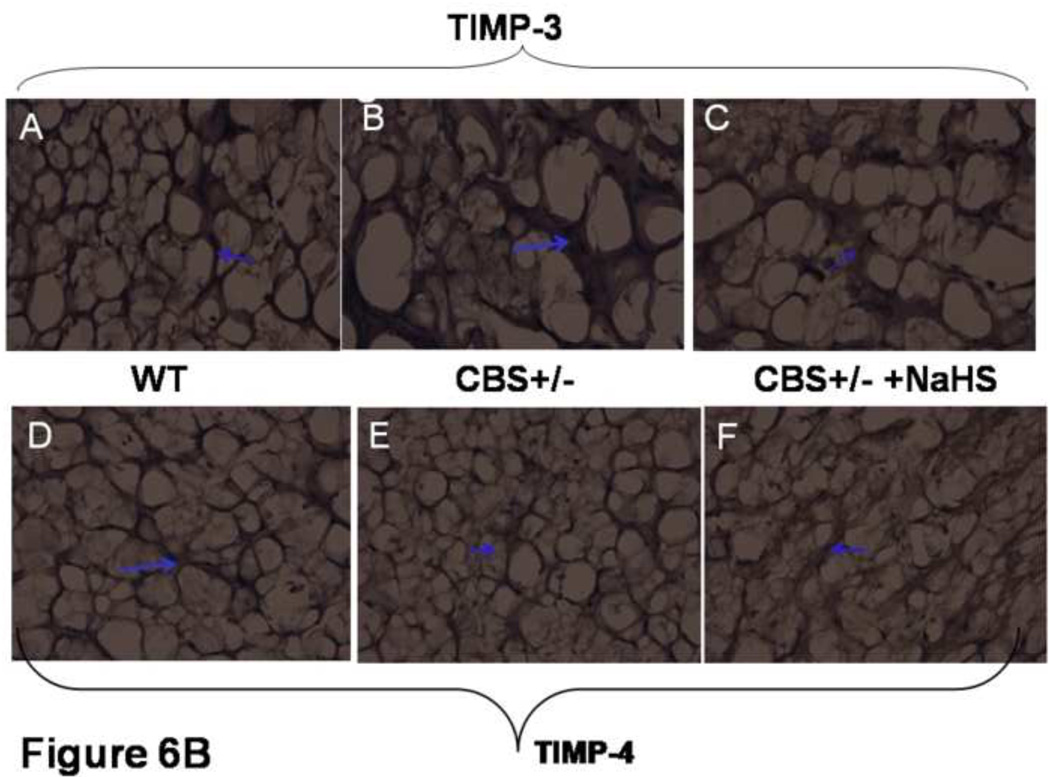
To localize the MMP-9 and TIMP-3 and -4, brain sections were stained from each group with DAB chromogen. There was a significant increase in MMP-9 in CBS (−/+) mice as compared to WT (Figure 6A). The treatment with H2S decreased MMP-9 expression. Similarly, there was an increase in TIMP-3 expression in CBS (−/+) mice as compared to WT and treatment with NaHS significantly decreased TIMP-3 levels in CBS (−/+) mice (Figure 6B). There was a decrease in the expression of TIMP-4 in CBS (−/+) as compared to WT mice. CBS−/+ mice treated with NaHS showed a significant increase in TIMP-4 expression compared to the CBS−/+ mice (Figure 6B). These results suggested increase in TIMP-3 and decrease in TIMP-4 in HHcy. The treatment with NaHS ameliorated the changes in TIMPs.
Figure 6.
