Abstract
Although the exact mechanism that makes bupropion hydrochloride (ZybanR) effective as a smoking cessation aid has not been fully elucidated, studies have found that bupropion and nicotine share behavioral and neurophysiological properties suggesting that bupropion might serve as a substitute for nicotine. In fact, bupropion prompts nicotine-appropriate responding in operant and Pavlovian drug discrimination studies with rats. A majority of the literature examining this substitution pattern has been done with an operant paradigm. The present research extended this literature by further characterizing the behavioral and neuropharmacological properties underlying the substitution for a nicotine conditioned stimulus (CS). Examination of the dose-effect function and temporal dynamics of this substitution pattern showed that bupropion (20 mg/kg) produced conditioned responding similar to nicotine (0.4 mg base/kg) (ED50=9.9 mg/kg) at 15 and 30 min after injection and partially substituted 5 and 60 min post-injection. Bupropion produced a pattern of conditioned responding similar to nicotine during a 60-min extinction test. Additionally, it has been hypothesized that bupropion and nicotine have an overlapping dopaminergic mechanism. We tested the effects of bupropion pretreatment the nicotine dose-effect function and the ability of dopamine antagonist to block the substitution of bupropion for nicotine. Pretreatment with doses of bupropion that did not substitute for the nicotine stimulus (5 and 10 mg/kg) did not effect nicotine conditioned responding; pretreatment with 20 mg/kg attenuated nicotine-evoked responding. Pretreatment with the dopamine antagonists SCH-23390 and eticlopride blocked the substitution. Finally, S,S-hydroxybupropion, the major metabolite of bupropion in humans, did not substitute for the nicotine CS.
Keywords: conditioned stimulus, discriminative stimulus, dopamine, drug discrimination, nicotinic acetylcholine receptors, hydroxybupropion, Pavlovian conditioning, Zyban
Introduction
Smoking is the number one preventable cause of premature death in the United States with approximately 440,000 dying each year (CDC, 2006). More than $75 billion in annual medical costs are directly attributed to smoking. In spite of these facts, 70.3 million people in the US reported smoking in the past month (NIDA, 2006). Most smokers (70%) express a desire to quit (CDC, 2006), but most relapse within the first few months (NIDA, 2006). There are several behavioral and pharmacological interventions that improve cessation rates. Nicotine replacement therapies, such as gum or the patch, provide small doses of nicotine, the addictive component in tobacco, to alleviate withdrawal symptoms (NIDA, 2006). Bupropion (ZybanR) and varenicline (ChantixR) are the only non-nicotine pharmacotherapies approved by the Federal Drug Administration for smoking cessation. Both drugs decrease cigarette cravings and increase the rate of quitting compared to placebo (Hurt et al., 1997; Swan et al., 2003; Gonzales et al., 2006; Jorneby et al., 2006). The effectiveness of varenicline purportedly reflects its action as a partial agonist for α4β2 containing (α4β2*) nicotinic acetylcholine receptors (nAChR) (Mihalak et al., 2006); however, the mechanism that makes bupropion an effective smoking cessation aid is still not well understood [see Dwoskin et al. (2006) for a review].
Although bupropion was first marketed as the antidepressant WellbutrinR, its behavioral and neurophysiological properties seem more similar to psychomotor stimulants. For example, in rodents acute administration of bupropion produces dose-dependent increases in locomotor activity (Cooper et al., 1980; Ortmann, 1985; Nomikos et al., 1992; Wilkinson et al., 2006; Wilkinson and Bevins, 2007) that is enhanced with repeated administration (Nomikos et al., 1992). Similar to other psychomotor stimulants, bupropion produces a conditioned place preference in rats (Ortmann, 1985) and is self-administered by rats (Tella et al., 1997) and primates (Lamb and Griffiths, 1990). Bupropion also produces increases in extracellular dopamine in the nucleus accumbens shell (Nomikos et al., 1992), an effect shared with cocaine, amphetamine, and nicotine (Carboni et al., 1989; Nisell et al., 1997).
Along with these rewarding and locomotor activating effects, stimulant drugs also have subjective (interoceptive) effects that contribute to the addiction processes. In operant and Pavlovian discrimination tasks designed to study these subjective effects, rats can be trained to discriminate nicotine from saline. Of interest to the present report is the finding that bupropion shares interoceptive stimulus properties with nicotine as evidenced by its ability to prompt nicotine-appropriate responding in operant discrimination tasks (Wiley et al., 2002; Young and Glennon, 2002). Notably, there is very limited literature about the ability of bupropion to substitute for nicotine in a Pavlovian discrimination task. Besheer et al. (2004) showed that bupropion substitutes for a nicotine conditioned stimulus (CS) in an appetitive Pavlovian discrimination task, but this substitution pattern has not been further explored.
Investigation of Pavlovian learning processes underlying smoking behavior has lead to a greater understanding of some key features of smoking behavior (Bevins and Palmatier, 2004; Conklin and Tiffany, 2002). Traditionally in these paradigms, nicotine is considered the unconditioned stimulus (US) which is paired with stimuli such as lighters, ashtrays, odor of smoke). After repeated pairings, these CSs come to evoke a conditioned response (CR). Previous studies have also shown that nicotine can also serve as a CS. For example, when nicotine is repeatedly paired with availability of sucrose, it comes to evoke an anticipatory food-seeking CR (Besheer et al., 2004; Wilkinson et al., 2006; Bevins et al., 2007; Murray and Bevins 2007a, 2007b). Further elucidation of the role of nicotine as both a US and CS in Pavlovian conditioning paradigms is important for fully understanding the variety of factors which influence nicotine addiction (Bevins and Palmatier, 2004). It is possible that the processes underlying nicotine’s ability to function as a CS are somewhat different from those underlying the discriminative stimulus properties (Murray and Bevins, 2007b).
The goal of the present study was to extend the findings of bupropion substitution patterns for a nicotine cue to a Pavlovian conditioning paradigm. In doing so, we also sought to extend literature by examining properties of bupropion substitution for nicotine that have not previously been examined. The temporal dynamics of bupropion substitution for a nicotine CS have not been examined. Drug states can be conceptualized as complex multimodal stimuli in which its’ constitute elements are the perceptible neurobiological effects of the drug at any given moment in time (cf. Balster, 1988). Given that such neurobiological effects change across time via pharmacokinetic and pharmacodynamic processes, a better understanding of these changes in the stimulus properties of bupropion across time will be important. Accordingly, two different testing procedures were utilized to fill this gap in our knowledge. First we assessed bupropion substitution for nicotine after different injection-to-placement intervals using brief tests that are standard in drug discrimination research. Second, we examined bupropion substitution for nicotine across a 60-min session. Because this test was conducted without sucrose, it provided us with an opportunity to determine whether 60 min of extinction with bupropion was similar to receiving extinction with nicotine. Substitution of extinction learning across drug stimuli has been investigated.
Previous reports have found that dopamine agonists substitute and dopaminergic antagonists block the discriminative stimulus properties of bupropion when rats are trained to discriminate bupropion from saline in an operant discrimination task (Spealman and Bergman, 1988; Terry and Katz, 1997). Although several reports have suggested that bupropion substitution for nicotine might be also mediated through this dopaminergic mechanism (Wiley et al., 2002; Rauhut et al., 2003; Shoaib et al., 2003; Wilkinson et al., 2006), this hypothesis has never been directly tested. Therefore, we tested whether the dopamine D1 antagonist SCH-23390 or the D2/3 antagonist eticlopride blocked bupropion substitution for nicotine. Because bupropion also has nAChR antagonist properties (Fryer et al., 1999; Slemmer et al., 2000; Miller et al., 2002; Damaj et al., 2004) we assessed whether mecamylamine, a central and peripheral nAChR antagonist, substituted for nicotine.
