Abstract
The major product of oxidative base damage is 8-oxo-7,8-dihydro-2′-deoxyguanine (8odG). The coding potential of this lesion is modulated by its glycosidic torsion angle that controls whether its Watson-Crick or Hoogsteen edge is utilized for base pairing. The 2.0 Å structure of DNA polymerase (pol) β bound with 8odGTP opposite template adenine indicates that the modified nucleotide assumes the mutagenic syn-conformation and that the non-mutagenic anti-conformation would be incompatible with efficient DNA synthesis.
In addition to DNA, DNA precursors (nucleotide pools) are also susceptible to oxidative damage from reactive oxygen species. It is generally believed that this genotoxic assault contributes to mutagenesis, carcinogenesis, and aging1. The major product of oxidative base damage is the highly mutagenic lesion 8odG. Unmodified deoxyguanine preferentially assumes an anti-conformation that readily forms a Watson-Crick base pair. In contrast, 8odG prefers a mutagenic syn-conformation that base pairs with adenine2,3 (Supplementary Fig. 1). This lesion is removed from DNA by the base excision repair pathway.
DNA polymerases are often confronted with modified substrates. Cells have developed a surveillance system that hydrolyzes 8odGTP to the monophosphate form4. DNA polymerases generally prefer to insert 8odGTP opposite adenine (Supplementary Table 1). This is primarily due to a strong discrimination against insertion opposite cytosine and an enhanced insertion opposite adenine (relative to dGTP). Left unrepaired, 8odGTP misinserted opposite adenine can result in an A to C transversion. Polβ is an X-family polymerase with a primary role in gap-filling DNA synthesis during base excision repair5.
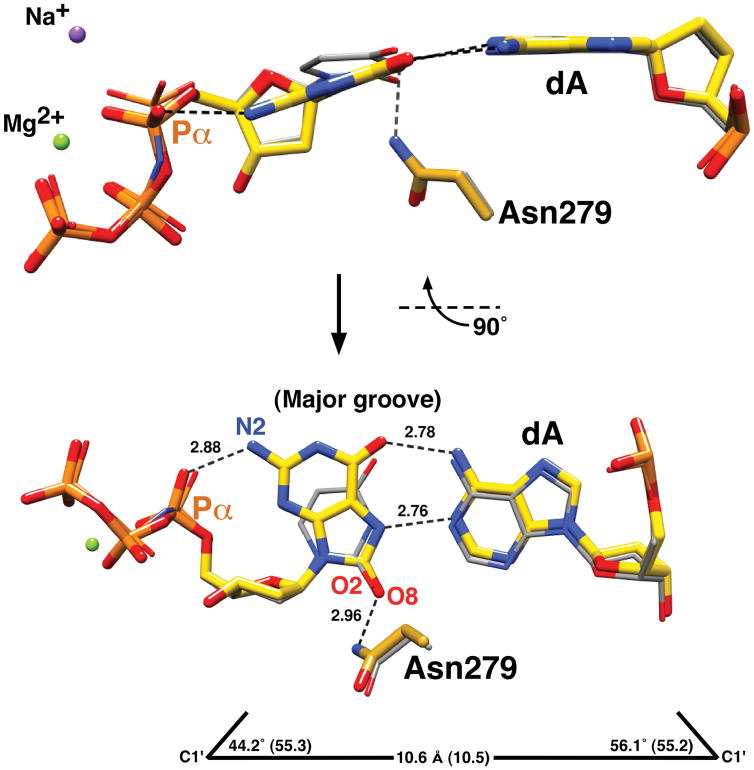
We determined the crystallographic ternary complex structure of human pol β to 2.00 Å with an incoming 8odGTP paired with a templating adenine (Supplementary Table 2 and Supplementary Methods). The structure reveals that the polymerase assumes a closed conformation typically observed with a Watson-Crick nascent base pair (Supplementary Fig. 2). In contrast to a staggered arrangement of bases with previously reported structures of pol β with active site mismatches6,7, the 8odGTP—dA mispair is planar (Fig. 1). This planar arrangement is accommodated because 8odGTP assumes the syn-conformation while dA remains in the anti-conformation so that the geometry is similar to that of a Watson-Crick base pair (Fig. 1). The density for the oxygen at C8 is clearly visible (Supplementary Fig. 3). The syn-conformation is stabilized through Hoogsteen hydrogen bonding with the templating adenine and a hydrogen bond with Asn279. Alanine substitution at residue 279 has been shown to interfere with 8odGTP insertion opposite adenine consistent with destabilizing the syn-conformation8. With a Watson-Crick base pair in the active site, Asn279 is a hydrogen bond donor to O2 of pyrimidines or N3 of purines. As has been noted previously3, syn-8odG positions O8 in the DNA minor groove in a similar position as O2 for a Watson-Crick base pair. Additionally, an intra-molecular hydrogen bond between N2 and a non-bridging oxygen on Pα (pro-SP) of 8odGTP could stabilize the syn-conformer (Fig. 1). Such an intra-molecular stabilizing interaction was postulated to occur when a templating 8odG was modeled in the syn-conformation in the pol β active site9.
