Abstract
Singlet oxygen is produced by absorption of red light by the phthalocyanine dye, Pc 4, followed by energy transfer to dissolved triplet oxygen. Mitochondria pre-incubated with Pc 4 were illuminated by red light and the damage to mitochondrial structure and function by the generated singlet oxygen was studied. At early illumination times (3–5 min. of red light exposure), state 3 respiration was inhibited (50%) while state 4 activity increased, resulting in effectively complete uncoupling. Individual complex activities were measured and only complex IV activity was significantly reduced and exhibited a dose response while the activities of electron transport complexes I, II and III were not significantly affected. Cyt c release was an increasing function of irradiation time with 30% being released following 5 min. of illumination. Mitochondrial expansion along with changes in the structure of the cristae were observed by transmission electron microscopy following 5 min. of irradiation with an increase of large vacuoles and membrane rupture occurring following more extensive exposures.
Keywords: Singlet oxygen, Mitochondria, Cytochrome c oxidase, Electron transport complexes, Cytochrome c release, Photodynamic therapy
Introduction
Singlet oxygen (1O2) is a unique reactive oxygen species 1(ROS) which can be generated by various mechanisms in biological systems [1–3]. When generated in tissues, 1O2 has the potential to react with most biological molecules. The ensuing oxidative damage has been shown to induce necrosis or apoptosis [4, 5].
1O2 may be intentionally generated in abnormal tissues by photodynamic therapy (PDT). PDT is a rapidly advancing treatment for cancer and certain benign conditions that utilizes a photosensitive dye and light to produce 1O2 in a specific location determined both by the localization of the light and the dye [6, 7]. As the primary mediator of PDT effects, 1O2 oxidizes biomolecules near its site of formation due to its reactivity which reduces its potential to diffuse over longer distances. Most photosensitizers for PDT are hydrophobic planar dyes built on a porphyrin or phthalocyanine platform. These molecules tend to localize in the membranes of one or more organelles, including mitochondria, endoplasmic reticulum (ER)/Golgi, lysosomes, or plasma membranes [8].
At Case Western Reserve University, we have been studying the silicon phthalocyanine Pc 4 for several years. Pc 4 was first synthesized here [9] and is currently being studied at University Hospitals Case Medical Center in clinical trials for skin malignancies and psoriasis. In cancer cells maintained in cell culture, Pc 4 localizes to mitochondria and ER [8, 10, 11], such that upon red light-illumination there is evidence for direct photodamage to mitochondrial substituents followed by the release of cytochrome c (Cyt c) into the cytoplasm and the induction of apoptosis, as characterized by the activation of caspases [5], the cleavage of specific caspase substrates, the fragmentation of DNA, and the condensation of nuclear chromatin into the characteristic features of morphological apoptosis [12, 13]. To better understand the steps in the process that are critical for the induction of apoptosis by PDT, we have sought to identify the initial mitochondrial targets of PDT-induced 1O2-mediated oxidative damage. We found that two anti-apoptotic proteins of the mitochondrial outer membrane, Bcl-2 and Bcl-xL, undergo a type of photodamage that leads to their complexation with other membrane constituents, as revealed on western blots by the loss of the native proteins in a manner roughly equivalent to the loss of reproductive integrity of the cells, the release of Cyt c, and the induction of apoptosis [7, 14, 15]. Further, we have determined that both cardiolipin and Cyt c can be oxidized by the 1O2 generated by red-illumination of Pc 4 present in both liposomes and isolated mitochondria (submitted companion paper).
Mitochondria are essential cellular organelles, responsible for final steps of oxidative metabolism and generation of ATP as well as important biosynthetic pathways. Mitochondria also provide the key initiators for the intrinsic apoptosis pathway, which is triggered upon the mitochondria receiving signals from other cellular sites or upon direct damage within this organelle. In the mitochondrial (intrinsic) pathway for apoptosis, Cyt c, as well as several other proteins of the intermembrane space, is released into the cytoplasm. Once released to the cytosol, Cyt c interacts with cytoplasmic proteins to form the apoptosome which results in the activation of a cascade of caspases. Mitochondrial functional damage by 1O2 has been studied using various photosensitizers where defects in mitochondrial respiration [16, 17], inactivation of electron transport chain (ETC) complexes, primarily cytochrome oxidase [16, 18–20], and induction of the mitochondrial permeability transition [21] have been reported. The importance of mitochondrial damage in porphyrin-based PDT has been recently reviewed [22]. The in vivo effect of disrupting mitochondrial energy metabolism has been verified by imaging tumor ATP decreases following PDT [23]. Herein we studied functional and structural damage to rat heart mitochondria by Pc 4-generated 1O2 using various analyses in a time-dependent manner with the intent of identifying the initial mitochondrial targets of 1O2.
Experimental
Materials
All chemicals were purchased from major commercial sources. Pc 4 was a generous gift of Dr. Malcolm E. Kenney (Chemistry Department, CWRU, Cleveland). Mitochondria were isolated from male Fisher 344 rats, aged 6 months [24] and used within 5 h of isolation. Mitochondria concentrations were determined by protein assay. Millipore water (18 MΩ) was used throughout.
Pc 4-mediated photodamage to rat heart mitochondria
Mitochondria (0.5 mg protein/mL) in a buffer containing 100 mM KCl, 50 mM 3-(N-morpholino) propanesulfonic acid (MOPS) and 0.5 mM EGTA were incubated with 200 nM Pc 4 at room temperature for 10 min. The suspension was illuminated with 100 mW/cm2 red light (λmax 670–675 nm) produced by a light-emitting diode array (EFOS, Mississauga, Ontario, Canada) at room temperature for varied times and otherwise kept in the dark for the remaining time course. Control mitochondria were incubated with Pc 4 and kept in the dark for the whole time course at room temperature. After irradiation, the mitochondrial suspensions were centrifuged at 2250×g for 10 min., and the pellets were kept on ice for analysis. When measuring the activities of individual ETC complexes and mitochondrial respiration rates, the mitochondrial pellet was resuspended in the same buffer to give 20 mg/mL protein and kept on ice for the assays.
