Abstract
Continuous, rhythmic beating of the heart requires exquisite control of expression, localization and function of cardiac ion channels – the foundations of the cardiac myocyte action potential. Disruption of any of these processes can alter the shape of the action potential, predisposing to cardiac arrhythmias. These arrhythmias can manifest in a variety of ways depending on both the channels involved and the type of disruption (i.e., gain or loss of function). As much as 1% of the population of developed countries is affected by cardiac arrhythmia each year, and a detailed understanding of the mechanism of each arrhythmia is crucial to developing and prescribing the proper therapies. Many of the antiarrhythmic drugs currently on the market were developed before the underlying cause of the arrhythmia was known, and as a result lack specificity, causing side effects. The majority of the available drugs target the conductance of cardiac ion channels, either by blocking or enhancing current through the channel. In recent years, however, it has become apparent that specific targeting of ion channel conductance may not be the most effective means for treatment. Here we review increasing evidence that suggests defects in ion channel trafficking play an important role in the etiology of arrhythmias, and small molecule approaches to correct trafficking defects will likely play an important role in the future of arrhythmia treatment.
Keywords: arrhythmia, atrial fibrillation, hERG, KCNQ1, Kv1.5, LQTS, surface expression, trafficking
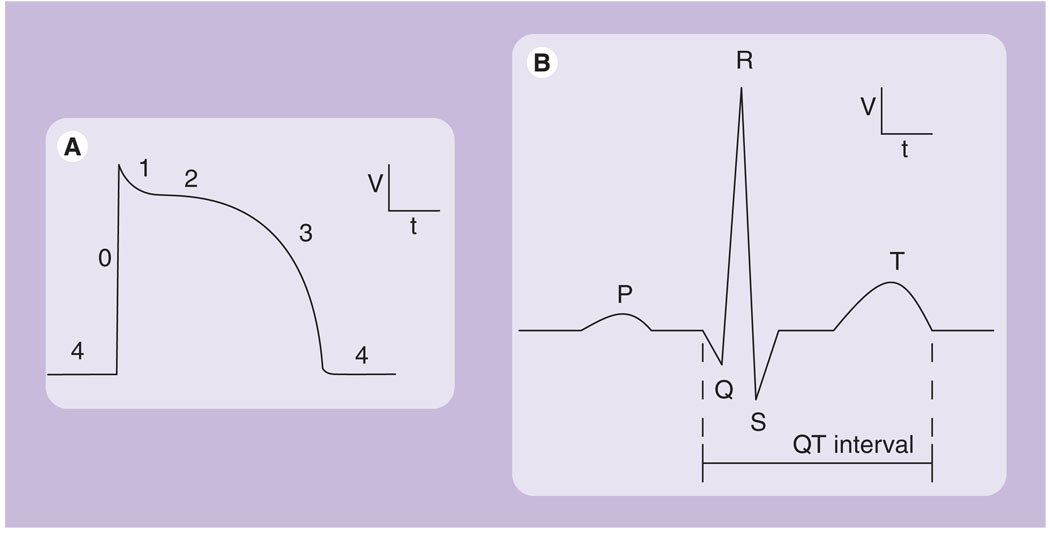
Execution of an action potential requires the coordinated activity of numerous cardiac ion channels (Figure 1A) [1]. The initial, depolarizing upstroke of an action potential is generated by the inward movement of positively charged sodium ions through voltage-gated sodium (Nav) channels, primarily Nav1.5 (SCNSA; Phase 0). These channels rapidly inactivate, and the cell depolarization triggers the opening of voltage-gated calcium (Cav) channels (Phase 1). Calcium influx, in addition to calcium release from intracellular stores, facilitates myocardial contraction. Cellular depolarization also triggers voltage-gated potassium (Kv) channels to open, allowing an efflux of potassium down its electrochemical gradient, beginning myocyte repolarization. The initial transient outward current (Ito) is generated by Kv channels such as Kv4.3 (KCND3; Phase 1). Repolarization continues with the delayed rectifier currents (IKr and IKs), generated by the human ether-a-go-go-related gene (hERG; KCNH2) and Kv7.1 (KCNQ1). The balance of calcium influx and potassium efflux generates the plateau phase of the action potential (Phase 2). Repolarization finishes and the action potential ends as IKs and IKr reach peak density and inward rectifying channels (Kir), such as Kir2.1, open, allowing potassium efflux to overcome the calcium influx (Phase 3). These channels and their associated currents are tightly regulated to ensure proper action potential morphology [2]. Additionally, some currents are specific to certain regions of the heart, such as expression of the ultra-rapid delayed rectifier (IKur) current in human atria but not ventricles (Phase 1) [3–5]. Dysfunction in any of the channels responsible for the cardiac action potential can lead to cardiac arrhythmia [6].
Figure 1. Representative cardiac waveforms.
(A) Typical human ventricular myocyte action potential, with phases 0–4 labeled. (B) Surface ECG from a normal heartbeat, including the P wave, QRS complex and T wave.
Cardiac arrhythmias can manifest in a number of ways. The best studied example is an arrhythmia due to a delay in ventricular repolarization and an increase in action potential duration (APD). Increased APD presents as an increased QT interval (Figure 1B) on an ECG, known as long QT syndrome (LQTS) [7]. While the presentation, a lengthened QT interval, is quite simple, the underlying cause can be more complicated. A delay in repolarization can be due to defects in Nav1.5 that result in increased sodium flux. An increase in sodium current can have a number of causes, including increased conductance, impaired inactivation, increased surface expression, or spontaneous opening and early reopening. A repolarization delay can also be caused by a decrease in potassium flux. Thus, loss-of-function mutations in both hERG and KCNQ1, which reduce repolarization capacity, are known to cause LQTS. On the other hand, atrial fibrillation (AF), the most common cardiac arrhythmia, can result from an increase in repolarization capacity and thus a decrease in APD [8]. This is largely due to reduced L-type Ca2+ current, although rare gain-of-function mutations in KCNQ1 [9], hERG [10], Kir2.1 [11] and KCNE ancillary subunits [12] have also been associated with AF due to increases in channel conductance. Various mutations in other cardiac ion channels and associated proteins are linked to a number of other arrhythmias, including Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia, short-QT syndrome and Wolf–Parkinson–White syndrome.
Treating and correcting the cause of these disorders is preferable to merely masking the symptoms, and it is clear that an understanding of the underlying genetic mechanism is necessary to achieve this. Unfortunately, most of the available antiarrhythmic drugs lack the necessary specificity and have numerous adverse effects [13,14]. For example, drugs used to treat AF, such as quinidine and sotalol, block the hERG potassium channel and increase the APD. While this is desirable for AF, the lack of atrial specificity often leads to prolonged ventricular QT interval, causing the potentially fatal polymorphic ventricular tachycardia Torsades de Pointes (TdP) [15]. In fact, most drugs are now screened for hERG blockade due to this side effect. Other commonly used drugs, such as amiodarone, have a broad range of effects including sodium, calcium and potassium channel blockade as well as noncompetitive β-blockade. These numerous effects make the drug surprisingly effective at treating AF; however, the list of side effects is quite extensive. More recent efforts have focused on developing drugs with increased specificity. A promising target for the treatment of AF is Kv1.5, which is responsible for the atrial-specific (in human heart) IKur. Many new drugs are currently in clinical trials, but they have yet to achieve the desired selectivity, efficacy, bioavailability and safety [16]. Fortunately, evidence is now emerging that suggests that targeting the pathways that control ion channel surface density may provide an alternative to traditional antiarrhythmic therapies that target channel conductance. In this article, we review the association of LQTS and ion channel trafficking, the best understood association of abnormal trafficking with arrhythmia, and we then present Kv1.5 (KCNA5) as an example of an emerging target in channel trafficking (see Table 1 for a more comprehensive list).
Table 1.
