Abstract
Purpose of review
In this mini-review, we have highlighted the recent breakthroughs in growth factor signaling that have made conceptual changes in our understanding of how blood vessels are formed.
Recent findings
Studies conducted over the past few years have focused on understanding the cell biology of vascular morphogenesis. The major themes include characterization of the different cell types that comprise a vascular sprout, as well as the regulatory influence of cell-cell and cell-matrix interactions on signaling outcomes. In addition, novel trends have emerged, including non-conventional ways in which VEGF contributes to cell survival and metabolic balance.
Summary
The growth of new capillary sprouts from a pre-existent vascular network requires a highly coordinated cellular response to both growth factors and morphogens. This response is sensed and triggered by cell surface receptors responsible for the activation of an intracellular cascade that efficiently initiates migration and proliferation programs. While the chief molecular players have been identified, great strides have been made in understanding their specific contributions to endothelial function during the angiogenic process.
Keywords: angiogenesis, capillaries, endothelial cells, VEGF, vascular system
1. Introduction
During the last two decades, the exploration of genetic targeting in mice has advanced our understanding of vascular morphogenesis. The resultant findings have identified a subset of signaling pathways that are essential for angiogenic growth during development and in the adult. These pathways include VEGF-VEGFR, Notch-DSL, Tie-Angiopoietin, VE-cadherin and Ephrin-Eph. Other prominent signaling pathways that contribute to further patterning and vascular remodeling also include plexins, TGF-beta, PDGF, and integrins.
This “genetic phase” of discovery has been recently followed by strategies that merge sophisticated cell biology with genetic modifications. The outcome has resulted in a plethora of publications that have refined the independent and combined contributions of these pathways in angiogenesis.
In this review we present breakthroughs in growth factor signaling during the last three years. Our emphasis focuses on the evidence that offers an in-depth and mechanistic understanding of how signaling pathways interact towards the organization of vascular sprouts. We have also made an effort to identify novel trends and highlight the unsuspected contribution of VEGF in metabolism.
2. The cellular anatomy of the vascular sprout
Information from cell biological studies and genetic deletion in mice has revealed that the cellular composition of a growing vascular sprout is molecularly and functionally heterogeneous. The endothelial cells at the invasive front exhibit unique properties and can be distinguished from their neighbors by the expression of a specific subset of molecules.
Growing vessel
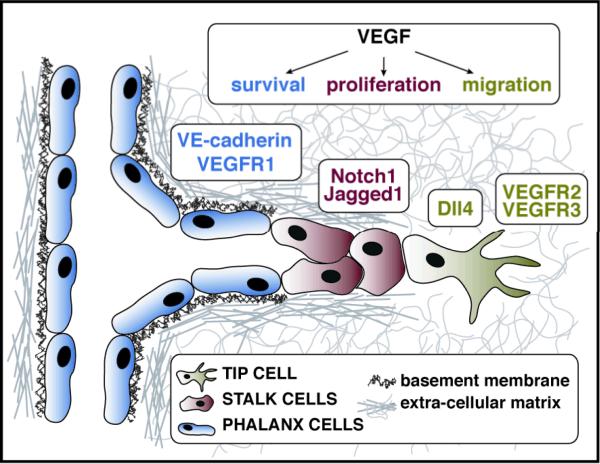
The vascular sprout is composed of a tip cell, at the leading front followed by stalk cells. The later cells comprise the body of the sprout and are responsible for the elongation of the new vascular branch (Figure 1). Tip cells were first described as functionally specialized and distinct endothelial cells by Gerhardt and colleagues [1]. These investigators characterized tip cells as non-proliferative, with dynamic filopodial protrusions that express high levels of VEGFR2. Tip cells sense and respond to gradients of VEGF and maintain an active migratory phenotype. On the other hand, stalk cells proliferate in response to VEGF, promoting the physical extension of the vessel [1]. The concept of tip and stalk cells is fully accepted today, but has gained a more clear molecular identity. Studies published over the past three years have underscored the importance of crosstalk between the VEGF and Notch signaling pathways in determining the identity and number of tip and stalk cells.
Figure 1.
