Abstract
Singlet oxygen, 1O2, is produced by absorption of red light by the phthalocyanine dye, Pc 4, followed by energy transfer to dissolved triplet molecular oxygen, 3O2. In tissues, Pc 4 concentrates in lipid bilayers, and particularly in mitochondrial membranes due to its positive charge. Illumination of cells and tissues with red light following uptake of Pc 4 results in cell death. The potential initial chemical steps that result in cellular dysfunction have been characterized in this study. Both unsaturated acyl chains of phospholipids and proteins are identified as targets of oxidation. Tetra-linoleoyl cardiolipin was oxidized in both liposomes and mitochondria following Pc 4 mediated 1O2 generation. Evidence for formation of both mono and bis hydroperoxide adducts of single linoleoyl side chains is provided by ESI-MS and ESI-MS/MS. Similarly illumination of Pc 4 into liposomes and mitochondria resulted in cytochrome c oxidation as detected by oxidation of H26 in the peptide, H26*KTGPNLHGLFGK, further supporting the potential use of this peptide as a biomarker for the presence of mitochondrial oxidative stress characteristic of 1O2 in vivo [1]. These observations provide evidence that formation of lipid hydroperoxides and/or protein oxidation can be the initial chemical steps in Pc 4 mediated induction of apoptosis in PDT.
Keywords: photodynamic therapy, cardiolipin, singlet oxygen, cytochrome c, mass spectrometry, mitochondria
Introduction
Oxidative stress is believed to contribute to the decrease in cellular function that accompanies aging [2] and that is associated with numerous degenerative diseases and even certain types of cancers [3-5]. Oxidative stress commonly involves reactive oxygen species 1 (ROS) which may oxidize almost every class of biomolecules, including DNA, RNA, lipids, carbohydrates and proteins. Singlet oxygen (1O2) is a highly reactive oxygen specie which may be generated in biological systems via several different procedures: by excitation of endogenous photosensitizers [6], by the activity of peroxidase enzymes [7], or by the decomposition of lipid peroxidation products which are formed during ischemia-reperfusion injury [8] or by other mechanisms [9-11].
1O2 can be generated intentionally by photodynamic therapy (PDT) to induce cell death as part of treatments for cancer and other pathological conditions. During PDT, 1O2 and other ROS are generated by illuminating a tissue region that has a natural or exogenously applied photosensitizer [12, 13]. Pc 4, a silicon phthalocyanine (Si-Pc) sensitizer [14], effectively generates 1O2 when exposed to red light in aerobic solutions, having a quantum yield of ~0.4 [15] which is not effectively different in liposomes containing polyunsaturated acyl groups such as linoleic acid [16]. While 1O2 is the only detected product in these purified in vitro systems, depending on environment the photochemistry of Si-Pcs can be altered, e.g. in the presence of an oxidizable porphyrin, a Si-Pc can accept an electron potentially both oxidizing the donor and subsequently reducing O2 to superoxide [17] and in the presence of an electron acceptor the Si-Pc can be oxidized[18]. These alternative modes of reaction make photoinduced chemistry observed in biological matrices less certain than in dilute aerobic solutions.
As an exogenous photosensitizer, Pc 4 has shown excellent treatment response in several different tumor models, including human tumor xenografts in immunocompromised mice [19-25], and is currently in a Phase I human clinical trial of PDT for the treatment of cutaneous tumors. In PDT, Pc 4 localizes in mitochondrial and endoplasmic reticulum/Golgi membranes [26] and induces apoptosis in many types of cells and tumors [27]. The primary apoptotic mechanism triggered by Pc 4-PDT is the mitochondrial (intrinsic) pathway involving release of cytochrome c (Cyt c) from mitochondria into the cytosol [28, 29], and the activation of a cascade of apoptosis-mediating caspases [13].
However the limitation in detecting 1O2 in biological systems makes it hard to prove the role of 1O2 in oxidative stress. We have recently discovered 1O2-derived oxidative products from His residues in Cyt c [1]. When oxidized by 1O2, His is converted to oxidative products characteristic of 1O2; mass increments of +14 and +32, e.g., His 26 in H26KTGPNLHGLFGR38 peptide. His 26 in this peptide is believed to form a crosslink with Lys 27. This unique crosslinked structure has significant potential to be used as a biomarker suggesting the presence of 1O2 in many biological systems. Because any protein containing the sequence of HK would potentially generate an analogous product, looking for this specific modification from tissue subjected to oxidative stress will provide evidence for the involvement of 1O2. His+14 also might be used to monitor the presence of 1O2 in biological systems by mass spectrometry.
Cardiolipin (CL) is highly localized to the mitochondrial inner membrane, where it interacts specifically with proteins of the electron transport chain (ETC). The negatively charged CL anchors Cyt c to the cytoplasmic side of the inner membrane. Because CL’s constituent fatty acids are linoleic and other polyunsaturated acids, it is also a likely target for oxidation by 1O2 [30].
Evidence for the role of CL oxidation during PDT sensitized by protoporphyrin IX was obtained both by mass spectrometry and by suppression of hydroperoxide formation [31]. Additionally, evidence suggests that oxidized CL is less able to interact with Cyt c [3, 31]. Thus, a consequence of CL oxidation is a decrease in the association of Cyt c with the inner mitochondrial membrane and an increase in the pool of free Cyt c in the intermembrane space [32]. This pool of Cyt c is available for release into the cytoplasm through pores formed in a process dependent upon the pro-apoptotic proteins Bax and/or Bak. Oxidation of CL may be further enhanced when Cyt c acquires peroxidase activity by its own oxidation [32].
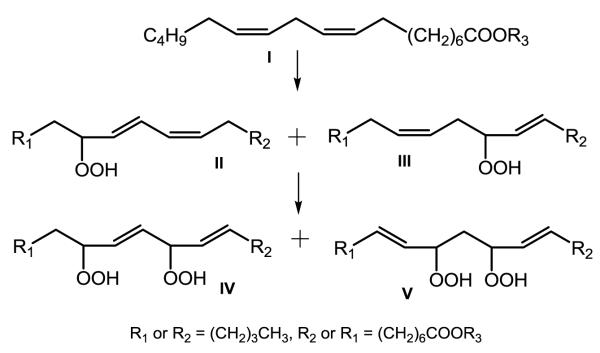
The structures of oxidized CL have been studied from cells exposed to H2O2 [31] and from mouse intestine exposed in vivo to γ-irradiation [33]. Peroxidation of either of the terminal carbons of the homoconjugated cis, cis double bonds present in linoleic acid (LA) results in the pseudosymmetric structures shown in Scheme 1 that have been chemically determined [34] and serve as a model of the anticipated modifications of the LA acyl groups of CL.