Mutations associated with trafficking defects in cardiac arrhythmia.
| Gene | Disease | Mutations | Proposed mechanism | Ref. |
|---|---|---|---|---|
| KCNQ1 | LQT1 | Y148X | Channel truncation | [55] |
| A178fs/105 | † | [56] | ||
| L191P | Exposure of a hydrophobic residue | [102] | ||
| H258R | † | [59] | ||
| E261D | † | [57] | ||
| E261K | † | [57] | ||
| L273F | † | [57] | ||
| F275S | † | [103] | ||
| ΔS276 | † | [55] | ||
| Q357R | † | [104] | ||
| R518X | † | [57] | ||
| M520R | Impaired calmodulin interaction | [105] | ||
| Q530X | † | [57] | ||
| T587M | Blocks interaction with hERG | [61] | ||
| G589D | Disruption of a trafficking motif | [106] | ||
| R591H | Disruption of a trafficking motif | [106] | ||
| R594Q | Disruption of a trafficking motif | [106] | ||
| ΔV595 | Impaired subunit assembly | [58] | ||
| R863X | Channel truncation | [107] | ||
| P631fs/19 | Generation of an RXR retention motif | [58] | ||
| 1008delC | † | [57] | ||
| KCNH2 | LQT2 | I31S | † | [33] |
| T65P | PAS domain disruption | [108] | ||
| del234–241 | † | [109] | ||
| A422T | † | [33] | ||
| A429P | † | [109] | ||
| D456Y | † | [33] | ||
| F463L | † | [77] | ||
| N470D | † | [33] | ||
| T474I | † | [33] | ||
| Y493F | † | [109] | ||
| R534C | † | [33] | ||
| A561T | † | [33] | ||
| A561V | † | [33] | ||
| H562P | † | [33] | ||
| I571L | † | [33] | ||
| G572S | † | [33] | ||
| KCNH2 | LQT2 | I593R | Activates the UPR pathway | [37] |
| P596R | † | [33] | ||
| G601S | Altered chaperone interactions that promote degradation |
[31] | ||
| G604S | † | [110] | ||
| Y611H | † | [33] | ||
| V612L | † | [33] | ||
| A614V | † | [33] | ||
| T623I | † | [33] | ||
| N629D | † | [33] | ||
| N629S | † | [33] | ||
| V630A | † | [33] | ||
| V630L | † | [33] | ||
| F640V | † | [33] | ||
| R725W | Strengthened interaction with ER-associated chaperones |
[40] | ||
| Q725X | Channel truncation with impaired subunit assembly |
[111] | ||
| R744fs | † | [112] | ||
| 2398+1G>C | 18 amino acid insertion that disrupts CNBD | [113] | ||
| F805C | † | [33] | ||
| S818L | † | [33] | ||
| V822M | † | [33] | ||
| R823W | † | [33] | ||
| p.Pro872fs | Channel truncation that retains WT channel intracellularly |
[114] | ||
| A915fs+47X | † | [115] | ||
| R1014X | Channel truncation | [45] | ||
| 1122fs/147 | † | [116] | ||
| SCN5A | Brugada | R282H | † | [117] |
| T353I | † | [118] | ||
| E1053K | Impaired interaction with ankyrin-G | [119] | ||
| R1232W/ T1620M |
† | [120] [121] |
||
| G1406R | † | [122] | ||
| R1432G | † | [123] | ||
| G1734R | † | [124] | ||
| M1766L | † | [125] | ||
| CCD | 5280delG | Misfolding | [126] | |
| SCN5A | CCD | P1008S | † | [127] |
| SCN3B | IVF | V54G | Intracellular retention of Nav1.5 | [128] |
| GPD1- L | Brugada | A280V | Intracellular retention of Nav1.5 | [129] |
| ANKB | LQT4 | E1425G | Loss of ion channel coordination | [68] |
| KCNE1 | LQT5 | L51H | Misfolding and retention by quality control mechanisms |
[130] |
| T58P/L59P | Disrupted KCNQ1 interaction | [131] | ||
| R98W | Disrupted KCNQ1 interaction | [131] | ||
| KCNJ2 | Andersen syndrome |
Δ95–98 | † | [62] |
| C101R | † | [132] | ||
| V302M | Loss of PIP2 binding | [63] | ||
| Δ314–315 | † | [62] | ||
| CACNA1C | LQT8 | A39V | † | [66] |
| Cav-3 | LQTS | T78K | † | [67] |
| HCN4 | SND | G480R | † | [133] |
| D533N | † | [134] | ||
| CACNB2b | CCD | D601E | † | [127] |
Mechanism is unknown.
CCD: Cardiac conduction disorder; CNBD: Cyclic nucleotide binding domain; ER: Endoplasmic reticulum; IVF: Idiopathic ventricular fibrillation; LQT1: Long QT syndrome type 1; LQT2: Long QT syndrome type 2; LQTS: Long QT syndrome; PAS: Per–Arnt–Sim; PIP2: Phosphatidylinositol-4,5-bisphosphate; SND: Sinus node dysfunction; UPR: Unfolded protein response; WT: Wild-type.
Trafficking & LQTS
Ion channel biogenesis, trafficking and degradation are quite complex, and there are many steps where disruption of the system can occur. As with all proteins, newly transcribed channel subunit mRNA associates with ribosomes in the cytosol and begins translation. As the nascent peptide exits the ribosome, a signal sequence in the peptide binds a signal-recognition peptide (SRP) that targets the complex to the endoplasmic reticulum (ER). There it binds the translocon, a complex that forms an aqueous pore through which the nascent peptide either passes into the ER or integrates into the ER membrane [17]. It is here that the channel’s transmembrane topology is defined [18], and the ER membrane also aids in folding, tetramerization and ancillary subunit assembly [19–21]. A number of mechanisms then control release from the ER, including retention/retrieval signals in the protein [22], anterograde signals [23], phosphorylation state [22,24] and association with other accessory proteins [25]. Channels then pass through the Golgi apparatus and on to the plasma membrane via kinesin motors [26]. Finally, channels can be endocytosed and either recycled or degraded [27,28]. For a more comprehensive review on channel ontogeny, see [29,30].
Human ether-a-go-go-related gene
Long QT syndrome commonly results from either a loss of function of KCNQ1 or hERG or a gain of function of Nav1.5. Each of these defects can result in a delay in ventricular repolarization, prolonging the QT interval and leading to TdP, ventricular fibrillation (VF), syncope and sudden death [7]. To date, over 200 KCNH2 mutations have been identified that associate with LQTS [31]. While inherited mutations sufficient to cause LQTS are rare due to negative selection, benign mutations that evade selection are often present that lead to QT prolongation in the presence of a drug, a condition known as drug-induced TdP (diTdP). While the most common cause of diTdP is blockade of cardiac Kv channels (reviewed in [32]), increasing evidence suggests alteration of channel trafficking plays an important role in the hereditary form of this disease. In fact, a study by Anderson et al. in 2006 examined 34 missense mutations associated with LQTS and identified trafficking deficiency as the most common mechanism for hERG-associated LQTS [33]. Furthermore, in most cases, the deficiency could be rescued by treatment with E4031 (a hERG blocker), thapsigargin (a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase [SERCA] inhibitor) or incubation at 27°C, although the effectiveness of each depended on the particular mutation present. Importantly, it was demonstrated that hERG blockade is not necessary for pharmacologic rescue. Terfenadine and fexofenadine are H1 receptor antagonists that are structurally similar, and each rescues the hERG trafficking mutant N470D. While terfenadine causes significant hERG blockade in addition to H1 antagonism, fexofenadine retains its H1 antagonist function with much less hERG inhibition. It is able to restore trafficking at a concentration that is 350-fold less than the IC50 for hERG inhibition[34].
Evidence suggests that most hERG missense mutations result in the production of an immature, misfolded form of the protein that is retained in the ER, where quality control mechanisms target the channel for degradation by the proteasome [35,36]. Indeed, it has been demonstrated that hERG trafficking mutants activate the unfolded protein response (UPR), an ER stress pathway associated with numerous disease states [37]. In some cases, the mutation present prevents normal tetrameric channel assembly, and rescue by channel blockers is unsuccessful. When the channel tetramer forms properly, it is likely that rescue occurs through a chaperone mechanism that stabilizes the protein and promotes surface expression, such as with E4031. Once inserted into the membrane, the (unblocked) channels appear to function normally [38,39]. Retention in the ER can also be facilitated by interaction with chaperones such as heat-shock protein (Hsp) 70 and Hsp90. Mutations such as R752W and G601S strengthen the interaction of hERG with Hsp70 and Hsp90. In these cases, lower incubation temperature or drug binding can interfere with Hsp interaction, releasing the channel and restoring proper trafficking. There is a fine balance, however, as Hsp90 interaction is still necessary for proper folding and maturation [40]. For example, the reactive oxygen species (ROS) generated during hypoxia have been shown to inhibit protein folding and maturation by interfering with the interaction between hERG and the Hsp90 chaperone complex [41]. Another important hERG chaperone has also been identified: FKBP38 [42]. Evidence suggests FKBP38 interacts with Hsp90, and the two may function in opposition to control hERG retention or release from the ER. Overexpression of FKBP38 was also able to partially rescue a hERG trafficking mutant, F805C.