Another important line of inquiry is the role of metabolites in bupropion’s action. Previous reports have found that the metabolites of bupropion have pharmacological properties (Martin et al, 1990; Bondarev et al., 2003; Damaj et al., 2004). It is possible that a metabolite also plays a role in the ability of bupropion to substitute for nicotine. In fact, a previous study found that the major active metabolite, S,S-hydroxybupropion, (Rotzinger et al., 1999) partially substituted for nicotine in an operant drug discrimination task (Bondarev et al., 2003). Therefore, we also tested the ability of S,S-hydroxybupropion to substitute for the nicotine CS.
Methods
Subjects
Thirty-two experimentally naïve male Sprague-Dawley rats obtained from Harlan (Indianapolis, IN, USA) were housed individually in clear plastic tubs lined with wood shavings in a temperature- and humidity-controlled colony. Water was continuously available in the home cage. Food access was restricted such that rats were maintained at 85% of their free-feeding body weights (341±13 g). The 85% target weight was increased by 2 g each month. All experimental sessions were conducted during the light portion of a 12 hr light:dark cycle. Protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee and followed the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996).
Apparatus
Eight conditioning chambers (ENV-008CT; Med Associates, Georgia, VT, USA) were used. Chambers measured 30.5 × 24.1 × 21 cm (l × w × h); the sidewalls were aluminum and the ceiling and front and back walls were made of clear polycarbonate. One aluminum side of each chamber had an opening to a dipper receptacle (5.2 × 5.2 × 3.8 cm; l × w × d). The end of the dipper arm had a 0.1-ml cup that allowed access to sucrose (26% w/v) in the receptacle when the arm was raised. An infrared emitter/detector unit, 1.2 cm within the receptacle and 3 cm from the floor of the chamber, monitored head entries. A separate infrared emitter/detector unit located on the front and back wall, 14.5 cm from each side wall and 4 cm from the floor, tracked general chamber activity. Each chamber was enclosed in a sound and light attenuating cubicle fitted with a fan that provided airflow and masked noise. A personal computer with Med Associates interface and software (Med-PC for Windows, version IV) controlled each session and recorded dipper entries and chamber activity.
Drugs
(−)-Nicotine hydrogen tartrate, bupropion hydrochloride, eticlopride hydrochloride, mecamylamine hydrochloride, SCH-23390 hydrochloride (Sigma, St. Louis, MO, USA), and S,S-hydroxybupropion (synthesized at the Research Triangle Institute) were dissolved in 0.9% saline. Nicotine was adjusted to a pH of 7.0±0.2 using a dilute NaOH solution. Nicotine (0.4 mg base/kg) and mecamylamine (0.5 and 1 mg salt/kg) were injected subcutaneously (SC). Bupropion (5–30 mg salt/kg), eticlopride (0.001–0.01 mg salt/kg), SCH-23390 (0.005–0.03 mg salt/kg), and S,S-hydroxybupropion (5–40 mg salt/kg) were injected intraperitoneally (IP). All injections were at a volume of 1 ml/kg and doses and injection routes for each ligand were based on previous work from our laboratory and others (Jones et al., 1980; Besheer et al., 1999; Bevins et al., 2001; Palmatier and Bevins, 2002; Young and Glennon, 2002; Bondarev et al., 2003; Besheer et al., 2004; Wilkinson et al., 2005; Murray and Bevins, 2007b).
Procedures: Experiment 1
Discrimination Training
For 3 days before the start of discrimination training, rats (n=16) were injected SC with nicotine (0.4 mg/kg) in the home cage to reduce the initial locomotor suppressant effects of nicotine (cf. Bevins et al., 2001). Discrimination training was conducted Monday through Friday. Sessions were constructed such that rats received 4 nicotine and 4 saline sessions in random order with the restriction that no more than 2 of one session type occurred in a row. On nicotine sessions, each rat received a SC injection of nicotine 5 min before placement in the chamber for 20 min; sucrose was presented 36 times (4 sec each) across the session. Four computer programs controlling nicotine sessions presented the first sucrose at different times (mean=137 sec, range=124–152 sec) and with different inter-sucrose intervals throughout the sessions (mean=25 sec, range=4–80 sec). Saline sessions were similar to nicotine sessions except saline replaced nicotine as the injected solution and no sucrose was available. Discrimination training lasted for 16 nicotine and 16 saline sessions.
Bupropion Substitution
Following discrimination training, rats were free-fed for 10 days, and then a new 85% weight was determined. Once this new target weight was attained, the bupropion substitution phase began. Testing was conducted in repeated 5-day cycles with 2 nicotine and 2 saline sessions randomly intermixed. Nicotine and saline sessions were identical to training. If rats met a performance criterion on these sessions (see later), then a test session was conducted on day 5. On the test, rats were administered bupropion IP (5, 10, 20, or 30 mg/kg) 15 min before placement, or 0.4 mg/kg nicotine or saline 5 min before chamber placement. The test session was 4 min and no sucrose was available. Each rat was assigned and tested with a unique random order of test solutions. Upon completing all solutions, rats were then tested with 20 mg/kg bupropion SC to test for injection route differences.
Bupropion Pretreatment
After the bupropion substitution phase, rats were assigned an intermixed order of saline, or 5 or 10 mg/kg bupropion followed by nicotine (0, 0.025, 0.05, 0.1, 0.2, or 0.4 mg/kg). Bupropion pretreatment was IP 15 min before placement; the second injection was SC 5 min before placement. There was a suggestive downward shift in conditioned responding with the 10 mg/kg bupropion pretreatment. Thus, after completing the entire initial pretreatment order rats were pretreated with 20 mg/kg bupropion followed by nicotine; only the 0.2 and 0.4 mg/kg doses of nicotine were tested. Two rats were removed from the experiment during this phase because the discrimination was not maintained.
Procedures: Experiment 2
Discrimination Training
Sixteen new rats were used in this study. Nicotine discrimination training was identical to Experiment 1.
Bupropion Injection-to-Placement Interval
The 5-day testing cycle was similar to Experiment 1. On the test day, rats were injected IP with 20 mg/kg bupropion (a dose that fully substituted for nicotine) 5, 15, 30, 60, 90, or 180 min before the 4-min test. As a benchmark, rats were also tested with saline or 0.4 mg/kg nicotine SC 5 min before placement. Unless otherwise noted, each rat was assigned a random order for testing.
Mecamylamine Substitution
In this phase, rats were tested with 0.5 or 1 mg/kg mecamylamine SC 25 min before placement, or 0.4 mg/kg nicotine or saline SC 5 min before placement.
Bupropion Substitution across 60 min
Following the mecamylamine substitution phase, rats were randomly assigned to receive bupropion (Bup Ext) or nicotine (Nic Ext) during a 60-min extinction session (i.e., no access to sucrose). The Nic Ext set (n=8) was injected with nicotine SC 5 min before placement; the Bup Ext set (n=8) was injected with 20 mg/kg bupropion IP 15 min before placement. The following day, all rats received nicotine 5 min before placement. This second extinction session was identical to the first in all other details.
