Abstract
The formation of new cell clusters is a histological hallmark of arthritic cartilage but the biology of clusters and their role in disease are poorly understood. This is the first comprehensive review of clinical and experimental conditions associated with cluster formation. Genes and proteins that are expressed in cluster cells, the cellular origin of the clusters, mechanisms that lead to cluster formation and the role of cluster cells in pathogenesis are discussed.
Introduction
Osteoarthritis (OA) is the most prevalent joint disease and aging is among its major risk factors (1). Aging-associated deficits in cartilage cell function have been widely documented (2). Yet, the tissue destruction and remodeling process in OA-affected articular cartilage is due to cell activation with production of extracellular matrix (ECM)-degrading enzymes, inflammatory mediators as well as new synthesis of ECM components which includes some that are not present in normal adult articular cartilage (3). A histological hallmark of OA cartilage is cell clusters (4–8) and these clusters express a broad range of activation and abnormal differentiation markers, suggesting that they contribute to pathogenesis. The objective of this review is to summarize information on patterns and mechanisms of cluster formation and their role in disease.
Cell arrangements and clusters in normal cartilage
The basic cellular unit in normal articular cartilage is the chondron which is defined as consisting of one or more cells and the surrounding pericellular matrix (8, 9). Multiple chondrons that are located in direct proximity to each other in a large lacuna are referred to as clusters. The pericellular matrix contains collagen types II, VI, IX and XI, hyaluronan, proteoglycans such as aggrecan, decorin and biglycan, and glycoproteins such as fibronectin, link protein and laminin (10). Cells bind via integrins and other receptors to these matrix components and this leads within the context of the territorial matrix structures to the different cell arrangements. The orientation and shape of the chondron reflects the local collagen architecture of the interterritorial matrix, which increases in thickness with depth from the tissue surface (11). Normal mature articular cartilage contains cell arrangements that are characteristic for each zone (Figure 1A). In the superficial zone cells are arranged in horizontal clusters parallel to the articular surface, in strings and pairs of cells and single cells dispersed among these patterns (12, 13). The cell arrangements in the superficial zone vary among joints, possibly related to different mechanical loading mechanisms (13). The middle and deep zones contain double or multiple chondrons arranged as vertical columns of cells (Figure 1B) (14–16). These cell patterns are thought derive from cell proliferation, probably also cell migration and the formation and specific organization of the ECM during joint development and maturation (11, 17, 18).
Figure 1. Schematic drawing and microscopic image of cell arrangements in normal cartilage.
A. Overview of cartilage zones.
1) Each zone has characteristic cell shape, morphology, orientation, and pericellular matrix (PM) deposition.
2) Superficial zone (SZ) cells are small, elongated in shape, parallel to the surface, and lack an extensive PM. These cells predominate the first 50 µm.
3) The middle zone (MZ) is distinguishable by rounded cells that do not exhibit an organized orientation relative to the surface, are within ECM rich in proteoglycans and show presence of PM.
4, 5) Deep zone (DZ) cells show an extensive PM deposition with chondrons in groups of three or more cells arranged in columns perpendicular to the surface. Modified from (138).
B. Horizontal sections of normal cartilage. Horizontal sections of normal human articular cartilage were prepared from the SZ to tide mark and stained with safranin O. As indicated on the saggital plane cross-section, image numbers 1–4 were cut at 20µm intervals starting from superficial to middle zone. Image numbers 5–8 represent sections from the MZ, DZ and tidemark at 400µm intervals. Bars: 200µm (large). 50µm (small).
Cell clusters in OA articular cartilage and other types of cartilage
Increased numbers and sizes of cell clusters are a hallmark histological feature of OA articular cartilage (Figure 2A) and can be detected in the majority of specimens (4, 5, 8, 19, 20). Clusters of chondrocytes are often localized near fissures and clefts of the upper cartilage layer. This shortens the diffusion distance for nutrients as well as for cell mediators from the synovial fluid but also of cluster-derived mediators to the synovial space. These chondrocyte clusters are characteristically round and located within a large lacuna, and can contain more than 20 cells (12). They are clearly different from the flattened, superficial and rounded, upper-middle zones chondrocytes from nonfibrillated human cartilage (13, 19). Chondrocytes in the middle and deep zones of severe OA have increased pericellular matrix with increased type X collagen (21–25). The concentration of type VI collagen may be reduced in the superficial zone of OA cartilage (22, 23) and a significant loss of mechanical properties is correlated with the loss of pericellular type VI collagen (26, 27).
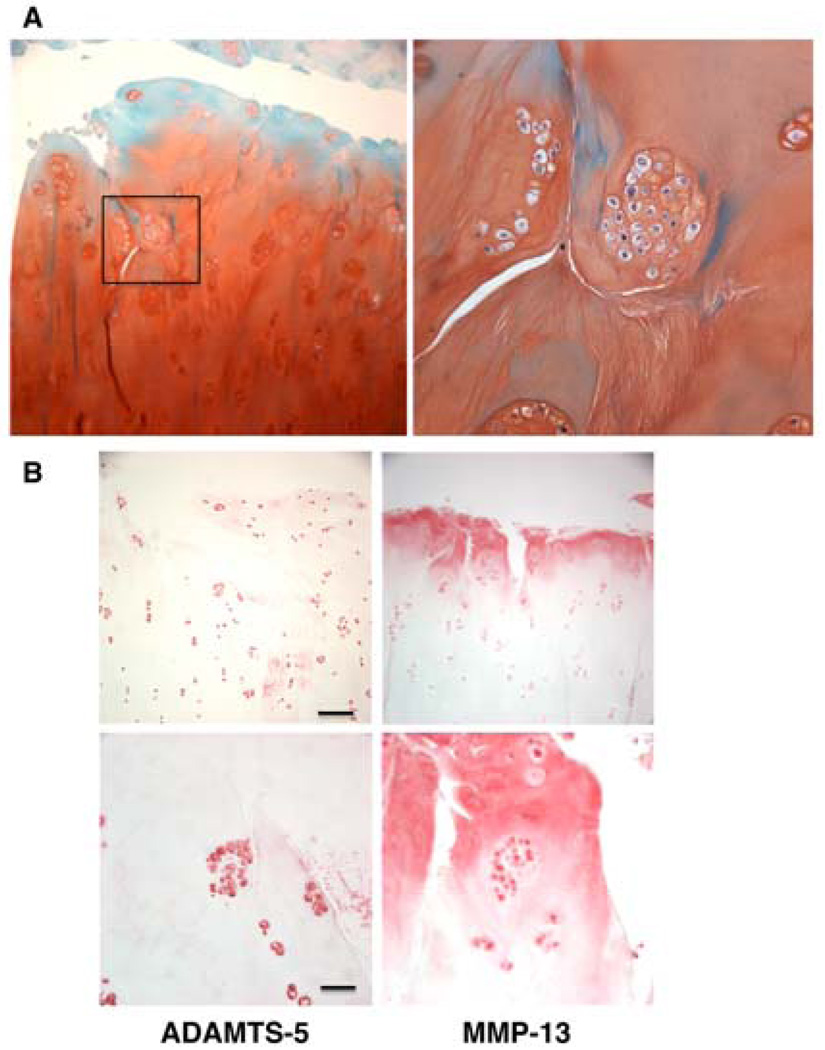
Figure 2. OA clusters.
Articular cartilage from a 75-year old male was stained with safranin O (A). The magnification was ×100 (left) and ×400 (right). (B) Articular cartilage from a 57-year old male was stained with ADAMTS-5 and MMP-13. Bar: 200µm (upper) and 50µm (lower).
Cell clusters not only form in OA-affected articular cartilage but also in the degenerated intervertebral disc (28–30), meniscus (31), fibrocartilaginous regions of tendons (32) and in cricoarytenoid cartilage (33).
Proliferation of cartilage cells in OA is a principal mechanism for the formation of clusters (34–40). This notion is supported by the incorporation of 3H-tymidine, the presence of two nuclei within the same chondron (41) and detection of proliferation-associated antigens such as PCNA and Ki67 (31, 42). The amount of proliferating chondrocytes increased during OA progression and cell division was activated specifically in cartilage with severe OA changes (43). Migration has been suggested as an alternative mechanism contributing to cluster formation based on changes in the chondrocyte cytoskeleton (19). Although chondrocytes can migrate in response to cytokines and growth factors in vitro (44, 45) information on this process in adult articular cartilage is sparse. Establishing information on the extent and patterns of cell migration in mature cartilage will potentially open avenues to better understanding of cartilage homeostasis and responses to injury.