Determination of Cyt c concentration in mitochondria
Proteins were extracted from the control and illuminated mitochondrial pellets using n-butanol and concentrated ammonium sulphate [25]. The protein extract was reduced by sodium dithionite, and the amount of Cyt c in the extract was measured using absorbance at 550 nm with 542 nm as reference (Δε = 19.6 mM−1cm−1).
Electron microscopy
After illumination, the treated and control mitochondria were fixed by adding an equal volume of phosphate-buffered quarter-strength Karnovsky’s fixative using the method reported elsewhere [26, 27] with little modification. Sections were stained with uranyl acetate and lead citrate and electron micrographs were obtained with a JEOL 1200EX electron microscope (Tokyo, Japan).
Measurement of electron transport chain complex activities
All assays were performed with a HP model 8452 diode array spectrophotometer. Complex I (NADH-Q reductase) activity was measured by following the decylubiquinone (DUQ)-dependent decrease in absorbance of NADH at 340 nm every 4 s for 1 min. [24, 28]. Twenty µg of mitochondria were dissolved in an assay buffer containing 0.2% bovine serum albumin (BSA), 0.015% asolectin, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.2 mM NADH, 0.002% antimycin A, 0.08% sodium cholate, and 50 mM KH2PO4 at pH 7.4. The reaction was started by addition of 0.15 mM DUQ.
Complex II (succinate dehydrogenase) activity was measured by following the decrease in absorbance of 2,6-dichloroindophenol (DCPIP) at 600 nm every 4 s for 1 min. using 20 µg of mitochondria in a buffer containing 0.3% BSA, 2 mM KCN, 0.5 mM phenazine ethosulfate, 0.025 mM DCPIP, 0.08% sodium cholate, and 100 mM Tris at pH 7.6 with 20 µg of mitochondria [29]. The reaction was started by adding 20 mM succinate.
Complex III (ubiquinol-Cyt c reductase) activity was measured by a modification of the method of Lesnefsky et al. [30]. Two µg of mitochondria were assayed in a buffer containing 0.1% bovine serum albumin, 0.1 mM EDTA, 60 µM oxidized Cyt c, 3 mM NaN3, 0.08% sodium cholate, and 50 mM KH2PO4 at pH 7.4. The reaction was started by the addition of 100 µM ubiquinol and the activity was measured by following the increase in the absorbance of reduced Cyt c at 550 nm every 4 s for 1 min.
Complex IV (Cyt c oxidase) activity was measured by following the decrease in the absorbance of reduced Cyt c at 550 nm every 4 s for 60 s [31]. The reaction was started by adding 2 µg of the mitochondria into a buffer containing 0.1 mM EDTA, 30 µM reduced Cyt c, 0.015% asolectin, 0.08% sodium cholate, and 50 mM KH2PO4 at pH 7.4 after a 3 min. preequilibration period.
Mitochondrial oxidative phosphorylation
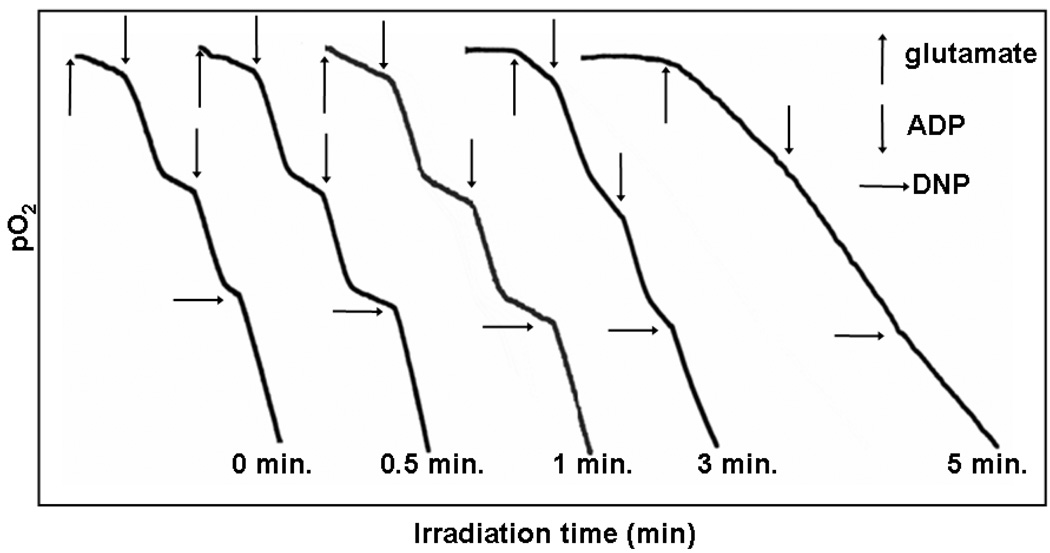
Oxygen consumption by mitochondria was measured using a Clark-type oxygen electrode at 30°C [26]. Control and illuminated mitochondria were suspended in an assay buffer containing 80 mM KCl, 50 mM MOPS, 1 mM ethyleneglycol tetraacetic acid, 5 mM KH2PO4, and 1 mg defatted, dialyzed BSA/mL at pH 7.4. ADP-dependent (state 3), ADP-independent (state 4) and maximal 2,4-dinitrophenol-stimulated respirations were measured with 500 µL of the mitochondrial suspension using either glutamate or succinate as substrates.