Figure 1.
Syn-conformation of 8odGTP paired with adenine. The structure of the substrate complex of pol β with a correct incoming nucleotide (dUMPNPP; PDB ID 2FMQ, gray carbons) was superimposed with the structure with an incoming 8odGTP paired with adenine (yellow carbons). Two views of the nascent base pair binding pocket illustrate the syn-conformation (χ = 51°) of 8odGTP forming a Hoogsteen base pair with adenine (dA). The geometry (C1′ distance and λ angles) of the mismatched base pair is shown at the bottom (values for a correct nascent base pair are in parentheses). The purple (Na+) and green (Mg2+) spheres represent modeled ions in the catalytic and nucleotide binding sites of the mismatched structure, respectively.
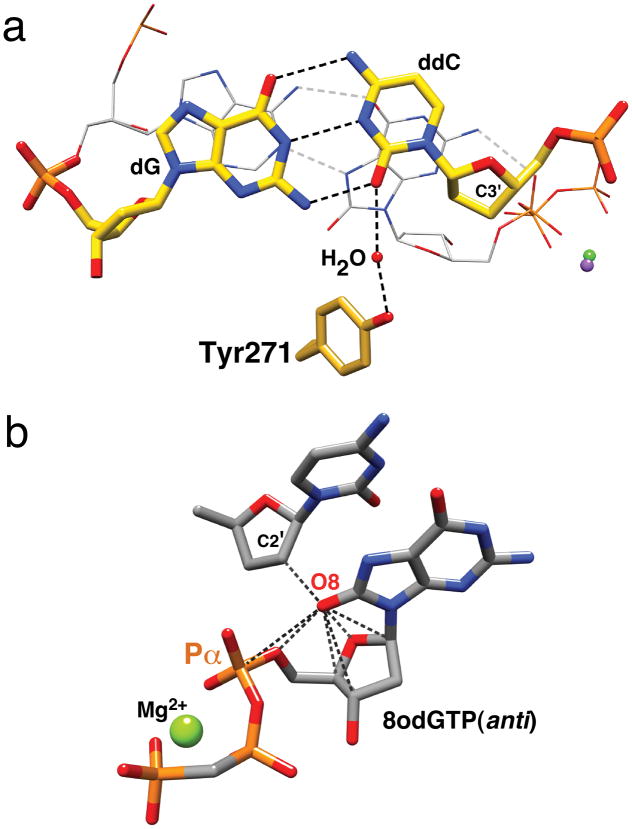
Although the nascent 8odGTP—dA base pair exhibits good geometry and minor groove hydrogen bonding, kinetic analysis indicates that insertion efficiency is reduced considerably relative to that observed for formation of a Watson-Crick base pair (Supplementary Table 1). The overall structure of the single-nucleotide gapped DNA is very similar to that observed with a correct nascent base pair (dUMPNPP—dA) (Supplementary Fig. 4). However, the primer terminus has moved toward the major groove thereby displacing C3′ away from Pα of the incoming 8odGTP (Fig. 2a). With a correct incoming nucleotide, the minor groove edge of the primer terminus (O2 of dCMP) is hydrogen bonded to Tyr271 (Supplementary Fig. 5). With an incoming 8odGTP, the altered primer terminus results in a loss of a direct interaction with Tyr271. A water molecule intervenes and bridges O2 (ddCMP) and Tyr271(OH). The primer terminus (C3′) is 4.5 Å from Pα of 8odGTP compared to 3.8 Å expected for a correct insertion10. Likewise, the altered geometry of the primer O3′ attack on Pα would reduce insertion.
Figure 2.
Structural features that discourage insertion of 8odGTP. (a) Although the 8odGTP(syn)—dA(anti) nascent base pair (gray carbons, wire representation) displays good geometry, the primer terminus base pair (yellow carbons) is displaced into the major groove in an attempt to maximize stacking interactions with 8odGTP(syn). This results in a loss of a direct hydrogen bond with Tyr271. Accordingly, the geometry between C3′ and Pα (8odGTP) is distorted. (b) Modeling a carbonyl at C8 of an incoming dGMPPCP(anti) paired with cytosine (PDB ID 2ISP) suggests that steric repulsion between the sugar-phosphate backbone and the sugar (C2′) of the primer terminus (dashed lines; distances tabulated in Supplementary Table 3) would reduce correct insertion of 8odGTP.