Diseases and experimental in vivo models associated with cartilage cell clusters
In addition to OA, cluster formation has also been demonstrated in articular cartilage from patients with rheumatoid arthritis and aseptic necrosis (46) but this has not been analyzed in detail.
Kashin-Beck disease which is endemic in certain regions of Asia, features OA-like joint pathology, and is caused by a combination of different environmental factors and selenium deficiency (47). Cartilage cell death or chondronecrosis is a principal pathogenetic mechanism but clusters form in response to the cell injury and express type X collagen in human disease and in an experimental model (48). Chondrocyte clusters in cartilage from patients with Kashin-Beck disease exhibited pericellular staining for types I, II, III and VI collagen (49) and expressed several regulatory factors, including FGF-2, VEGF, TGFβ and PTHrP (50).
Experimental OA can be induced by various approaches such as transection of ligaments or meniscectomy, or injection of joints with collagenase, leading to instability due to ligament damage. Experimental OA can also be induced by intraarticular injection of agents such as iodoacetate or papain that cause chondrocyte damage or death. Chondrocyte cluster formation is a feature of all mechanical and chemical OA models (51, 52). In a partial thickness cartilage defect model a repair response as indicated by chondrocyte proliferation in clusters and a decrease in defect size was observed in immature but not mature rabbits (53). Analysis of the temporal changes in a similar model in pigs showed that there was an initial phase of cell necrosis adjacent to the cut edges. This was followed by the formation of cell clusters that expressed MMP-13 (54). In the ‘groove model’ of OA experimental femoral cartilage lesions are induced by scraping grooves with a metal wire. This leads to fibrillations on the femoral condyle and the tibial plateaus. Histological analysis showed chondrocyte clusters around the cartilage lesions (55).
Deficient or excessive mechanical loading of cartilage is also associated with cluster formation. Joint immobilization in mature rabbits led to loss of glycosaminoglycans, and fissuring. There were acellular areas of cartilage and cell clusters at 28–42 days after immobilization (56). Cartilage from strenuously trained horses showed more fibrillation and chondrocyte clusters than did the more gently exercised animals (57). Inclusion of high impact exercise in a partial meniscectomy model in rats was associated with cluster formation (58, 59). Impact loading of rat femoral head articular cartilage induced chondrocyte cluster formation as early as 4 hours after injury (60). Since chondrocyte doubling times are on the order of 24 hours (61) and this possibly suggests cell migration. Chronic repetitive stresses in vivo induced by knee ligament transection are also associated with cluster formation (62, 63).
Osteochondral allograft transplantation is used to treat cartilage defects in younger individuals. A certain amount of chondrocyte death occurs during harvesting, cryopreservation and after implantation of the osteochondral grafts. In a goat model the formation of cell clusters in the implanted grafts was detected by 8 days and the clusters increased in size and cell numbers that exceeded 100 cells per cluster after one year. The cluster cells expressed type II collagen but not type I or type X (64, 65). This model does not feature OA-like pathology or joint inflammation which may explain why cells did not show the abnormal differentiation with type X collagen expression seen in OA. The formation of such clusters expressing type II collagen may represent an initial stage of cartilage regeneration. In a rabbit allograft transplantation model there was also a marked increase in ECM and formation of round and polygonal clusters of chondrocytes in the middle and deep zones of the grafted cartilage. The adjacent host articular cartilage remained normal and did not show cell clusters (66).
Quinolone antibiotics induce an arthropathy in juvenile animals and this appears to be due to an increased sensitivity of immature chondrocytes to toxic effects of quinolones (67). Animals with the arthropathy showed cavities in the middle zone of the articular cartilage containing necrotic chondrocytes. After 14 days, many of the lacunae in the areas of the defects contained chondrocyte clusters. When treated for 14 days and allowed a 14-day recovery period, territorial matrix had been deposited around individual chondrocytes within the clusters, indicating that in immature joints there is a certain degree of spontaneous repair by cluster cells (68).
Thus, diverse types of clinical and experimental cartilage lesions are all associated with the formation of chondrocyte clusters that contain more cells than clusters in normal cartilage. In most examples of cartilage damage the cell clusters express abnormal activation and differentiation markers that appear to be due to concomitant inflammation.
In vitro models and inducers of cluster formation
Chondrons, the cells with their intact pericellular matrix can be isolated using modifications of protocols that are used for cell isolation from cartilage (69, 70). When chondrons were cultured in alginate beads or agarose constructs, various types of cell clusters formed over time. The clusters differed in morphology and ECM deposition and these differences were related to the cartilage zone from which the chondrons were isolated (69). Chondrons cultured as pellets increased in size and weight over a 6-week period without apparent cell proliferation. Chondron pellets accumulated significantly more proteoglycan and type II collagen than did chondrocyte pellets, indicating an anabolic effect of the native pericellular matrix. After 5 weeks in culture, ECM remodeling was evident in the chondron pellets. Cells that had been uniformly distributed throughout the pellets began to cluster between large areas of interterritorial matrix rich in type II collagen. After 12 weeks, clusters were stacked in columns, suggesting cell migration (70). Such enzymatically-isolated chondrons cultured in a three-dimensional matrix serve as a useful model of cluster formation.
Chondrocytes cultured as suspensions in agarose or alginate adopt a round morphology and form clusters of cells reminiscent of chondrocyte clusters in intact cartilage (71, 72). In alginate scaffolds a higher cell seeding density led to formation of larger cell clusters that expressed type II but not type I collagen (73).
Several growth factors can stimulate chondrocyte cluster formation in vitro and FGF2 appears the most important. In a genetic screen where chondrocytes were transduced with genes that are expressed in OA cartilage and then cultured in agarose, FGF2 was the most potent inducer of cluster formation. Interestingly, all other genes that induced clusters also upregulated FGF2 expression, suggesting a common FGF2-dependent pathway (74). Similar effects of FGF2 were observed in other culture models. Addition of FGF2 to cartilage explants with surgical wound incisions induced proliferation with cluster morphology, expression of Notch-1 and the proteinases MMP-13 and ADAMTS-4 (75). In mandibular condylar cartilage of aging mice TGF-β induced numerous clusters (76) while TGF-α stimulated proliferation of chondrocytes and formation of cell clusters in rat osteochondral explants. In the same model TGF-α reduced type II collagen and aggrecan while increasing MMP-13 and cathepsin C (77). Mechanical stress on chondrocyte suspension cultures also stimulated cluster formation (78).
These observations from culture models suggest that isolated chondrons and chondrocytes spontaneously form cell clusters and this can be enhanced by the addition of mitogens such as FGF2 or TGF or by mechanical stress. An important unanswered question is whether cells can migrate from the clusters and populate acellular areas or form new interterritorial matrix similar to that in normal articular cartilage.
Cytoskeleton and cilia of cluster cells
In normal chondrocytes F-actin is typically organized as a dense cortical structure, predominantly located just inside the cell membrane. The cells in fibrillated OA cartilage displayed rearranged actin filaments (19). The vimentin intermediate filaments in superficial flattened chondrocytes assemble in the form of perinuclear bundles while in the middle zone chondrocyte vimentin was perinuclear as well as in the cell periphery. On the other hand, the intermediate filaments in the cluster cells in human OA cartilage were more disorganized and sometimes completely absent (19). In experimental OA in rat knees, all three major cytoskeletal elements underwent distinct reduction in stain intensity ranging from 5% for actin to almost 40% for vimentin (79). There was also significant cytoskeletal disorganization (80). The cytoskeletal changes in OA chondrocytes are presumably linked to their abnormal activation and differentiation patterns as a close association between cytoskeletal organization and chondrocyte differentiation has been documented in various in vitro models (81, 82).
The contractile actin isoform, α-smooth muscle actin mediates the contractile behavior of cells and may be beneficial in tissue repair responses. The original observation that chondrocytes were able to contract three-dimensional matrix structures (83) led to the discovery of α-smooth muscle actin in certain types of chondrocytes (84). In normal articular cartilage 75% of cells in the superficial zone but only 10% in the deep zone express this actin isoform. In OA cartilage clusters the majority of cells expressed α-smooth muscle actin (85). It is at present unknown whether normal chondrocytes can de novo express α-smooth muscle actin or whether the increase in cells expressing α-smooth muscle actin in OA is the result of proliferation of this subset. The strong differentiation potential of α-smooth muscle actin positive cells indicates that they may represent immature chondrocytes or chondroprogenitor cells (86).