RESULTS
Cyt c release from mitochondria
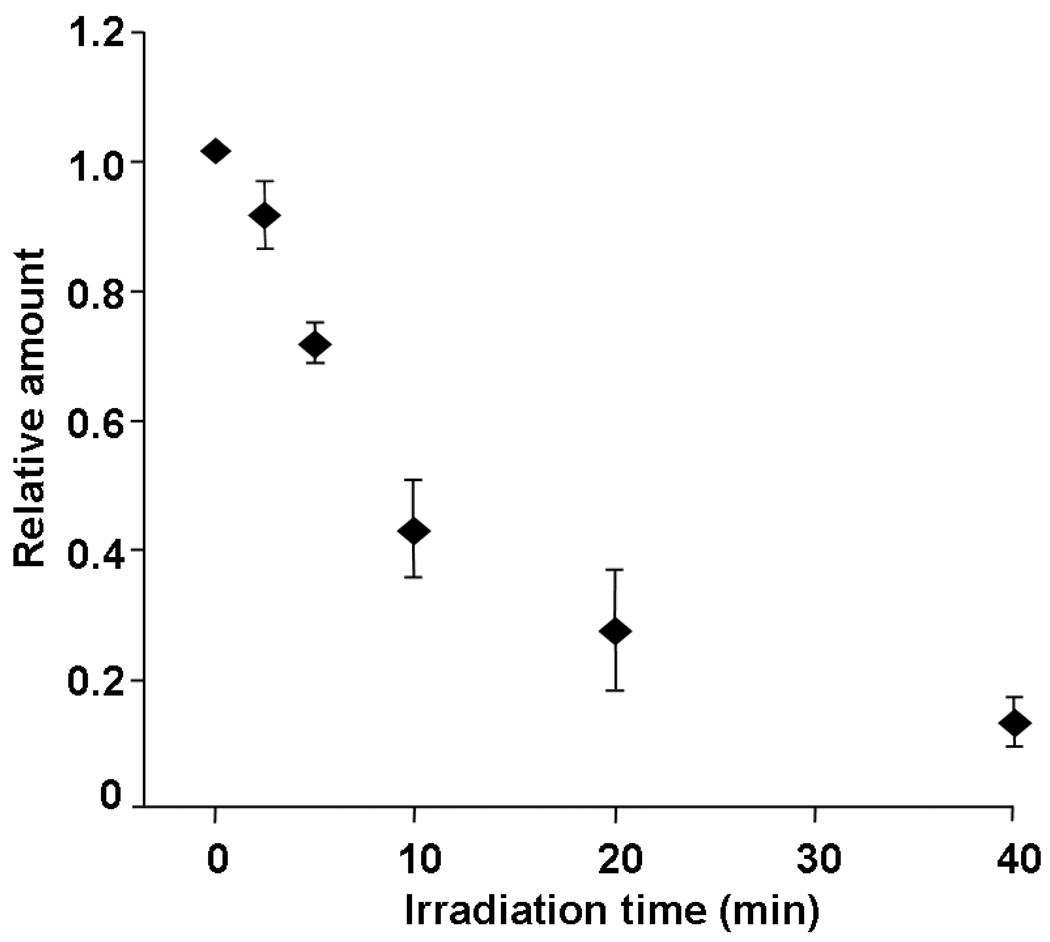
Proteins were extracted from control and illuminated mitochondrial pellets, and the amounts of Cyt c in the extracts were measured using absorbance of reduced Cyt c. The amounts of Cyt c extracted from the illuminated mitochondria were normalized to the amount extracted from the control mitochondria (Figure 1). The results show that after only 5 min. of illumination, 30% of Cyt c was released and after 40 min. ~ 90% had been released from the mitochondria. This Cyt c release upon irradiation was also confirmed by SDS-PAGE (data not shown). The release of Cyt c was found to occur mainly during irradiation because the continued release of Cyt c was negligible while mitochondria were being kept in the dark following irradiation.
Figure 1.
Relative amount of Cyt c extracted from mitochondria pellet. Cyt c was extracted from mitochondria pellet illuminated for 0 to 40 min. and quantified using absorbance. The quantity was normalized to 0 min. sample.
Effect of Pc 4 red-illumination on mitochondrial morphology
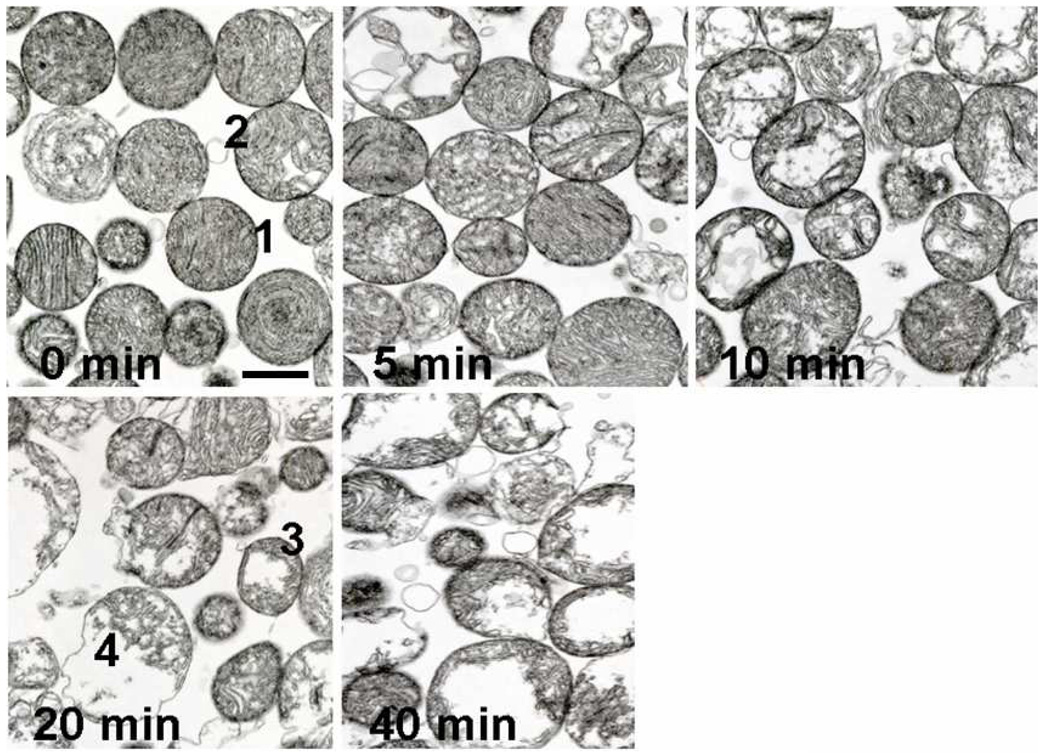
Electron micrographs of mitochondria confirm that the outer membrane is still visibly intact until over 10 min. of irradiation, even though the inner structure of the mitochondria was significantly altered at earlier times. Figure 2 shows the gradual changes in mitochondrial structure attendant to Pc 4-PDT-generated 1O2. Mitochondria began to swell and lose cristae at 5 min., and the damage became more significant at 10 min. Following 20 min. of illumination, some mitochondria lost the integrity of the inner membrane and at 40 min. a majority of the mitochondria were destroyed.