Dopamine Antagonism of Bupropion Substitution
Rats that met the test criterion during continued discrimination training were pretreated with SCH-23390 (0.005, 0.01, 0.03 mg/kg), eticlopride (0.001, 0.0025, 0.005, 0.01, 0.03 mg/kg), or saline before receiving 20 mg/kg bupropion. The pretreatment injection was IP 30 min before the test; bupropion was given IP 15 min before testing. For a comparison with training conditions, rats were also tested with 0.4 mg/kg nicotine or saline. One rat was removed from the experiment during this phase because the discrimination was not maintained.
S,S-hydroxybupropion Substitution
We were interested in the role of a major active metabolite of bupropion, S,S-hydroxybupropion, in the ability of bupropion to substitute for the nicotine stimulus. The eight rats that demonstrated the highest level of conditioned responding to 20 mg/kg bupropion during the 15 min injection-to-placement interval test were used in this phase. Training continued as described above. Rats that qualified for testing were injected with S,S-hydroxybupropion (5, 10, or 20 mg/kg) IP 30 min before placement, or 0.4 mg/kg nicotine or saline SC 5 min before placement. After testing on all these solutions, all rats were then tested with 40 mg/kg S,S-hydroxybupropion.
Dependent Measures and Testing Criterion
The main dependent measure was dipper entries per sec before the first sucrose delivery during nicotine sessions, or an equivalent time during saline and 4-min test sessions (see later for 60-min sessions). Dipper entries per sec were reported because the time before the first sucrose delivery varied between sessions. Further, only entries before the first sucrose delivery were used to prevent the influence of sucrose delivery on any measure (cf. Besheer et al., 2004). General activity was defined as the number of chamber beam breaks per sec during the time before the sucrose delivery or an equivalent time during saline and test sessions. Because no sucrose was available during the 60-min substitution tests of Experiment 2, dipper entries and activity were measured in 2-min intervals for the entire session.
Before a rat tested, its discrimination performance for the first four days of the test cycle had to meet criterion. The testing criterion required that on each of the nicotine sessions dipper entries had to be at least 0.01 per sec greater than during both of the saline sessions (cf. Murray and Bevins, 2007b). Rats that did not meet criterion remained in the home cage for that test session.
Data Analysis
For discrimination training, a t-test was used to compare the mean dipper entries and activity on the last three nicotine sessions to the mean of the corresponding saline sessions. A one-way ANOVA was used for substitution and pretreatment tests with post-hoc Fishers Least Significant Difference (LSD) tests prompted by a significant main effect. Median effective doses (ED50s) were calculated using the linear portion of the ascending limb of the dose-effect curve. A paired t-test was used to compare dipper entries and general activity for the 20 mg/kg bupropion IP versus SC administration. A paired t-test was also used to compare 40 mg/kg S,S hydroxybupropion versus baseline nicotine and saline responding because this dose was tested after the other doses of hydroxybupropion. The bupropion pretreatment data were analyzed using a repeated measures ANOVA with Pretreatment and Nicotine doses as the repeated within-subject factors. Because the 20 mg/kg bupropion pretreatment was tested after the other doses, it was analyzed separately using paired t-tests. A mixed two-way ANOVA with Drug as the between subjects factor and Time as the repeated within subject factor was used to analyze dipper entries and activity during the 60-min substitution test. Statistical significance was declared using a two-tailed rejection region of 0.05 for all tests.
Results
Experiment 1
Discrimination Training
Dipper entries evoked by the interoceptive stimulus effects of nicotine were elevated compared to the corresponding saline session on session 5 and remained higher throughout discrimination training (data not shown). Mean dipper entry rates across the last 3 nicotine sessions (0.150 ± 0.014) were significantly elevated compared to saline (0.038 ± 0.003), t(15)=8.11, p<0.001.
Bupropion Substitution
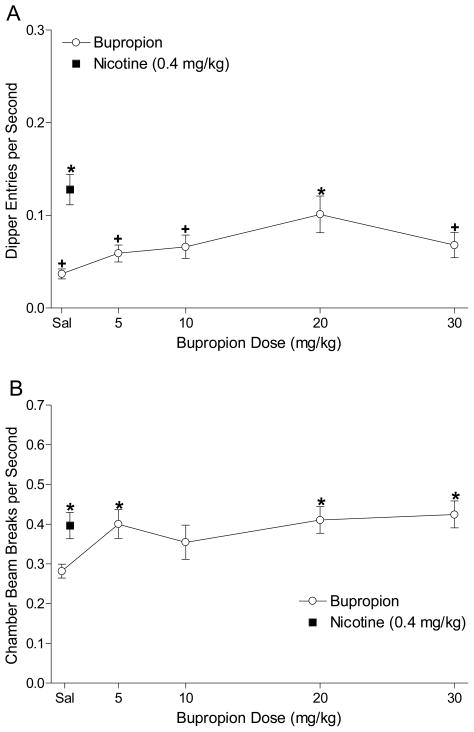
Figure 1A shows the dipper entry rates for the bupropion substitution phase. There was a significant main effect of Dose, F(4,48)=3.12, p=0.023. Follow-up analyses revealed that nicotine and 20 mg/kg bupropion produced elevated conditioned responding compared to saline; dipper entry rates were below nicotine levels for saline and all doses of bupropion except 20 mg/kg, LSDmmd=0.041. This pattern indicates that only 20 mg/kg bupropion fully substituted for the nicotine stimulus. The ED50 for bupropion substitution was 9.9 mg/kg. Figure 1B shows general locomotor activity in the chamber for the bupropion substitution phase. There was a main effect of Dose, F(4,48)=3.60, p=0.012. Activity was elevated above saline for 0.4 mg/kg nicotine, 5, 20, and 30 mg/kg bupropion; activity was not different from nicotine for any of the bupropion doses tested, LSDmmd=0.105. A pair-wise t-test comparing conditioned responding after 20 mg/kg bupropion was injected IP (mean=0.101±0.020) versus SC (mean=0.069±0.023) found no difference, t<1. There was also no difference in activity induced by the IP (mean=0.42±0.039) versus SC (mean=0.45±0.030) injection, t<1.
Figure 1.
Panel A shows the mean (±1 SEM) dipper entry rates during bupropion substitution testing (n=16). Panel B shows the mean (±1 SEM) activity per sec for the bupropion substitution testing. For both panels, * denotes a significant difference (p<0.05) from saline; + denotes a significant difference from nicotine.