The primary cilium was initially described in motile cells but is now known to be a highly conserved, single cytoplasmic organelle in virtually all eukaryotic cells. The chondrocyte primary cilium projects into the pericellular matrix of the chondron and through integrins and other receptors interacts with matrix components such as collagens type II and VI (87–90). In addition to cell migration, the chondrocyte cilium is thought to play a role in mechanotransduction (91). Defects in perichondrial, chondroblast, and chondrocyte primary cilia have recently been implicated in both skeletal patterning and growth plate abnormalities (92–96).
In normal cartilage, the length of superficial zone chondrocyte cilia was shorter than deep zone cilia. Cilia showed a clear orientation in relation to the zone of origin; superficial zone chondrocyte cilia were consistently oriented away from the articular surface. In both mild and severe OA tissue, the proportion of ciliated cells increased markedly from the articulating surface to the tidemark. The percentage of cilia detected on chondrocytes at the joint surface increased with OA severity (97). There were also significant differences between the incidence of cilia in deep zone cells, both between normal and mild OA and mild and severe OA. The increased presence of cilia, centrioles and filopodia in and near clusters under the fibrillated region of the OA cartilage suggests that they could be motile cells (7, 19). The filopodia may also be involved in cell-to-cell contact and communication between paired chondrocytes in the superficial zone of articular cartilage (98). Small cytoplasmic projections were detected between some chondrocyte pairs in the superficial zone of adult rabbit articular cartilage. Such cell-cell interactions are likely to also occur in cell clusters but this has not been formally demonstrated.
Proteins and genes expressed in clusters
Immunohistochemical analyses of OA cartilage have drawn attention to clusters because they express a very large number of induced proteins that are not detected in normal cartilage cells (99–118). A representative list of induced proteins is shown in Table 1. Although a systematic analysis of gene or protein expression in OA clusters such as using clusters that are isolated and separated from other cartilage cells has not yet been performed, the available information suggests an activation pattern that prominently includes ECM-degrading enzymes and inflammatory mediators (Figure 2B). A variety of growth factors that is expressed in the clusters (50, 119) presumably drive the cell proliferation in the clusters and possibly in the adjacent cartilage areas. Cell clusters in OA cartilage also show changes in cell signaling proteins that mediate the cell activation. An additional pattern is that of abnormal differentiation. This is based on hypertrophy markers such as collagen X (120), osteocalcin (21), osteopontin (121), MMP-13 and Runx2 that are co-localized in nearly all chondrocytes in clusters in fibrillated OA cartilage (122). Cluster cells also express markers of immature or de-differentiated chondrocytes. Pleiotrophin is abundant in fetal or juvenile cartilage, but rarely detected in normal adult tissues. In human OA cartilage pleiotrophin was expressed in clusters of superficial chondrocytes (123). It needs to be determined whether these activation markers are shared by all cluster cells or whether some cells express inflammatory mediators while others express growth factors.
Table 1.
Gene and protein expression patterns in OA cartilage cell clusters.
| Receptors, Kinases, Transcription factors |
Sox9(73,138) |
| Runx2(122) | |
| C-maf (99) | |
| Leptin/ Leptin receptor (28) | |
| c-Fos,c-Jun(AP-1)(30) | |
| HMGB1 (100) | |
| Cytokines, Growth factors |
BMP-7(OP-1)(101) |
| FGF2(102) | |
| BMP-2,−4,−6(103) | |
| PGE2, COX-1,−2(104) | |
| Histamine (105) | |
| S-100(106) | |
| IL-1β,TNFa(107) | |
| Pleiotrophin(123) | |
| WISP3 (110) | |
| ECM, ECM-degrading Enzymes |
Aggrecan (72,73) |
| Procollagen (64) | |
| Col I (72) | |
| Col II (64, 69, 72, 73) | |
| Col VI (23) | |
| Col X (120) | |
| TIHP-1 (54) | |
| 5ZP(12) | |
| MMP-1,−8,−13(107) | |
| MMP-2, −3, −8,−9(108) | |
| ADAMTS-10(109) | |
| Cathepsin K (113), D (114), B (115) | |
| Suits (116) | |
| Syndecan-4(116) | |
| Cell Death | Apoptosis (103,111) |
| Autophagy (111, 112) | |
| Progenitor Cell Markers |
Notch-1 (118,138) |
| Stro-1 (138, 116, 141) | |
| VCAM-1 (138) | |
| Ann ex in VI (42) | |
| Plelotrophln(123) |
These findings indicate that OA cluster cells are activated and represent an important source of pathogenetic mediators. At present the relative contribution of cells in the original cartilage areas versus of those in the expanded clusters is unknown but it is apparent that cluster cells are a major contributor to the overall increase in gene and protein expression observed in OA cartilage as detected in DNA array and proteomics analyses (124–126).
Cell death and crystal deposition in clusters
A series of studies examined apoptosis in cartilage from human and experimental OA and consistently reported apoptotic cells in the clusters (127–129). It is unclear what induces cluster cell apoptosis but the selective death of only some cells in each cluster argues against general mechanisms such as nutrient or oxygen deprivation. Potential mechanisms are expression of programmed cell death receptors and ligands that may in part be related to the abnormal hypertrophic differentiation program.
A scenario that encompasses cluster cell hypertrophy, cell death and matrix calcification can be proposed. Crystal deposition was described in the pericellular regions near the surface zone of OA articular cartilage and this was coupled with increased numbers of matrix vesicles and alkaline phosphatase activity (130). There is abundant evidence for the expression of hypertrophic markers in the clusters (see above). In addition, cluster cells express pyrophosphate-generating enzymes (131). Matrix calcification in growth plate physiologically involves cell death and the generation of matrix vesicles and chondrocyte-derived apoptotic bodies can serve similar functions (132). Apoptotic chondrocytes in OA cartilage showed abundant alkaline phosphatase-rich matrix vesicles budding from the plasma membrane with hydroxyapatite microcrystals on their surface (133). Staining of cartilage sections showed co-localization of clusters containing apoptotic cells adjacent to calcium deposits (131) (Figure 3). In OA-affected menisci the presence of type X collagen and deposition of calcium as detected with alizarin red also suggested the possibility that mineralization of the extracellular matrix surrounding the cell clusters was occurring (31).
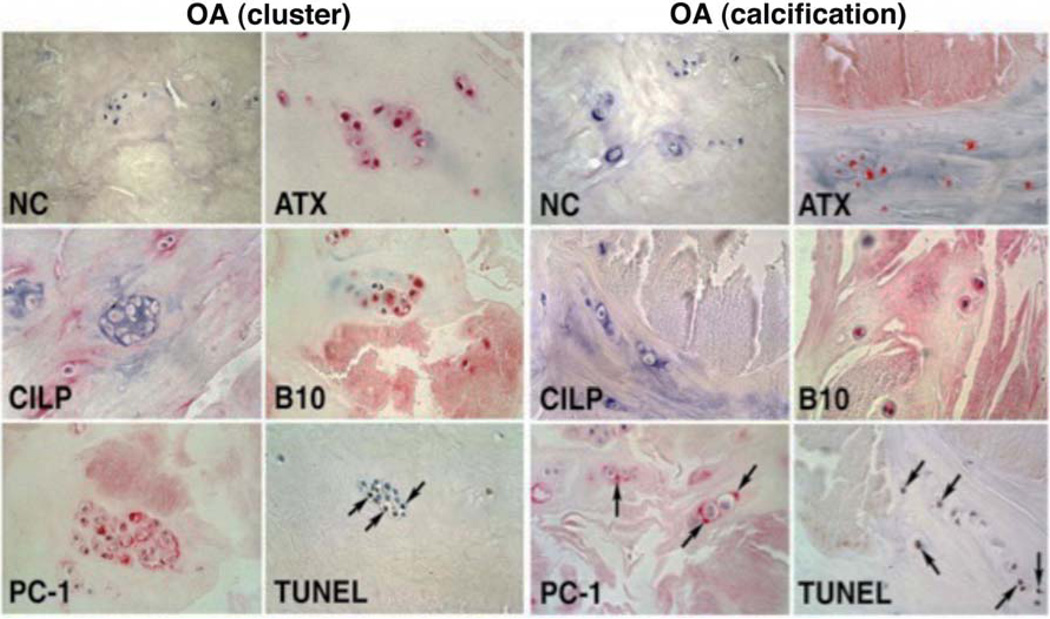
Figure 3. OA cluster cell apoptosis and calcification.