Figure 2.
Time course of mitochondrial structural changes observed by transmission electron microscopy. Isolated rat cardiac mitochondria, pretreated with Pc 4, and continuously illuminated for various times (minutes) were then prepared for electron microscopy. Control Pc 4 treated mitochondria (0). Structural changes were quantified by subdividing mitochondria into four classes with increasing disruption; typical members of each quartile are numbered 1–4 in the 0 and 20 min micrographs.
The alteration of mitochondrial morphology following 5 min. of illumination was closely examined by comparison with control mitochondria. Electron micrographs were taken from three different spots of each sample, both control and illuminated mitochondria. Individual mitochondria in these pictures were categorized into four groups from 1 to 4 with 1 being intact or minimally damaged and 4 being most severely damaged. A typical example of each group is marked in Figure 2. The number of mitochondria counted in the each field was between 65 and 76 and the percentage of mitochondria in each category is listed in Table 1. The common apparent damage occurring to these heart mitochondria during 5 min of illumination is a loss of cristae and generation of vacuoles, presumably by disruption of the mitochondrial inner structure, including alterations to the inner membrane, which may contribute to the observed complete uncoupling of respiration during the initial short illumination times.
Table 1.
Changes in mitochondrial structural integrity following 5 min. of irradiation. For this analysis mitochondria were divided into 4 classes demonstrating decreasing structural integrity as shown in Figure 2.
| time (min.) | 1 (%) | 2 (%) | 3 (%) | 4 (%) |
|---|---|---|---|---|
| 0 | 26 ± 3 | 44 ± 2 | 23 ± 4 | 6.4 ± 3 |
| 5 | 4.8 ± 2 | 58 ± 6 | 30 ± 7 | 7.3 ± 1 |
ETC complex activities
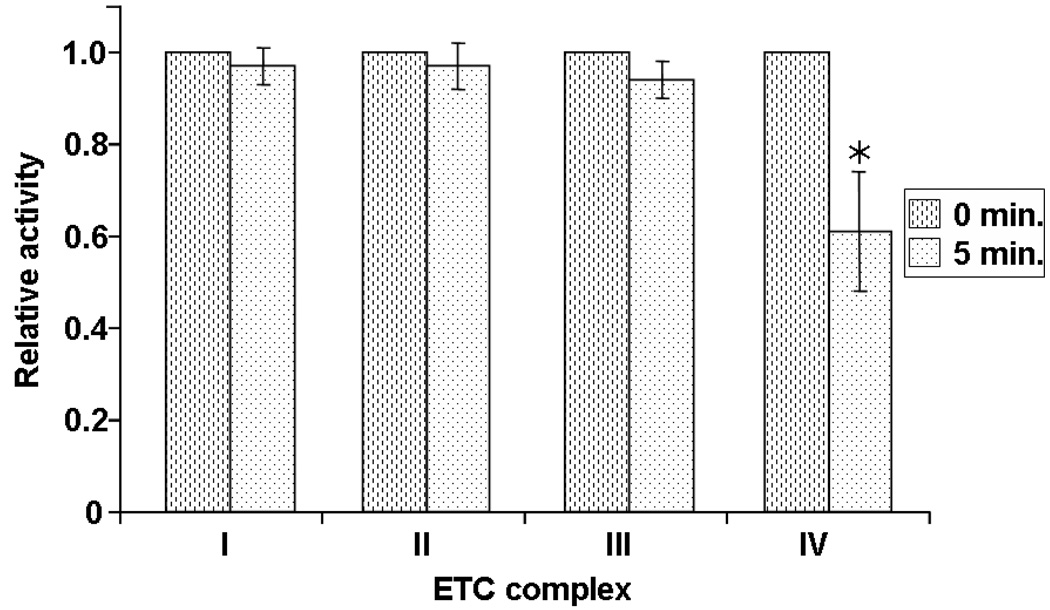
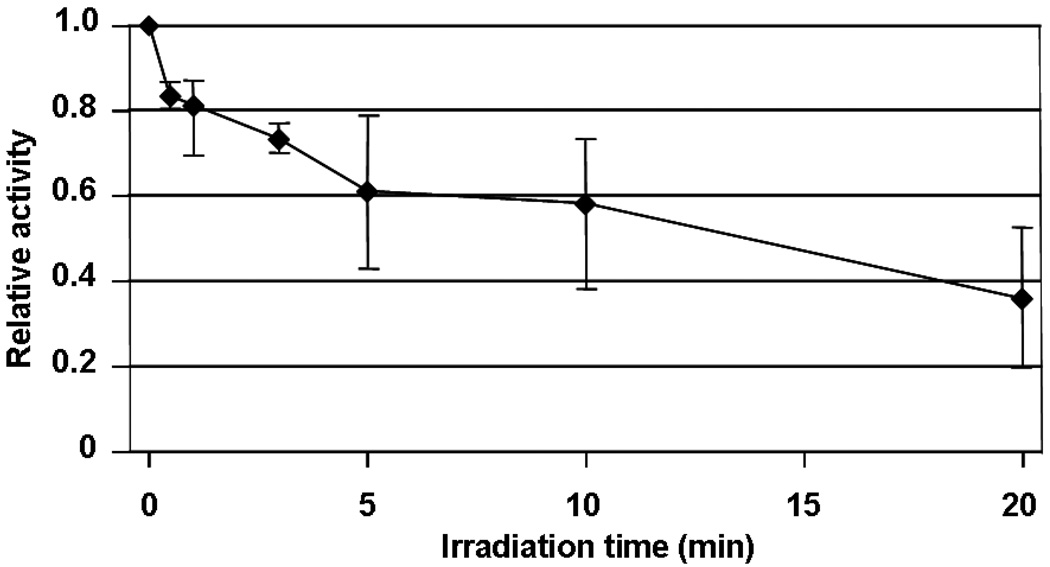
Functional damage to the individual mitochondrial ETC complexes was also studied in an attempt to identify the initial oxidative events that are induced by PDT. The activities of ETC complexes I, II, III and IV of mitochondria exposed to 1O2 for 5 min. were assayed by solubilizing the mitochondria with cholate buffers. The results showed that the individual activities of solubilized complexes I, II, and III were essentially preserved following 5 min. of photo-oxidation (Figure 3) and showed only modest losses at 20 min. In contrast, complex IV activity was severely affected; the deficit was significant (20% decrease) following as little as 30 s, and after 20 min. of photo-oxidation the activity was diminished by 65% (Figure 4). These data indicate that there is a specific Pc 4-PDT-induced alteration of complex IV in rat heart mitochondria.
