Abstract
Rationale
Fast-transient outward K+ (Ito,f) and ultra-rapid delayed rectifier K+ currents (IKur or IK,slow) contribute to mouse cardiac repolarization. Gender studies on these currents have reported conflicting results.
Objective
One key missing piece information in these studies is the animals’ estral stage. We decided to revisit gender-related differences in K+ currents, taking into consideration the females’ estral stage.
Methods and Results
We hypothesized that changes in estrogen levels during the estral cycle could play a role in determining the densities of K+ currents underlying ventricular repolarization. Peak total K+ current (IK,total) densities (pA/pF, at +40 mV) were much higher in males (48.6±3.0) than in females at estrus (27.2±2.3) but not at diestrus-2 (39.1±3.4). Underlying this change, Ito,f and IK,slow were lower in females at estrus vs males and diestrus-2 (IK,slow: male 21.9±1.8, estrus 14.6±0.6, diestrus-2 20.3±1.4; Ito,f: male 26.8±1.9, estrus 14.9±1.6, diestrus-2 22.1±2.1). The lower IK,slow in estrus was only due to IK,slow1 reduction without changes of IK,slow2. Estrogen treatment of ovariectomized mice decreased IK,total (46.4±3.0 to 28.4±1.6), Ito,f (26.6±1.6 to 12.8±1.0) and IK,slow (22.2±1.6 to 17.2±1.4). Transcript levels of Kv4.3 and Kv1.5 (underlying Ito,f and IK,slow, respectively) were lower in estrus vs. diestrus-2 and male. In ovariectomized mice, estrogen treatment resulted in downregulation of Kv4.3 and Kv1.5, but not Kv4.2, KChIP2 and Kv2.1 transcripts. K+ current reduction in high estrogenic conditions were associated with prolongation of the action potential duration and corrected QT interval.
Conclusion
Downregulation of Kv4.3 and Kv1.5 transcripts by estrogen are one mechanism defining gender-related differences in mouse ventricular repolarization.
Keywords: Estrogen; Ito; IK,slow; estrus; diestrus; gender; Kv4.3; Kv1.5
INTRODUCTION
Gender-based differences in cardiac repolarization have been previously shown in humans, with females having longer action potentials and higher susceptibility to arrhythmias than same-aged males1. In mouse however, studies on gender-related differences in right and left ventricular myocytes have yielded different results when examining fast transient outward K+ current (Ito,f) and ultra rapid delayed rectifier K+ current (IKur or IK,slow), which play an important role in cardiac repolarization2, 3. Reduction of IK,slow but not Ito,f4, reduction of Ito,f5, or no change at all6 in females vs. males were reported. One common feature in all these studies was the lack of explicit report of the stage of females’ estral cycle. During the estral cycle there are changes in plasma hormone levels, especially of estrogen (E2)7, that have been shown to downregulate cardiac Kv4.3 transcript 8 which is one of the molecular correlates of Ito,f. In rats, the estral cycle lasts ~4 days with an estrogen peak at proestrus, hours before estrus; hence, the estrus stage reflects the effects of the high E2 surge at proestrus. These effects decay towards diestrus-2, when the animal has been exposed to low E2 levels7 for the longest time (~3 days). Here, we revisited in detail gender differences and E2-mediated changes in K+ repolarizing currents by initially measuring E2 levels during the mouse estral cycle, and correlated E2 levels with outward K+ currents, action potential duration (APD) and corrected QT (QTc) interval. We measured total outward K+ current (IK,total) densities as well as their main components IK,slow and Ito,f in males and in females at estrus (under the influence of high estrogen of the preceding proestrus), diestrus-2 (under a prolonged exposure to low estrogen levels), and in E2-treated ovariectomized (OVX) mice. Our data revealed that IK,total, Ito,f and IK,slow densities were lower in animals under the influence of high E2 (estrus and E2-treated OVX) when compared with low E2 levels (diestrus-2, males and OVX sham). Lower K+ current densities in high estrogenic conditions were associated with downregulation of Kv4.3 and Kv1.5 transcript levels, providing a molecular correlate for the prolongation of both APD and QTc interval in female mice under the influence of high estrogen.
METHODS
2.1. Animals and hormonal treatment
Male, female and ovariectomized (OVX) mice were used (C57BL/6). Protocols received institutional review and committee approval. The female estral cycle and the treatment of OVX mice with E2 are described in the online data supplement.
2.2. Chemicals
Tetrodotoxin, tetraethylammonium (TEA) and 4-aminopyridine (4-AP) were from Sigma and estrogen pellets were from Innovative Research of America.
2.3. Isolation of Ventricular Myocytes, electrophysiology and Electrocardiograms
Whole-cell, voltage-, and current-clamp recordings were obtained at room temperature from right ventricular myocytes. Cell isolation, recording solutions, the pulse protocols and the details of the ECG recordings and analysis are provided in the online data supplement.
2.4. Real-Time PCR
Total RNA from mouse heart right ventricle was isolated using Trizol (Invitrogen) and was reverse transcribed with gene specific primers using Omniscript RT kit (Qiagen). See online data supplement for details.
2.5. Statistics
ANOVA followed by Tukey-Kramer Multiple-Comparison test was used for statistical analysis. Repeated Measures ANOVA was used for I–V curves. Probability values <0.05 were considered statistically significant. Values are mean±SE.