In contrast with hERG missense mutations, nonsense mutations generate a stop codon that results in a truncation of the channel’s C-terminus. These mutations typically result in nonfunctional channels, and they can exert a dominant-negative effect on the wild-type (WT) channel subunits. Depending on the length of the truncation, the channel may either reach the cell surface but lack proper function (shorter truncations) or be retained in the ER (longer truncations). It has been reported that the cyclic nucleotide binding domain (residues 750–870) is necessary for proper trafficking of hERG, and mutations in this region result in ER retention. Indeed, all LQTS-associated mutations, both missense and nonsense, in this region result in trafficking defects [43]. In the case of nonsense mutations, however, simple release from the ER will not restore function. For these channels, restoring the full-length protein requires an intervention that will result in read through of the premature stop codon. Yao et al. have recently reported success in correcting some nonsense mutations using aminoglycoside antibiotics, a class of drugs known to reduce the accuracy of translation [44]. Using G-418 and gentamicin, they demonstrate rescue of protein truncation in the W927X and R1014X mutant hERG channels [45]. While the treatment was not successful in rescuing all of the tested mutations, it represents a proof-of-principle that trafficking defects due to protein truncation could also be corrected.
hERG trafficking has also been observed to be disrupted by cardiac glycosides [46]. These compounds act by interfering with Na+/K+ pumps and not through direct interaction with hERG. While a number of signaling pathways are activated by Na+/K+ pump inhibition, none is connected to hERG trafficking. By contrast, cardiac glycosides affect hERG trafficking by decreasing intracellular potassium concentrations, and normal potassium levels appear necessary for proper folding of hERG [47]. The authors suggest K+ is likely to bind to the conductive filter early in channel biogenesis and help form a properly folded protein. The lower K+ levels lead to misfolding in the filter region (and possibly in the rest of the protein), resulting in ER retention. Furthermore, Guo et al. demonstrate that low serum [K+] leads to increased internalization and degradation of hERG by the lysosomal pathway [48]. Their study suggests that a serum [K+] associated with clinical hypokalemia results in increased ubiquitination and endocytic degradation of hERG. This recent information provides a potential alternative mechanistic explanation for the association of hypokalemia and LQTS [49]. It will be interesting to see how other LQTS mutations associated with hypokalemia, such as hERG V822M [50] or T10M in the hERG ancillary subunit KCNE2 [51], fit this new model.
KCNQ1
Another Kv channel α subunit associated with LQTS is KCNQ1, which generates cardiac myocyte IKs. Many KCNQ1 mutations have been identified that associate with inherited arrhythmias such as Jervell and Lange-Nielsen syndrome (JLNS) and Romano–Ward syndrome (RWS), and most of these mutations result in impaired channel function [52]. Early reports suggested KCNQ1 mutations resulted in channels that reached the surface but were nonfunctional [53]; however, recent evidence suggests that KCNQ1 trafficking defects may also play a role in disease. Subsequent reports identified that the mutations T587M, ΔS276 and A178fs/105 each result in trafficking deficiency [54–56]. A more thorough report by Wilson et al. examined numerous mutations associated with either JLNS or RWS and discovered that a majority of them led to some degree of ER retention [57]. The mutations occurred throughout the protein and ranged from simple point mutations to frameshift nonsense mutations, so the retention is likely due to quality control mechanisms. While still not as extensively studied as hERG trafficking, emerging research studies continue to examine the contributions of KCNQ1 trafficking to cardiac arrhythmia.
Sato et al. describe two KCNQ1 mutations identified in a LQTS family: one mutant results in the deletion of a valine at position 595 (delV595) and the other, owing to the insertion of a cytosine, results in a frameshift after residue 631 that causes 19 novel amino acids (P631fs/19) [58]. In the case of ΔV595, there is impaired subunit assembly, which leads to a loss of surface expression. This also suggests that subunit assembly is necessary for proper trafficking. In the case of P631fs/19, the inserted amino acids generated an RXR motif that resulted in ER retention. These both represent novel mechanisms for trafficking dysfunction. Another mutation, KCNQ1-H258R, when coexpressed with the ancillary subunit KCNE1, forms channels with accelerated activation, slowed deactivation and a negatively shifted voltage dependence, all of which would be predicted to cause short QT syndrome. However, these effects are counteracted by an additional effect on channel trafficking, as the mutation also results in intracellular retention [59]. At slow rates or low plateau voltages, the gain-of-function in channel kinetics is balanced by the trafficking defect. However, at faster rates, such as those seen during exercise, the altered kinetic properties of the channel become saturated and the trafficking defect dominates, leading to QT prolongation. This demonstrates the importance of examining all aspects of channel function when evaluating the effects of novel mutations.
Importantly, once seemingly disparate Kv channel α subunits can also impart trafficking defects on each other. Ehrlich et al. previously described an interaction between KCNQ1 and hERG that promoted hERG membrane expression [60], and it has now been demonstrated that mutations in KCNQ1 can result in a decrease in hERG membrane expression [61]. The KCNQ1-T587M mutation results in a loss of membrane trafficking, and while the mutant can still interact with hERG, it no longer promotes membrane expression. This coordinated decrease could account for the severe clinical phenotype seen with this particular mutant. This discovery further underscores the complexity of channel trafficking and arrhythmias.
Kir2.1, Cav1.2, Cav-3 & Ankyrin-B
While relatively little is known about how trafficking defects in other LQTS-associated proteins contribute to the disease, some research does suggest a role. Mutations in the inward rectifier potassium channel Kir2.1 associated with Andersen–Tawil syndrome (ATS) were characterized, and some were found to result in intracellular retention [62]. The V302M mutation appears to result in misfolding and improper maturation, and deletion of residues 314–315 also resulted in a loss of membrane trafficking. Mutations in this region appear to interfere with phosphatidylinositol 4,5-bisphosphate (PIP2) binding, which is necessary for proper channel function [63]. Another mutation, M307I, was identified in Korean ATS patients and also appears to interfere with PIP2 binding and channel trafficking[64]. This interaction may also be targeted by certain drugs. Indeed, tamoxifen, an estrogen receptor antagonist used to treat breast cancer, was recently discovered to inhibit Kir2.1 indirectly by interfering with the PIP2-channel interaction [65].
In addition to Kir2.1, other LQTS proteins have also been associated with trafficking dysfunction. The A39V mutation in Cav1.2 (CACNA1C), a calcium channel associated with LQTS and other cardiac arrhythmias, results in a loss of surface trafficking [66]. A T78K mutation in Cav-3 (CAV3), a caveolin important in muscle physiology, also causes intracellular retention [67]. Finally, an E1425G mutation in Ankyrin-B (ANKB), an adapter protein important for proper channel targeting, has been associated with a loss of ion channel coordination [68]. While these mutations have yet to be directly associated with LQTS, they all follow a common theme of disrupted trafficking in cardiac arrhythmia.
Trafficking & AF
Kv1.5
Much like LQTS, AF is a complex disease potentially arising from a number of causes. Under normal sinus rhythm, an electrical impulse from the sinus node triggers contraction of the atria. This signal is then passed through the atrioventricular (AV) node and ventricular contraction occurs. During AF, this coordinated electrical signaling is replaced by multiple re-entrant waves in the atria that result in disorganized, rapid and repetitive atrial contraction (reviewed in [13,69]). Studies of AF patient populations have identified mutations in numerous ion channels, including hERG, KCNQ1 and Kir2.1, that associate with the disease [9,11,70–72]. As discussed previously, loss-of-function mutations in these genes can lead to an increase in APD and QT prolongation; however, in the AF populations, evidence suggests that the identified mutations lead to an increase in function and shortening of the action potential, which can predispose to AF. Unfortunately, targeting many of these channels leads to numerous unintended effects. One of the most widely used AF drugs, amiodarone, blocks sodium, calcium and potassium channels, in addition to β-blockade. Even if channel subunit specificity is achieved, expression of these channels in the ventricles as well as the atria means effective AF correction will likely cause an increase in ventricular APD. Owing to the off-target effects seen with current AF therapies, much research has focused on identifying and developing atrial-specific therapeutics. A key emerging target is Kv1.5 (KCNA5), which underlies the IKur current in humans. Importantly, this current has atrial-specific expression in the heart, making it a very promising target [69,73]. Over the last decade, many small molecule inhibitors for Kv1.5 have been developed in the hope of providing a safer, more effective treatment for AF. Ford and Milnes review many of the more recent entries into clinical development, but none have been able to achieve all of the characteristics desired, including selectivity, efficacy, bioavailability and safety [16]. Clearly, there is still an enormous unmet need for AF therapy, and to achieve that, we need a stronger understanding of Kv1.5 function. Fortunately, the research efforts relating to Kv1.5 biology as it relates to AF are steadily increasing [16], and, much like hERG and KCNQ1, evidence is beginning to suggest an important role for Kv1.5 trafficking in the development and maintenance of AF.
The first association of Kv1.5 and AF came from the discovery that chronic AF patients had a more than 50% reduction in Kv1.5 expression in the atria [74]. This reduction in expression did not correlate with a decrease in mRNA levels, however, suggesting regulation at the post-transcriptional level [75]. These results suggested that the change was a consequence of AF rather than a cause. The first report suggesting a causal relationship came when a nonsense mutation in KCNA5 was identified in an AF family [76]. The mutation, E375X, generates a premature stop codon, and the truncated protein had a dominant-negative effect on the expression of IKur. The mutation increased the susceptibility to early afterdepolarization and arrhythmogenic activity. This was also the first report to suggest that an increase in atrial APD could also lead to AF. Additional KCNA5 mutations were later discovered that also linked a loss of function with AF [77]. By contrast, overexpression of Kv1.5 in rat cardiomyocytes significantly decreases APD [78], which agrees with other reports describing gain-of-function mutations in Kv channels that lead to AF [11,12,79]. These studies highlight the importance of tightly controlled Kv1.5 surface expression; indeed, both increases and decreases in function can lead ostensibly to the same disease, and proper ion channel density is regulated by a fine balance between trafficking to and removal from the cell surface. Additionally, this underscores the importance of understanding the genetic basis for disease on a case-by-case basis, as Kv1.5 inhibitors, currently being pursued for AF therapy, will likely be ineffective in patients with a loss of channel function.