Figure 3.
Activities of mitochondrial ETC complexes upon irradiation. Individual complex activities were measured in mitochondria illuminated for 5 min. in the presence of Pc 4. Complex I, II and III activities were essentially preserved for 5 min. but complex IV activity declined to 60 % of control mitochondria. *p < 0.01 compared to control mitochondria.
Figure 4.
Complex IV activity as a function of irradiation time up to 20 min.
Pc 4-PDT-induced deficits in mitochondrial oxidative phosphorylation
As shown in Table 2, following 5 min. of illumination of Pc 4-loaded mitochondria, the state 3 respiration rate decreased to ~35% of control. In contrast state 4 respiration increased by ~50%, bringing the respiratory control ratio to near unity; i.e., the mitochondria have become effectively uncoupled. Illumination for longer than 5 min. decreased both state 3 and state 4 respiration rates by the same extent. Succinate-stimulated respiration activities also showed similar results; the state 3 respiration rate decreased while the state 4 respiration increased after 3 min. (data not shown).
Table 2.
Mitochondrial respiration following 5 min. of irradiation
| min. | state 3 | state 4 | DNP | RCR |
|---|---|---|---|---|
| 0 | 136 ±9.2 | 29 ±0.7 | 178 ±30.4 | 4.7 |
| 5 | 48 ±19.1 | 45 ±18.4 | 48 ±26.9 | 1.1 |
To identify the initial changes in mitochondrial function, mitochondrial respiration with shorter illumination times was closely observed. Typical changes in respiration are shown in Figure 5. At times as short as 1 minute, state 4 respiration could be observed increasing. In this replicate, after 3 min. of illumination, state 4 respiration increased by 100% while the state 3 respiration was unchanged. After 5 min. of irradiation, both the state 4 and state 3 respiration rates started decreasing.
Figure 5.
Effect of irradiation on oxygen consumption by rat heart mitochondria. Mitochondria were preincubated with Pc 4 for 10 min then illuminated for up to 5 min. State 4 respiration was increased while state 3 respiration remained intact in short irradiation time. With longer irradiation time, both state 3 and 4 respiration rates decreased and are almost same which is a sign of completely uncoupling. Arrows indicate the times when substrates were added.
DISCUSSION
Pc 4-mediated PDT
The mechanism of Pc 4-mediated PDT in cells has focused for several years on mitochondrial effects, although few direct studies of PDT mediated damage on intact isolated mitochondria have been reported. A major advantage of the controlled time of illumination experiment is that the effective dose can be varied as the time of exposure, allowing the crucial functional deficits to be decoupled from the collateral damage that occurs at higher doses. A significant difficulty with characterizing PDT mediated photo-oxidation is comparing the effective doses in vitro, in cell culture and in vivo.
Varied oxygen concentrations in different media make it even harder to compare the effective doses of ROS generated. Oxygen is an essential component for any photo-oxidation mediated by 1O2. Not surprisingly, the change in oxygen concentration during PDT also affects the efficiency of PDT [6, 32]. For experiments using mitochondria in vitro, the concentration of mitochondria is important in order to have sufficient oxygen present during irradiation so that the quantum yield of 1O2 is not adversely affected. In concentrated suspension (over ~5 mg protein/mL) with limited surface area, it is routine that by oxidizing endogenous substrates, mitochondria consume oxygen faster than oxygen diffuses through the air-water interface into the solution, generating hypoxic conditions. The concentration used in this study (0.5 mg/mL) was chosen to minimize the rate of endogenous oxygen consumption, hence maintaining the necessary oxygen for effective PDT.
In interpreting the data from the reported experiments, we are assuming that the 5 min. exposure to the red light source produces a PDT effect at least as great as that required to induce apoptosis in cell culture and to be effective in oxygenated tissue in vivo. This assumption is based on the observations that at this time the mitochondria are effectively uncoupled, rendering them unable to generate ATP and have released a significant fraction of Cyt c to the medium. Furthermore 5 min. corresponds to the time required to induce apoptosis in cell culture using the same LED array in the presence of Pc 4, although it is recognized that the effective concentrations of Pc 4 at the critical mitochondrial sites may differ in cells vs. the present isolated mitochondrial system. With this assumption the results indicate that the primary functional deficits that we detected were the uncoupling of respiration and specific inactivation of complex IV.
Pc 4-PDT damage to the mitochondrial ETC
The mitochondrial ETC, which consists of a series of redox active protein complexes, is responsible for more than 85% of the oxygen consumed by cells [33] and mitochondrial oxidative phosphorylation generates 95% of the energy utilized by most cells [34]. ETC complexes deliver electrons to oxygen while pumping protons into the mitochondrial intermembrane space, generating a potential gradient across the inner membrane. Using this proton gradient, ATP synthase converts ADP to ATP. Damage to one or more of these components in ETC may cause malfunction of the ETC, which in turn leads to cell death.