Bupropion Pretreatment
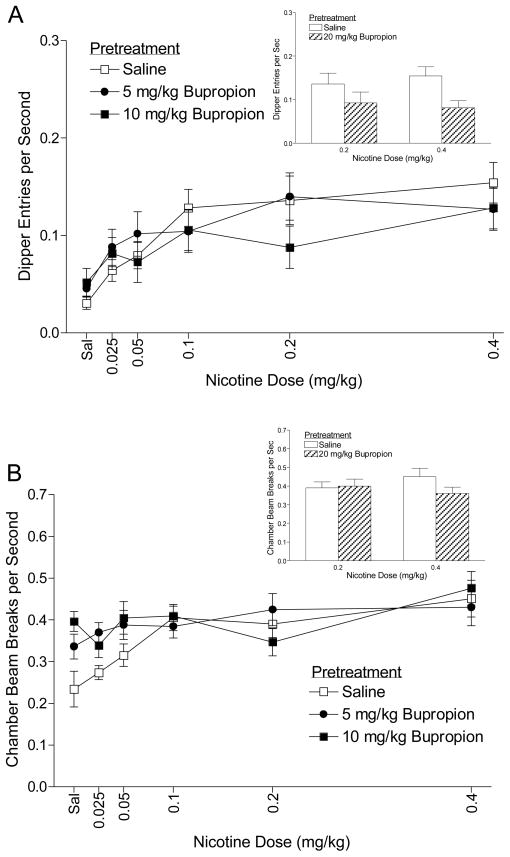
Figure 2A shows the dipper entry rates for rats pretreated with saline, 5, or 10 mg/kg bupropion and then injected with varying doses of nicotine. There was a main effect of Dose, F(5,60)=15.10, p<0.001, suggesting that dipper entries increased as nicotine dose increased. Despite the suggestive downward shift in conditioned responding at 10 mg/kg, there was no effect of Pretreatment, F(2,24)=1.19, p=0.322, nor a Dose × Pretreatment interaction, F(10,120)=1.64, p=0.103, indicating that pretreatment with 5 or 10 mg/kg bupropion had no effect on the nicotine-dose effect function: ED50=0.099 mg/kg (saline pretreatment), 0.094 mg/kg (5 mg/kg bupropion pretreatment), and 0.057 mg/kg (10 mg/kg bupropion pretreatment). Dipper entries per sec after pretreatment with 20 mg/kg bupropion followed by 0.2 or 0.4 mg/kg nicotine are shown in the embedded bar graph of Figure 2A. For comparison purposes, dipper entries for 0.2 and 0.4 mg/kg nicotine after a saline pretreatment (values from the line graph) are also included. There was a main effect of Pretreatment, F(1,12)=9.33, p=0.01, suggesting that 20 mg/kg bupropion decreased nicotine-evoked conditioned responding. The main effect of Dose and the Pretreatment × Dose interaction were not significant, Fs≥1.28, ps≤0.28.
Figure 2.
Panel A shows the mean (±1 SEM) dipper entry rates for rats pretreated with saline or 5 or 10 mg/kg bupropion and then tested with nicotine (n=14). The mean (+1 SEM) dipper entry rates for rats pretreated with saline or 20 mg/kg bupropion and then nicotine are shown in the embedded bar graph. Panel B shows the mean (±1 SEM) activity per sec for the bupropion pretreatment phase. The mean (+1 SEM) activity per sec after pretreatment with saline or 20 mg/kg bupropion then nicotine are shown in the embedded bar graph.
Locomotor activity for the bupropion pretreatment phase is shown in Figure 2B. There was a main effect of Dose, F(5,60)=6.57, p<0.001, but no main effect of Pretreatment, F(2,24)=3.20, p=0.059. The Dose × Pretreatment interaction was significant, F(10,120)=2.41, p=0.012, indicating that bupropion pretreatment differentially affected activity. Follow-up analyses indicated that pretreatment with 5 mg/kg bupropion showed elevated activity compared to saline pretreatment when followed by saline or 0.025 mg/kg nicotine. Pretreatment with 10 mg/kg bupropion elevated activity at saline and the 0.025 and 0.05 mg/kg nicotine test doses, LSDmmd=0.074. Locomotor activity for the 20 mg/kg bupropion pretreatment tests is shown in the embedded bar graph of Figure 2B. There was no main effect of Pretreatment or Dose, and no Pretreatment × Dose interaction, Fs≥2.25, ps≤0.159. These results indicate that nicotine-induced locomotor activity was not affected by pretreatment with 20 mg/kg bupropion.
Experiment 2
Discrimination Training
Dipper entries evoked by the nicotine state were elevated compared to saline on session 5 and remained higher throughout discrimination training (data not shown). Mean dipper entry rates on the last 3 nicotine sessions (0.133 ± 0.011) were significantly elevated compared to saline (0.058 ± 0.006), t(15)=6.49, p<0.001, indicating that the discrimination was acquired.
Bupropion Injection-to-Placement Interval
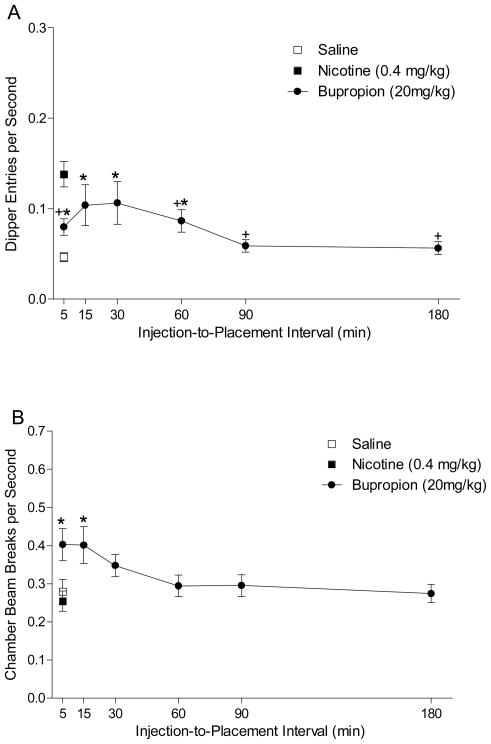
Figure 3A shows the dipper entries per sec after various injection-to-placement intervals for substitution tests with 20 mg/kg bupropion. A one-way ANOVA revealed a main effect of Interval, F(5,75)=2.33, p=0.047. Conditioned responding was significantly higher than saline at 5, 15, 30, and 60-min intervals and significantly lower than nicotine at 5, 60, 90, and 180 min, LSDmmd=0.031. This data pattern indicates that bupropion fully substitutes for the nicotine stimulus at 15 and 30 min. Figure 3B shows the chamber activity. There was a main effect of Interval, F(5,75)=3.741, p<0.01, indicating that locomotor activity decreased as the injection-to-placement interval increased. Bupropion-induced activity was elevated compared to saline and nicotine after 5 and 15 min, LSDmmd=0.083.
Figure 3.
Panel A shows mean (+1 SEM) dipper entry rates during the bupropion injection-to-placement interval testing (n=16). Panel B shows mean (±1 SEM) activity per sec during bupropion injection to placement interval testing. For both panels, * denotes a significant difference (p<0.05) from saline; + denotes a significant difference from nicotine.
Mecamylamine Substitution
Table 1 shows dipper entries and chamber activity during the mecamylamine substitution tests. A one-way ANOVA examining dipper entries showed no effect of dose, F<1, indicating that mecamylamine did not evoke responding above saline levels. Mecamylamine also had no effect on general activity, F(2,28)=2.41, p=0.109.
Table 1.