Despite little evidence of a direct connection between Kv1.5 trafficking and AF, it is clear that improper channel expression and surface density can lead to a disease state, and it is likely that controlling expression levels therapeutically will play an important role in the future of AF treatment. Fortunately, numerous reports are emerging that have begun to develop a picture of Kv1.5 regulation. Potassium channel ancillary subunits, such as the KCNE family of proteins, are known to be involved in channel regulation; however, they have also been implicated in atrial disease states. In fact, a gain-of-function mutation, R27C, in KCNE2 was identified in two AF families that caused an increase in KCNQ1 current [12]. In addition, we discovered that KCNE2 can also interact with Kv1.5 and promote its targeted surface expression in vivo in ventricular myocytes. In Kcne2−/− mice, we observed a complete loss of Kv1.5 trafficking to the ventricular intercalated discs and a predisposition to QT prolongation [80]. By contrast, KChIP2, another potassium channel ancillary subunit, has been shown to decrease the surface expression of Kv1.5, probably by causing retention in the ER [81]. The mechanism of action and role in AF that these ancillary subunits have is not yet clear, but disruption of channel surface expression certainly has a role in the etiology of cardiac arrhythmias.
In addition to potassium channel ancillary subunits, a number of other interacting proteins have been identified that may regulate Kv1.5 trafficking. Eldstrom et al. discovered an increase in Kv1.5 current with the addition of SAP97, a PDZ domain protein that can help anchor proteins at the membrane [82]. SAP97 likely interacts with Caveolin-3, a scaffolding protein present in lipid rafts, and together they recruit Kv1.5 to specific microdomains [83]. Once Kv1.5 reaches these lipid raft microdomains, interactions with cholesterol modify the steady-state kinetics of the channel [84]. In addition, caveolin trafficking mutants can retain Kv1.5 intracellularly. Indeed, caveolin mutants have been associated with LQTS and pacemaker dysfunction [85,86]. Another scaffolding protein, four and a half LIM protein 1 (FHL1), was also shown to interact with Kv1.5 and promote surface expression [87]. It is clear that numerous protein–protein interactions are necessary for proper Kv1.5 expression, and disruptions in any could potentially lead to cardiac dysfunction. Along with these protein interactors, some post-translational modifications have been identified that regulate Kv1.5 surface expression. Phosphorylation by Src tyrosine kinase leads to current suppression [88]; however, PKA activity can increase basal Kv1.5 currents [89]. S-acylation, the attachment of fatty acid side chains to cysteine residues, promotes surface expression, and inhibition of S-acylation results in proteasomal degradation of the channel [90].
Ion channel surface density can be regulated by a multitude of other mechanisms. The kinesin I isoform Kif5b, a molecular motor, has been shown to increase surface expression of Kv1.5, and a dominant-negative form of the protein blocks surface expression, suggesting an important role in forward trafficking [26]. Mutations in other kinesin isoforms have already been associated with a disease state [91], and it is likely that Kif5b mutations could lead to cardiac arrhythmia. Once in the membrane, channels can be regulated by endocytosis and either degradation or recycling. Endocytosis occurs under normal conditions, but recent evidence suggests some antiarrhythmic drugs may also promote the process [92]. An increase in Kv1.5 internalization was observed following treatment with the antiarrhythmic drug quinidine, and while acute treatment was reversible, chronic treatment led to channel degradation. These data are similar to those seen with drug-induced hERG internalization, as mentioned previously. Once internalized to early endosomes, Kv1.5 has been shown to interact with the dynein motor complex for transport along microtubules [93], suggesting dynein dysfunction as well as microtubule disruption could be a contributing factor in arrhythmia, especially considering cytoskeletal changes are seen in myocytes of patients with congestive heart failure [94]. Internalized Kv1.5 can then either be targeted for degradation by the Nedd4–2 ubiquitin ligase [27] or recycled back to the membrane in a process mediated by the small GTPases Rab4 and Rab11 [28].
Channel trafficking as a therapeutic target
It was only a decade ago that Furutani et al. described the first trafficking defect associated with cardiac arrhythmia. The hERG glycine-to-serine missense mutation (G601S) identified in a LQTS family results in the production of an immature form of the channel that fails to exit the ER and therefore does not reach the plasma membrane [95]. Importantly, it was then shown that some hERG channel blockers, including cisapride, E4031 and quinidine, were able to rescue hERG surface trafficking defects [38]. While most of these drugs are much more effective as channel blockers than chaperones, some, such as C6-TEA and C8-TMA, were efficient chaperones and poor blockers. This suggested that channel blockade and trafficking can be independently targeted.
On the other hand, instead of rescuing defective hERG surface trafficking, a number of drugs are also known to decrease hERG surface density. Ketoconazole, an antifungal, and fluoxetine, a selective serotonin reuptake inhibitor, have been shown to reduce surface density by at least 50% after 48 h of treatment, in addition to their channel-blocking properties. Other drugs, however, have been shown to reduce surface expression without blocking conductance through the channel. Pentamidine, an antiprotozoal agent, and probucol, a cholesterol-lowering drug, have long been known to induce LQTS and TdP, and the mechanism of action was assumed to be channel blockade. Recently, it was demonstrated that clinically relevant concentrations of these drugs cause a reduction in hERG surface expression, which is the most likely cause of the QT prolongation [96,97]. These studies demonstrate that pharmacological treatments can both cause and correct channel trafficking defects. They also highlight the necessity of encompassing trafficking issues in pharmacogenomic considerations for cardiac arrhythmia treatment.
Expert commentary
Defects in protein trafficking have been implicated in many different human diseases, including cystic fibrosis, diabetes and Alzheimer’s disease [98–100]. More recently, protein trafficking has also been recognized as a cause for cardiac arrhythmia. Early work identified mutations in the hERG potassium channel that lead to intracellular retention. Numerous following studies discovered additional mutations with similar defects, and trafficking dysfunction is now suggested as the most common mechanism for hERG-induced LQTS. A considerable number of trafficking mutations have also been identified for KCNQ1, but most other arrhythmia-associated proteins have yet to be fully explored in this respect. While research is currently being conducted on the trafficking mechanisms of many arrhythmia-related proteins, in this article, we chose to highlight the known trafficking mechanisms associated with LQTS, and then focus on the emerging field of Kv1.5 trafficking research. The channel’s atrial-specific expression in human heart makes it an important target for AF therapy, and a better understanding of how channel localization is regulated will surely have a role in future drug development. Additionally, many of the known mechanisms controlling Kv1.5 surface expression will likely be conserved across other channels and proteins, making it an excellent example (Figure 2).
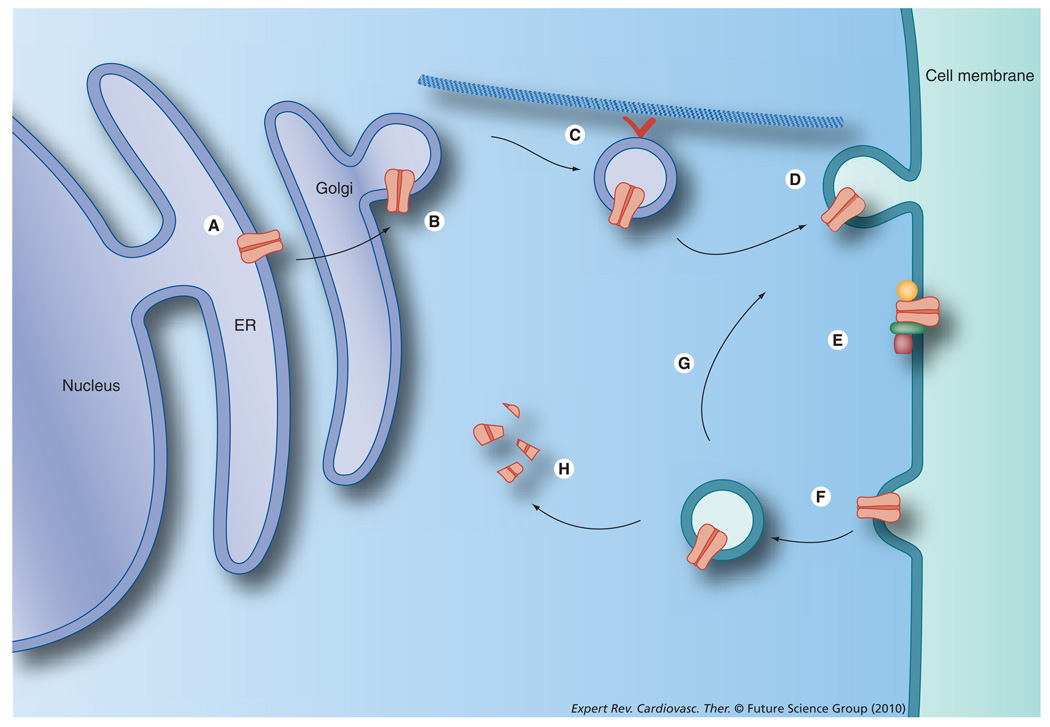
Figure 2. Some of the possible ways Kv1.5 trafficking is regulated.