Scheme 1.
Pseudosymmetric structures of linoleate peroxidized by 1O2 (adapted from reference 34).
In the current work, Cyt c and CL were exposed to Pc 4 generated ROS in liposomes and mitochondria to test their potential use as markers of PDT induced oxidative stress and potentially for 1O2. The results indicate that photosensitization produces an effective oxidant of CL and Cyt c and that the oxidized products can be readily identified in isolated mitochondria exposed to Pc 4-mediated PDT conditions.
Experimental
Materials
The commercial sources of experimental materials were as follows: Cyt c, α-cyano-4-hydroxycinnamic acid (CHCA), and CL – Sigma-Aldrich (St. Louis, MO, USA); sequencing-grade modified trypsin - Promega (Madison, WI, USA); microcon centrifugal concentrators and ZipTip microscale C18 solid phase extraction tips - Millipore (Bedford, MA, USA); dimyristoylphosphatidyl choline (DMPC) - Avanti Polar Lipids (Alabaster, AL); Whatman TLC plates - Fisher Sci. (Pittsburgh, PA). Pc 4 was a generous gift of Dr. Malcolm Kenney (CWRU, Cleveland). Mitochondria were isolated from male Fisher 344 rats, aged 6 months [35], and used within 5 h of isolation. Millipore water (18 MΩ) was used throughout.
Mass Spectrometry
Analyses of Cyt c tryptic digests were performed with a Bruker BiFlex III MALDI-TOF-MS equipped with a pulsed N2 laser (3 ns pulse at 337 nm) and XTOF acquisition software. The reflectron mode was used with an accelerating voltage of −20 kV. LC-MS/MS analyses of digested Cyt-c were performed with a Thermo Finnigan LCQ orbitrap spectrometer in the Case Center for Proteomics (Cleveland, OH, USA). Analysis of CL was performed with a Thermo Finnigan LCQ Advantage spectrometer. The MS and MS/MS spectra were obtained by direct infusion in the negative ion mode with a collision energy of 35 eV.
Liposome preparation and Pc 4 incorporation
Large unilamellar vesicles were prepared with a Mini-Extrudor from Avanti Polar Lipids, Inc. (Whatman® membrane diameter ≅ 100 nm). Typically, an aliquot of lipid solution in absolute ethanol was evaporated to form a thin film of lipid which was rehydrated by the addition of 2.5 mL of PBS (pH = 7.4, 0.1 M NaH2PO4 and K2HPO4, and 0.15 M NaCl). Aliquots of the mixture were extruded at 45 °C. Pc 4 was incorporated into liposomes e by addition of a Pc 4 stock solution in THF/EtOH (1:1) and incubating for 30 min. at 45 °C with stirring. Finally Cyt c in aqueous solution was added and stirred gently at 37 °C for 20 min. The final concentration of lipid was 2.1 mM with 20 % of CL for CL oxidation and 20 or 50% of CL for Cyt c oxidation.
Pc 4-mediated photo-oxidation of Cyt c and CL in liposomes
Liposomes (2 mL each) incubated with 5 μM Cyt c and 6 μM Pc 4 were pipetted into 3.5-cm diameter tissue culture dishes, resulting in solution depths of about 2 mm. The liposomes were exposed to 100 mW/cm2 red light produced by a light-emitting diode array (EFOS, Mississauga, Ontario, Canada, λmax 670–675 nm) at room temperature for 40 min while control liposomes were kept in the dark. CL was extracted from 0.5 mL of the DMPC+CL liposome solution using 1.5 mL CHCl3:MeOH (2:1). The extract was dried under a stream of N2 and redissolved in 20 μL CHCl3. The solution was dispensed on silica gel TLC plates, developed with CHCl3:MeOH:acetic acid:H2O (3: 0.52: 0.36: 0.12) [36] and visualized by I2. The CL spot was scraped into a 1.5-mL Eppendorf tube and extracted using 100 μL CHCl3:MeOH (1:1). The CHCl3:MeOH solution of CL was analyzed by negative ion ESI-MS with direct infusion. Cyt c in the illuminated and control liposomes was digested using trypsin in solution without separation from the liposomes or other purification, and the Cyt c digest was analyzed both by MALDI-TOF-MS with CHCA as a matrix and by LC-MS/MS.
Pc 4 mediated photo-oxidation of rat heart mitochondria
Mitochondria (0.5 mg/mL) in a buffer containing 100 mM KCl, 50 mM MOPS and 0.5 mM EGTA were incubated with 200 nM Pc 4 at room temperature for 10 min. The suspension was illuminated for the indicated time while control mitochondria were incubated with Pc 4 and kept in the dark for the whole time course at room temperature. After irradiation, the mitochondrial suspensions were centrifuged at 2250×g for 10 min, and the pellets and supernates were saved separately.
Cyt c and CL isolation from mitochondria
CL was isolated from control and illuminated mitochondrial pellets by suspending 1.0 mg of mitochondria in 0.5 mL of saturated NaCl. The CL was then extracted using the same method described above as that used for extraction of CL from the liposome. Proteins were also extracted from the control and illuminated mitochondrial pellets using n-butanol and concentrated ammonium sulphate [37]. The protein extract was fractionated by filtration through a YM-50 microcon and the filtrate concentrated with a YM-3 microcon. The retentate, which contained the Cyt c, was digested using trypsin in solution. Cyt c in the supernates from the mitochondrial pellets was similarly isolated and digested.