Localization of TUNEL-positive cells, calcium deposits and pyrophosphate-generating enzymes in menisci from OA-affected human knees. The left panel shows apoptotic cells, many in clusters, in the vicinity of (alizarin red-positive) calcified areas. The right panel shows cells immediately bordering calcifications. Staining for plasma cell membrane glycoprotein (PC-1), autotaxin (ATX) and B10 is also prominent at sites of calcification and in areas with TUNEL-positive cells. CILP: cartilage intermediate layer protein. Modified from (131).
Stem cell markers in cartilage and clusters
The abnormal activation and differentiation pattern of cluster cells in OA cartilage has been interpreted as chondrocyte de-dedifferentiation (3) where the differentiated articular chondrocytes change gene expression patterns in response to the different extracellular signaling environment (134). An alternative scenario is that cluster formation is the function of a unique subpopulation of progenitor cells. A series of recent studies suggests the presence of cells with phenotypic markers and functions of progenitor cells in mature articular cartilage (135–137). Cell surface markers such as Stro-1 or Notch-1 were most strongly expressed in the superficial zone (136–138). Notch-1 was also densely localized in the deeper zone of articular cartilage (138, 139). A prominent feature of OA cartilage is the strong expression of stem cell markers in the cell clusters (138) (Figure 4). Surgical injury to articular cartilage is also associated with proliferation of progenitor cells that produce new extracellular matrix (140). Migratory chondrogenic progenitor cells were recently described in OA joints (141) and they resemble cells that are seen in the clusters. A systematic analysis of the fate of cells expressing progenitor markers during the development of OA is required to address this hypothesis.
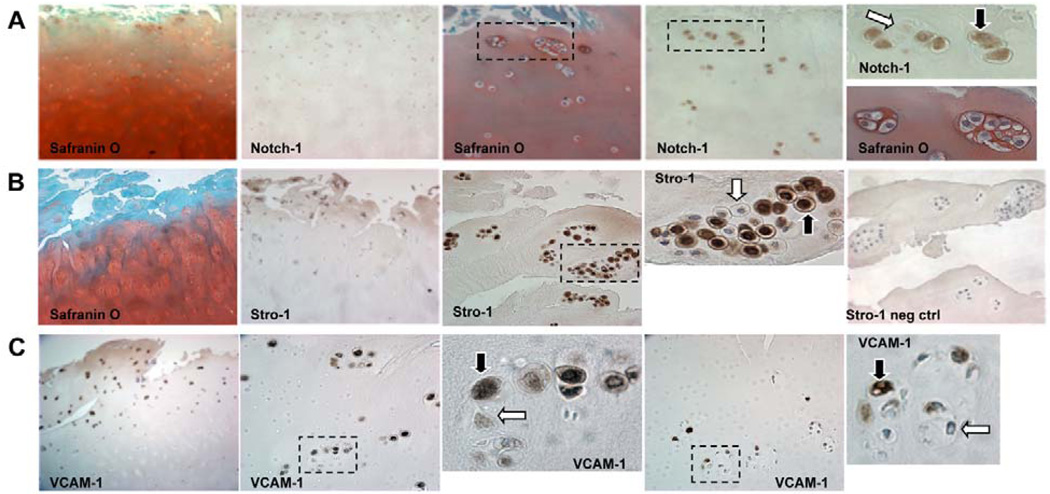
Figure 4. OA cluster staining for stem cell markers.
A majority of cells in clusters (69 to 79%) are positive for Notch-1, Stro-1 and VCAM-1. Clusters located in the DZ had significantly reduced frequencies of Stro-1 positive cells. (A) Safranin O and Notch-1 staining in clusters (×10 and ×40). (B) Safranin O and Stro-1 staining of OA cartilage sections (×10 and ×40). (C) OA cartilage sections immunostained for VCAM-1 (×10 and ×40). Positive staining indicated by black arrows and negative with white arrows. Modified from (138).
Summary and Conclusions
Cell organization in clusters represents an interesting and as yet not well-characterized phenomenon in normal articular cartilage, in tissue responses to chemical or mechanical injury and prominently in cartilage affected by diseases such as OA. Cluster formation also occurs readily in culture models suggesting an intrinsic tendency and capacity of certain cartilage cells to assume this cellular organization.
It can be proposed that repair of cartilage lesions that include both ECM damage and cell death would require replication of some of the cells adjacent to the damaged area, followed by migration, differentiation and new matrix formation (140). It appears that cluster formation represents the first phase of this response. Clusters are seen with a remarkable uniformity in cartilage exposed to a broad range of injuries that are always associated with some degree of cell death. Whether any surviving cell or only certain subsets of cells are able to proliferate and form clusters is unknown. An interesting hypothesis is that it is a function of immature or progenitor cells. The triggers for cell proliferation may include loss of cell-cell interactions due to cell death. Liberation of growth factors such as FGF2 from damaged tissue appears to be an important mechanism for the generation of mitogenic stimuli at least during the initial stages of cluster formation. The rate and size of cluster formation depend on the type of injury and maturity of cartilage. Cells within clusters have certain features suggesting that they can migrate but whether cells do indeed migrate from the clusters to acellular areas of cartilage is an important unresolved question of importance to understanding intrinsic cartilage repair as well as to cartilage engineering approaches. Movements of single cells or collective movements of interconnected groups or clusters of cells have recently been characterized as important mechanisms in tissue and organ development (142). Cell-cell adhesions, cell-matrix interactions, contractility and the ability of cells to form protrusions and migrate as described for the cluster cells contribute to active motility.
Tissue engineering of chondral and osteochondral grafts is being actively pursued as a potential method of surgically repairing cartilage lesions. Cluster formation occurs in three-dimensional cultures of chondrocytes and needs to be carefully evaluated in engineered constructs using scaffolds with stem cells. Spontaneous chondrocyte cluster formation has also been noted in osteochondral allografts in several animal models (66). These clusters did not appear to express the abnormal markers seen in OA clusters. However, it must be noted that these studies were conducted in tissue or cells from young adult animals and in joints that did not have an inflammatory response. Collectively, these findings raise a concern for tissue engineering during the pre-implantation cell proliferation and matrix generation phases as well as the postoperative phase after implantation in the chondral lesion.
Clusters that form in arthritic articular cartilage contain cells that express a large number of pathogenic mediators and thus appear the site of much of the increased biosynthetic activity that characterizes OA cartilage. The activation patterns are those of a disordered or mixed differentiation phenotype. This may be due to the influence of inflammatory mediators produced in the clusters or by other inflamed joint tissues. The activation of cluster cells and their products appear to contribute to the manifestations of cartilage diseases such as ECM degradation and calcification, and joint inflammation. The cluster cells illustrate the ability of a subpopulation of cartilage cells to undergo activation and proliferation even in older individuals. Correcting the abnormal differentiation and harnessing the reparative potential of these cells pharmacologically may offer new approaches to cartilage repair and OA therapy.