Endothelial cells in a growing vascular sprout are functionally and molecularly distinct. A growing sprout is composed of tip cells (green), stalk cells (red), and phalanx cells (blue). Each cell type is characterized by a unique molecular signature, resulting in a differential response to VEGF. Tip cells exhibit a migratory response to VEGF, and show an upregulation of Dll4, VEGFR3, and VEGFR2. Stalk cells undergo proliferation and show upregulation of Notch1 and Jagged1. VEGF signaling in phalanx cells leads to a survival response mediated by increased levels of VE-cadherin and VEGFR1.
One of the hallmark achievements in the field in recent years has been the identification of the Dll4-Notch1 pathway as the instructive regulator of tip versus stalk cell fate. The findings concurrently emerged from several groups using three distinct experimental models of angiogenesis including solid tumor growth in mice [2,3], postnatal mouse retinas [4-6], and zebrafish embryos [7,8]. In all these models, blockade of Dll4 activity, using pharmacological inhibitors or genetic inactivation of one allele, led to high vascular density due to an increase in the number of tip cells and excessive vascular sprouting. On the other hand, activation of Notch signaling led to a reduction in the number of tip cells and decreased vascular density [4,7]. Furthermore, VEGF-VEGFR2 signaling was shown to upregulate expression of Dll4 in tip cells, allowing Dll4 to activate Notch1 in the adjacent stalk cells, causing suppression of the tip cell phenotype. This coordinated regulation ensures the selection of a single cell as the leader (i.e., tip) while the adjacent cells become followers (stalk cells).
How might Notch signaling suppress the tip cell phenotype? Additional mechanistic insights have revealed that Notch inactivation through deletion of Dll4 results in a reduction of VEGFR1 levels [5,6]. This suggests that Notch signaling may block tip cell formation by regulating the levels of VEGFR1, a decoy receptor that antagonizes VEGFR2 function [9,10]. Another mechanism through which Notch signaling may suppress the tip cell phenotype is through transcriptional downregulation of VEGFR3. VEGFR3 expression has been localized to the leading tip cells of endothelial sprouts in the zebrafish embryo [7], the postnatal retina, and in tumor vasculature [11], where VEGFR2 is also highly expressed. However, in the absence of Notch signaling, VEGFR3 becomes ectopically expressed throughout the sprout [7,11]. Consistent with these findings, inhibition of VEGFR3 signaling through genetic targeting, or with monoclonal antibody blockade, both result in decreased vessel density due to a reduction in the number of sprouts, branching points, and proliferating cells, suggesting that VEGFR3 is a positive regulator of tip cell selection. Importantly, the excessive sprouting that occurs upon genetic or pharmacological disruption of Dll4/Notch signaling is extinguished upon blocking VEGFR3 signaling [11].
More recently, the Notch ligand Jagged1 has been shown to act as an antagonist of Dll4-Notch signaling in sprouting angiogenesis [12]. While Dll4 is expressed highly in tip cells, Jagged1 expression is low. Instead, Jagged1 has increased expression in stalk cells [13]. Deletion of Jagged1 in endothelial cells led to a decrease in the number of tip cells and filopodia and reduced vessel density in postnatal mouse retinas. When Notch was glycosylated by the Fringe family of glycoslytransferases, Jagged1 was able to act as an antagonist of Dll4/Notch signaling. In addition, Jagged1 inactivation in postnatal endothelial cells led to reduced expression of VEGFR3 in tip cells in the retina, providing a mechanistic explanation for impaired sprouting in the absence of Jagged1 [12].
Quiescent vessel
Adding to the cellular heterogeneity of a vessel, recent work by Carmeliet and colleagues describes a third type of endothelial cell, restricted to quiescent vessels, which they refer to as the phalanx cell [14]. Using tumor blood vasculature as their experimental model, the authors showed that heterozygous deficiency of the prolyl-hydoxylase domain protein 2 (PHD2) normalized the endothelial lining without affecting tumor vessel density or lumen size. In wild type mice, the endothelial cells lining the tumor vessel walls were disorganized and irregular. However, the vessel wall in the PHD2 +/- mice formed a uniform “cobblestone” formation with clearly defined boundaries and branching points [14]. They described the endothelial cells that adopted this quiescent phenotype as a phalanx cell. Phalanx cells maintained lumen patency and formed a tightly aligned endothelial cell layer.
Mechanistically, maintenance of the quiescent endothelial cell phenotype depended on the HIF-mediated upregulation of soluble VEGFR1 and VE-cadherin. VE-cadherin is the main component of endothelial cell adherens junctions, and was recently shown to upregulate claudin-5, an endothelial specific tight junction protein [15]. Strengthening both adherens and tight junctions would serve as a mechanism for tightening the endothelial cell barrier, and promoting the quiescent, normalized phenotype.