Dipper entries and chamber beam breaks during mecamylamine substitution
| Drug (mg/kg) | Mean (±1 SEM) Dipper Entries per Sec | Mean (±1 SEM) Beam Breaks per Sec |
|---|---|---|
| 0.4 Nicotine | 0.140 (0.015) | 0.244 (0.027) |
| Saline | 0.046 (0.005) | 0.279 (0.032) |
| 0.5 Mec | 0.047 (0.011) | 0.190 (0.093) |
| 1 Mec | 0.050 (0.011) | 0.235 (0.115) |
Mec= Mecamylamine
Bupropion Substitution across 60-min
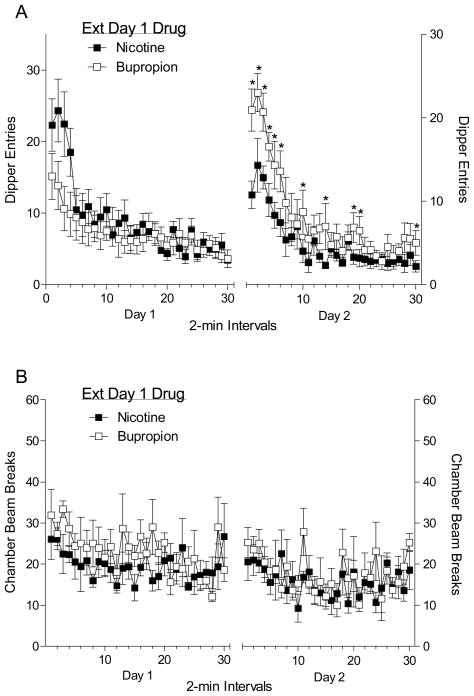
The left graph in Panel A of Figure 4 shows dipper entries in 2-min intervals during the first extinction session. A two-way ANOVA revealed a main effect of Time, F(29,406)=9.997, p<0.001. Although dipper entries appeared lower for bupropion (20 mg/kg) in the early portion of the session, neither the main effect of Group nor Group × Time interaction was significant, Fs<1. This data pattern suggests that conditioned responding evoked by bupropion was similar to nicotine across the 60-min session (i.e., full substitution). The right graph in Panel B of Figure 4 shows the dipper entries from the second day of extinction when both groups received nicotine. A two-way ANOVA revealed a main effect of Time, F(29,406)=23.13, p<0.001, and Group, F(1,14)=6.72, p<0.001, as well as a significant Time × Group interaction, F(29,406)=2.70, p<0.001. Fisher’s LSD test showed that dipper entries were elevated for rats in the bupropion group during intervals 1–6, 10, 14, 19, 20, and 30, LSDmmd=2.88. This outcome suggests that extinction learning with bupropion does not necessarily transfer to nicotine. The left graph of Panel A shows the activity in 2-min time blocks during the first extinction session. A two-way ANOVA revealed a main effect of Time, F(29,406)=1.62, p=0.025. The main effect of Group and the Time × Group interaction were not significant, Fs≤1.01, ps≥0.234. Activity during the second extinction session is shown in the right graph of Panel B. A two-way ANOVA revealed a main effect of Time, F(29,406)=1.92, p=0.003. The main effect of Group and Group × Time interaction were not significant, Fs≤1.19, ps≥0.293.
Figure 4.
The left graph of Panel A shows the mean (±1 SEM) dipper entries in 2-min intervals for the first 60-min session (n=8). The right graphs show the mean (±1 SEM) dipper entries for the second 60-min session when all rats received nicotine. Panel B shows the mean (±1 SEM) activity in 2-min bins for the first (left graph) and the second 60-min session (right graph). In all graphs the filled squares represent rats that received nicotine during the first 60-min session, and open squares represent rats that received bupropion during the first session. * denotes a significant difference (p<0.05) between the groups.
Dopamine Antagonism
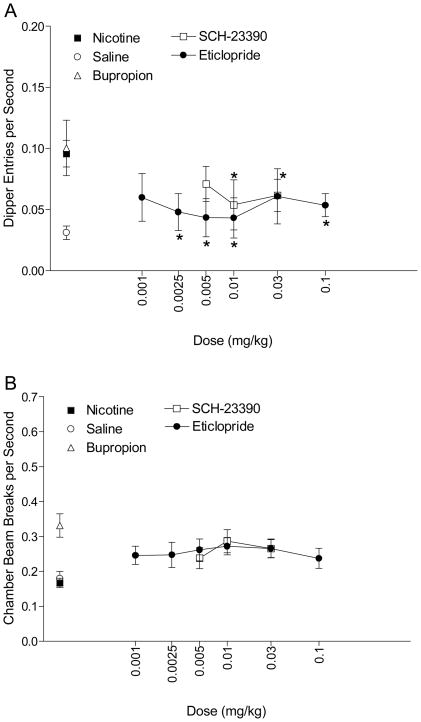
Dipper entries from the antagonism of bupropion substitution for nicotine phase are shown in Figure 5A. A one-way ANOVA revealed a main effect of SCH-23390, F(3,42)=3.52, p=0.023. Fisher’s LSD tests found that conditioned responding was decreased after pretreatment with 0.01 and 0.03 mg/kg SCH-23390, LSDmmd=0.031. The main effect of eticlopride was also significant, F(6,84)=2.29, p=0.042. Dipper entry rates were decreased after pretreatment with 0.0025, 0.005, and 0.1 mg/kg eticlopride compared to saline pretreatment, LSDmmd=0.038. Activity from this phase is shown in Figure 5B. There was no effect of Dose for either drug, Fs≤1.34, ps≥0.267.
Figure 5.
Panel A shows the mean (±1 SEM) dipper entry rates during dopamine antagonist testing (n=15). * denotes a significant difference (p<0.05) from bupropion responding. Panel B shows the mean (±1 SEM) activity per sec for the dopamine antagonist testing.
S,S-hydroxybupropion
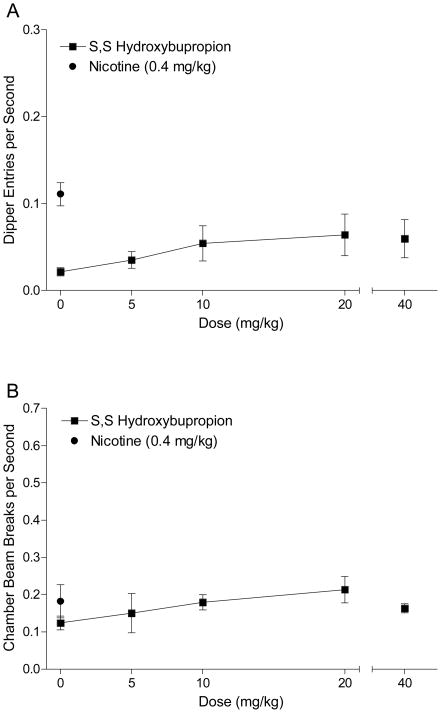
Figure 6A shows dipper entries for S,S-hydroxybupropion substitution testing. There was a main effect of Dose, F(3,21)=4.04, p=0.02, but follow-up analyses indicated that none of the doses tested produced responding above saline levels (LSDmmd=0.09). In addition, 40 mg/kg did not produce elevated dipper entries compared to saline, t(7)=2.08, p=0.08. This pattern suggests that S,S-hydroxybupropion did not substitute for the interoceptive stimulus effects of nicotine. Activity for S,S-hydroxybupropion testing is shown in Figure 6B. There was no effect of Dose, F(3,21)=2.24, p=0.11, and the paired t-test comparing 40 mg/kg hydroxybupropion to saline was not significant, t<1.
Figure 6.
Panel A shows the mean (±1 SEM) dipper entry rates during S,S-hydroxybupropion substitution testing (n=8). Panel B shows the mean (±1 SEM) activity per sec for the S,S-hydroxybupropion substitution testing.
Discussion
The research in the present report clearly show that pharmacological properties of bupropion substitute for those of a nicotine CS in a Pavlovian drug discrimination task. These findings extend in important ways the Pavlovian (Besheer et al., 2004) and operant conditioning (Wiley et al., 2002; Young and Glennon, 2002) research investigating bupropion substitution for the interoceptive stimulus effects of nicotine. This substitution pattern seen in both paradigms likely reflects their shared behavioral and physiological properties. Both drugs are self-administered by rats (Clarke, 1969; Tella et al., 1997; Bruijnzeel and Markou, 2003; Rauhut et al., 2003) and produce a conditioned place preference (Ortmann, 1985; Wilkinson and Bevins, 2008). Indeed bupropion and nicotine increase dopamine in the nucleus accumbens (Nomikos et al., 1992; Nisell et al., 1997; Zocchi et al., 2003) and lower intracranial self-stimulation thresholds (Huston-Lyon and Kornetsky, 1992; Cryan et al., 2003) suggesting activation of the reward or incentive motivational pathways in the central nervous system. These results, combined with the present research, suggest that part of bupropion’s effectiveness as a smoking cessation aid might be its ability to substitute for the stimulus effects of nicotine.