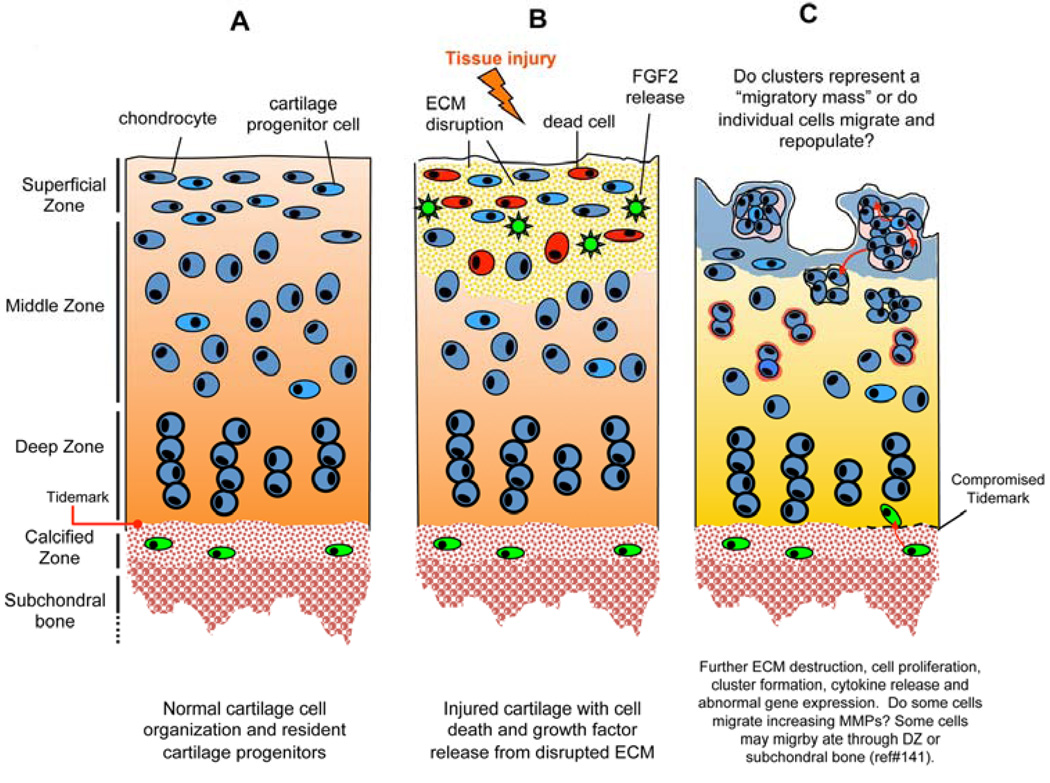
Figure 5. Schematic drawing of mechanisms and phases of cluster formation.
(A). Normal cartilage cell organization and resident cartilage progenitors. (B). Injured cartilage with cell death and growth factor release (e.g. FGF2) from disrupted ECM. (C). Further ECM destruction, cell proliferation, cluster formation, cytokine release and abnormal gene expression. Some cells may migrate through DZ or subchondral bone (141).
Acknowledgments
Supported by NIH grants AG007996, AR058954, AR044058, AR054135, AR051565 the Sam and Rose Stein Endowment Fund, the Arthritis Foundation, and the Veterans Administration Research Service.
REFERENCES
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3(2):107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins . The pathology of articular and spinal diseases. Edward Arnold & Co; 1949. p. 331. [Google Scholar]
- 5.Mankin HJ, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970;52(3):424–434. [PubMed] [Google Scholar]
- 6.Poole CA, Matsuoka A, Schofield JR. Chondrons from articular cartilage. III. Morphologic changes in the cellular microenvironment of chondrons isolated from osteoarthritic cartilage. Arthritis Rheum. 1991;34(1):22–35. doi: 10.1002/art.1780340105. [DOI] [PubMed] [Google Scholar]
- 7.Kouri JB, Arguello C, Luna J, Mena R. Use of microscopical techniques in the study of human chondrocytes from osteoarthritic cartilage: an overview. Microsc Res Tech. 1998;40(1):22–36. doi: 10.1002/(SICI)1097-0029(19980101)40:1<22::AID-JEMT4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Lee GM, Paul TA, Slabaugh M, Kelley SS. The incidence of enlarged chondrons in normal and osteoarthritic human cartilage and their relative matrix density. Osteoarthritis Cartilage. 2000;8(1):44–52. doi: 10.1053/joca.1999.0269. [DOI] [PubMed] [Google Scholar]
- 9.Poole CA, Wotton SF, Duance VC. Localization of type IX collagen in chondrons isolated from porcine articular cartilage and rat chondrosarcoma. Histochem J. 1988;20(10):567–574. doi: 10.1007/BF01002611. [DOI] [PubMed] [Google Scholar]
- 10.Poole CA. Articular cartilage chondrons: form, function and failure. J Anat. 1997;191(Pt 1):1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youn I, Choi JB, Cao L, Setton LA, Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage. 2006;14(9):889–897. doi: 10.1016/j.joca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Schumacher BL, Su JL, Lindley KM, Kuettner KE, Cole AA. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat Rec. 2002;266(4):241–248. doi: 10.1002/ar.10063. [DOI] [PubMed] [Google Scholar]
- 13.Rolauffs B, Williams JM, Grodzinsky AJ, Kuettner KE, Cole AA. Distinct horizontal patterns in the spatial organization of superficial zone chondrocytes of human joints. J Struct Biol. 2008;162(2):335–344. doi: 10.1016/j.jsb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tissue Res. 1988;18(3):223–234. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- 15.Brighton CT, Kitajima T, Hunt RM. Zonal analysis of cytoplasmic components of articular cartilage chondrocytes. Arthritis Rheum. 1984;27(11):1290–1299. doi: 10.1002/art.1780271112. [DOI] [PubMed] [Google Scholar]
- 16.Kuettner KE, Aydelotte MB, Thonar EJ. Articular cartilage matrix and structure: a minireview. J Rheumatol Suppl. 1991;27:46–48. [PubMed] [Google Scholar]
- 17.Morrison EH, Ferguson MW, Bayliss MT, Archer CW. The development of articular cartilage: I. The spatial and temporal patterns of collagen types. J Anat. 1996;189(Pt 1):9–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Chi SS, Rattner JB, Matyas JR. Communication between paired chondrocytes in the superficial zone of articular cartilage. J Anat. 2004;205(5):363–370. doi: 10.1111/j.0021-8782.2004.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouri JB, Jimenez SA, Quintero M, Chico A. Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthritis Cartilage. 1996;4(2):111–125. doi: 10.1016/s1063-4584(05)80320-6. [DOI] [PubMed] [Google Scholar]
- 20.Goyal N, Gupta M, Joshi K, Nagi ON. Osteoarthritic femoral articular cartilage of knee joint in man. Nepal Med Coll J. 2006;8(2):88–92. [PubMed] [Google Scholar]
- 21.Pullig O, Weseloh G, Ronneberger D, Kakonen S, Swoboda B. Chondrocyte differentiation in human osteoarthritis: expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif Tissue Int. 2000;67(3):230–240. doi: 10.1007/s002230001108. [DOI] [PubMed] [Google Scholar]
- 22.Hambach L, Neureiter D, Zeiler G, Kirchner T, Aigner T. Severe disturbance of the distribution and expression of type VI collagen chains in osteoarthritic articular cartilage. Arthritis Rheum. 1998;41(6):986–996. doi: 10.1002/1529-0131(199806)41:6<986::AID-ART5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Pullig O, Weseloh G, Swoboda B. Expression of type VI collagen in normal and osteoarthritic human cartilage. Osteoarthritis Cartilage. 1999;7(2):191–202. doi: 10.1053/joca.1998.0208. [DOI] [PubMed] [Google Scholar]
- 24.Horikawa O, Nakajima H, Kikuchi T, Ichimura S, Yamada H, Fujikawa K, et al. Distribution of type VI collagen in chondrocyte microenvironment: study of chondrons isolated from human normal and degenerative articular cartilage and cultured chondrocytes. J Orthop Sci. 2004;9(1):29–36. doi: 10.1007/s00776-003-0737-4. [DOI] [PubMed] [Google Scholar]
- 25.McDevitt CA, Lipman JM, Ruemer RJ, Sokoloff L. Stimulation of matrix formation in rabbit chondrocyte cultures by ascorbate. 2. Characterization of proteoglycans. J Orthop Res. 1988;6(4):518–524. doi: 10.1002/jor.1100060407. [DOI] [PubMed] [Google Scholar]
- 26.Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng. 2003;125(3):323–333. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005;38(3):509–517. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Zhao CQ, Liu D, Li H, Jiang LS, Dai LY. Expression of leptin and its functional receptor on disc cells: contribution to cell proliferation. Spine (Phila Pa 1976) 2008;33(23):E858–E864. doi: 10.1097/BRS.0b013e31818338e5. [DOI] [PubMed] [Google Scholar]
- 29.Sowa G, Vadala G, Studer R, Kompel J, Iucu C, Georgescu H, et al. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008;33(17):1821–1828. doi: 10.1097/BRS.0b013e31817e2ce3. [DOI] [PubMed] [Google Scholar]
- 30.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju E. Oncoprotein c-Fos and c-Jun immunopositive cells and cell clusters in herniated intervertebral disc tissue. Eur Spine J. 2002;11(5):452–458. doi: 10.1007/s00586-001-0383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellio Le Graverand MP, Sciore P, Eggerer J, Rattner JP, Vignon E, Barclay L, et al. Formation and phenotype of cell clusters in osteoarthritic meniscus. Arthritis Rheum. 2001;44(8):1808–1818. doi: 10.1002/1529-0131(200108)44:8<1808::AID-ART318>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin M, Tyers RN, Ralphs JR. Age-related changes in tendon fibrocartilage. J Anat. 1991;179:127–136. [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen FP, Tillmann BN. Osteoarthritis in cricoarytenoid joint. Osteoarthritis Cartilage. 1999;7(6):505–514. doi: 10.1053/joca.1999.0253. [DOI] [PubMed] [Google Scholar]
- 34.Mankin HJ. Localization of Tritiated Thymidine in Articular Cartilage of Rabbits: I. Growth In Immature Cartilage. J Bone Joint Surg Am. 1962;44(4):682–688. [Google Scholar]
- 35.Mankin HJ. Localization of Tritiated Thymidine in Articular Cartilage of Rabbits: II. Repair in Immature Cartilage. J Bone Joint Surg Am. 1962;44(4):688–698. [Google Scholar]