RESULTS
Pc 4-mediated photo-oxidation of CL in liposomes
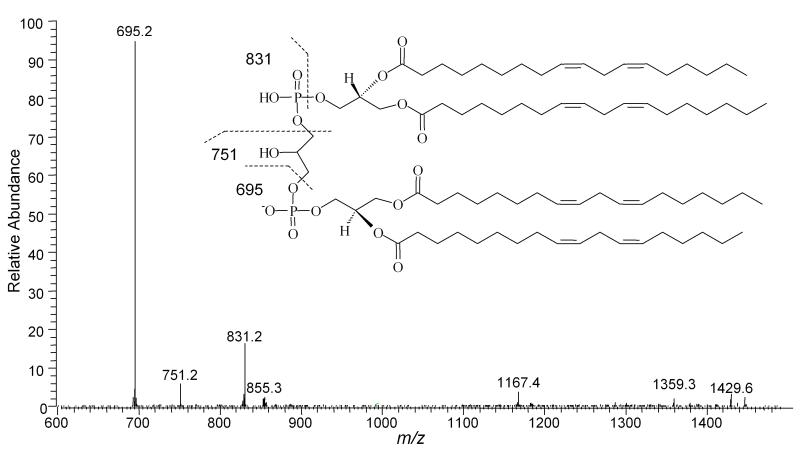
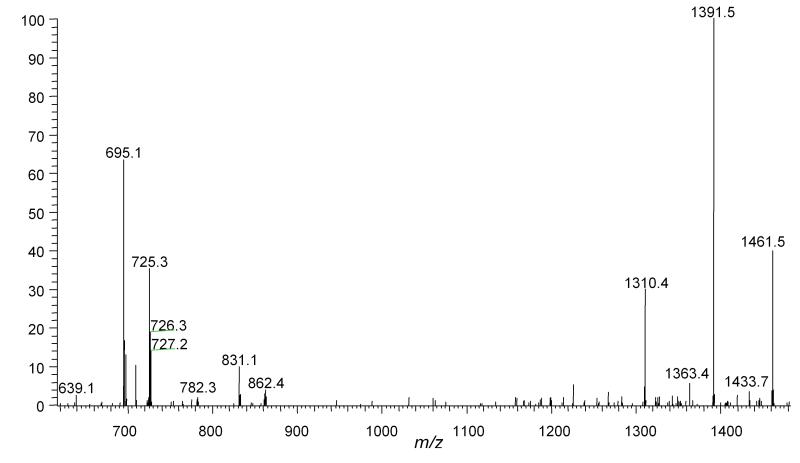
Oxidation of CL present in liposomes was investigated using ESI-MS and ESI-MS/MS. The nominal monoisotopic mass for standard tetra-linoleoyl-CL ((18:2)4CL) [M-H]- is 1447 and the ESI-MS and ESI-MS/MS spectra have been characterized by Lesnefsky et al. [38]. The tandem mass spectrum of standard CL with m/z 1447 shows three major fragment ions, m/z 695, 751, and 831 (Figure 1). The mass spectrum of oxidized CL isolated from the Pc 4-containing liposomes showed several new peaks with nominal monoisotopic m/z [1447+32]- and [1447+64]- (Figure 2). The structures of ions [1447+32]-, [1447+64]-, presumed to have incorporated one and two O2 molecules, respectively, were further characterized by ESI-MS/MS. The tandem mass spectrum of the [1447+32]- ion is consistent with the formation of two pseudosymmetric hydroperoxide structural isomers labeled II in Scheme 1 as found in the oxidation of LA by 1O2. The structures of the predicted fragment ions derived from these products are shown in Figure 3. The presence of peaks at m/z 727 and 709 (derived from dehydration of 727) and absence of an m/z 711 peak strongly support the assignment of the major product with m/z [1447+32]- as a monohydroperoxide.
Figure 1.
ESI-MS/MS spectrum of control CL with m/z 1447.7. Major fragment with m/z 695.2 is phosphatidic acid containing two C18:2 residues and m/z 831.2 is glycerophosphatidic acid containing two C18:2 residues. The fragment with m/z 751.2 is glycerol added to the fragment with m/z 695.2.
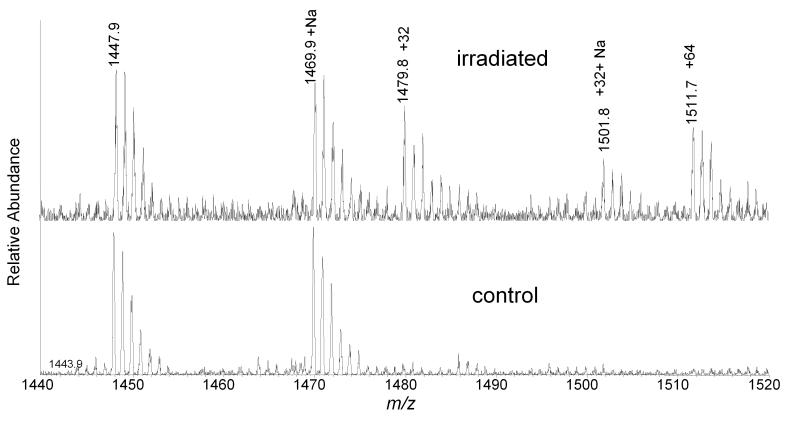
Figure 2.
ESI-MS spectra of CL extracted from liposomes. Control was kept in the dark in the presence of Pc 4 while the sample was illuminated with light for 40 min. The observed oxidative modifications are identified by their mass increments, +32 and +64, are indicated by the increment in mass of the monoisotopic peak.
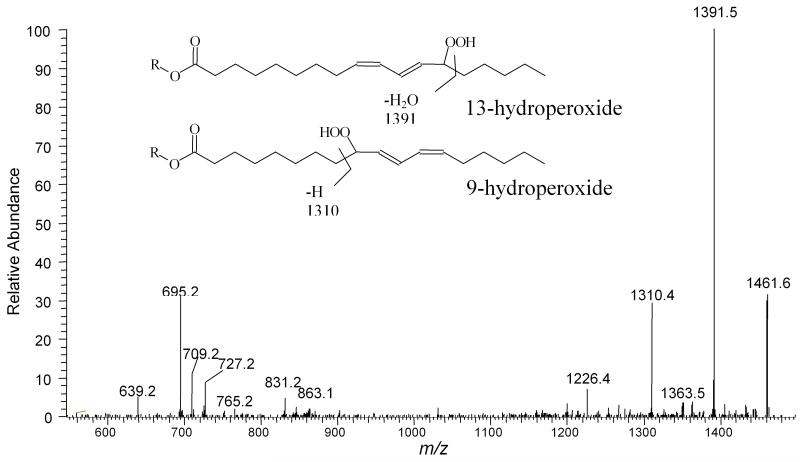
Figure 3.