Effect of hydrogen sulfide on interstitial deposition of MMPs/TIMPs shown by confocal microscopy. A: Representative immunohistochemistry of MMP-9 activity in brain sections, stained with DAB chromogen. The arrow indicate increase in brown color (MMP-9) in CBS (−/+) mice. Significant decrease in MMP-9 activity in CBS (−/+) treated with NaHS mice. Magnifications 40×. B: Immunohistochemistry of TIMP-3 and TIMP-4 expression in brain sections, stained with DAB chromogen. TIMP-3 activity was increase in CBS+/− section as compared to WT whereas TIMP-4 activity was decrease, indicated by arrow brown color density. Treatment of NaHS reversed the levels of MMP and TIMPs. Magnification 40 ×
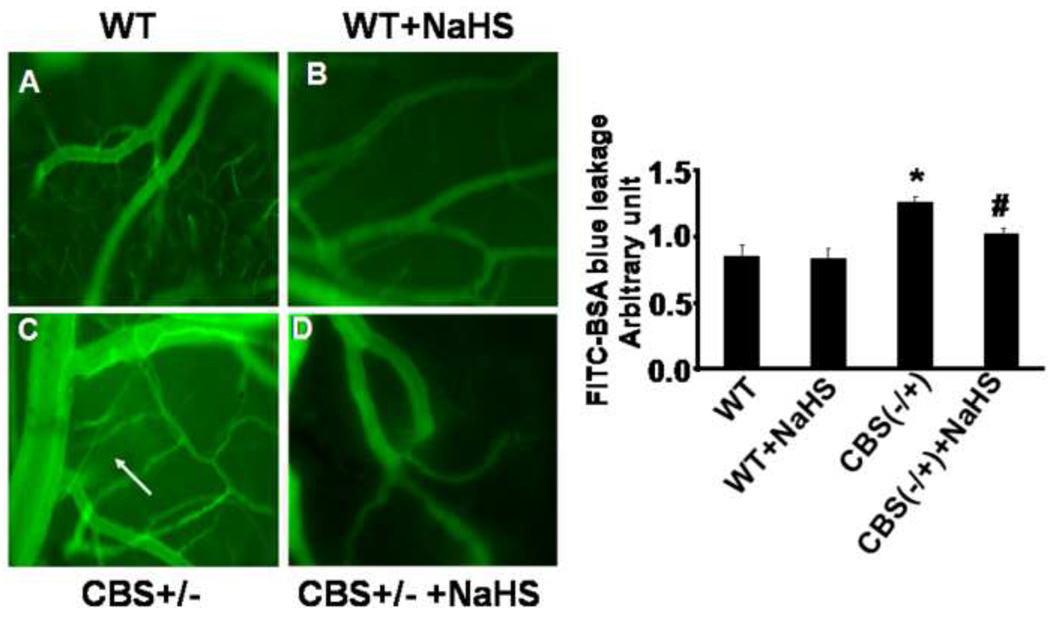
To determine whether H2S mitigated the blood brain barrier (BBB) permeability in CBS−/+ mice, we measured interstitial diffusion of BSA-FITC in brains of WT, WT+NaHS, CBS−/+, CBS−/+ +NaHS mice by intravital microscropy. We observed that BBB permeability was significantly higher in CBS−/+ compared to WT mice. Interestingly, NaHS treatment mitigated BBB permeability in CBS−/+ mice (Figure 7). These results suggested that HHcy was associated with increased permeability in the brain interstitial parenchyma and this damage was partially prevented by H2S supplementation with NaHS (Figure 7).
Figure 7.
Photograph taken on an in-vivo fluorescent microscope (60 ×). The effect of H2S on microcirculation in brain: Representative data shows blood flow, permeability in WT (A); WT treated with NaHS (B); CBS (−/+) (C); CBS (−/+) treated with NaHS (D). The flow was measured for 10 minutes. The arrow represents an increase in the leakage of FITC in brain interstitial parenchyma in CBS (−/+) mice. The bar graphs represent the leakage in mean±SEM from n=4 in each group. Significant *p<0.05 compared to WT. ≠p<0.05 compared to CBS (−/+) mice.
5. DISCUSSION
Although the expression of CSE was increased by NaHS (Figure 1), the basal expression of CSE in the brain was under the detectable level (Ishii et al., 2004). Therefore, it was difficult to understand the involvement of CSE in Hcy metabolism in the brain. Interestingly, both the CBS and CSE enzymes were responsible for endogenous production of H2S. We found that in the presence of NaHS, CSE mRNA was increased, but there was no change in CBS (Figure 1). The mRNA expression of CSE enzyme was elevated with exogenous supplementation of NaHS, a donor of H2S, in CBS−/+ mice. This was associated with mitigation of the increase in MMP-2 and -9 in CBS−/+ mouse brain by H2S supplementation. In addition, TIMP-3 increased and TIMP-4 decreased in CBS−/+ mouse brain, and this differential expression of TIMP-3 versus TIMP-4 was normalized by H2S. Interestingly, it was known that TIMP-3 induced vascular apoptosis (Baker et al, 1998) and TIMP-4 protected (Tummalapalli et al, 2001). These data were corroborated with the brain damage, as evidenced by increased vascular permeability.