Interestingly, PHD2 -/+ phalanx cells showed a reduced migratory and mitogenic response, but an enhanced survival response to VEGF. This response may be regulated by an increase in the levels of VE-cadherin, which is required for VEGF-mediated survival of endothelial cells [16].
3. Cell-cell interactions as modulators of angiogenic signals
Studies on signaling in vascular cells have not deviated from similar analysis in other cell types, in that the focus has been on one growth factor at a time. In the last three years, however, a multitude of papers have dissected the intricacies of dual and/or tripartite interactions. Analysis of these associations has exposed an unsuspected and sophisticated hierarchy of cellular responses during angiogenesis. Furthermore, these studies have revealed that the same players might assume distinct roles depending upon the status of endothelial cell confluency versus subconfluency, (perhaps analogous to phalanx versus tip cells) (Figure 2).
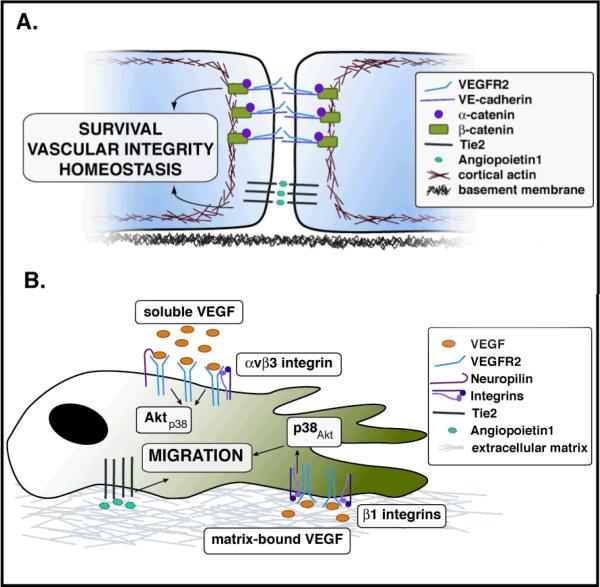
Figure 2.
Location and confluency status dictate the distribution of signaling mediators during vascular morphogenesis. The maintenance of vascular integrity is partially mediated through the tripartite interaction of VEGFR2, VE-cadherin, and beta-catenin (A). Interactions between Tie2 and Angiopoietin1 at cell-cell contacts also contribute to the homeostasis of quiescent endothelial cells (A), while Tie2-Angiopoietin1 signaling in the context of cell-matrix interactions leads to migration of endothelial cells (B). The association status of VEGF with the matrix dictates differential responses by VEGFR2. Activation of VEGFR2 by matrix-bound VEGF leads to its association with beta1-integrins and activation of p38. Soluble VEGF binding leads to VEGFR2 association with Neuropilins or beta3-integrins, leading to activation of Akt (B).
In quiescent endothelial cells, VE-cadherin promotes stabilization of the differentiated vascular wall, inhibits proliferation and decreases cell permeability [17,18]. Recent information has revealed that VE-cadherin binds to VEGFR2, and together with beta-catenin forms a multiprotein complex that promotes vascular stability and survival [19,20]. Interestingly, this tripartite association prevents phosphorylation and internalization of VEGFR2 in the presence of ligand [19]. The findings indicate that engagement of cell-cell interactions promotes a functional change in the response of VEGFR2 to its ligand. Furthermore, VE-cadherin, through the activation of Rho-kinase and myosin light chain 2, was shown to coordinate the stabilization of cell-cell junctions and suppression of a migratory phenotype [20]. Overall the combined information suggests that activation of cell-cell interactions inhibits the angiogenic response through the regulation of the cytoskeleton and by taming the response to VEGF (Figure 2A).
Not only cell-cell, but also cell-matrix interactions contribute to regulate the responses mediated by VEGFR2. A series of recent experiments have found that activation of VEGFR2 by matrix-bound VEGF results in prolonged phosphorylation of this receptor and preferential p38, rather than Akt, activation [21] (Figure 2B). Association of VEGF with matrix also induced the binding of VEGFR2 to beta1 integrins and disfavored its interaction with Neuropilin1 [21]. In contrast, activation of VEGFR2 by VEGF when in its soluble form, i.e., not associated with matrix proteins, triggers the formation of a complex that includes Neuropilin1 and beta3 integrins [22,23]. This complex is functionally and molecularly distinct from the one that is activated by matrix-bound VEGF and results in two distinct forms of vascular expansion: sprouting growth (matrix–bound VEGF) or vascular hyperplasia (soluble VEGF) [24].