ESI-MS/MS spectrum of m/z 1479.7 precursor ion. The presence of the hydroperoxide leads to prominent ions associated with acyl chain cleavage. As indicated, the fragment ions at m/z 1310.4 and 1391.5 are attributed to the 9 and 13-hydroperoxides derived from peroxidation of the terminal carbons of the homoconjugated cis, cis double bonds of LA, respectively (R = lyso CL).
Treating the oxidized CL with NaBH4 resulted in the elimination of the peak at m/z [1447+64]- with concomitant increase in the peak at m/z [1447+16]-, while the intensity of [1447+32]- was moderately decreased. The MS/MS spectrum of the [1447+32]- obtained following NaBH4 treatment showed a different fragmentation pattern from that observed in Figure 3. An m/z 711 peak was present while peaks at m/z 704, 1310 and 1391, were absent; suggesting that this different species with a monoisotopic mass at m/z [1447+32]- is dihydroxy CL generated by reduction of dihydroperoxyl CL (data not shown).
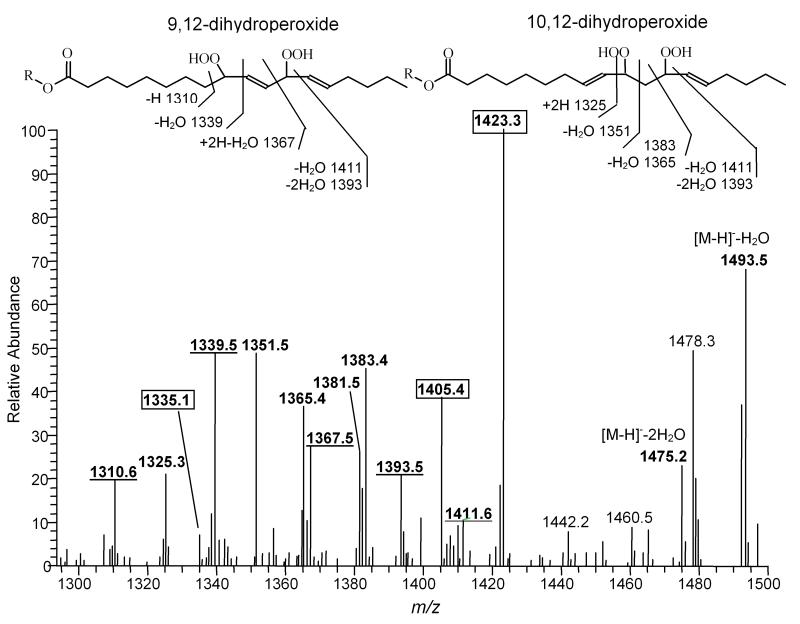
The tandem mass spectrum of m/z [1447+64]- is more complicated. Oxidative addition of two oxygen molecules could occur to one side chain or to two different side chains. If two separate acyl groups are oxidized, these two side chains may be attached to the same or different glycerol moieties, and both cases are suggested by peaks at m/z 727 and 759 in MS/MS spectrum (data not shown). The major species observed by ESI-MS/MS were CL(18:2)2(18:2 13-OOH)2 with fragment peaks at 1423, 1405, and 1335, as shown in Figure 4.
Figure 4.
ESI-MS/MS spectrum of oxidized CL with m/z 1511.7. CL was isolated from - illuminated liposomes containing Pc 4. The identified fragment peaks derived from predicted acyl chain cleavage sites are labeled in bold. The peaks with m/z 1393 and 1411, fragment ions are consistent with the structure of the 9,12-dihydroperoxide and 10,12-bis hydroperoxide. The peaks with m/z 1310, 1339 and 1367 are consistent with the former and 1325, 1351, 1383 and 1365 with the latter. The boxed peaks with m/z 1423, 1405 and 1335 are predicted fragments from species with two 13-hydroperoxides on separate linoleates. The unassigned peak at m/z 1478 has the potential to be derived from a 1O2 specific reaction product (R = lyso CL).
When two hydroperoxide groups are present in a single chain, there are three possible structural isomers predicted by the reaction mechanism: 9,12-dihydroperoxide, 10,12-dihydroperoxide and 10,13-dihydroperoxide, as shown in Scheme 1. The major fragment ions in the m/z region from 1250-1500 are well matched with the theoretical fragments from only the 9,12-dihydroperoxide and 10,12-dihydroperoxide (Figure 4). The peak at m/z 1423 may derive from 10,13-dihydroperoxide; however, the lack of additional correlating peaks and the presence of the peak at m/z 1335 strongly suggest that the m/z 1423 ion may derive from the fragmentation of a species containing two 13-hydroperoxy acyl chains. This interpretation indicates that there is a significant propensity for a single acyl chain to incorporate both O2 molecules. However, potentially different ionization efficiencies of each isomer and fragmentation patterns make the comparison of relative intensities inappropriate to quantify relative yields of the oxidized products.