(A) ER retention, (B) post-translational modifications, (C) forward trafficking along microtubules, (D) membrane insertion, (E) protein–protein interactions, (F) internalization, (G) recycling and (H) degradation.
ER: Endoplasmic reticulum.
Many of the current treatment options for cardiac arrhythmia rely on small molecule inhibitors of ion channels. Unfortunately, these inhibitors often lack specificity, and their effectiveness is quite variable. Much of that variability is likely due to the unpredictable nature of the disease. Indeed, inhibition of a channel that is no longer trafficked properly to the surface is not likely to be successful. Fortunately, channel trafficking is now being recognized as an important factor in arrhythmia pathogenesis, and targeting trafficking rather than conductance is emerging as a viable alternative for arrhythmia therapy. Small molecules have already been demonstrated to both inhibit and promote channel surface expression. As these drugs were not originally designed or tested to target trafficking, it is certainly possible that a more directed approach could lead to even better results. Of course, a more complete understanding of the trafficking mechanisms is necessary before improved treatment options can be fully explored.
Five-year view
As has been discussed here and elsewhere, pharmacogenomic considerations are necessary if the specificity and efficacy of current arrhythmia treatments are to be fully addressed. The underlying mechanisms of cardiac arrhythmia vary greatly, and a single arrhythmogenic phenotype can be caused by both gain-of-function and loss-of-function mutations in the various different channels underlying cardiac action potentials. This variability cannot easily be addressed by the broad treatments currently available. Successful therapy will require the use of treatments targeting the underlying cause of the disease; proper identification of these causes, both as general mechanisms and specific disruptions in patients, is necessary for assigning the right treatment to the right person. To achieve this, we first need to identify more of the proteins and processes involved in channel trafficking. While much work is already underway to achieve this goal, advances in the way we study trafficking will also be important. In particular, studying trafficking in heterologous systems suffers from the possibility that necessary interacting proteins that are present in the native environment are absent from the study system. Experiments using isolated myocytes address these concerns; however, introducing exogenous DNA into these systems still proves technically challenging. Often, isolated myocytes will undergo significant dedifferentiation during the transfection process, and new techniques that allow the use of healthy, fully differentiated myocytes will greatly aid in the study of protein trafficking. Further advances in imaging techniques will also aid in trafficking studies, as current technology often limits the spatial resolution of fluorescent proteins. Finally, the use of new PCR techniques will aid in identifying novel arrhythmia mutations [101]. Once identified, we will need to utilize advances in diagnostic genotyping to decide which patients receive which treatments. There have already been great advances made in the past 5 years, and surely those made in the next 5 years will bring us even closer to achieving our goal.
Key issues
Cardiac arrhythmias affect millions of people and are potentially lethal.
The underlying causes of arrhythmia are quite variable, but most current treatment options do not account for this variability.
Numerous mutations have been identified in arrhythmia patients that alter ion channel subunit trafficking.
Defects in hERG trafficking are the most common cause of long QT syndrome type II.
hERG trafficking defects can be both caused and corrected by pharmacological treatment.
Kv1.5 is a key emerging target for atrial fibrillation therapy, and evidence is beginning to suggest that targeting Kv1.5 trafficking may be a viable alternative to targeting channel conductance.
A more complete understanding of channel trafficking mechanisms will be necessary for future drug development.
Advances in technology will further facilitate the study of protein trafficking in native cardiac myocyte environments.
Acknowledgments
Geoffrey W Abbott is supported by the National Heart, Lung and Blood Institute, National Institutes of Health (R01 HL079275; R01HL101190), the American Heart Association (0855756D), and an Irma T Hirschl Career Scientist Award. William T Harkcom is supported by a National Institutes of Health Predoctoral Training Grant (T32GM073546).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Grant AO. Cardiacion channels. Circ. Arrhythm. Electrophysiol. 2009;2(2):185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 2.Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J. Physiol. 2000;525(2):285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5 K+ channel protein in explanted cardiac tissue. J. Clin. Invest. 1995;96(1):282–292. doi: 10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amos GJ, Wettwer E, Metzger F, Li Q, Himmel HM, Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J. Physiol. 1996;491(Pt 1):31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ. Res. 1993;73(6):1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 6.Charpentier F, Demolombe S, Escande D. Cardiac channelopathies: from men to mice. Ann. Med. 2004;36(s1):28–34. doi: 10.1080/17431380410032508. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman MJ. The long QT syndrome: ion channel diseases of the heart. Mayo Clin. Proc. 1998;73(3):250–269. doi: 10.4065/73.3.250. [DOI] [PubMed] [Google Scholar]
- 8.Otway R, Vandenberg JI, Fatkin D. Atrial fibrillation - a new cardiac channelopathy. Heart Lung Circ. 2007;16(5):356–360. doi: 10.1016/j.hlc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y-H, Xu S-J, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 10.McPate MJ, Duncan RS, Milnes JT, Witchel HJ, Hancox JC. The N588K–hERG K+ channel mutation in the ‘short QT syndrome’: mechanism of gain-in-function determined at 37 °C. Biochem. Biophys. Res. Commun. 2005;334(2):441–449. doi: 10.1016/j.bbrc.2005.06.112. [DOI] [PubMed] [Google Scholar]
- 11.Xia M, Jin Q, Bendahhou S, et al. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem. Biophys. Res. Commun. 2005;332(4):1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Xia M, Jin Q, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am. J. Hum. Genet. 2004;75(5):899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page RL, Roden DM. Drug therapy for atrial fibrillation: where do we go from here? Nat. Rev. Drug Discov. 2005;4(11):899–910. doi: 10.1038/nrd1876. [DOI] [PubMed] [Google Scholar]
- 14.Ruan Y, Liu N, Napolitano C, Priori SG. Therapeutic strategies for long-QT syndrome: does the molecular substrate matter? Circ. Arrhythm. Electrophysiol. 2008;1(4):290–297. doi: 10.1161/CIRCEP.108.795617. [DOI] [PubMed] [Google Scholar]
- 15.Roden DM. Cellular basis of drug-induced torsades de pointes. Br. J. Pharmacol. 2008;154(7):1502–1507. doi: 10.1038/bjp.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford JWP, Milnes JTP. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I kur): rationale, pharmacology and evidence for potential therapeutic value. J. Cardiovasc. Pharmacol. 2008;52(2):105–120. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 17. Johnson AE, van Waes MA. The translocon: a dynamic gateway at the ER membrane. Annu. Rev. Cell Dev. Biol. 1999;15(1):799–842. doi: 10.1146/annurev.cellbio.15.1.799.. • Demonstrates that channel trafficking and conductance can be targeted separately.