Cell-cell and cell-matrix interactions have also been noted to distinctively modulate responses to Angiopoetin1 through the Tie2 receptor [25,26] (Figure 2). Two elegant publications convincingly showed that in confluent endothelial cells, Angiopoeitin1 bridges Tie2 at cell-cell contacts. This interaction results in trans-association of Tie2 and downstream activation of Foxo1 and eNOS [25]. Clustering of Tie2 at the cell-cell interface also results in binding to VE-PTP [26]. The interaction results in a marked decrease in endothelial permeability and provides a mechanistic explanation for the phenotype of the Tie2 and Angiopoietin null mice [27-29]. In the absence of cell contacts, Angiopoetin1 induces a translocation of Tie2 to the rear of the cell in clusters similar to, but distinct from, focal adhesions, without the association of VE-PTP [26].
4. Regulation of endothelial cell survival
VEGF signaling has long been regarded as a necessary factor for endothelial cell survival. In recent years, several new aspects through which the VEGF family of ligands mediates the survival of endothelial cells, independent of classical VEGF signaling, have come to light.
It was recently demonstrated that endothelial cells produce VEGF, and that autocrine VEGF signaling is required for endothelial cell survival in a cell autonomous manner [30]. Deletion of VEGF specifically from endothelial cells resulted in progressive endothelial degeneration. While autocrine VEGF signaling is dispensable angiogenesis, it is crucial for the maintenance and homeostasis of blood vessels. Furthermore, circulating and exogenous VEGF was unable to compensate for the loss of VEGF in endothelial cells [30]. Endogenous VEGF signaling has also been shown to mediate survival in other cell types, in particular müller cells and photoreceptors [31].
Another growth factor with significance to endothelial cell survival is VEGF-B. VEGF-B is not required for blood vessel growth, but is necessary for the survival of not only endothelial cells, but also vascular smooth muscle cells and pericytes. The effect of VEGF-B on survival was mediated, in part, by an upregulation of VEGFR1 and Neuropilin1 [32].
5. Contribution of VEGF signaling to metabolic balance
The response of VEGF to hypoxia has been acknowledged and well supported in the literature. VEGF is transcriptionally regulated by HIFs, and changes in oxygen tension result in a concurrent increase in VEGF expression and vascular density [33]. During the past few years, we have expanded our understanding of the regulatory effects of oxygen sensing proteins on VEGF in both the endothelium and other cell types. Combined, the information seems to reveal that the VEGF family might act as a global sensor of metabolic stress in a large variety of tissues.
Recent advances have linked the regulation of oxygen consumption by other cell types to the activity of VEGF in the vasculature. Intracellular levels of oxygen directly control metabolic pathways and regulate a cohort of transcription factors that provide further homeostatic support favoring aerobic metabolism. Amongst the transcription factors, HIFs and ARNT are constitutively expressed, but are kept separate and therefore inactive by prolyl hydroxylase domain proteins (PHD1-3) under normoxic conditions. PHDs target HIFs for proteasomal degradation under normal oxygen tension. Hypoxia downregulates PHD activity, enabling accumulation of HIFs [34]. Genetic studies in mice have recently highlighted additional functions for PHD proteins in both the endothelium and surrounding tissues.
In the absence of PHD2, the oxygen sensing machinery of endothelial cells is altered in a way that appears to precondition the cells to better adapt to hypoxia. In particular, inactivation of the oxygen sensor PHD2 caused endothelial cells to readjust their shape and molecular signature to restore oxygen supply to the vasculature. Haplodeficiency of PHD2 resulted in normalization of tumor endothelial cells into a quiescent phalanx cell phenotype [14]. Tumor blood vessels are considered “abnormal” in that they are leaky and are inefficient in oxygenation, resulting in hypoxia, which induces the production of growth factors and cytokines by the surrounding tumor cells. This in turn promotes tumor invasion and metastasis. Therefore, the concept of vessel “normalization” has gained significant interest in the field as a means of controlling the tumor vasculature to improve drug delivery [35]. One would hypothesize that reducing the levels of endothelial PHD2 would lead to an increase in tumor metastasis due to the upregulation of the HIF signaling pathways. In contrast, the tumors in PHD2 +/- mice were less invasive and metastatic due to blood vessel normalization. In addition, the response of phalanx cells to VEGF induced endothelial survival, rather than migration or proliferation [14].