Pc 4 mediated photo-oxidation of Cyt c in liposomes
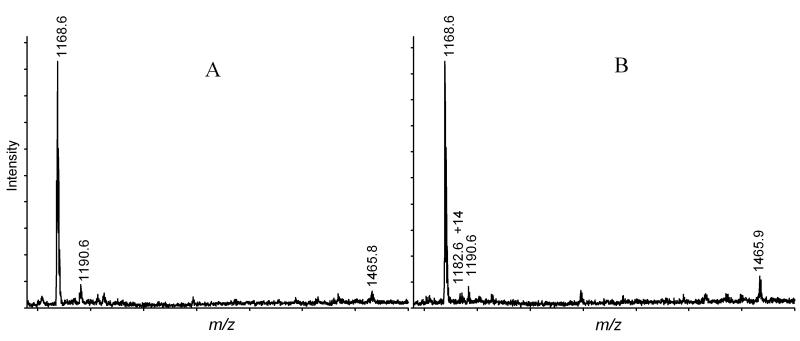
Cyt c was exposed to ROS generated by illumination of Pc 4 incorporated into DMPC liposomes with or without CL. Following the illumination, the Cyt c was digested and analyzed using MALDI-TOF-MS. The mass spectra showed that two peptides previously identified as Pc 4 mediated oxidation products, the H26KTGPNLHGLFGK +32 Da and the TGPNLH33GLFGK +14 Da peptides [1], were present (Figure 5). Modifications of the His residue in these peptides were further confirmed by LC-MS/MS. These two peptides have been proposed to be products attributed to 1O2. As in previous studies, the H26KTGPNLHGLFGK +32 Da tryptic peptide is the most intense peak associated with a modified peptide. The presence of CL in the liposomes was thought to potentially reduce or eliminate the oxidation of Cyt c by scavenging ROS produced in the interior of the liposome bilayer. This anticipated decrease, however, may be offset by an increase in association of Cyt c with the liposomes in the presence of CL. Under the conditions employed the Cyt c should have largely associated with the CL-containing liposomes. The presence of CL (20 or 50%) did not reduce the observed modification of Cyt c. However, 50% of CL rather resulted in enhanced modification of His26 as shown in Figure 5, where the relative intensity of the H26KTGPNLHGLFGK +32 Da peak to the unmodified TGPNLHGLFGK peak has increased from 4% to 10%. This increase may be attributed either to the association of Cyt c with the liposome or alternatively to the excess CL solubilizing the Pc 4 in a second hexagonal phase [39]. In either case, it is clear that the Pc 4 generated oxidant is not completely scavenged by the presence of the linoleic acyl groups of the CL.
Figure 5.
MALDI spectra of Cyt c digest. 5 μM Cyt c was illuminated in the DMPC liposome (A) or DMPC and CL (1:1) liposome (B) in the presence of 6 μM Pc 4 for 40 min. The total concentration of liposome was 200 μM. The peak at m/z 1465.9 represents H26*KTGPNLHGLFGK peptide with H26 modified. The intensity of this oxidized peptide peak relative to the unmodified peptide has increased from 4% in spectrum A to 10% in B.
Pc 4 mediated photo-oxidation of CL and Cyt c in mitochondria
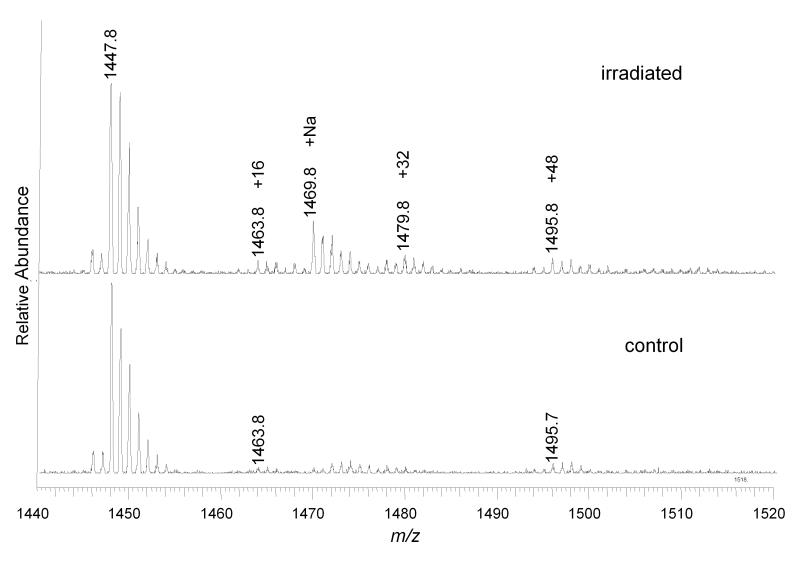
CL was also extracted from mitochondria subjected to Pc 4-mediated photo-oxidation for 20 min and fractionated by TLC using the same method employed to purify CL from liposomes. CL samples from control and illuminated mitochondria were analyzed by direct infusion ESI-MS and MS/MS. The MS spectrum shows that the +32 peak, corresponding to the incorporation of O2 into CL, was detectable from illuminated mitochondria, but its relative intensity remained at less than 10% of the unmodified CL (Figure 6). The tandem mass spectrum of the +32 peak revealed effectively the same fragment pattern as the +32 peak of CL isolated from illuminated liposomes (Figure 7). However, the +64 peak, found following photo-oxidation of CL–containing liposomes, was not enhanced by irradiation (Figure 4). Following 5 min. of irradiation, any oxidized CL products were present only at levels below our limits of detection (data not shown).
Figure 6.
ESI-MS spectra of CL extracted from control and illuminated mitochondria. Unmodified CL (1447.8), +16 and +48 peaks are present in both spectra. But +32 (1469.8) peak is significantly increased by 20 min of irradiation in the presence of Pc 4.
Figure 7.
ESI-MS/MS spectrum of oxidized CL with m/z 1479.7. CL was isolated from illuminated mitochondria. The intensities and patterns of the peaks are similar to the spectrum of CL isolated from Pc 4- illuminated liposomes.
Proteins were extracted and fractionated from mitochondria illuminated in the presence of Pc 4. The protein mixture containing Cyt c was digested in solution, and the digest was analyzed by MALDI-TOF and LC-MS/MS. Although MALDI spectra confirmed the presence of Cyt c, none of the characteristic oxidized peaks were observed with a signal greater than 1.5-times background. However, with the greater sensitivity afforded by LC-MS/MS, the modified H26KTGPNLHGLFGK +32 peptide in the Cyt c digest extracted from mitochondria illuminated for 40 min. was easily detected (Figure 8). A difficulty found in studying mitochondrial Cyt c oxidation by Pc 4 illumination was that a significant amount of Cyt c was released into the supernate following even short irradiation times. The released Cyt c was analyzed by tryptic digestion and LC-MS.
Figure 8.
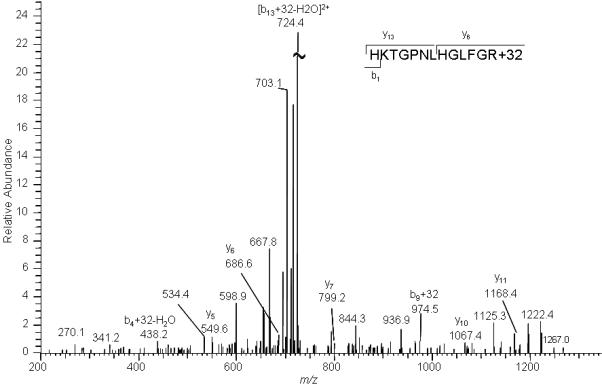
LC- MS/MS spectrum of precursor ions whose m/z corresponds to that predicted for the doubly + charged ion of H26KTGPNLHGLFGR peptide+32 (733.4). Cyt c was extracted from the pellet fraction of mitochondria that had been illuminated for 40 min in the presence of Pc 4.