- 36.Telhag H. Mitosis of chondrocytes in experimental "osteoarthritis" in rabbits. Clin Orthop Relat Res. 1972;86:224–229. doi: 10.1097/00003086-197207000-00034. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell AG, Bentley G. Chondrocyte multiplication in osteoarthritic articular cartilage. J Bone Joint Surg Br. 1973;55(3):588–594. [PubMed] [Google Scholar]
- 38.Hirotani H, Ito T. Chondrocyte mitosis in the articular cartilage of femoral heads with various diseases. Acta Orthop Scand. 1975;46(6):979–986. doi: 10.3109/17453677508989287. [DOI] [PubMed] [Google Scholar]
- 39.Havdrup T, Telhag H. Scattered mitosis in adult joint cartilage after partial chondrectomy. A histological, autoradiographical and biochemical study in rabbits. Acta Orthop Scand. 1978;49(5):424–429. doi: 10.3109/17453677808993256. [DOI] [PubMed] [Google Scholar]
- 40.Rotzer A, Mohr W. [3H-thymidine incorporation into chondrocytes of arthritic cartilage] Z Rheumatol. 1992;51(4):172–176. [PubMed] [Google Scholar]
- 41.Lee DA, Bentley G, Archer CW. The control of cell division in articular chondrocytes. Osteoarthritis Cartilage. 1993;1(2):137–146. doi: 10.1016/s1063-4584(05)80029-9. [DOI] [PubMed] [Google Scholar]
- 42.Pfander D, Swoboda B, Kirsch T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am J Pathol. 2001;159(5):1777–1783. doi: 10.1016/S0002-9440(10)63024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfander D, Kortje D, Weseloh G, Swoboda B. [Cell proliferation in human arthrotic joint cartilage] Z Orthop Ihre Grenzgeb. 2001;139(5):375–381. doi: 10.1055/s-2001-17977. [DOI] [PubMed] [Google Scholar]
- 44.Chang C, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthritis Cartilage. 2003;11(8):603–612. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 45.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26(10):1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 46.Kusuzaki K, Sugimoto S, Takeshita H, Murata H, Hashiguchi S, Nozaki T, et al. DNA cytofluorometric analysis of chondrocytes in human articular cartilages under normal aging or arthritic conditions. Osteoarthritis Cartilage. 2001;9(7):664–670. doi: 10.1053/joca.2001.0463. [DOI] [PubMed] [Google Scholar]
- 47.Moreno-Reyes R, Suetens C, Mathieu F, Begaux F, Zhu D, Rivera MT, et al. Kashin-Beck osteoarthropathy in rural Tibet in relation to selenium and iodine status. N Engl J Med. 1998;339(16):1112–1120. doi: 10.1056/NEJM199810153391604. [DOI] [PubMed] [Google Scholar]
- 48.Downey CM, Horton CR, Carlson BA, Parsons TE, Hatfield DL, Hallgrimsson B, et al. Osteo-chondroprogenitor-specific deletion of the selenocysteine tRNA gene, Trsp, leads to chondronecrosis and abnormal skeletal development: a putative model for Kashin-Beck disease. PLoS Genet. 2009;5(8):e1000616. doi: 10.1371/journal.pgen.1000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo X, Thomas A, Pirkko L. [A study on abnormal chondrocyte differentiation and abnormal expression of collagen types in articular cartilage from patients with Kaschin-Beck disease] Zhonghua Bing Li Xue Za Zhi. 1998;27(1):19–22. [PubMed] [Google Scholar]
- 50.Guo X, Zuo H, Cao CX, Zhang Y, Geng D, Zhang ZT, et al. Abnormal expression of Col X, PTHrP, TGF-beta, bFGF, and VEGF in cartilage with Kashin-Beck disease. J Bone Miner Metab. 2006;24(4):319–328. doi: 10.1007/s00774-006-0690-3. [DOI] [PubMed] [Google Scholar]
- 51.Moriizumi T, Yamashita N, Okada Y. Papain-induced changes in the guinea pig knee joint with special reference to cartilage healing. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(6):461–474. doi: 10.1007/BF02899052. [DOI] [PubMed] [Google Scholar]
- 52.van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. Development of osteoarthritic lesions in mice by "metabolic" and "mechanical" alterations in the knee joints. Am J Pathol. 1989;135(6):1001–1014. [PMC free article] [PubMed] [Google Scholar]
- 53.Bos PK, Verhaar JA, van Osch GJ. Age-related differences in articular cartilage wound healing: a potential role for transforming growth factor beta1 in adult cartilage repair. Adv Exp Med Biol. 2006;585:297–309. doi: 10.1007/978-0-387-34133-0_20. [DOI] [PubMed] [Google Scholar]
- 54.Hembry RM, Dyce J, Driesang I, Hunziker EB, Fosang AJ, Tyler JA, et al. Immunolocalization of matrix metalloproteinases in partial-thickness defects in pig articular cartilage. A preliminary report. J Bone Joint Surg Am. 2001;83-A(6):826–838. doi: 10.2106/00004623-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Marijnissen AC, van Roermund PM, TeKoppele JM, Bijlsma JW, Lafeber FP. The canine 'groove' model, compared with the ACLT model of osteoarthritis. Osteoarthritis Cartilage. 2002;10(2):145–155. doi: 10.1053/joca.2001.0491. [DOI] [PubMed] [Google Scholar]
- 56.Okazaki R, Sakai A, Nakamura T, Kunugita N, Norimura T, Suzuki K. Effects of transforming growth factor beta s and basic fibroblast growth factor on articular chondrocytes obtained from immobilised rabbit knees. Ann Rheum Dis. 1996;55(3):181–186. doi: 10.1136/ard.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray RC, Zhu CF, Goodship AE, Lakhani KH, Agrawal CM, Athanasiou KA. Exercise affects the mechanical properties and histological appearance of equine articular cartilage. J Orthop Res. 1999;17(5):725–731. doi: 10.1002/jor.1100170516. [DOI] [PubMed] [Google Scholar]
- 58.Lozoya KA, Flores JB. A novel rat osteoarthrosis model to assess apoptosis and matrix degradation. Pathol Res Pract. 2000;196(11):729–745. [PubMed] [Google Scholar]
- 59.Appleton CT, McErlain DD, Henry JL, Holdsworth DW, Beier F. Molecular and histological analysis of a new rat model of experimental knee osteoarthritis. Ann N Y Acad Sci. 2007;1117:165–174. doi: 10.1196/annals.1402.022. [DOI] [PubMed] [Google Scholar]
- 60.Henson FM, Vincent TA. Alterations in the vimentin cytoskeleton in response to single impact load in an in vitro model of cartilage damage in the rat. BMC Musculoskelet Disord. 2008;9:94. doi: 10.1186/1471-2474-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Favaretto AL, Lins CE, Felipe MS, Da Cruz WB. [Characterization of a chondrocyte primary culture from rib cartilage of the rat] Rev Bras Biol. 1989;49(3):731–736. [PubMed] [Google Scholar]
- 62.Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4(2):87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]
- 63.D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54(6):1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 64.Muldrew K, Chung M, Novak K, Schachar NS, Zernicke RF, McGann LE, et al. Evidence of chondrocyte repopulation in adult ovine articular cartilage following cryoinjury and long-term transplantation. Osteoarthritis Cartilage. 2001;9(5):432–439. doi: 10.1053/joca.2000.0409. [DOI] [PubMed] [Google Scholar]
- 65.Muldrew K, Novak K, Studholme C, Wohl G, Zernicke R, Schachar NS, et al. Transplantation of articular cartilage following a step-cooling cryopreservation protocol. Cryobiology. 2001;43(3):260–267. doi: 10.1006/cryo.2001.2349. [DOI] [PubMed] [Google Scholar]
- 66.Makino T, Fujioka H, Kurosaka M, Matsui N, Yoshihara H, Tsunoda M, et al. Histologic analysis of the implanted cartilage in an exact-fit osteochondral transplantation model. Arthroscopy. 2001;17(7):747–751. doi: 10.1053/jars.2001.24705. [DOI] [PubMed] [Google Scholar]
- 67.Yabe K, Murakami Y, Nishida S, Sekiguchi M, Furuham K, Goryo M, et al. A non-arthropathic dose and its disposition following repeated oral administration of ofloxacin, a new quinolone antimicrobial agent, to juvenile dogs. J Vet Med Sci. 2001;63(8):867–872. doi: 10.1292/jvms.63.867. [DOI] [PubMed] [Google Scholar]
- 68.Sharpnack DD, Mastin JP, Childress CP, Henningsen GM. Quinolone arthropathy in juvenile New Zealand white rabbits. Lab Anim Sci. 1994;44(5):436–442. [PubMed] [Google Scholar]