- 18.Sato Y, Sakaguchi M, Goshima S, Nakamura T, Uozumi N. Integration of Shaker-type K+ channel, KAT1, into the endoplasmic reticulum membrane: Synergistic insertion of voltage-sensing segments, S3-S4, and independent insertion of pore-forming segments, S5-S6. Proc. Natl Acad. Sci. USA. 2002;99(1):60–65. doi: 10.1073/pnas.012399799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosolapov A, Deutsch C. Folding of the voltage-gated K+ channel T1 recognition domain. J. Biol. Chem. 2003;278(6):4305–4313. doi: 10.1074/jbc.M209422200. [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Robinson JM, Edwards D, Deutsch C. T1-T1 interactions occur in ER membranes while nascent Kv peptides are still attached to ribosomes. Biochemistry. 2001;40(37):10934–10946. doi: 10.1021/bi010763e. [DOI] [PubMed] [Google Scholar]
- 21.Nagaya N, Papazian DM. Potassium channel α and β subunits assemble in the endoplasmic reticulum. J. Biol. Chem. 1997;272(5):3022–3027. doi: 10.1074/jbc.272.5.3022. [DOI] [PubMed] [Google Scholar]
- 22.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J. Neurosci. 2001;21(9):3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Takimoto K, Levitan ES. Surface expression of Kv1 channels is governed by a C-terminal motif. J. Biol. Chem. 2000;275(16):11597–11602. doi: 10.1074/jbc.275.16.11597. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Sroubek J, Krishnan Y, Li Y, Bian J, McDonald TV. PKA phosphorylation of hERG protein regulates the rate of channel synthesis. Am. J. Physiol. Heart Circ. Physiol. 2009;296(5):H1244–H1254. doi: 10.1152/ajpheart.01252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology. 2001;41(6):714–723. doi: 10.1016/s0028-3908(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 26.Zadeh AD, Cheng Y, Xu H, et al. Kif5b is an essential forward trafficking motor for the Kv1.5 cardiac potassium channel. J. Physiol. 2009;587(19):4565–4574. doi: 10.1113/jphysiol.2009.178442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehmer C, Laufer J, Jeyaraj S, et al. Modulation of the voltage-gated potassium channel Kv1.5 by the SGK1 protein kinase involves inhibition of channel ubiquitination. Cell. Physiol. Biochem. 2008;22(5–6):591–600. doi: 10.1159/000185543. [DOI] [PubMed] [Google Scholar]
- 28.McEwen DP, Schumacher SM, Li Q, et al. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J. Biol. Chem. 2007;282(40):29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch C. Potassium channel ontogeny. Annu. Rev. Physiol. 2002;64(1):19–46. doi: 10.1146/annurev.physiol.64.081501.155934. [DOI] [PubMed] [Google Scholar]
- 30.Steele DF, Eldstrom J, Fedida D. Mechanisms of cardiac potassium channel trafficking. J. Physiol. 2007;582(1):17–26. doi: 10.1113/jphysiol.2007.130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker VE, Wong MJH, Atanasiu R, Hantouche C, Young JC, Shrier A. Hsp40 chaperones promote degradation of the hERG potassium channel. J. Biol. Chem. 2009;285(5):3319–3329. doi: 10.1074/jbc.M109.024000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott GW, Roepke TK. Pharmacogenetics of drug-induced arrhythmias. Expert Rev. Clin. Pharmacol. 2008;1(1):93–104. doi: 10.1586/17512433.1.1.93. [DOI] [PubMed] [Google Scholar]
- 33. Anderson CL, Delisle BP, Anson BD, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113(3):365–373. doi: 10.1161/CIRCULATIONAHA.105.570200.. •• Evidence that most long QT syndrome-associated hERG mutations cause trafficking defects
- 34.Rajamani S, Anderson CL, Anson BD, January CT. Pharmacological rescue of human K+ channel long-QT2 mutations: human ether-a-go-go-related gene rescue without block. Circulation. 2002;105(24):2830–2835. doi: 10.1161/01.cir.0000019513.50928.74. [DOI] [PubMed] [Google Scholar]
- 35.Ficker E, Dennis AT, Obejero-Paz CA, Castaldo P, Taglialatela M, Brown AM. Retention in the endoplasmic reticulum as a mechanism of dominant-negative current suppression in human long QT syndrome. J. Mol. Cell. Cardiol. 2000;32(12):2327–2337. doi: 10.1006/jmcc.2000.1263. [DOI] [PubMed] [Google Scholar]
- 36.Gong Q, Keeney DR, Molinari M, Zhou Z. Degradation of trafficking-defective long QT syndrome type II mutant channels by the ubiquitin-proteasome pathway. J. Biol. Chem. 2005;280(19):19419–19425. doi: 10.1074/jbc.M502327200. [DOI] [PubMed] [Google Scholar]
- 37.Keller S, Platoshyn O, Yuan J. Long QT syndrome-associated I593R mutation in hERG potassium channel activates ER stress pathways. Cell Biochem. Biophys. 2005;43(3):365–377. doi: 10.1385/CBB:43:3:365. [DOI] [PubMed] [Google Scholar]
- 38.Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (hERG) mutations. J. Biol. Chem. 2002;277(7):4989–4998. doi: 10.1074/jbc.M107345200. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman ES, Ficker E. Is restoration of intracellular trafficking clinically feasible in the long QT syndrome? J. Cardiovasc. Electrophysiol. 2003;14(3):320–322. doi: 10.1046/j.1540-8167.2003.02363.x. [DOI] [PubMed] [Google Scholar]
- 40.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel hERG. Circ. Res. 2003;92(12):e87–e100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 41.Nanduri J, Bergson P, Wang N, Ficker E, Prabhakar NR. Hypoxia inhibits maturation and trafficking of hERG K+ channel protein: role of Hsp90 and ROS. Biochem. Biophys. Res. Commun. 2009;388(2):212–216. doi: 10.1016/j.bbrc.2009.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker VE, Atanasiu R, Lam H, Shrier A. Co-chaperone FKBP38 promotes hERG trafficking. J. Biol. Chem. 2007;282(32):23509–23516. doi: 10.1074/jbc.M701006200. [DOI] [PubMed] [Google Scholar]
- 43.Akhavan A, Atanasiu R, Noguchi T, Han W, Holder N, Shrier A. Identification of the cyclic-nucleotide-binding domain as a conserved determinant of ion-channel cell-surface localization. J. Cell Sci. 2005;118(13):2803–2812. doi: 10.1242/jcs.02423. [DOI] [PubMed] [Google Scholar]
- 44.Burke JF, Mogg AE. Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucl. Acids Res. 1985;13(17):6265–6272. doi: 10.1093/nar/13.17.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao Y, Teng S, Li N, Zhang Y, Boyden PA, Pu J. Aminoglycoside antibiotics restore functional expression of truncated hERG channels produced by nonsense mutations. Heart Rhythm. 2009;6(4):553–560. doi: 10.1016/j.hrthm.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Wible BA, Wan X, Ficker E. Cardiac glycosides as novel inhibitors of human ether-a-go-go-related gene channel trafficking. J. Pharmacol. Exp. Ther. 2007;320(2):525–534. doi: 10.1124/jpet.106.113043. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Dennis AT, Trieu P, et al. Intracellular potassium stabilizes human ether-à-go-go-related gene channels for export from endoplasmic reticulum. Mol. Pharmacol. 2009;75(4):927–937. doi: 10.1124/mol.108.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo J, Massaeli H, Xu J, et al. Extracellular K+ concentration controls cell surface density of IKr in rabbit hearts and of the hERG channel in human cell lines. J. Clin. Invest. 2009;119(9):2745–2757. doi: 10.1172/JCI39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roden DM, Thompson KA, Hoffman BF, Woosley RL. Clinical features and basic mechanisms of quinidine-induced arrhythmias. J. Am. Coll. Cardiol. 1986;8(1 Suppl. A):73A–78A. doi: 10.1016/s0735-1097(86)80032-8. [DOI] [PubMed] [Google Scholar]
- 50.Berthet M, Denjoy I, Donger C, et al. C-terminal hERG mutations: The role of hypokalemia and a KCNQ1-associated mutation in cardiac event occurrence. Circulation. 1999;99(11):1464–1470. doi: 10.1161/01.cir.99.11.1464. [DOI] [PubMed] [Google Scholar]
- 51.Gordon E, Panaghie G, Deng L, et al. A KCNE2 mutation in a patient with cardiac arrhythmia induced by auditory stimuli and serum electrolyte imbalance. Cardiovasc. Res. 2008;77(1):98–106. doi: 10.1093/cvr/cvm030. [DOI] [PubMed] [Google Scholar]
- 52.Lehnart SE, Ackerman MJ, Benson DW, Jr, et al. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116(20):2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- 53.Bianchi L, Priori SG, Napolitano C, et al. Mechanisms of IKs suppression in LQT1 mutants. Am. J. Physiol. Heart Circ. Physiol. 2000;279(6):H3003–H3011. doi: 10.1152/ajpheart.2000.279.6.H3003. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita F, Horie M, Kubota T, et al. Characterization and subcellular localization of KCNQ1 with a heterozygous mutation in the C terminus. J. Mol. Cell. Cardiol. 2001;33(2):197–207. doi: 10.1006/jmcc.2000.1300. [DOI] [PubMed] [Google Scholar]
- 55.Gouas L, Bellocq C, Berthet M, et al. New KCNQ1 mutations leading to haploinsufficiency in a general population: defective trafficking of a KvLQT1 mutant. Cardiovasc. Res. 2004;63(1):60–68. doi: 10.1016/j.cardiores.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Aizawa Y, Ueda K, Wu L-m, et al. Truncated KCNQ1 mutant, A178fs/105, forms hetero-multimer channel with wild-type causing a dominant-negative suppression due to trafficking defect. FEBS Lett. 2004;574(1–3):145–150. doi: 10.1016/j.febslet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Wilson AJ, Quinn KV, Graves FM, Bitner-Glindzicz M, Tinker A. Abnormal KCNQ1 trafficking influences disease pathogenesis in hereditary long QT syndromes (LQT1) Cardiovasc. Res. 2005;67(3):476–486. doi: 10.1016/j.cardiores.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 58. Sato A, Arimura T, Makita N, et al. Novel mechanisms of trafficking defect caused by KCNQ1 mutations found in long QT syndrome. J. Biol. Chem. 2009;284(50):35122–35133. doi: 10.1074/jbc.M109.017293.. • Describes two novel mechanisms for trafficking defects in KCNQ1 mutants