Besheer et al. (2004) found that nicotine served as a CS in an appetitive Pavlovian drug discrimination task. These CS effects of nicotine were dose dependent (ED50=0.113 mg/kg). We replicated this finding with an ED50 of 0.099 mg/kg. Further Besheer et al. (2004) found that bupropion (20 mg/kg) produced partial substitution for a 0.4 mg/kg nicotine CS. In the present study, we found full substitution using the same training dose of nicotine. The training protocols between these studies were similar except the present experiments provided more sucrose deliveries per session (8 versus 36). Wilkinson et al. (2006) found that the increase in number of sucrose deliveries increased the conditioned response magnitude and resistance to extinction. Perhaps this increase in conditioned excitation with more nicotine-sucrose pairings changed somewhat the nature of the interoceptive stimulus effects of nicotine (cf. Bevins, in press). Alternatively, Besheer et al. (2004) only tested eight rats at this bupropion dose providing limited statistical power. Regardless, full substitution of bupropion for the nicotine CS was observed repeatedly in different phases and in different sets of rats in the present study. Further, this result is consistent with most operant drug discrimination studies that report that bupropion fully substitutes for a nicotine discriminative stimulus (Wiley et al., 2002; Young and Glennon, 2002). Additionally, the bupropion ED50 of 9.9 mg/kg found in the present study falls within the wide range reported in the operant drug discrimination studies: ED50=22.5 mg/kg (Wiley et al., 2002) and ED50=5.5 mg/kg (Young and Glennon, 2002).
As detailed earlier, the behavioral and neurobiological research indicates that bupropion and nicotine are acting on similar mechanisms. As such, one might expect that pretreatment with bupropion to shift the nicotine dose-effect curve. In the present study, pretreatment with doses of bupropion (5 or 10 mg/kg) that did not substitute for nicotine had no systematic effect on conditioned responding evoked by the nicotine CS. Direct comparisons of these results with previous finds warrant caution as a result of potentially important differences in the nature of the nicotine stimulus (i.e., CS versus discriminative stimulus). Regardless, this result is consistent with Wiley et al. (2002) who found that pretreatment with doses of bupropion that did not substitute for nicotine when given alone (i.e., 2.5, 5, or 10 mg/kg), had no effect on the percent of nicotine-appropriate bar-pressing at the training dose of nicotine (0.4 mg/kg). In contrast, Young and Glennon (2002) reported that a low dose of bupropion (i.e., 3 mg/kg) that did not substitute for nicotine shifted the nicotine dose-response curve to the left. That is, bupropion increased the proportion of nicotine-appropriate bar-pressing to low doses of nicotine.
In addition to the very nature of the discrimination task, there are several other key procedural differences between the present study and that of Young and Glennon (2002) that might account for the differences in results. First, Young and Glennon used a higher training dose of nicotine (0.6 mg/kg). Both the present study and Wiley et al. (2002) used 0.4 mg/kg nicotine. Perhaps the interoceptive stimulus properties of these doses of nicotine differ. Consistent with this suggestion is the finding by Murray and Bevins (2007b) that 0.6 mg/kg nicotine does not fully generalize for a 0.4 mg/kg nicotine CS. Differences in the stimulus properties of the training drug could affect the ability of doses of bupropion to shift the nicotine dose effect curve. Second, Young and Glennon (2002) injected bupropion SC 30 min before testing [compared to IP 15 min before testing in the present study and SC 10 min before testing in Wiley et al. (2002)] and nicotine 15 min before placement [compared to 5 min in both the present study and Wiley et al. (2002)]. These differences in injection routes and intervals might have produced differences in the interoceptive drug state during testing. This suggestion is consistent with the earlier discussion on the temporal nature of drug stimuli and is supported by the present research showing that substitution of bupropion for nicotine changes with the injection-to-placement interval (see Figure 3). Finally, there were differences in the schedules of reinforcement. We used a variable time (VT) 25-sec schedule of sucrose delivery, whereas Young and Glennon used a variable interval (VI) 15-sec schedule with sweetened milk; Wiley et al. (2002) used a fixed-ratio (FR) 10 schedule with 45-mg food pellets. Clearly, future research will need to focus on potential behavioral factors that alter the interaction between the interoceptive stimulus effects of nicotine and bupropion.
Although pretreatment with 10 mg/kg bupropion did not significantly alter conditioned responding at any of the nicotine doses tested, there was a trend for a decrease in dipper entries at 0.2 and 0.4 mg/kg. This trend prompted us to test 20 mg/kg bupropion. Interestingly, pretreatment with this dose of bupropion that fully substituted for the nicotine CS significantly decreased conditioned responding evoked by 0.2 and 0.4 mg/kg nicotine. Locomotor activity was not affected making it unlikely that reduced conditioned responding resulted from stereotypy or some non-specific motor impairment. One explanation for the decreased responding is that the combination of 20 mg/kg bupropion with either 0.2 or 0.4 mg/kg nicotine produced a somewhat unique interoceptive stimulus, therefore, reducing conditioned responding. Another possibility is that the cueing effects of the two drugs combine to act like a higher dose of nicotine. Besheer et al. (2004; see also Murray and Bevins, 2007b) showed that conditioned responding decreased when the dose of nicotine was increased (0.6 mg/kg) compared to the training dose (0.4 mg/kg). This suppressed conditioned responding, however, is typically accompanied by a decrease in general activity complicating the interpretation of the conditioned responding (Murray and Bevins, 2007b). Combining 20 mg/kg bupropion with nicotine did not produce this decrease in activity. Finally, past research has shown that bupropion has some action as a nAChR antagonist (Fryer et al., 1999; Slemmer et al., 2000; Miller et al., 2002; Damaj et al., 2004). Past research from our laboratory has shown that nicotine-evoked conditioned responding is mediated by central nAChRs. That is, the central and peripheral nAChR antagonist mecamylamine, but not the mostly peripheral nAChR antagonist hexamethonium, blocked conditioned responding evoked by the nicotine CS (Besheer et al., 2004). According to this account, pretreatment with 20 mg/kg bupropion could have produced a decrease in conditioned responding at 0.2 and 0.4 mg/kg nicotine through its action as a nAChR antagonist. As the present discussion suggests, our enthusiasm for the combined nicotine and bupropion being like a higher dose of nicotine is diminished by the activity data (i.e., no decrease in locomotor activity). Beyond this, the results of the current study do not allow us to dissociate among these alternatives.
In the initial test for bupropion substitution (see Figure 1), a 15-min injection-to-placement interval was used based on previous data from our laboratory showing that bupropion produced some nicotine conditioned responding at this interval (Besheer et al., 2004). Jones et al. (1980) found that when bupropion (not nicotine) was trained as a discriminative stimulus, bupropion-appropriate lever pressing was greatest 15 min after injection and similar to saline levels after 90 min. In the present study, we found that the stimulus effects of bupropion overlapped with those of nicotine for at least 60 min. This overlap was complete at 15 and 30 min and partial at 60 min. This is the first report of the temporal dynamics of bupropion substitution for nicotine. Notably, these results are corroborated by a second test that examined bupropion substitution for nicotine in a 60-min extinction session. During this test, bupropion and nicotine produced similar rates of conditioned responding across the 60-min session.