Vascular resistance was significantly decreased in carotid artery of CBS (−/+) mice (Kumar et al, 2008). Hcy has been shown to decrease NO-mediated vascular relaxation and therefore, increased blood pressure and decreased blood flow. The decrease in blood flow activated the MMPs and caused cerebral vascular permeability. H2S behaved as a cerebrovascular dilator (Zhao et al, 2001). Vascular contractility was regulated by endogenous and exogenous supply of H2S (Elrod et al, 2007). At physiologically-relevant concentrations, H2S dilated arterioles via activation of ATP-sensitive K+ channels (Tang et al, 2005; Yang et al, 2005). This function was known to promote apoptosis of vascular smooth muscle cells and inhibit proliferation associated with vascular remodeling (Kubo et al, 2007). Intravital microscopy data suggested an increase in BSA-FITC leakage in brain interstitium (Figure 7) of CBS (−/+) mice. These results coincided with the earlier reports that Hcy increased albumin leakage through the endothelial cell monolayer in pial vessels (Lominadze et al, 2006; Tyagi et al, 2007a; Tyagi et al, 2007b). Hcy-induced BSA-FITC leakage drastically decreased with NaHS (H2S donor) treatment.
Previously, we showed that Hcy increased MMP-9 activity in brain endothelial cells (Tyagi et al, 2009). However, the role of H2S in activation of MMP-9 was not defined. In the present study, for the first time, we demonstrated a significant increase in the MMP-2 and MMP-9 protein expression and MMP-9 activity in CBS−/+ mice (Figures 2 and 3). MMP-9 activity was suppressed in CBS−/+ mice treated with NaHS (Figure 3). Others have suggested that endogenous H2S may decrease the level of MMP-13 and TIMP-1 in rats (Li et al, 2009). The increased MMP-2,-9 protein, mRNA levels and MMP-9 activity caused degradation of matrix proteins and increased BBB permeability. The H2S reversed the effect of Hcy on cerebral vascular injury, in part, inhibiting MMPs and increasing TIMP-4.
MMPs activities were regulated by activation of the precursor zymogens and inhibition by the endogenous inhibitors, TIMPs. Thus, the balance between MMPs and TIMPs were critical for the ECM remodeling (Wald et al, 2002) that was essential for developmental and morphogenetic processes (Dollery et al, 1999; Nagase et al, 2006). MMPs degraded proteins that compose the arterial matrix (collagen, laminin, elastin and fibronectin). The expression level of TIMP-4 significantly decreased in CBS−/+ mice. While, TIMP-3 increased in these mice (Figure 4). TIMP-3 induced vascular apoptosis (Baker et al, 1998) and TIMP-4 protected (Tummalapalli et al, 2001). In the present study, we observed an increase in mRNA levels of TIMP-3, MMP-2, MMP-9 and decrease in the mRNA level of TIMP-4 in CBS−/+ mice (Figure 5). The treatment of NaHS inhibited the HHcy-induced sub-endothelial matrix remodeling, suggesting a protective role of H2S in cerebral vascular remodeling. HHcy inactivated TIMP-4 and increased activities of MMP-2 and MMP-9 in hearts of hyperhomocysteinmic rats (Sood et al, 2002). TIMP-3 is known to promote apoptosis in normal cells through increased death receptor signaling (Ahonen et al, 2003). Present study along with earlier reports, suggested substantial increase in MMP-2 and MMP-9 with increased or decreased expression of their inhibitors (TIMP-1, TIMP-3) (Refsum et al, 1998). Brain sections stained for MMP-9 and TIMP-3 showed more interstitial deposition, whereas TIMP-4 staining showed less deposition (Figure 6A and B). This may be a strong indication that Hcy affected MMPs and TIMPs balance, secondary to decrease in H2S.