DISCUSSION
Photo-Oxidation of CL and Cyt c by Pc 4 Illumination
CL is an unusual phospholipid mainly localized to mitochondrial inner membranes. The four constituent acyl chains are enriched in polyunsaturated fatty acids, with linoleic acid being the most prominent. A special enzyme-catalyzed exchange results in the primary CL species containing four linoleic acid moieties, CL(18:2 (9Z,12Z))4 [38]. CL is required for the optimal functioning of the electron transport chain complexes and appears specifically bound at the protein-lipid interface in crystal structures. Consequently, oxidation of CL may lead to functional changes in the mitochondria.
When exposed to ROS generated by illumination of Pc 4 embedded in DMPC liposomes, CL(18:2 (9Z,12Z))4 was readily oxidized, resulting in species with mass increments of +32, and +64. Chemical derivatization coupled with tandem mass spectrometry are consistent with the +32 and +64 species being derived from lipid hydroperoxides as major products. The mass spectrum of CL from illuminated mitochondria equilibrated with Pc 4 showed only one CL(18:2 (9Z,12Z))4 oxidized peak, which had an m/z increment of +32. Tandem mass spectrometry indicated that this ion was comprised of the same two structural isomers identified in the liposome oxidation reactions (compare Figures 3 & 7). The similar intensities and pattern of fragment ions in the tandem mass spectra strongly suggest that photo-oxidation of CL in mitochondria occurred by a mechanism similar to that which occurs in the simpler liposome model. While the absolute concentrations of Pc 4 present in both the liposome and mitochondrial models are comparable and the light flux is similar, the quantum yield of 1O2 cannot be assumed to be identical. This limits a quantitative comparison. However, this demonstrates that the presence of Pc 4 in mitochondrial membranes exposed to red light generates sufficient 1O2 to react with the CL present. However it has not been determined that the oxidation products from the reaction with 1O2 will be different from free radical-initiated lipid peroxidation, although initial studies with LA suggest that the 10,12- dihydroperoxides formed as a result of reaction with 1O2 will not be formed by free radical-mediated peroxidation [34]. And the 10,12- dihydroperoxide identified with oxidation of CL in liposomes by 1O2 was not observed from Pc 4-mediated photo-oxidation of CL in mitochondria. Careful characterization of the tandem mass spectra of this form of CL may yet provide a distinctive fragment ion that would permit its presence to be identified or quantified in the presence of more abundant CL-dihydroperoxides
The isolation of CL hydroperoxides from mitochondria immediately following photo-oxidation indicates that when generated by Pc 4 illumination, these species are generated more rapidly than they are metabolically removed. The observation of accumulating hydroperoxides indicates that mitochondrial antioxidants, e.g. α-tocopherol, either competed unsuccessfully or were overwhelmed by the 1O2 flux. Additionally the CL hydroperoxides were not removed either by reaction with glutathione other endogenous nucleophiles or with alkyl hydroperoxide reductases (peroxiredoxins) which would convert the hydroperoxides to alcohols [40]. Further, the absence of either mono hydroxyl acyl chains or cleaved acyl chains suggests that no detectable one electron reduction of the hydroperoxides in Fenton type reactions occurred in these isolated mitochondria, as that process leads to a wide variety of chain shortened products.
The unique His oxidation originally found in solution was also identified in Cyt c oxidation by 1O2 associated with liposomes and in photo-illuminated mitochondria. The fact that this crosslinked product is still the most intense oxidized peptide peak further establishes its potential to be used as a biomarker of short-term 1O2 exposure.
The oxidation of both CL and Cyt c by Pc 4 present both in liposomes and mitochondria during irradiation validate these molecules as potential targets of the 1O2 generated during PDT of cells and tissues. The similarity of the results suggests that the liposome model can be a relevant vehicle for studying the products of Pc 4-mediated photo-oxidation.
Role of 1O2 in mitochondrial dysfunction
Mitochondria are suggested to play a key role in PC 4 mediated apoptosis. Mitochondria in illuminated cells showed mitochondrial inner membrane permeabilization resulting in mitochondrial depolarization, loss of membrane potential, swelling and released Cyt c [28, 29]. Using the same irradiation conditions used for these studies, illuminated mitochondria showed significant functional and structural deficits, including Cyt c release, complete decoupling, activity loss of complex IV and swelling, within 5 min. (companion manuscript). The current results suggest that lipid hydroperoxides formed from unsaturated fatty acyl groups contribute to these mitochondrial functional and structural deficits. Consistent with this role for CL hydroperoxides, exogenous peroxidized CL has been shown to induce Cyt c release, mitochondrial permeability transition, swelling, and complex IV activity loss [41, 42]. However, it is still a challenge to correlate the extent of lipid and CL oxidation to the functional and structural damage of mitochondria. If structural changes of CL initiate gain or change of function of critical bio-molecules, this gain/change in function could lead to any of the deficits found with a minimal extent of oxidation. The effects of oxidation of CL by 1O2 on the functional and structural damages of mitochondria will be pursued.
SUMMARY
1O2 has been generated in situ in phospholipid bilayers by irradiation of Pc 4. The 1O2 generated reacts both with CL and Cyt c. The oxidized CL obtained from liposomes, as characterized by ESI-MS and ESI-MS/MS, consisted of two major ions with mass increments of +32 and +64, while the oxidized CL isolated from illuminated mitochondria had a single prominent ion with a +32 mass increment. The +32 ions are both attributed to formation of hydroperoxides at either C9 or C13. Cyt c oxidation by 1O2 generated by Pc 4 in liposomes and mitochondria was also studied by MALDI-TOF and LC-MS/MS. Modifications of His26 and His33 were detected with and without CL present in the liposomes as well as in the mitochondrial samples by MALDI and LC-MS/MS. Modification of His26, present as an intra-peptide crosslink, was detectable by LC-MS/MS following in situ 1O2 generation by Pc 4. This modification indicates that CL does not effectively scavenge all of the 1O2 generated in the liposomes or mitochondria by illumination of Pc 4. The 1O2 mediated oxidation of phospholipid and/or protein are proposed to be the initiating steps in Pc 4 photodynamic therapy.