That 60-min substitution test for nicotine and bupropion was conducted in extinction (i.e., no sucrose). As such, this experimental design provided us with a unique opportunity to examine whether extinction with bupropion was the same as extinction with nicotine. As noted above, the pattern of bupropion substitution for the nicotine CS was similar across the 60-min extinction session. Based on this similarity in conditioned responding, one might predict comparable levels of extinction learning when subsequently tested with the nicotine CS. This expected outcome did not occur. Rather, rats extinguished with bupropion and then tested with nicotine had higher levels of conditioned responding on the test than rats extinguished and tested with nicotine. Most learning theorists now recognize that loss of conditioned responding across extinction reflects, at least in part, new associative learning that competes with or inhibits the original learning (Pavlov, 1927; Rescorla, 1997; Bouton, 2002). With this in mind, we suggest that what was learned during extinction with bupropion was not the same as that with nicotine and hence did not generalize sufficiently when tested with nicotine. This conclusion leads to the interesting possibility that stimulus substitution based on neuropharmacological processes does not necessarily predict substitution based on learning processes (i.e., “associative substitution”). What do different patterns of results mean for the underlying neuropharmacological processes? Future research will need to pursue these ideas further. We should note that there might have been partial transfer of extinction in the bupropion-treated group. However, we cannot assess this possibility given the opportunist nature of the present design (i.e., the original purpose was to test the temporal pattern of bupropion substitution). Research explicitly designed to assess transfer of extinction learning will need to include a no extinction (saline) group.
Bupropion substitution for a nicotine CS suggests at least partial overlap of the neurobiological processes responsible for their interoceptive stimulus effects. Nicotine exposure quickly desensitizes certain nAChRs (Dani and Heinemann, 1996; Dani and De Biasi, 2001). This desensitization can be conceptualized as a functional antagonism and may be an important part of the stimulus properties that nicotine and bupropion share. Indeed, bupropion has nAChR antagonist properties (Fryer et al., 1999; Slemmer et al., 2000; Miller et al., 2002; Damaj et al., 2004;). Mecamylamine, a nAChR antagonist which blocks nicotine-evoked conditioned responding when given in combination with nicotine (Besheer et al., 2004), did not elevate conditioned responding above saline levels when given alone. This finding suggests that the CS effects of nicotine are not driven by the nAChR functional antagonism process.
Nicotine and bupropion produce an increase in dopamine in the nucleus accumbens (Nisell et al., 1997; Nomikos et al., 1992; Zocchi et al., 2003). Bupropion and nicotine both lower intracranial self-stimulation thresholds (Huston-Lyons and Kornetsky, 1992; Cryan et al., 2003) indicating action in the reward pathway. In fact, dopamine agonist substitute for and dopamine antagonists block the discriminative stimulus properties of bupropion when rats are trained to discriminate bupropion from saline in an operant drug discrimination task (Spealman and Bergman, 1988; Terry and Katz, 1997). These results suggest that dopaminergic processes in the incentive-salience pathway might be a possible mechanism for bupropion’s effectiveness as a smoking cessation aid (Wiley et al., 2002; Bruijnzeel and Markou, 2003; Cryan et al., 2003; Rauhut et al., 2003; Dwoskin et al., 2006; Wilkinson et al., 2006). In support of these speculations, we found that the dopamine D1 antagonist SCH- 23390 (0.01 and 0.03 mg/kg) and the D2/3 antagonist eticlopride (0.0025–0.01 mg/kg) both decreased bupropion substitution for the nicotine CS without altering general motor activity. To our knowledge, this is the first direct test of the hypothesis that the ability of bupropion to substitute for nicotine is mediated through a dopaminergic mechanism.
S,S-hydroxybupropion is one of the major metabolites of bupropion in humans (Schoeder, 1983; Rotzinger et al., 1999). Bondarev et al. (2003) reported that S,S-hydroxybupropion (11 mg/kg) produced partial substitution for 0.6 mg/kg nicotine and fully substituted for 1 mg/kg amphetamine (ED50=4.4 mg/kg) in an operant drug discrimination task using rats. However, in that experiment, behavior was disrupted (i.e., a decrease in overall lever pressing) at 11 mg/kg S,S-hydroxybupropion. S,S-hydroxybupropion (5–40 mg/kg) did not substitute for the nicotine CS in the present experiment. Further, general activity was not affected even at the high dose of S,S-hydroxybupropion. As discussed earlier, the processes underlying nicotine’s ability to serve as a CS might be somewhat distinct from those underlying the discriminative stimulus properties resulting in different substitution patterns (cf. Murray and Bevins, 2007b). Additionally, perhaps these differences are a result of training procedures. For example, the present study used 0.4 mg/kg nicotine while Bondarev et al., (2003) used 0.6 mg/kg nicotine. As detailed earlier, these doses of nicotine might have different interoceptive stimulus properties (Murray and Bevins, 2007b) that produce differences in substitution patterns.
In the current study, general locomotor activity in the chamber was measured along with conditioned responding (i.e., dipper entries). Several laboratories have reported that bupropion is a potent locomotor stimulant (Cooper et al., 1980; Nomikos et al., 1992; Ortmann, 1985; Wilkinson et al., 2006; Wilkinson and Bevins, 2007). We replicated this finding in the present study. One possible explanation for bupropion substitution for the nicotine CS is that a general increase in locomotor activity produced a concomitant increase in dipper entries. This explanation is unlikely considering that another potent locomotor stimulant (i.e., amphetamine) does not substitute for the nicotine CS (Besheer et al., 2004). Further, the current results also show a clear dissociation between bupropion-induced locomotor activity and substitution for the nicotine CS (see Figure 2). For example, most doses of bupropion produced elevated locomotor activity, yet full substitution was only observed at 20 mg/kg bupropion. Further, bupropion produced full substitution for the nicotine CS after a 15 or 30-min injection-to-placement interval, yet locomotor activity was elevated only at the 5 and 15-min injection-to-placement intervals. These results clearly indicate a dissociation of the locomotor stimulant effects from the nicotine-like stimulus effects of bupropion.
This study addressed several important gaps in our understanding of bupropion substitution for a nicotine CS along with extending previous operant drug discrimination studies that showed bupropion substitution for a nicotine discriminative stimulus. For example, the stimulus properties of bupropion substitute for nicotine up to 60 min. Additionally, the dopaminergic properties of bupropion, and not the nAChR antagonist properties were necessary for the substitution. This conclusion supports suggestions by several researchers that the overlapping dopaminergic process between bupropion and nicotine might be at least partially responsible for the efficacy of bupropion as a smoking cessation aid (Shoaib, 1998; Rauhut et al., 2003; Shoaib et al., 2003; Wilkinson et al., 2006; see also Dwoskin et al., 2006 for a review). Interestingly, the major metabolite of bupropion in humans, S,S hydroxybupropion, did not evoke nicotine-appropriate responding. Importantly, the present study is also the first to examine transfer of extinction learning (i.e., associative substitution). Although bupropion produced a similar pattern of responding to nicotine during an extinction session, this learning did not generalize back to the original nicotine CS. Considering the importance of extinction learning to relapse and in many smoking cessation programs, it will be important that this pattern be further explored.