The efficiency of 8odGTP insertion opposite cytosine is approximately 11–25-fold lower than opposite adenine8,11. This is consistent with the inability to crystallize a complex with 8odGTP paired with a templating cytosine. Using a crystallographic structure of pol β with a dGTP analogue paired with cytosine (PDB ID 2ISP)12, a carbonyl was added to C8 to provide insight to the structural repercussions. The model of 8odGTP(anti) paired with cytosine suggests that steric repulsion between O8 and its deoxyribose-phosphate would distort the active site conformation (Fig. 2b). It has long been recognized that 8odG prefers the syn-conformation due to steric repulsion with the deoxyribose backbone that the anti-conformation could impose13,14. Additionally, crystallographic structures of DNA polymerases with 8odG as the templating nucleotide indicate that the deoxyribose phosphate backbone conformation can modulate the glycosidic preference of the modified template base7,15,16. Whereas pol β can modify the backbone position of a templating 8odG for insertion of dCTP without appreciable loss of insertion efficiency, it is not surprising that repositioning or distorting Pα of an incoming 8odGTP in an anti-conformation would result in a dramatic loss of insertion. Since the conformation of the incoming nucleotide for most DNA polymerases where structures are available are very similar17, most DNA polymerases would be expected to discriminate against 8odGTP(anti) as highlighted by the preferential insertion of this analog opposite adenine (Supplemental Table 1).
Supplementary Material
Acknowledgments
This research was supported by Research Project Number Z01-ES050158 in the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences and was in association with the National Institutes of Health Grant 1U19CA105010.
Footnotes
Accession codes. Protein Data Bank: Coordinates and structure factors for the pol β-DNA-8odGTP complex have been deposited with accession code 3MBY.
Author contributions. E.W.H. and R.P. expressed and purified protein. L.C.P. contributed to structure refinement. V.K.B. prepared, crystallized, and solved the structure of the 8odGTP–dA pol β complex. V.K.B., W.A.B., and S.H.W. analyzed data and wrote the manuscript.
References
- 1.Ames BN, Gold LS. Mutat Res. 1991;250:3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 2.Kouchakdjian M, et al. Biochemistry. 1991;30:1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- 3.McAuley-Hecht KE, et al. Biochemistry. 1994;33:10266–10270. doi: 10.1021/bi00200a006. [DOI] [PubMed] [Google Scholar]
- 4.Maki H, Sekiguchi M. Nature. 1992;355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 5.Beard WA, Wilson SH. Chem Rev. 2006;106:361–382. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- 6.Batra VK, Beard WA, Shock DD, Pedersen LC, Wilson SH. Mol Cell. 2008;30:315–324. doi: 10.1016/j.molcel.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krahn JM, Beard WA, Wilson SH. Structure (Camb) 2004;12:1823–1832. doi: 10.1016/j.str.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Miller H, Prasad R, Wilson SH, Johnson F, Grollman AP. Biochemistry. 2000;39:1029–1033. doi: 10.1021/bi991789x. [DOI] [PubMed] [Google Scholar]
- 9.Krahn JM, Beard WA, Miller H, Grollman AP, Wilson SH. Structure (Camb) 2003;11:121–127. doi: 10.1016/s0969-2126(02)00930-9. [DOI] [PubMed] [Google Scholar]
- 10.Batra VK, et al. Structure (Camb) 2006;14:757–766. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JA, Duym WW, Fowler JD, Suo Z. J Mol Biol. 2007;367:1258–1269. doi: 10.1016/j.jmb.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 12.Sucato CA, et al. Biochemistry. 2007;46:461–471. doi: 10.1021/bi061517b. [DOI] [PubMed] [Google Scholar]
- 13.Culp SJ, Cho BP, Kadlubar FF, Evans FE. Chem Res Toxicol. 1989;2:416–422. doi: 10.1021/tx00012a010. [DOI] [PubMed] [Google Scholar]
- 14.Uesugi S, Ikehara M. J Am Chem Soc. 1977;99:3250–3253. doi: 10.1021/ja00452a008. [DOI] [PubMed] [Google Scholar]
- 15.Hsu GW, Ober M, Carell T, Beese LS. Nature. 2004;431:217–221. doi: 10.1038/nature02908. [DOI] [PubMed] [Google Scholar]
- 16.Rechkoblit O, et al. PLoS Biol. 2006;4:e11. doi: 10.1371/journal.pbio.0040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beard WA, Wilson SH. Structure (Camb) 2003;11:489–496. doi: 10.1016/s0969-2126(03)00051-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.