ACKNOWLEDGMENTS
This work was supported by NIH/NIA grant P01 AI55739-01 to V.E.A and by NIH/NCI grant R01 CA106491 to N.L.O.
Footnotes
- CL
- cardiolipin (an adoption of the lipid maps notation identifies the four acyl chains in parentheses following the acronym CL, e.g. CL(18:2 (9Z,12Z))4 is tetralineoyl-CL)
- CHCA
- α-cyano-4-hydroxycinnamic acid
- Cyt c
- cytochrome c
- DMPC
- dimyristoylphosphatidyl choline
- ESI-MS
- electrospray ionization mass spectrometry
- LA
- linoleic acid
- PDT
- photodynamic therapy
- ROS
- reactive oxygen species
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kim J, Rodriguez ME, Guo M, Kenney ME, Oleinick NL, Anderson VE. Oxidative modification of cytochrome c by singlet oxygen. Free Radic Biol Med. 2008;44:1700–1711. doi: 10.1016/j.freeradbiomed.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- [3].Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta. 2000;1502:139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- [4].Wallace DC. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247–263. doi: 10.1002/0470868694.ch20. discussion 263-246. [DOI] [PubMed] [Google Scholar]
- [5].Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- [6].Cadet J, Ravanat JL, Martinez GR, Medeiros MH, Di Mascio P. Singlet oxygen oxidation of isolated and cellular DNA: product formation and mechanistic insights. Photochem Photobiol. 2006;82:1219–1225. doi: 10.1562/2006-06-09-IR-914. [DOI] [PubMed] [Google Scholar]
- [7].Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochem Photobiol Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- [8].Miyamoto S, Martinez GR, Medeiros MH, Di Mascio P. Singlet molecular oxygen generated from lipid hydroperoxides by the russell mechanism: studies using 18(O)-labeled linoleic acid hydroperoxide and monomol light emission measurements. J Am Chem Soc. 2003;125:6172–6179. doi: 10.1021/ja029115o. [DOI] [PubMed] [Google Scholar]
- [9].Kanofsky JR, Wright J, Miles-Richardson GE, Tauber AI. Biochemical requirements for singlet oxygen production by purified human myeloperoxidase. J Clin Invest. 1984;74:1489–1495. doi: 10.1172/JCI111562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- [11].Khan AU, Kasha M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc Natl Acad Sci U S A. 1994;91:12365–12367. doi: 10.1073/pnas.91.26.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- [14].Oleinick NL, Antunez AR, Clay ME, Rihter BD, Kenney ME. New phthalocyanine photosensitizers for photodynamic therapy. Photochem Photobiol. 1993;57:242–247. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- [15].He J, Larkin HE, Li YS, Rihter D, Zaidi SI, Rodgers MA, Mukhtar H, Kenney ME, Oleinick NL. The synthesis, photophysical and photobiological properties and in vitro structure-activity relationships of a set of silicon phthalocyanine PDT photosensitizers. Photochem Photobiol. 1997;65:581–586. doi: 10.1111/j.1751-1097.1997.tb08609.x. [DOI] [PubMed] [Google Scholar]
- [16].Sholto A, Ehrenberg B. Hydrophobicity, topography in membranes and photosensitization of silicon phthalocyanines with axial ligands of varying lengths. Photochem Photobiol Sci. 2008;7:344–351. doi: 10.1039/b716377k. [DOI] [PubMed] [Google Scholar]
- [17].Tannert S, Ermilov EA, Vogel JO, Choi MT, Ng DK, Roder B. The influence of solvent polarity and metalation on energy and electron transfer in porphyrin-phthalocyanine heterotrimers. J Phys Chem B. 2007;111:8053–8062. doi: 10.1021/jp0724222. [DOI] [PubMed] [Google Scholar]
- [18].Martin-Gomis L, Ohkubo K, Fernandez-Lazaro F, Fukuzumi S, Sastre-Santos A. Synthesis and photophysical studies of a new nonaggregated C60-silicon phthalocyanine-C60 triad. Org Lett. 2007;9:3441–3444. doi: 10.1021/ol701444d. [DOI] [PubMed] [Google Scholar]
- [19].Whitacre CM, Feyes DK, Satoh T, Grossmann J, Mulvihill JW, Mukhtar H, Oleinick NL. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4 of SW480 human colon cancer xenografts in athymic mice. Clin Cancer Res. 2000;6:2021–2027. [PubMed] [Google Scholar]
- [20].Colussi VC, Feyes DK, Mulvihill JW, Li YS, Kenney ME, Elmets CA, Oleinick NL, Mukhtar H. Phthalocyanine 4 (Pc 4) photodynamic therapy of human OVCAR-3 tumor xenografts. Photochem Photobiol. 1999;69:236–241. [PubMed] [Google Scholar]
- [21].Whitacre CM, Satoh TH, Xue L, Gordon NH, Oleinick NL. Photodynamic therapy of human breast cancer xenografts lacking caspase-3. Cancer Lett. 2002;179:43–49. doi: 10.1016/s0304-3835(01)00853-9. [DOI] [PubMed] [Google Scholar]
- [22].George JE, 3rd, Ahmad Y, Varghai D, Li X, Berlin J, Jackowe D, Jungermann M, Wolfe MS, Lilge L, Totonchi A, Morris RL, Peterson A, Lust WD, Kenney ME, Hoppel CL, Sun J, Oleinick NL, Dean D. Pc 4 photodynamic therapy of U87-derived human glioma in the nude rat. Lasers Surg Med. 2005;36:383–389. doi: 10.1002/lsm.20185. [DOI] [PubMed] [Google Scholar]