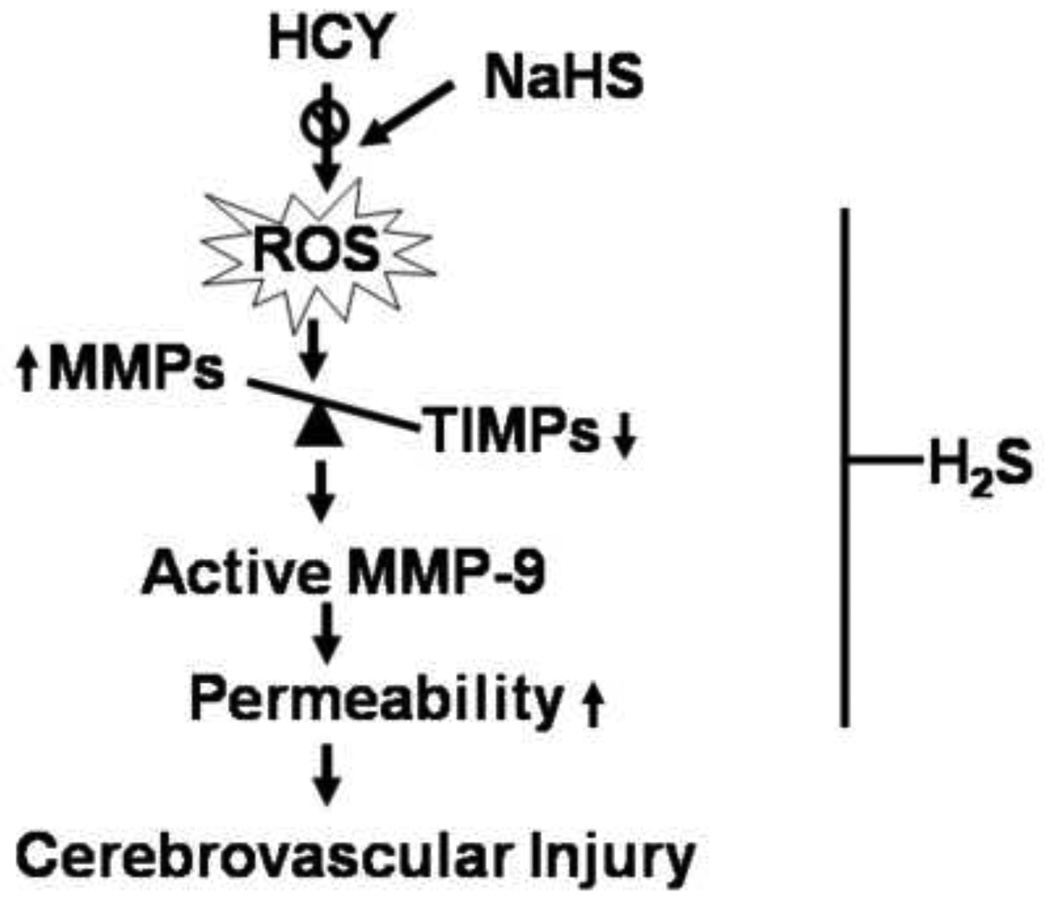
Our observation defined a novel role of H2S in HHcy induced cerebral vascular injury and supported the hypothesis presented in Figure 8. In summary, we have shown that elevation of plasma Hcy level induced disbalance of MMP/TIMP ratio, causing cerebral vascular permeability. H2S supplementation, however, showed the reversal of permeability. Thus, our study suggested that H2S could be a beneficial therapeutic candidate for the treatment of HHcy-associated pathologies, such as stroke and neurological disorders.
Figure 8.
Schematic presentation of proposed mechanism for the protective effect of H2S against Hcy-induced cerebral vascular injury. Due to decrease in CBS and CSE activity, Hcy is increased. This leads to oxidative stress and MMP/TIMP axis imbalance, causing cerebrovascular permeability and dementia.
Limitations
It was known that at higher concentration, millimolar range, H2S can be toxic (Truong et al, 2006; Qu et al, 2008). However, the physiological range (30 µMol/L) of H2S was protective (Zhao et al, 2001). Although the toxic effects of Hcy were associated with increase in oxidative stress. Interestingly, if we could convert Hcy to H2S by CBS and CSE double gene transfer, we may change the paradigm of Hcy-mediated toxicity to Hcy-mediated H2S production and protection. H2S has different benefit other than Vitamin C or E or anthocyanins. Vitamin C or E or anthocyanins were antioxidant, while H2S was blood pressure regulator (Wagner et al, 2009; Yang et al, 2008) and antioxidant. When low concentrations of H2S combined with antioxidant agents, such as SOD or vitamin C, may synergistically increase their antioxidant effects (Yan et al, 2006; Tyagi et al, 2009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A part of this study was supported by NIH grants NS-51568, HL-71010, HL-88012, and HL-74185.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE, Kahari VM. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene. 2003;22:2121–2134. doi: 10.1038/sj.onc.1206292. [DOI] [PubMed] [Google Scholar]
- Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101(6):1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- Dollery CM, McEwan JR, Wang M, Sang QA, Liu YE, Shi YE. TIMP-4 is regulated by vascular injury in rats. Ann. N. Y. Acad. Sci. 1999;878:740–741. doi: 10.1111/j.1749-6632.1999.tb07777.x. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia NW, Reiffenstein RJ, Dowling G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: post-mortem studies and two case reports. J. Anal. Toxicol. 1989;13:105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, Kimura H. Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem. J. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007;232:138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, Givvimani S, Tyagi SC. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem. Int. 2008;53:214–219. doi: 10.1016/j.neuint.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jin H, Bin G, Wang L, Tang C, Du J. Endogenous hydrogen sulfide regulates pulmonary artery collagen remodeling in rats with high pulmonary blood flow. Exp. Biol. Med. (Maywood) 2009;234:504–512. doi: 10.3181/0807-RM-230. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am. J. Physiol Heart Circ. Physiol. 2006;290:H1206–H1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J. Biol. Chem. 2004;279:29871–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Qu K, Lee SW, Bian JS, Low CM, Wong PT. Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int. 2008 Jan;52(1–2):155–165. doi: 10.1016/j.neuint.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Rabaneda LG, Carrasco M, Lopez-Toledano MA, Murillo-Carretero M, Ruiz FA, Estrada C, Castro C. Homocysteine inhibits proliferation of neuronal precursors in the mouse adult brain by impairing the basic fibroblast growth factor signaling cascade and reducing extracellular regulated kinase 1/2-dependent cyclin E expression. FASEB J. 2008;22:3823–3835. doi: 10.1096/fj.08-109306. [DOI] [PubMed] [Google Scholar]
- Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Richardson CJ, Magee EA, Cummings JH. A new method for the determination of sulphide in gastrointestinal contents and whole blood by microdistillation and ion chromatography. Clin. Chim. Acta. 2000;293:115–125. doi: 10.1016/s0009-8981(99)00245-4. [DOI] [PubMed] [Google Scholar]
- Robert K, Pages C, Ledru A, Delabar J, Caboche J, Janel N. Regulation of extracellular signal-regulated kinase by homocysteine in hippocampus. Neuroscience. 2005;133:925–935. doi: 10.1016/j.neuroscience.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Valenzuela M, Wang XL, Looi JC, Brodaty H. Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology. 2002;58:1539–1541. doi: 10.1212/wnl.58.10.1539. [DOI] [PubMed] [Google Scholar]
- Santiard-Baron D, Aupetit J, Janel N. Plasma homocysteine levels are not increased in murine models of Alzheimer's disease. Neurosci. Res. 2005;53:447–449. doi: 10.1016/j.neures.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De GR, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu. Rev. Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-beta-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am. J. Physiol Cell Physiol. 2007;293:C1779–C1787. doi: 10.1152/ajpcell.00207.2007. [DOI] [PubMed] [Google Scholar]
- Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am. J. Physiol Renal Physiol. 2009;297:F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An Analysis of Variance Test for Normality (Complete Samples) Biometrika. 1965 Dec.Vol. 52(No. 3/4):591–611. [Google Scholar]
- Sood HS, Cox MJ, Tyagi SC. Generation of nitrotyrosine precedes activation of metalloproteinase in myocardium of hyperhomocysteinemic rats. Antioxid. Redox. Signal. 2002;4:799–804. doi: 10.1089/152308602760598954. [DOI] [PubMed] [Google Scholar]
- Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc. Res. 2008;79:632–641. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- Tang G, Wu L, Liang W, Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br. J. Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O'Brien PJ. Drug Metabolism Reviews. Molecular Mechanisms of Hydrogen Sulfide Toxicity. 2006;38:733–744. doi: 10.1080/03602530600959607. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Lominadze D, Gillespie W, Moshal KS, Sen U, Rosenberger DS, Steed M, Tyagi SC. Differential expression of gamma-aminobutyric acid receptor A (GABA(A)) and effects of homocysteine. Clin. Chem. Lab Med. 2007a;45:1777–1784. doi: 10.1515/CCLM.2007.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Moshal KS, Sen U, Vacek TP, Kumar M, Hughes WM, Jr., Kundu S, Tyagi SC. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid. Redox. Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Moshal KS, Tyagi SC, Lominadze D. gamma-Aminbuturic acid A receptor mitigates homocysteine-induced endothelial cell permeability. Endothelium. 2007b;14:315–323. doi: 10.1080/10623320701746164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummalapalli CM, Heath BJ, Tyagi SC. Tissue inhibitor of metalloproteinase-4 instigates apoptosis in transformed cardiac fibroblasts. J Cell Biochem. 2001;80(4):512–521. doi: 10.1002/1097-4644(20010315)80:4<512::aid-jcb1005>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Wagner CA. Hydrogen sulfide: a new gaseous signal molecule and blood pressure regulator. J Nephrol. 2009 Mar–Apr;22(2):173–176. [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, Moore PK. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351(2):485–491. doi: 10.1016/j.bbrc.2006.10.058. 15. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008 Oct 24;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yang G, Jia X, Wu L, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005;569:519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagli G, Patacchini R, Trevisani M, Abbate R, Cinotti S, Gensini GF, Masotti G, Geppetti P. Hydrogen sulfide inhibits human platelet aggregation. Eur. J. Pharmacol. 2007;559:65–68. doi: 10.1016/j.ejphar.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]