Acknowledgments
We thank Jessica Linkugel, Jennifer Murray, Carmela Reichel, and Amanda Struthers for their thoughtful comments on an earlier version of this report. We thank Linda Dwoskin for many insightful conversations including her suggestion to test mecamylamine for substitution. We also thank Imad Damaj for facilitating the collaboration reflected in this research. The research and R. A. Bevins were supported by United States Public Health Service grant DA018114. Jamie Wilkinson was supported by Nebraska Tobacco Settlement Biomedical Research Enhancement Funds while preparing this manuscript for publication. Ivy Carroll was supported by grant DA019377. All MED-PC programs used in the present article are available upon request.
Footnotes
Conflict of Interest
None.
References
- Balster RL. Drugs as chemical stimuli. In: Colpaert FC, Balster RL, editors. Psychopharmacology Series 4: Transduction Mechanisms of Drug Stimuli. Springer; New York: 1988. pp. 3–11. [PubMed] [Google Scholar]
- Besheer J, Jensen HC, Bevins RA. Dopamine antagonism in a novel-object preference and a novel-object place conditioning preparation with rats. Behav Brain Res. 1999;103:35–44. doi: 10.1016/s0166-4328(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacol. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA. In: Bevins RA, Caggiula AR, editors. Altering the motivational function of nicotine through conditioning processes; The motivational impact of nicotine and its role in tobacco use: The 55th Nebraska Symposium on Motivation; Springer, NY. in press. [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Beh Cog Neurosci Rev. 2004;3:143–58. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal tracking task with rats. Behav Brain Res. 2007;177:134–41. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharm. 2003;474:85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette Smoking Among Adults United States. Morbidity and Mortality Weekly Report [serial online] 2002. 2000;51(29):642–645. [cited 2006 Nov 06]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5129a3.htm. [PubMed]
- Clark MS. Self-administered nicotine solutions preferred to placebo by the rat. B J Pharmacol. 1969;35:367P. [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Hester TJ, Maxwell RA. Behavioral and biochemical effects of the antidepressant bupropion (Welbutrin): Evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exper Therap. 1980;215:127–134. [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacol. 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of metabolites of bupropion on behavior and on function of momoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–82. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Phamcol Biochem Behav. 2001;70:439–46. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–08. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Reviews. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exper Therap. 1999;288:88–92. [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. J Am Medical Assoc. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking-cessation. The New England Journal of Medicine. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Jones CN, Howard JL, McBennett ST. Stimulus properties of antidepressants in the rat. Psychopharmacol. 1980;67:111–118. doi: 10.1007/BF00431964. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained release bupropion for smoking cessation: a randomized controlled trial. J Am Med Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR. Self-administration in baboons and the discriminative stimulus effects in rats of bupropion, nomifensine, diclofensine and imipramine. Psychopharmacol. 1990;102:183–190. doi: 10.1007/BF02245920. [DOI] [PubMed] [Google Scholar]
- Martin P, Massol J, Colin JN, Lacomblez L, Puech AJ. Antidepressant profile of bupropion and three metabolites in mice. Pharmacopsychiatry. 1990;23:187–194. doi: 10.1055/s-2007-1014505. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Miller DK, Sumithran SP, Dwoskin LP. Bupropion inhibits nicotine-evoked [(3)H] overflow from rat striatal slices preloaded with [(3)H]dopamine and from rat hippocampal slices preloaded with [(3)H]norepinephrine. J Pharmacol Exper Therap. 2002;302:1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol. 2007a;18:707–16. doi: 10.1097/FBP.0b013e3282f14ec6. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007b;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Cigarettes and other tobacco products. NIDA Info Facts. 2006 July; Available from: www.drugabuse.gov/pdf/infofacts/Tobacco06.
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nisell M, Marcus M, Nomikos GG, Svensson TH. Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J Neural Transmission. 1997;104:1–10. doi: 10.1007/BF01271290. [DOI] [PubMed] [Google Scholar]
- Nomikos GC, Damsma G, Wenkstern D, Fibiger HC. Effect of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacol. 1992;7:7–14. [PubMed] [Google Scholar]
- Ortmann R. The conditioned place preference paradigm in rats: Effect of bupropion. Life Sciences. 1985;37:2021–2027. doi: 10.1016/0024-3205(85)90033-5. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Examination of GABAergic and dopaminergic compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology. 2002;45:87–94. doi: 10.1159/000048682. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Anrep GV, translator. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacol. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Bourin M, Akimoto Y, Coutts RT, Baker GB. Metabolism of some “second” and “fourth” generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine. Cell Mol Neurobiol. 1999;19:427–442. doi: 10.1023/a:1006953923305. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery after Pavlovian conditioning with multiple outcomes. Animal Learn Behav. 1997;25:99–107. [Google Scholar]
- Rotzinger S, Bourin M, Akimoto Y, Coutts RT, Baker GB. Metabolism of some “second”- and “fourth”-generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine. Cell Mol Neurobiol. 1999;19:427–442. doi: 10.1023/a:1006953923305. [DOI] [PubMed] [Google Scholar]
- Schoeder DH. Metabolism and kinetics of bupropion. J Clin Psychiatry. 1983;44:79–81. [PubMed] [Google Scholar]
- Shoaib M. Is dopamine important in nicotine dependence? J Physiol. 1998;92:229–233. doi: 10.1016/s0928-4257(98)80024-7. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharm. 2003;165:405–12. doi: 10.1007/s00213-002-1277-x. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exper Therap. 2000;295:321–327. [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, Bergman K. Effectiveness of bupropion sustained release for smoking cessation in a health care setting. Archives of Internal Medicine. 2003;163:2337–2344. doi: 10.1001/archinte.163.19.2337. [DOI] [PubMed] [Google Scholar]
- Tella SE, Ladenheim B, Lud Cadet J. Differential Regulation of dopamine transporter after chronic self-administration of bupropion and nomifensine. J Pharmacol Exper Therap. 1997;281:508–513. [PubMed] [Google Scholar]
- Terry P, Katz JL. Dopaminergic mediation of the discriminative stimulus effects of bupropion in rats. Psychophamacol. 1997;134:201–212. doi: 10.1007/s002130050443. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Martin BR, Damaj MI. Nicotine-like discriminative stimulus effects of bupropion in rats. Experimental and Clinical Psychopharmacol. 2002;10:129–135. doi: 10.1037//1064-1297.10.2.129. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Palmatier MI, Bevins RA. Pre-exposure to nicotine alters subsequent locomotor stimulant effects of bupropion in rats. Nicotine Tob Res. 2006;184:470–81. doi: 10.1080/14622200500484642. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, Bevins RB. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as a function of number of conditioning trials and unpaired sucrose deliveries. Behav Pharmacol. 2006;17:161–72. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Bevins RA. Bupropion hydrochloride produces conditioned hyperactivity in rats. Physio Behav. 2007;90:790–796. doi: 10.1016/j.physbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Bevins RA. Intravenous nicotine conditions a place preference in rats using an unbiased design. Pharmacol Biochem Behav. 2008;88:256–64. doi: 10.1016/j.pbb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Glennon RA. Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol. 2002;443:113–118. doi: 10.1016/s0014-2999(02)01554-6. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Girlanda E, Varnier G, Sartori I, Zanetti L, et al. Dopamine responsiveness to drugs of abuse: A shell-core investigation in the nucleus accumbens in the mouse. Synapse. 2003;50:293–302. doi: 10.1002/syn.10271. [DOI] [PubMed] [Google Scholar]