- [23].Fei B, Wang H, Meyers JD, Feyes DK, Oleinick NL, Duerk JL. High-field magnetic resonance imaging of the response of human prostate cancer to Pc 4-based photodynamic therapy in an animal model. Lasers Surg Med. 2007;39:723–730. doi: 10.1002/lsm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bai L, Guo J, Bontempo FA, Iii, Eiseman JL. The Relationship of Phthalocyanine 4 (Pc 4) Concentrations Measured Noninvasively to Outcome of Pc 4 Photodynamic Therapy in Mice. Photochem Photobiol. 2009 doi: 10.1111/j.1751-1097.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- [25].Lee RG, Vecchiotti MA, Heaphy J, Panneerselvam A, Schluchter MD, Oleinick NL, Lavertu P, Alagramam KN, Arnold JE, Sprecher RC. Photodynamic therapy of cotton-tailed rabbit papilloma virus-induced papillomas in a severe combined immunodeficient mouse xenograft system. The Laryngoscope. 2009 doi: 10.1002/lary.20709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney ME, Samia AC, Burda C, Oleinick NL. Fluorescence resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Res. 2003;63:5194–5197. [PubMed] [Google Scholar]
- [27].Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:S146–156. [PubMed] [Google Scholar]
- [28].Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379–47386. doi: 10.1074/jbc.M107678200. [DOI] [PubMed] [Google Scholar]
- [29].Chiu SM, Oleinick NL. Dissociation of mitochondrial depolarization from cytochrome c release during apoptosis induced by photodynamic therapy. Br J Cancer. 2001;84:1099–1106. doi: 10.1054/bjoc.2000.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frankel EN. Secondary products of lipid oxidation. Chem Phys Lipids. 1987;44:73–85. doi: 10.1016/0009-3084(87)90045-4. [DOI] [PubMed] [Google Scholar]
- [31].Kriska T, Korytowski W, Girotti AW. Role of mitochondrial cardiolipin peroxidation in apoptotic photokilling of 5-aminolevulinate-treated tumor cells. Arch Biochem Biophys. 2005;433:435–446. doi: 10.1016/j.abb.2004.09.025. [DOI] [PubMed] [Google Scholar]
- [32].Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, DeKosky ST, Greenberger JS, Shvedova AA, Kagan VE. Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochim Biophys Acta. 2006;1757:648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- [33].Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- [34].Zhang W, Sun M, Salomon RG. Preparative singlet oxygenation of linoleate provides doubly allylic dihydroperoxides: putative intermediates in the generation of biologically active aldehydes in vivo. J Org Chem. 2006;71:5607–5615. doi: 10.1021/jo0605795. [DOI] [PubMed] [Google Scholar]
- [35].Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- [36].Ventrella A, Catucci L, Mascolo G, Corcelli A, Agostiano A. Isolation and characterization of lipids strictly associated to PSII complexes: focus on cardiolipin structural and functional role. Biochim Biophys Acta. 2007;1768:1620–1627. doi: 10.1016/j.bbamem.2007.03.024. [DOI] [PubMed] [Google Scholar]
- [37].Scarlett JL, Murphy MP. Release of apoptogenic proteins from the mitochondrial intermembrane space during the mitochondrial permeability transition. FEBS Lett. 1997;418:282–286. doi: 10.1016/s0014-5793(97)01391-4. [DOI] [PubMed] [Google Scholar]
- [38].Lesnefsky EJ, Stoll MS, Minkler PE, Hoppel CL. Separation and quantitation of phospholipids and lysophospholipids by high-performance liquid chromatography. Anal Biochem. 2000;285:246–254. doi: 10.1006/abio.2000.4783. [DOI] [PubMed] [Google Scholar]
- [39].Ahmed-Adrar NS, Collin F, Couturier M, Vitrac H, Bonnefont-Rousselot D, Jore D, Gardes-Albert M. Radiolytic yield of cardiolipin peroxidation by gamma rays in large unilamellar vesicles of phosphatidylcholine. Radiat Res. 2009;171:622–630. doi: 10.1667/RR1473.1. [DOI] [PubMed] [Google Scholar]
- [40].Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- [41].Petrosillo G, Casanova G, Matera M, Ruggiero FM, Paradies G. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett. 2006;580:6311–6316. doi: 10.1016/j.febslet.2006.10.036. [DOI] [PubMed] [Google Scholar]
- [42].Musatov A. Contribution of peroxidized cardiolipin to inactivation of bovine heart cytochrome c oxidase. Free Radic Biol Med. 2006;41:238–246. doi: 10.1016/j.freeradbiomed.2006.03.018. [DOI] [PubMed] [Google Scholar]
- [43].Varnes ME, Chiu SM, Xue LY, Oleinick NL. Photodynamic therapy-induced apoptosis in lymphoma cells: translocation of cytochrome c causes inhibition of respiration as well as caspase activation. Biochem Biophys Res Commun. 1999;255:673–679. doi: 10.1006/bbrc.1999.0261. [DOI] [PubMed] [Google Scholar]
- [44].Lee JW, Miyawaki H, Bobst EV, Hester JD, Ashraf M, Bobst AM. Improved functional recovery of ischemic rat hearts due to singlet oxygen scavengers histidine and carnosine. J Mol Cell Cardiol. 1999;31:113–121. doi: 10.1006/jmcc.1998.0850. [DOI] [PubMed] [Google Scholar]
- [45].Cai Q, Takemura G, Ashraf M. Antioxidative properties of histidine and its effect on myocardial injury during ischemia/reperfusion in isolated rat heart. J Cardiovasc Pharmacol. 1995;25:147–155. doi: 10.1097/00005344-199501000-00023. [DOI] [PubMed] [Google Scholar]
- [46].Sun S, Bao Z, Ma H, Zhang D, Zheng X. Singlet oxygen generation from the decomposition of alpha-linolenic acid hydroperoxide by cytochrome c and lactoperoxidase. Biochemistry. 2007;46:6668–6673. doi: 10.1021/bi700178u. [DOI] [PubMed] [Google Scholar]
- [47].Foote CS, Wexler S. Olefin oxidations with excited singlet molecular oxygen. J Am Chem Soc. 1964;86:3879–3880. [Google Scholar]
- [48].Di Mascio P, Bechara EJ, Medeiros MH, Briviba K, Sies H. Singlet molecular oxygen production in the reaction of peroxynitrite with hydrogen peroxide. FEBS Lett. 1994;355:287–289. doi: 10.1016/0014-5793(94)01224-5. [DOI] [PubMed] [Google Scholar]