- 59.Labro AJ, Boulet IR, Timmermans J-P, Ottschytsch N, Snyders DJ. The rate-dependent biophysical properties of the LQT1 H258R mutant are counteracted by a dominant negative effect on channel trafficking. J. Mol. Cell. Cardiol. 2009;48(6):1096–1104. doi: 10.1016/j.yjmcc.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Ehrlich JR, Pourrier M, Weerapura M, et al. KvLQT1 Modulates the distribution and biophysical properties of hERG. J. Biol. Chem. 2004;279(2):1233–1241. doi: 10.1074/jbc.M309087200. [DOI] [PubMed] [Google Scholar]
- 61.Biliczki P, Girmatsion Z, Brandes RP, et al. Trafficking-deficient long QT syndrome mutation KCNQ1-T587M confers severe clinical phenotype by impairment of KCNH2 membrane localization: Evidence for clinically significant IKr-IKs [α]-subunit interaction. Heart Rhythm. 2009;6(12):1792–1801. doi: 10.1016/j.hrthm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Bendahhou Sd, Donaldson MR, Plaster NM, Tristani-Firouzi M, Fu Y-H, Ptácek LJ. Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. J. Biol. Chem. 2003;278((51)):51779–51785. doi: 10.1074/jbc.M310278200. [DOI] [PubMed] [Google Scholar]
- 63.Soom M, Schönherr R, Kubo Y, Kirsch C, Klinger R, Heinemann SH. Multiple PIP2 binding sites in Kir2.1 inwardly rectifying potassium channels. FEBS Lett. 2001;490(1–2):49–53. doi: 10.1016/s0014-5793(01)02136-6. [DOI] [PubMed] [Google Scholar]
- 64.Choi B-O, Kim J, Suh BC, et al. Mutations of KCNJ2 gene associated with Andersen-Tawil syndrome in Korean families. J. Hum. Genet. 2007;52(3):280–283. doi: 10.1007/s10038-006-0100-7. [DOI] [PubMed] [Google Scholar]
- 65.Ponce-Balbuena D, Lopez-Izquierdo A, Ferrer T, Rodriguez-Menchaca AA, Arechiga-Figueroa IA, Sanchez-Chapula JA. Tamoxifen inhibits inward rectifier K+ 2.x family of inward rectifier channels by interfering with phosphatidylinositol 4,5-bisphosphate-channel interactions. J. Pharmacol. Exp. Ther. 2009;331(2):563–573. doi: 10.1124/jpet.109.156075. [DOI] [PubMed] [Google Scholar]
- 66.Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115(4):442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Traverso M, Gazzerro E, Assereto S, et al. Caveolin-3 T78M and T78K missense mutations lead to different phenotypes in vivo and in vitro. Lab. Invest. 2008;88(3):275–283. doi: 10.1038/labinvest.3700713. [DOI] [PubMed] [Google Scholar]
- 68.Mohler PJ, Schott J-J, Gramolini AO, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 69.Nattel S. Therapeutic implications of atrial fibrillation mechanisms: can mechanistic insights be used to improve AF management? Cardiovasc. Res. 2002;54(2):347–360. doi: 10.1016/s0008-6363(01)00562-4. [DOI] [PubMed] [Google Scholar]
- 70.Bellocq C, van Ginneken ACG, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109(20):2394–2397. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 71.Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in hERG. Circulation. 2004;109(1):30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 72.Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ. Res. 2005;96(7):800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 73.Li G-R, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ. Res. 1996;78(4):689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- 74.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ. Res. 1997;80(6):772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 75.Brundel BJJM, Van Gelder IC, Henning RH, et al. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J. Am. Coll. Cardiol. 2001;37(3):926–932. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 76.Olson TM, Alekseev AE, Liu XK, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006;15(14):2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Li J, Lin X, et al. Novel KCNA5 loss-of-function mutations responsible for atrial fibrillation. J. Hum. Genet. 2009;54(5):277–283. doi: 10.1038/jhg.2009.26. [DOI] [PubMed] [Google Scholar]
- 78.Tanabe Y, Hatada K, Naito N, et al. Over-expression of Kv1.5 in rat cardiomyocytes extremely shortens the duration of the action potential and causes rapid excitation. Biochem. Biophys. Res. Commun. 2006;345(3):1116–1121. doi: 10.1016/j.bbrc.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 79.Hong K, Piper DR, Diaz-Valdecantos A, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc. Res. 2005;68(3):433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 80.Roepke TK, Kontogeorgis A, Ovanez C, et al. Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f) FASEB J. 2008;22(10):3648–3660. doi: 10.1096/fj.08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Guo W, Mellor RL, Nerbonne JM. KChIP2 modulates the cell surface expression of Kv1.5-encoded K+ channels. J. Mol. Cell. Cardiol. 2005;39(1):121–132. doi: 10.1016/j.yjmcc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Eldstrom J, Choi WS, Steele DF, Fedida D. SAP97 increases Kv1.5 currents through an indirect N-terminal mechanism. FEBS Lett. 2003;547(1–3):205–211. doi: 10.1016/s0014-5793(03)00668-9. [DOI] [PubMed] [Google Scholar]
- 83.Folco EJ, Liu G-X, Koren G. Caveolin-3 and SAP97 form a scaffolding protein complex that regulates the voltage-gated potassium channel Kv1.5. Am. J. Physiol. Heart Circ. Physiol. 2004;287(2):H681–H690. doi: 10.1152/ajpheart.00152.2004. [DOI] [PubMed] [Google Scholar]
- 84.McEwen DP, Li Q, Jackson S, Jenkins PM, Martens JR. Caveolin regulates Kv1.5 trafficking to cholesterol-rich membrane microdomains. Mol. Pharmacol. 2008;73(3):678–685. doi: 10.1124/mol.107.042093. [DOI] [PubMed] [Google Scholar]
- 85.Vatta M, Ackerman MJ, Ye B, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114(20):2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 86.Ye B, Balijepalli RC, Foell JD, et al. Caveolin-3 associates with and affects the function of hyperpolarization-activated cyclic nucleotide-gated channel 4. Biochemistry. 2008;47(47):12312–12318. [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Z, Browning CF, Hallaq H, et al. Four and a half LIM protein 1: a partner for KCNA5 in human atrium. Cardiovasc. Res. 2008;78(3):449–457. doi: 10.1093/cvr/cvn038. [DOI] [PubMed] [Google Scholar]
- 88.Holmes TC, Fadool DA, Ren R, Levitan IB. Association of src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274(5295):2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 89.Mason HS, Latten MJ, Godoy LD, Horowitz B, Kenyon JL. Modulation of Kv1.5 currents by protein kinase A, tyrosine kinase, and protein tyrosine phosphatase requires an intact cytoskeleton. Mol. Pharmacol. 2002;61(2):285–293. doi: 10.1124/mol.61.2.285. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L, Foster K, Li Q, Martens JR. S-acylation regulates Kv1.5 channel surface expression. Am. J. Physiol. Cell Physiol. 2007;293(1):C152–C161. doi: 10.1152/ajpcell.00480.2006. [DOI] [PubMed] [Google Scholar]
- 91.Ebbing B, Mann K, Starosta A, et al. Effect of spastic paraplegia mutations in KIF5A kinesin on transport activity. Hum. Mol Genet. 2008;17(9):1245–1252. doi: 10.1093/hmg/ddn014. [DOI] [PubMed] [Google Scholar]
- 92. Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR. Antiarrhythmic drug-induced internalization of the atrial-specific K+ channel Kv1.5. Circ. Res. 2009;104(12):1390–1398. doi: 10.1161/CIRCRESAHA.108.192773.. • Demonstrates that antiarrhythmic drugs can, in addition to pore blockade, cause channel internalization
- 93.Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 Surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ. Res. 2005;97(4):363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- 94.Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J. Heart Lung Transplant. 2004;23(8):954–963. doi: 10.1016/j.healun.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 95. Furutani M, Trudeau MC, Hagiwara N, et al. Novel mechanism associated with an inherited cardiac arrhythmia: defective protein trafficking by the mutant hERG (G601S) potassium channel. Circulation. 1999;99(17):2290–2294. doi: 10.1161/01.cir.99.17.2290.. • First paper to describe a trafficking mutation underlying long QT syndrome
- 96.Cordes JS, Sun Z, Lloyd DB, et al. Pentamidine reduces hERG expression to prolong the QT interval. Br. J. Pharmacol. 2005;145(1):15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo J, Massaeli H, Li W, et al. Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J. Pharmacol. Exp. Ther. 2007;321(3):911–920. doi: 10.1124/jpet.107.120931. [DOI] [PubMed] [Google Scholar]