This study showed that the mitochondrial ETC is highly sensitive to 1O2. Using Pc 4-PDT, 5 min. of illumination significantly damaged the mitochondrial respiration system resulting in a complete uncoupling and a loss of state 3 respiration by 65%. These results are similar to those recently reported for isolated rat brain mitochondria where uncoupling resulted in a decrease in the respiratory control ratio [35] and are consistent with the results obtained in vivo where PDT results in dramatic decreases in both complex IV and in ATP [23]. Uncoupling of the ETC leads to depolarization of the mitochondrion. This loss of membrane potential may result in mitophagy though a pink1-parkin mediated pathway [36]. This mitophagy is an early response to oxidative stress [32a].
It is interesting that only complex IV activity decreased following 5 min. of irradiation while the activities of the complexes I, II and III were essentially preserved. Longer irradiation decreased the activities of complex I, II and III as well, but complex IV more severely. We have shown that 1O2 generated by illumination of Pc 4 loaded mitochondria can oxidize Cyt c, and presumably other sensitive proteins as well (submitted). Whether complex IV has a higher affinity for Pc 4 and is damaged due to its proximity to the site of 1O2 generation or is inherently more reactive to 1O2 is unresolved by these experiments.
Effect of complex IV dysfunction
Complex IV is the terminal enzyme in the ETC where a metal center with His ligands accumulates four electrons to effect the complete reduction of O2 to H2O in a single reaction. The propensity of 1O2 to react with His residues makes it interesting to speculate that it is this center that is oxidized by 1O2. The inhibition of complex IV is common to many photosensitizers considered for PDT including photofrin, tetra(m-hydroxyphenyl) chlorin, chalcogenapyrylium dyes as well as phthalocyanins. The independence of inhibition on photosensitizer structure suggests that it is the localized generation of ROS, not a specific association with complex IV that is responsible for the generality of inhibition. Independent of the exact site of modification, impairment of complex IV activity will limit the electron flow causing electron storage in ETC. It has been hypothesized that the decreased activity of complex IV leads to the electron leak to surrounding oxygen to generate other ROS that will cause additional damage to the mitochondrial ETC [37]. Other studies have shown that copper toxicity induces mitochondria-mediated apoptosis by decreasing complex IV activity, which was suggested to be responsible for the structural alteration of mitochondria and Cyt c release [37, 38].
Another important aspect of the observed complex IV inactivation, is that some forms of complex IV dysfunction result directly in uncoupling; i.e. reducing oxygen to water without concomitant proton pumping. This malfunction is not a characteristic of complex I or complex III [39]. Mutations of the side chain of certain amino acids at the active site or along the proton channels of complex IV have been shown to induce uncoupling [40–42]. In most cases, the uncoupling involved electron transport activity loss, but mutation of N131D in subunit 1 showed uncoupling with preserved O2 reduction activity [42]. The increased state 4 rate found following 3 min. of irradiation in our study may be attributable to a similar alteration of complex IV activities. Damage responsible for the loss of complex IV activity (~30% after 3 min. of illumination) also may have initiated the uncoupling found at this time.
However the extent of complex IV activity loss found in this study seems not to be enough to cause the initial state 3 respiration loss observed after 5 min. The faster uncoupled respiration rate indicates that complex IV activity would be sufficient to support faster state 3 respiration. Additionally complex IV does not usually limit state 3 respiration, e.g. 50% inhibition of complex IV activity causes only a 10 % decrease of state 3 respiration [43]. Accompanied with the complex IV activity loss and Cyt c release, alteration of mitochondrial inner structure observed by EM may also have some effect on the reduced state 3 respiration rate. The role of the organization of the ETC complexes within the cristae has drawn attention recently [44] and the changes observed in the mitochondrial organization may have a significant effect on respiration rates without altering the individual rates measured in the assays in the presence of homogenizing detergent.
Oxidation of CL and Cyt c in Mitochondrial ETC
CL oxidation may be responsible for the structural and functional damage found in this study. Yamaok et al. showed that mitochondria with decreased CL had a lower state 3 respiration rate [45]. It is also reported that CL is essential to maintain complex IV activity [46]. Complex IV activity defects can be produced both by oxidation and depletion of CL [47, 48]. Because CL’s constituent fatty acids are unsaturated, it is readily oxidized by 1O2. Using the same condition for mitochondria photo-irradiation in the presence of Pc 4, 20 min. of irradiation oxidized about 8 % of total CL content in mitochondria as determined by mass spectrometry (companion manuscript). Because oxidized side chains of phospholipids may be extruded from the bilayer [49], CL oxidation could result in rearrangements in the phospholipid bilayer which would allow H+ diffusion across the membrane, providing a mechanism of uncoupling mitochondria.
Cyt c is also a target of 1O2 and provides unique oxidized structures only observed following generation of 1O2 [50]. But mitochondrial release of Cyt c is found to be more readily detected than oxidation of Cyt c in Pc 4-PDT-induced mitochondrial oxidation (submitted), suggesting that oxidation of Cyt c is not responsible for the mitochondrial functional damage observed in this study.
Cyt c release
Following 5 min of illumination, mitochondria released about 30% of Cyt c. Most of this Cyt c has not been oxidized. This early loss of Cyt c does not adversely affect the uncoupled rate of respiration suggesting that this loss is not the primary cause for the mitochondrial functional damage observed in this short time of illumination. The Cyt c release however is presumed to be an important event in PDT-induced apoptosis [51]. However, oxidation of CL found at short irradiation times may play an important role in Cyt c release as suggested elsewhere [52, 53].