- 69.Ross JM, Sherwin AF, Poole CA. In vitro culture of enzymatically isolated chondrons: a possible model for the initiation of osteoarthritis. J Anat. 2006;209(6):793–806. doi: 10.1111/j.1469-7580.2006.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graff RD, Kelley SS, Lee GM. Role of pericellular matrix in development of a mechanically functional neocartilage. Biotechnol Bioeng. 2003;82(4):457–464. doi: 10.1002/bit.10593. [DOI] [PubMed] [Google Scholar]
- 71.Kolettas E, Buluwela L, Bayliss MT, Muir HI. Expression of cartilage-specific molecules is retained on long-term culture of human articular chondrocytes. J Cell Sci. 1995;108(Pt 5):1991–1999. doi: 10.1242/jcs.108.5.1991. [DOI] [PubMed] [Google Scholar]
- 72.Lin YJ, Yen CN, Hu YC, Wu YC, Liao CJ, Chu IM. Chondrocytes culture in three-dimensional porous alginate scaffolds enhanced cell proliferation, matrix synthesis and gene expression. J Biomed Mater Res A. 2009;88(1):23–33. doi: 10.1002/jbm.a.31841. [DOI] [PubMed] [Google Scholar]
- 73.Yen CN, Lin YR, Chang MD, Tien CW, Wu YC, Liao CJ, et al. Use of porous alginate sponges for substantial chondrocyte expansion and matrix production: effects of seeding density. Biotechnol Prog. 2008;24(2):452–457. doi: 10.1021/bp0702828. [DOI] [PubMed] [Google Scholar]
- 74.Quintavalla J, Kumar C, Daouti S, Slosberg E, Uziel-Fusi S. Chondrocyte cluster formation in agarose cultures as a functional assay to identify genes expressed in osteoarthritis. J Cell Physiol. 2005;204(2):560–566. doi: 10.1002/jcp.20345. [DOI] [PubMed] [Google Scholar]
- 75.Khan IM, Bishop JC, Gilbert S, Archer CW. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthritis Cartilage. 2009;17(4):518–528. doi: 10.1016/j.joca.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Livne E. In vitro response of articular cartilage from mature mice to human transforming growth factor beta. Acta Anat (Basel) 1994;149(3):185–194. doi: 10.1159/000147575. [DOI] [PubMed] [Google Scholar]
- 77.Appleton CT, Usmani SE, Bernier SM, Aigner T, Beier F. Transforming growth factor alpha suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis. Arthritis Rheum. 2007;56(11):3693–3705. doi: 10.1002/art.22968. [DOI] [PubMed] [Google Scholar]
- 78.Maeda S, Nishida J, Sato T, Inomata Y, Shimamura T, Horiuchi S. Changes in microstructure and gene expression of articular chondrocytes cultured in a tube under mechanical stress. Osteoarthritis Cartilage. 2005;13(2):154–161. doi: 10.1016/j.joca.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 79.Capin-Gutierrez N, Talamas-Rohana P, Gonzalez-Robles A, Lavalle-Montalvo C, Kouri JB. Cytoskeleton disruption in chondrocytes from a rat osteoarthrosic (OA) -induced model: its potential role in OA pathogenesis. Histol Histopathol. 2004;19(4):1125–1132. doi: 10.14670/HH-19.1125. [DOI] [PubMed] [Google Scholar]
- 80.Lambrecht S, Verbruggen G, Verdonk PC, Elewaut D, Deforce D. Differential proteome analysis of normal and osteoarthritic chondrocytes reveals distortion of vimentin network in osteoarthritis. Osteoarthritis Cartilage. 2008;16(2):163–173. doi: 10.1016/j.joca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 82.Woods A, Wang G, Beier F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol. 2007;213(1):1–8. doi: 10.1002/jcp.21110. [DOI] [PubMed] [Google Scholar]
- 83.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, et al. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18(11):769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 84.Kim AC, Spector M. Distribution of chondrocytes containing alpha-smooth muscle actin in human articular cartilage. J Orthop Res. 2000;18(5):749–755. doi: 10.1002/jor.1100180511. [DOI] [PubMed] [Google Scholar]
- 85.Povysil C, Kana R, Dundr P, Tvrdik D, Horak M, Vaculik J, et al. Distribution of chondrocytes containing alpha-smooth muscle actin in human normal, osteoarthrotic, and transplanted articular cartilage. Pathol Res Pract. 2008;204(12):883–890. doi: 10.1016/j.prp.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Kinner B, Gerstenfeld LC, Einhorn TA, Spector M. Expression of smooth muscle actin in connective tissue cells participating in fracture healing in a murine model. Bone. 2002;30(5):738–745. doi: 10.1016/s8756-3282(02)00695-6. [DOI] [PubMed] [Google Scholar]
- 87.Wilsman NJ, Farnum CE, Reed-Aksamit DK. Incidence and morphology of equine and murine chondrocytic cilia. Anat Rec. 1980;197(3):355–361. doi: 10.1002/ar.1091970309. [DOI] [PubMed] [Google Scholar]
- 88.Poole CA, Jensen CG, Snyder JA, Gray CG, Hermanutz VL, Wheatley DN. Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol Int. 1997;21(8):483–494. doi: 10.1006/cbir.1997.0177. [DOI] [PubMed] [Google Scholar]
- 89.Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, et al. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28(2):101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 90.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54(9):1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 91.Poole CA, Flint MH, Beaumont BW. Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil. 1985;5(3):175–193. doi: 10.1002/cm.970050302. [DOI] [PubMed] [Google Scholar]
- 92.Gouttenoire J, Valcourt U, Bougault C, Aubert-Foucher E, Arnaud E, Giraud L, et al. Knockdown of the intraflagellar transport protein IFT46 stimulates selective gene expression in mouse chondrocytes and affects early development in zebrafish. J Biol Chem. 2007;282(42):30960–30973. doi: 10.1074/jbc.M705730200. [DOI] [PubMed] [Google Scholar]
- 93.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, et al. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134(2):307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 94.McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007;26(4):234–246. doi: 10.1016/j.matbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, et al. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134(16):2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- 96.Kaushik AP, Martin JA, Zhang Q, Sheffield VC, Morcuende JA. Cartilage abnormalities associated with defects of chondrocytic primary cilia in Bardet-Biedl syndrome mutant mice. J Orthop Res. 2009;27(8):1093–1099. doi: 10.1002/jor.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McGlashan SR, Cluett EC, Jensen CG, Poole CA. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn. 2008;237(8):2013–2020. doi: 10.1002/dvdy.21501. [DOI] [PubMed] [Google Scholar]
- 98.Thompson AM, Stockwell RA. An ultrastructural study of the marginal transitional zone in the rabbit knee joint. J Anat. 1983;136(Pt 4):701–713. [PMC free article] [PubMed] [Google Scholar]
- 99.Li T, Xiao J, Wu Z, Qiu G. Over-expression of c-maf by chondrocytes in osteoarthritis. J Int Med Res. 2009;37(1):129–135. doi: 10.1177/147323000903700115. [DOI] [PubMed] [Google Scholar]
- 100.Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106(4):1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chubinskaya S, Merrihew C, Cs-Szabo G, Mollenhauer J, McCartney J, Rueger DC, et al. Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem. 2000;48(2):239–250. doi: 10.1177/002215540004800209. [DOI] [PubMed] [Google Scholar]