- 98. Aridor M, Hannan LA. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic. 2000;1(11):836–851. doi: 10.1034/j.1600-0854.2000.011104.x.. •• Excellent review of diseases associated with defective intracellular transport
- 99.Aridor M, Hannan LA. Traffic jams II: an update of diseases of intracellular transport. Traffic. 2002;3(11):781–790. doi: 10.1034/j.1600-0854.2002.31103.x. [DOI] [PubMed] [Google Scholar]
- 100. Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ. Res. 2004;94(11):1418–1428. doi: 10.1161/01.RES.0000128561.28701.ea.. •• Excellent early review of ion channel trafficking in cardiac arrhythmia
- 101.Li J, Makrigiorgos GM. COLD-PCR: a new platform for highly improved mutation detection in cancer and genetic testing. Biochem. Soc. Trans. 2009;37(2):427–432. doi: 10.1042/BST0370427. [DOI] [PubMed] [Google Scholar]
- 102.Pan N, Sun J, Lv C, Li H, Ding J. A hydrophobicity-dependent motif responsible for surface expression of cardiac potassium channel. Cell. Signal. 2009;21(2):349–355. doi: 10.1016/j.cellsig.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Wang Q-f, Du R, et al. Congenital long QT syndrome caused by the F275S KCNQ1 mutation: mechanism of impaired channel function. Biochem. Biophys. Res. Commun. 2009;380(1):127–131. doi: 10.1016/j.bbrc.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 104.Boulet IR, Raes AL, Ottschytsch N, Snyders DJ. Functional effects of a KCNQ1 mutation associated with the long QT syndrome. Cardiovasc. Res. 2006;70(3):466–474. doi: 10.1016/j.cardiores.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 105.Schmitt N, Calloe K, Nielsen NH, et al. The novel C-terminal KCNQ1 mutation M520R alters protein trafficking. Biochem. Biophys. Res. Commun. 2007;358(1):304–310. doi: 10.1016/j.bbrc.2007.04.127. [DOI] [PubMed] [Google Scholar]
- 106.Kanki H, Kupershmidt S, Yang T, Wells S, Roden DM. A structural requirement for processing the cardiac K+ channel KCNQ1. J. Biol. Chem. 2004;279(32):33976–33983. doi: 10.1074/jbc.M404539200. [DOI] [PubMed] [Google Scholar]
- 107.Teng S, Ma L, Dong Y, et al. Clinical and electrophysiological characterization of a novel mutation R863X in hERG C-terminus associated with long QT syndrome. J. Mol. Med. 2004;82(3):189–196. doi: 10.1007/s00109-003-0504-1. [DOI] [PubMed] [Google Scholar]
- 108.Paulussen Ae, Raes A, Matthijs G, Snyders DJ, Cohen N, Aerssens J. A novel mutation (T65P) in the PAS domain of the human potassium channel hERG results in the long QT syndrome by trafficking deficiency. J. Biol. Chem. 2002;277(50):48610–48616. doi: 10.1074/jbc.M206569200. [DOI] [PubMed] [Google Scholar]
- 109.Keller D, Grenier J, Christ G, et al. Characterization of novel KCNH2 mutations in type 2 long QT syndrome manifesting as seizures. Can. J. Cardiol. 2009;25(8):455–462. doi: 10.1016/s0828-282x(09)70117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huo J, Zhang Y, Huang N, et al. The G604S–hERG mutation alters the biophysical properties and exerts a dominant-negative effect on expression of hERG channels in HEK293 cells. Pflügers Arch. 2008;456(5):917–928. doi: 10.1007/s00424-008-0454-0. [DOI] [PubMed] [Google Scholar]
- 111.Gong Q, Keeney DR, Robinson JC, Zhou Z. Defective assembly and trafficking of mutant hERG channels with C-terminal truncations in long QT syndrome. J. Mol. Cell. Cardiol. 2004;37(6):1225–1233. doi: 10.1016/j.yjmcc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 112.Hsueh C-H, Chen W-P, Lin J-L, Liu Y-B, Su M-J, Lai L-P. Functional studies on three novel HCNH2 mutations in Taiwan: identification of distinct mechanisms of channel defect and dissociation between glycosylation defect and assembly defect. Biochem. Biophys. Res. Commun. 2008;373(4):572–578. doi: 10.1016/j.bbrc.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 113.Gong Q, Zhang L, Moss AJ, et al. A splice site mutation in hERG leads to cryptic splicing in human long QT syndrome. J. Mol. Cell. Cardiol. 2008;44(3):502–509. doi: 10.1016/j.yjmcc.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paulussen ADC, Raes A, Jongbloed RJ, et al. hERG mutation predicts short QT based on channel kinetics but causes long QT by heterotetrameric trafficking deficiency. Cardiovasc. Res. 2005;67(3):467–475. doi: 10.1016/j.cardiores.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 115.Christé G, Thériault O, Chahine M, et al. A new C-terminal hERG mutation A915fs+47X associated with s ymptomatic LQT2 and auditory-trigger syncope. Heart Rhythm. 2008;5(11):1577–1586. doi: 10.1016/j.hrthm.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sasano T, Ueda K, Orikabe M, et al. Novel C-terminus frameshift mutation, 1122fs/147, of hERG in LQT2: additional amino acids generated by frameshift cause accelerated inactivation. J. Mol. Cell. Cardiol. 2004;37(6):1205–1211. doi: 10.1016/j.yjmcc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 117.Poelzing S, Forleo C, Samodell M, et al. SCN5A polymorphism restores trafficking of a Brugada syndrome mutation on a separate gene. Circulation. 2006;114(5):368–376. doi: 10.1161/CIRCULATIONAHA.105.601294. [DOI] [PubMed] [Google Scholar]
- 118.Pfahnl AE, Viswanathan PC, Weiss R, et al. A sodium channel pore mutation causing Brugada syndrome. Heart Rhythm. 2007;4(1):46–53. doi: 10.1016/j.hrthm.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohler PJ, Rivolta I, Napolitano C, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc. Natl Acad. Sci. USA. 2004;101(50):17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baroudi G, Acharfi S, Larouche C, Chahine M. Expression and intracellular localization of an SCN5A double mutant R1232W/T1620M implicated in Brugada syndrome. Circ. Res. 2002;90(1):e11–e16. [PubMed] [Google Scholar]
- 121.Makita N, Mochizuki N, Tsutsui H. Absence of a trafficking defect in R1232W/T1620M, a double SCN5A mutant responsible for Brugada syndrome. Circ. J. 2008;72(6):1018–1019. doi: 10.1253/circj.72.1018. [DOI] [PubMed] [Google Scholar]
- 122.Tan B-H, Valdivia CR, Song C, Makielski JC. Partial expression defect for the SCN5A missense mutation G1406R depends on splice variant background Q1077 and rescue by mexiletine. Am. J. Physiol. Heart Circ Physiol. 2006;291(4):H1822–H1828. doi: 10.1152/ajpheart.00101.2006. [DOI] [PubMed] [Google Scholar]
- 123.Baroudi G, Pouliot V, Denjoy I, Guicheney P, Shrier A, Chahine M. Novel mechanism for Brugada syndrome: defective surface localization of an SCN5A mutant (R1432G) Circ. Res. 2001;88(12):e78–e83. doi: 10.1161/hh1201.093270. [DOI] [PubMed] [Google Scholar]
- 124.Valdivia CR, Tester DJ, Rok BA, et al. A trafficking defective, Brugada syndrome-causing SCN5A mutation rescued by drugs. Cardiovasc. Res. 2004;62(1):53–62. doi: 10.1016/j.cardiores.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 125.Ye B, Valdivia CR, Ackerman MJ, Makielski JC. A common human SCN5A polymorphism modifies expression of an arrhythmia causing mutation. Physiol. Genomics. 2003;12(3):187–193. doi: 10.1152/physiolgenomics.00117.2002. [DOI] [PubMed] [Google Scholar]
- 126.Herfst LJ, Potet F, Bezzina CR, et al. Na+ channel mutation leading to loss of function and non-progressive cardiac conduction defects. J. Mol. Cell. Cardiol. 2003;35(5):549–557. doi: 10.1016/s0022-2828(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 127.Hu D, Barajas-Martinez H, Nesterenko VV, et al. Dual variation in SCN5A and CACNB2b underlies the development of cardiac conduction disease without Brugada syndrome. Pacing Clin. Electrophysiol. 2010;33(3):274–285. doi: 10.1111/j.1540-8159.2009.02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Valdivia CR, Medeiros-Domingo A, Ye B, et al. Loss-of-function mutation of the SCN3B–encoded sodium channel {β}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc. Res. 2010;86(3):392–400. doi: 10.1093/cvr/cvp417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.London B, Michalec M, Mehdi H, et al. Mutation in glycerol-3-phosphate dehydrogenase 1-like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116(20):2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krumerman A, Gao X, Bian J-S, Melman YF, Kagan A, McDonald TV. An LQT mutant minK alters KvLQT1 trafficking. Am. J. Physiol. Cell Physiol. 2004;286(6):C1453–C1463. doi: 10.1152/ajpcell.00275.2003. [DOI] [PubMed] [Google Scholar]
- 131.Harmer SC, Wilson AJ, Aldridge R, Tinker A. Mechanisms of disease pathogenesis in long QT syndrome type 5. Am. J. Physiol. Cell Physiol. 2009;298(2):C263–C273. doi: 10.1152/ajpcell.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ballester LY, Benson DW, Wong B, et al. Trafficking-competent and trafficking-defective KCNJ2 mutations in Andersen syndrome. Hum. Mutat. 2006;27(4):388. doi: 10.1002/humu.9418. [DOI] [PubMed] [Google Scholar]
- 133.Nof E, Luria D, Brass D, et al. Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation. 2007;116(5):463–470. doi: 10.1161/CIRCULATIONAHA.107.706887. [DOI] [PubMed] [Google Scholar]
- 134.Ueda K, Nakamura K, Hayashi T, et al. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J. Biol. Chem. 2004;279(26):27194–27198. doi: 10.1074/jbc.M311953200. [DOI] [PubMed] [Google Scholar]