The detailed mechanism of Cyt c release is still not fully understood. However, the apparent intact outer membrane observed in the electron micrographs suggests that the release is not attributable to the physical destruction of mitochondrial outer membranes that was observed only following longer periods of 1O2 exposure. Two speculative mechanisms would explain the rapid release followed by minimal additional leakage. First that there is a threshold for damage after which all of the Cyt c in an individual mitochondrion is rapidly released as observed by Goldstein et al [54] or alternatively, that the mechanism for release is activated during the illumination but is rapidly repaired in the dark. In either case, release relies on the presence of Bax which is present on our isolated rat heart mitochondria (unpublished).
SUMMARY
The earliest event induced by illumination of Pc 4-loaded mitochondria that these studies detected was a decrease in state 3 respiration concomitant with a complete uncoupling of respiration. Additionally structural changes to some of the mitochondria could be detected by EM as well as a modest release of Cyt c. However the molecular causes of the mitochondrial uncoupling along with specificity of Complex IV inactivation require additional study. With longer irradiation times, increased Cyt c release, alteration of the mitochondrial structure and nearly complete complex IV activity losses were observed.
ACKNOWLEDGEMENT
This work was supported by NIH/NIA grant P01 AI55739-01 to V.E.A and by NIH/NCI grant R01 CA106491 to N.L.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: BSA, bovine serum albumin; CL, cardiolipin; Cyt c, cytochrome c; DCPIP, 2,6-dichloroindophenol; DUQ, decylubiquinone; ER, endoplasmic reticulum; ETC, electron transport chain; MOPS, (N-morpholino)propanesulfonic acid; PDT, photodynamic therapy; ROS, reactive oxygen species
REFERENCES
- 1.Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochem Photobiol Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 2.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 3.Miyamoto S, Martinez GR, Medeiros MH, Di Mascio P. Singlet molecular oxygen generated from lipid hydroperoxides by the russell mechanism: studies using 18(O)-labeled linoleic acid hydroperoxide and monomol light emission measurements. J Am Chem Soc. 2003;125:6172–6179. doi: 10.1021/ja029115o. [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 5.Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usuda J, Chiu SM, Azizuddin K, Xue LY, Lam M, Nieminen AL, Oleinick NL. Promotion of photodynamic therapy-induced apoptosis by the mitochondrial protein Smac/DIABLO: dependence on Bax. Photochem Photobiol. 2002;76:217–223. doi: 10.1562/0031-8655(2002)076<0217:poptia>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney ME, Samia AC, Burda C, Oleinick NL. Fluorescence resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Res. 2003;63:5194–5197. [PubMed] [Google Scholar]
- 9.Oleinick NL, Antunez AR, Clay ME, Rihter BD, Kenney ME. New phthalocyanine photosensitizers for photodynamic therapy. Photochem Photobiol. 1993;57:242–247. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 10.Ke MS, Xue LY, Feyes DK, Azizuddin K, Baron ED, McCormick TS, Mukhtar H, Panneerselvam A, Schluchter MD, Cooper KD, Oleinick NL, Stevens SR. Apoptosis mechanisms related to the increased sensitivity of Jurkat T-cells vs A431 epidermoid cells to photodynamic therapy with the phthalocyanine Pc 4. Photochem Photobiol. 2008;84:407–414. doi: 10.1111/j.1751-1097.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez ME, Zhang P, Azizuddin K, Delos Santos GB, Chiu SM, Xue LY, Berlin JC, Peng X, Wu H, Lam M, Nieminen AL, Kenney ME, Oleinick NL. Structural factors and mechanisms underlying the improved photodynamic cell killing with silicon phthalocyanine photosensitizers directed to lysosomes versus mitochondria. Photochem Photobiol. 2009;85:1189–1200. doi: 10.1111/j.1751-1097.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:S146–S156. [PubMed] [Google Scholar]
- 13.Kessel D, Luo Y. Photodynamic therapy: a mitochondrial inducer of apoptosis. Cell Death Differ. 1999;6:28–35. doi: 10.1038/sj.cdd.4400446. [DOI] [PubMed] [Google Scholar]
- 14.Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379–47386. doi: 10.1074/jbc.M107678200. [DOI] [PubMed] [Google Scholar]
- 15.Chiu SM, Oleinick NL. Dissociation of mitochondrial depolarization from cytochrome c release during apoptosis induced by photodynamic therapy. Br J Cancer. 2001;84:1099–1106. doi: 10.1054/bjoc.2000.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modica-Napolitano JS, Joyal JL, Ara G, Oseroff AR, Aprille JR. Mitochondrial toxicity of cationic photosensitizers for photochemotherapy. Cancer Res. 1990;50:7876–7881. [PubMed] [Google Scholar]
- 17.Salet C, Moreno G, Ricchelli F. Effects of photodynamic action on respiration in nonphosphorylating mitochondria. Arch Biochem Biophys. 1998;358:257–263. doi: 10.1006/abbi.1998.0863. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee SR, Possel H, Srivastava TS, Kamat JP, Wolf G, Devasagayam TP. Photodynamic effects induced by meso-tetrakis[4-(carboxymethyleneoxy)phenyl] porphyrin on isolated Sarcoma 180 ascites mitochondria. J Photochem Photobiol B. 1999;50:79–87. doi: 10.1016/S1011-1344(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 19.Gibson SL, Hilf R. Photosensitization of mitochondrial cytochrome c oxidase by hematoporphyrin derivative and related porphyrins in vitro and in vivo. Cancer Res. 1983;43:4191–4197. [PubMed] [Google Scholar]
- 20.Detty MR, Merkel PB, Hilf R, Gibson SL, Powers SK. Chalcogenapyrylium dyes as photochemotherapeutic agents. 2. Tumor uptake, mitochondrial targeting, and singlet-oxygen-induced inhibition of cytochrome c oxidase. J Med Chem. 1990;33:1108–1116. doi: 10.1021/jm00166a005. [DOI] [PubMed] [Google Scholar]