- 102.Khan IM, Palmer EA, Archer CW. Fibroblast growth factor-2 induced chondrocyte cluster formation in experimentally wounded articular cartilage is blocked by soluble Jagged-1. Osteoarthritis Cartilage. 2009 doi: 10.1016/j.joca.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 103.Garimella R, Bi X, Camacho N, Sipe JB, Anderson HC. Primary culture of rat growth plate chondrocytes: an in vitro model of growth plate histotype, matrix vesicle biogenesis and mineralization. Bone. 2004;34(6):961–970. doi: 10.1016/j.bone.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 104.Garbacki N, Angenot L, Bassleer C, Damas J, Tits M. Effects of prodelphinidins isolated from Ribes nigrum on chondrocyte metabolism and COX activity. Naunyn Schmiedebergs Arch Pharmacol. 2002;365(6):434–441. doi: 10.1007/s00210-002-0553-y. [DOI] [PubMed] [Google Scholar]
- 105.Tetlow LC, Woolley DE. Histamine stimulates the proliferation of human articular chondrocytes in vitro and is expressed by chondrocytes in osteoarthritic cartilage. Ann Rheum Dis. 2003;62(10):991–994. doi: 10.1136/ard.62.10.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen F, Kerner MB, Dorfman HD, Hamerman D. The distribution of S-100 protein in articular cartilage from osteoarthritic joints. J Rheumatol. 1990;17(12):1676–1681. [PubMed] [Google Scholar]
- 107.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 108.Gepstein A, Arbel G, Blumenfeld I, Peled M, Livne E. Association of metalloproteinases, tissue inhibitors of matrix metalloproteinases, and proteoglycans with development, aging, and osteoarthritis processes in mouse temporomandibular joint. Histochem Cell Biol. 2003;120(1):23–32. doi: 10.1007/s00418-003-0544-1. [DOI] [PubMed] [Google Scholar]
- 109.Chubinskaya S, Cs-Szabo G, Kuettner KE. ADAM-10 message is expressed in human articular cartilage. J Histochem Cytochem. 1998;46(6):723–739. doi: 10.1177/002215549804600604. [DOI] [PubMed] [Google Scholar]
- 110.Sen M, Cheng YH, Goldring MB, Lotz MK, Carson DA. WISP3-dependent regulation of type II collagen and aggrecan production in chondrocytes. Arthritis Rheum. 2004;50(2):488–497. doi: 10.1002/art.20005. [DOI] [PubMed] [Google Scholar]
- 111.Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis. 2010 doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 112.Caramés B, Taniguchi N, Otsuki S, Blanco F, Lotz M. Autophagy is a Protective Mechanism in Normal Cartilage and its Aging-related Loss is Linked with Cell Death and Osteoarthritis. Arthritis Rheum. 2009 doi: 10.1002/art.27305. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morko JP, Soderstrom M, Saamanen AM, Salminen HJ, Vuorio EI. Up regulation of cathepsin K expression in articular chondrocytes in a transgenic mouse model for osteoarthritis. Ann Rheum Dis. 2004;63(6):649–655. doi: 10.1136/ard.2002.004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vittorio N, Crissman JD, Hopson CN, Herman JH. Histologic assessment of cathepsin D in osteoarthritic cartilage. Clin Exp Rheumatol. 1986;4(3):221–230. [PubMed] [Google Scholar]
- 115.Hernandez-Vidal G, Jeffcott LB, Davies ME. Immunolocalization of cathepsin B in equine dyschondroplastic articular cartilage. Vet J. 1998;156(3):193–201. doi: 10.1016/s1090-0233(98)80122-6. [DOI] [PubMed] [Google Scholar]
- 116.Otsuki S, Taniguchi N, Grogan SP, D'Lima D, Kinoshita M, Lotz M. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008;10(3):R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15(9):1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 118.Hiraoka K, Grogan S, Olee T, Lotz M. Mesenchymal progenitor cells in adult human articular cartilage. Biorheology. 2006;43(3–4):447–454. [PubMed] [Google Scholar]
- 119.Omoto S, Nishida K, Yamaai Y, Shibahara M, Nishida T, Doi T, et al. Expression and localization of connective tissue growth factor (CTGF/Hcs24/CCN2) in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12(10):771–778. doi: 10.1016/j.joca.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 120.von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K, et al. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 121.Pullig O, Weseloh G, Gauer S, Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol. 2000;19(3):245–255. doi: 10.1016/s0945-053x(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 122.Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12(12):963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 123.Pufe T, Bartscher M, Petersen W, Tillmann B, Mentlein R. Pleiotrophin, an embryonic differentiation and growth factor, is expressed in osteoarthritis. Osteoarthritis Cartilage. 2003;11(4):260–264. doi: 10.1016/s1063-4584(02)00385-0. [DOI] [PubMed] [Google Scholar]
- 124.Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56(6):1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 125.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54(11):3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 126.Ruiz-Romero C, Carreira V, Rego I, Remeseiro S, Lopez-Armada MJ, Blanco FJ. Proteomic analysis of human osteoarthritic chondrocytes reveals protein changes in stress and glycolysis. Proteomics. 2008;8(3):495–507. doi: 10.1002/pmic.200700249. [DOI] [PubMed] [Google Scholar]
- 127.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41(9):1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 128.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 129.Chang J, Ma XC, Wu DC, Jiao YT. [Study on the characteristics of apoptosis in the condyles of osteoarthritic temporomandibular joints] Hua Xi Kou Qiang Yi Xue Za Zhi. 2004;22(5):353–356. [PubMed] [Google Scholar]
- 130.Ali SY. Apatite-type crystal deposition in arthritic cartilage. Scan Electron Microsc. 1985;(Pt 4):1555–1566. [PubMed] [Google Scholar]
- 131.Johnson K, Hashimoto S, Lotz M, Pritzker K, Goding J, Terkeltaub R. Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum. 2001;44(5):1071–1081. doi: 10.1002/1529-0131(200105)44:5<1071::AID-ANR187>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 132.Hashimoto S, Ochs RL, Rosen F, Quach J, McCabe G, Solan J, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Natl Acad Sci U S A. 1998;95(6):3094–3099. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kouri JB, Aguilera JM, Reyes J, Lozoya KA, Gonzalez S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. J Rheumatol. 2000;27(4):1005–1019. [PubMed] [Google Scholar]
- 134.Fukui N, Ikeda Y, Ohnuki T, Tanaka N, Hikita A, Mitomi H, et al. Regional differences in chondrocyte metabolism in osteoarthritis: a detailed analysis by laser capture microdissection. Arthritis Rheum. 2008;58(1):154–163. doi: 10.1002/art.23175. [DOI] [PubMed] [Google Scholar]
- 135.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50(5):1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 136.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 137.Hayes AJ, Dowthwaite GP, Webster SV, Archer CW. The distribution of Notch receptors and their ligands during articular cartilage development. J Anat. 2003;202(6):495–502. doi: 10.1046/j.1469-7580.2003.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11(3):R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ustunel I, Ozenci AM, Sahin Z, Ozbey O, Acar N, Tanriover G, et al. The immunohistochemical localization of notch receptors and ligands in human articular cartilage, chondroprogenitor culture and ultrastructural characteristics of these progenitor cells. Acta Histochem. 2008;110(5):397–407. doi: 10.1016/j.acthis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 140.Otsuki S, Grogan SP, Miyaki S, Kinoshita M, Asahara H, Lotz MK. Tissue neogenesis and STRO-1 expression in immature and mature articular cartilage. J Orthop Res. 2010;28(1):96–102. doi: 10.1002/jor.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4(4):324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 142.Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322(5907):1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]