In contrast to PHD2, loss of PHD1 does not cause abnormal angiogenesis, but instead has an effect on the metabolic program of skeletal muscle cells [36]. PHD1 deficiency lowers oxygen consumption in skeletal muscle through a shift from oxidative to a more anaerobic metabolic program. While under normal conditions the loss of PHD1 impairs oxidative muscle performance, under ischemic conditions the enhanced oxygen conservation in myofibers lacking PHD1 prevents excess oxygen damage by reducing necrosis. Overall it seems that the regulation of HIF, by PHD1 and 2 are cell-specific and that each protein offers alternative outcomes depending on the cell type.
Recent work has further linked the regulation of oxygen consumption by other cell types to the activity of VEGF in the vasculature. The metabolic regulator PGC-1alpha is increased in various cell types under nutrient and oxygen deprivation. Moreover, PGC-1alpha has been shown to regulate angiogenesis by inducing the expression of VEGF, PDGF-BB and Angiopoietin2 in skeletal muscle [37]. Interestingly, induction of VEGF by PGC-1alpha is independent of the HIF pathway, but instead requires PGC-1alpha interaction with estrogen-related receptor-alpha, a protein involved in fatty acid oxidation and oxidative phosphorylation. The emerging interactions between metabolic pathways and VEGF uncover an important and unsuspected contribution of this growth factor in the overall regulation of energy consumption by the cell.
6. Concluding remarks
Our understanding of vascular morphogenesis has continued to grow at an exponential rate. While the major focus of endothelial cell biology in the last decade has been on the identification of the central signaling cascades and characterization of their main functions, the last three years have been marked by an increased emphasis on the cell biology of vascular morphogenesis. In particular, the cellular dissection of the nascent vascular sprout into tip and stalk cells has provided a critical conceptual advance that permeates the field. This has been coupled with the critical and recent advances on how cell-cell and cell-matrix contacts modulate receptor tyrosine kinase signaling in endothelial cells. Finally, publications in the last three years have contributed to an emerging notion that the VEGF family of signaling molecules might contribute to metabolic sensing.
Where do we go from here? Clearly the cell-biological phase of vascular biology will continue to expand. The intricacies of binding partners and their biological effects will most certainly fill the framework of information on vascular network formation and remodeling. Additional trends, already appearing in the literature, are likely to include how organ-specific microenvironments impact endothelial differentiation and heterogeneity. Along these lines, the cell biological consequences of paracrine interactions in a growing vascular bed are likely to be critical in further completing the picture of signaling circuitry in the vascular endothelium.
Acknowledgements
We thank Dr. Ann Zovein for critical reading of this review and Florian Milde for assistance with the illustrations. We would also like to acknowledge the fact that we were unable to cite and discuss many other contributions due to the space constraints and focus of this review article. CMW is supported by the Vascular Biology Training Grant at UCLA (T32 HL69766) and MLIA is supported by grants from the NIH (CA126935, HL085618), CIRM and Leducq foundation.