- 21.Cosso RG, Turim J, Nantes IL, Almeida AM, Di Mascio P, Verces AE. Mitochondrial permeability transition induced by chemically generated singlet oxygen. J Bioenerg Biomembr. 2002;34:157–163. doi: 10.1023/a:1016075218162. [DOI] [PubMed] [Google Scholar]
- 22.Hilf R. Mitochondria are targets of photodynamic therapy. J Bioenerg Biomembr. 2007;39:85–89. doi: 10.1007/s10863-006-9064-8. [DOI] [PubMed] [Google Scholar]
- 23.Ceckler TL, Gibson SL, Kennedy SD, Hilf R, Bryant RG. Heterogeneous tumour response to photodynamic therapy assessed by in vivo localised 31P NMR spectroscopy. Br J Cancer. 1991;63:916–922. doi: 10.1038/bjc.1991.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- 25.Scarlett JL, Murphy MP. Release of apoptogenic proteins from the mitochondrial intermembrane space during the mitochondrial permeability transition. FEBS Lett. 1997;418:282–286. doi: 10.1016/s0014-5793(97)01391-4. [DOI] [PubMed] [Google Scholar]
- 26.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 27.Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electronic microscope. J. Cell Biol. 1965;27:137A. (Abstract). [Google Scholar]
- 28.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 29.Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987;80:71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 31.Kayser EB, Sedensky MM, Morgan PG, Hoppel CL. Mitochondrial oxidative phosphorylation is defective in the long-lived mutant clk-1. J Biol Chem. 2004;279:54479–54486. doi: 10.1074/jbc.M403066200. [DOI] [PubMed] [Google Scholar]
- 32.Wang HW, Putt ME, Emanuele MJ, Shin DB, Glatstein E, Yodh AG, Busch TM. Treatment-induced changes in tumor oxygenation predict photodynamic therapy outcome. Cancer Res. 2004;64:7553–7561. doi: 10.1158/0008-5472.CAN-03-3632. [DOI] [PubMed] [Google Scholar]
- 33.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial Dysfunction and Psychiatric Disorders. Neurochem Res. 2008 doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- 35.Medina WS, dos Santos NA, Curti C, Tedesco AC, dos Santos AC. Effects of zinc phthalocyanine tetrasulfonate-based photodynamic therapy on rat brain isolated mitochondria. Chem Biol Interact. 2009;179:402–406. doi: 10.1016/j.cbi.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi L, Lombardo MF, Ciriolo MR, Rotilio G. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem Res. 2004;29:493–504. doi: 10.1023/b:nere.0000014820.99232.8a. [DOI] [PubMed] [Google Scholar]
- 38.Rossi L, De Martino A, Marchese E, Piccirilli S, Rotilio G, Ciriolo MR. Neurodegeneration in the animal model of Menkes' disease involves Bcl-2-linked apoptosis. Neuroscience. 2001;103:181–188. doi: 10.1016/s0306-4522(00)00562-5. [DOI] [PubMed] [Google Scholar]
- 39.Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 40.Murphy MP. Slip and leak in mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 1989;977:123–141. doi: 10.1016/s0005-2728(89)80063-5. [DOI] [PubMed] [Google Scholar]
- 41.Lee HM, Das TK, Rousseau DL, Mills D, Ferguson-Miller S, Gennis RB. Mutations in the putative H-channel in the cytochrome c oxidase from Rhodobacter sphaeroides show that this channel is not important for proton conduction but reveal modulation of the properties of heme a. Biochemistry. 2000;39:2989–2996. doi: 10.1021/bi9924821. [DOI] [PubMed] [Google Scholar]
- 42.Pfitzner U, Hoffmeier K, Harrenga A, Kannt A, Michel H, Bamberg E, Richter OM, Ludwig B. Tracing the D-pathway in reconstituted site-directed mutants of cytochrome c oxidase from Paracoccus denitrificans. Biochemistry. 2000;39:6756–6762. doi: 10.1021/bi992235x. [DOI] [PubMed] [Google Scholar]
- 43.Lucas DT, Szweda LI. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc Natl Acad Sci U S A. 1999;96:6689–6693. doi: 10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stuart RA. Supercomplex organization of the oxidative phosphorylation enzymes in yeast mitochondria. J Bioenerg Biomembr. 2008;40:411–417. doi: 10.1007/s10863-008-9168-4. [DOI] [PubMed] [Google Scholar]
- 45.Yamaoka S, Urade R, Kito M. Cardiolipin molecular species in rat heart mitochondria are sensitive to essential fatty acid-deficient dietary lipids. J Nutr. 1990;120:415–421. doi: 10.1093/jn/120.5.415. [DOI] [PubMed] [Google Scholar]
- 46.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Serena D, Ruggiero FM. Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic Biol Med. 1999;27:42–50. doi: 10.1016/s0891-5849(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 47.Yamaoka-Koseki S, Urade R, Kito M. Cardiolipins from rats fed different dietary lipids affect bovine heart cytochrome c oxidase activity. J Nutr. 1991;121:956–958. doi: 10.1093/jn/121.7.956. [DOI] [PubMed] [Google Scholar]
- 48.Musatov A. Contribution of peroxidized cardiolipin to inactivation of bovine heart cytochrome c oxidase. Free Radic Biol Med. 2006;41:238–246. doi: 10.1016/j.freeradbiomed.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Witting LA. Lipid peroxidation in vivo. J Am Oil Chem Soc. 1965;42:908–913. doi: 10.1007/BF02632443. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Rodriguez ME, Guo M, Kenney ME, Oleinick NL, Anderson VE. Oxidative modification of cytochrome c by singlet oxygen. Free Radic Biol Med. 2008;44:1700–1711. doi: 10.1016/j.freeradbiomed.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agostinis P, Buytaert E, Breyssens H, Hendrickx N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem Photobiol Sci. 2004;3:721–729. doi: 10.1039/b315237e. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalvez F, Bessoule JJ, Rocchiccioli F, Manon S, Petit PX. Role of cardiolipin on tBid and tBid/Bax synergistic effects on yeast mitochondria. Cell Death Differ. 2005;12:659–667. doi: 10.1038/sj.cdd.4401585. [DOI] [PubMed] [Google Scholar]
- 53.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]