Abbreviations
- ARNT
aryl hydrocarbon receptor nuclear translocator
- Dll
delta-like ligand
- DSL
delta, serrate, lag2
- eNOS
endothelial nitric oxide synthase
- HIF
hypoxia inducible factor
- PDGF
platelet derived growth factor
- PGC
peroxisome-proliferator-activated receptor-gamma coactivator
- PHD
prolyl hydroxylase domain
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- VE-PTP
vascular endothelial protein tyrosine phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **2.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [Together with Noguera-Troise et al, this study demonstrates that blocking Notch signaling with neutralizing antibodies against Dll4 causes excessive endothelial cell sprouting and hyperproliferation. Tumor blood vessels were poorly perfused, resulting in a decrease in tumor growth.] [DOI] [PubMed] [Google Scholar]
- **3.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [Along with Ridgway et al, this article provides evidence that inhibition of Dll4/Notch inhibits tumor growth by causing excessive sprouting and branching of tumor blood vessels, rendering them nonproductive.] [DOI] [PubMed] [Google Scholar]
- **4.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [This study shows that Dll4 signaling from tip cells to Notch1 in stalk cells suppresses the tip cell phenotype, regulating the ratio of endothelial cell types required for proper vascular branching.] [DOI] [PubMed] [Google Scholar]
- 5.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [Using the zebrafish embryo as a model system, the authors show that Notch signaling inhibits the sprouting, tip cell phenotype. Furthermore, this article demonstrates that VEGFR3 is preferentially expressed in tip cells, but in the absence of Notch signaling, VEGFR3 becomes ectopically expressed throughout the sprout.] [DOI] [PubMed] [Google Scholar]
- 8.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 9.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 10.Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol. 2004;164:1531–1535. doi: 10.1016/S0002-9440(10)63711-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [This manuscript implicates VEGFR3 as a positive regulator of tip cell selection. Inhibition of VEGFR3 signaling in mouse retinas and in tumors resulted in a decrease in vessel sprouts and branching points. In addition, the authors show that the excessive sprouting induced by inhibition of Notch signaling can be reversed by inhibiting VEGFR3 signaling.] [DOI] [PubMed] [Google Scholar]
- **12.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [In this article, the authors provide evidence that Jagged1 acts as a positive regulator of tip cell specification by antagonizing Dll4-Notch signaling, under conditions where Notch is glycosylated by the Fringe family of proteins.] [DOI] [PubMed] [Google Scholar]
- 13.Hofmann JJ, Luisa Iruela-Arispe M. Notch expression patterns in the retina: An eye on receptor-ligand distribution during angiogenesis. Gene Expr Patterns. 2007;7:461–470. doi: 10.1016/j.modgep.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [This study demonstrates that haplodeficiency of PHD2 promotes “normalization” of tumor vasculature containing blood vessels comprised of phalanx cells, or a quiescent, tightly aligned endothelial cell layer. As a result, tumors from PHD heterozygous mice were less invasive and metastatic.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 17.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 18.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [Here, the authors show that VEGFR2 signaling and internalization occurs more efficiently when VE-cadherin is absent from cell-cell contacts, providing a mechanism through which VE-cadherin limits cell proliferation and promotes vascular stabilization.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19:668–674. doi: 10.1016/j.cub.2009.02.057. [This article demonstrates that VE-cadherin suppresses the migratory phenotype by stabilizing cell-cell junctions. Mechanistically, VE-cadherin antagonizes VEGFR2 signaling through the activation of Rho kinase and myosin light-chain 2.] [DOI] [PubMed] [Google Scholar]
- 21.Chen TT, Luque A, Lee S, Anderson SM, Segure T, Iruela-Arispe ML. Anchorage of VEGF to the Extracellular Matrix Conveys Differential Signaling Responses to Endothelial Cells. J Cell Biol. 2010 doi: 10.1083/jcb.200906044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura H, Li X, Goishi K, van Meeteren LA, Jakobsson L, Cebe-Suarez S, Shimizu A, Edholm D, Ballmer-Hofer K, Kjellen L, et al. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112:3638–3649. doi: 10.1182/blood-2007-12-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J Biol Chem. 2009;284:33966–33981. doi: 10.1074/jbc.M109.030700. [This article provides evidence that beta3-integrin can negatively regulate VEGF signaling by binding to Neuropilin1 and preventing its association with VEGFR2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **25.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [In this study, the authors show that Angiopoietin1-Tie2 signaling in the presence of either cell-cell contacts or cell-matrix contacts led to differences in signaling outcomes. While activation of Tie2 at cell-cell contacts led to activation of Akt to promote survival, cell-matrix activation of Tie2 led to Erk activation to promote migration.] [DOI] [PubMed] [Google Scholar]
- **26.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [This article demonstrated that Tie2 activation by Angiopoietin1 at cell-cell contacts led to association of Tie2 with VE-PTP and a decrease in endothelial permeability, while Tie2 activation by matrix-bound Angiopoietin1 resulted in increased cell motility.] [DOI] [PubMed] [Google Scholar]
- 27.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 28.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 29.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong L, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 34.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 36.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- *37.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [This study links VEGF signaling in the vasculature to metabolic regulation by surrounding tissues. The metabolic regulator PGC-1alpha can induce angiogenesis by upregulating the levels of VEGF in skeletal muscle in a mechanism that is independent of HIF but dependent on another protein in the metabolic pathway, estrogen-related receptor-alpha.] [DOI] [PubMed] [Google Scholar]