RESULTS
Plasma E2 concentration is highest in females at proestrus which is mimicked in E2-treated OVX mice
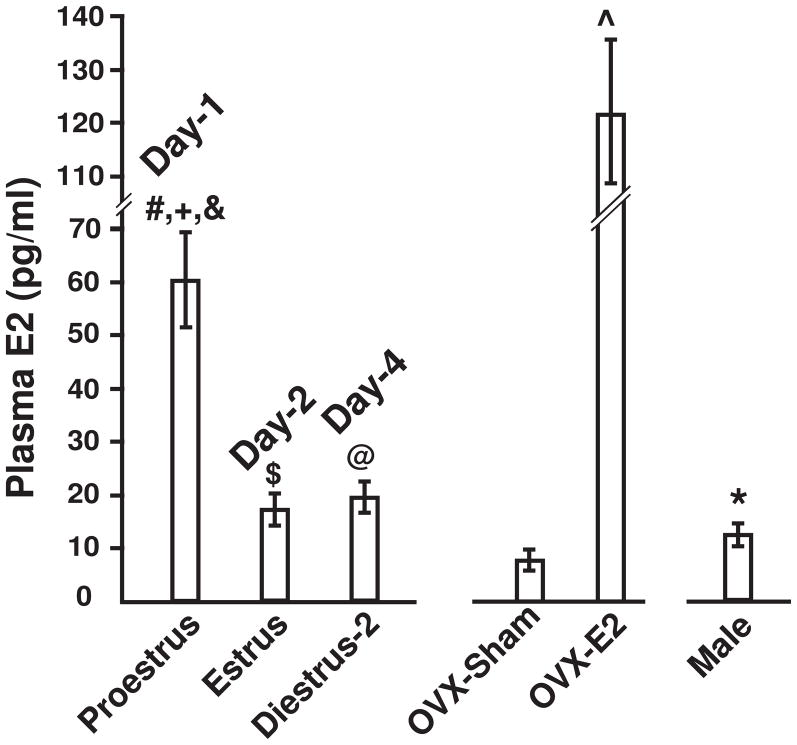
Similar to rats7, female mice have an estrous cycle of ~four days; proestrus, estrus, metaestrus/diestrus-1 and diestrus-2. E2 plasma concentrations [E2] were measured in male and female mice (proestrus, estrus and diestrus-2 identified from vaginal smears) and in OVX mice control (sham) and E2 treated (Fig. 1). Similarly to rats7, [E2] is maximal in the proestrus stage, reaching ~60 pg/ml, and is reduced to ~20 pg/ml at estrus and diestrus-2. [E2] in male mice is ~12 pg/ml, which is significantly lower than [E2] in dietrus-2 (P<0.001). As expected, ovariectomy decreased [E2] to ~7 pg/ml which increased to ~120 pg/ml after E2 treatment. Note that although [E2] is low in estrus and diestrus-2, the estrus stage is primed by a preceding (0.5–1 day) surge of estrogen in proestrus, while diestrus-2 is marked by a prolonged exposure (~3 days) to low estrogen levels. Therefore, in this work we used mice under the influence of high estrogen (estrus and E2-treated OVX) and low estrogen (diestrus-2, OVX sham, and males).
Figure 1. Plasma [E2] in females and males.
[E2] measurements were performed in females at different stages of the estral cycle, in OVX placebo treated (sham) or E2-treated, and in male mice. Values are (pg/ml): males 12.4±1.3 (n=10); proestrus 60±9 (n=5), estrus 17.1±2.5 (n=17), diestrus-2 19.6±1.5 (n=14), OVX sham 7.30±0.9 (n=6) and E2-treated 120.7±12.9 (n=3). Significantly different (+) proestrus vs. male (P<0.05), (#) proestrus vs. diestrus-2 (P<0.05), (&) proestrus vs. estrus (P<0.05), (*) male vs. diestrus-2 (P<0.001), (@) diestrus-2 vs. OVX-sham (P<0.001), ($) estrus vs. OVX-sham (p,0.001) and (^) E2-treated vs. OVX-sham (P<0.05).
Gender and estral cycle determine outward K+ current densities
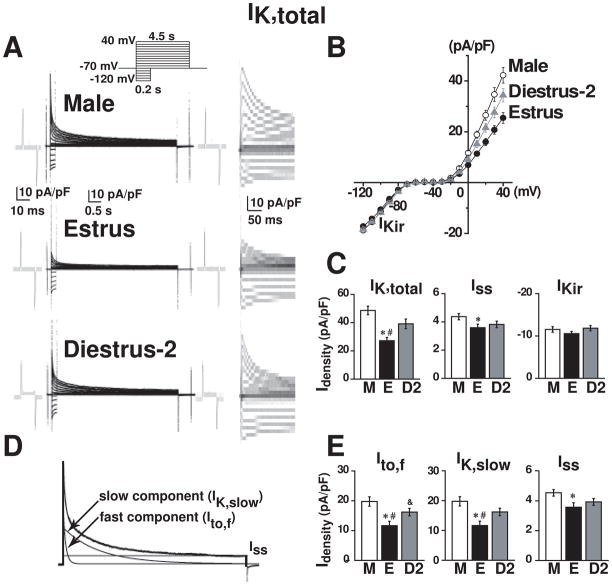
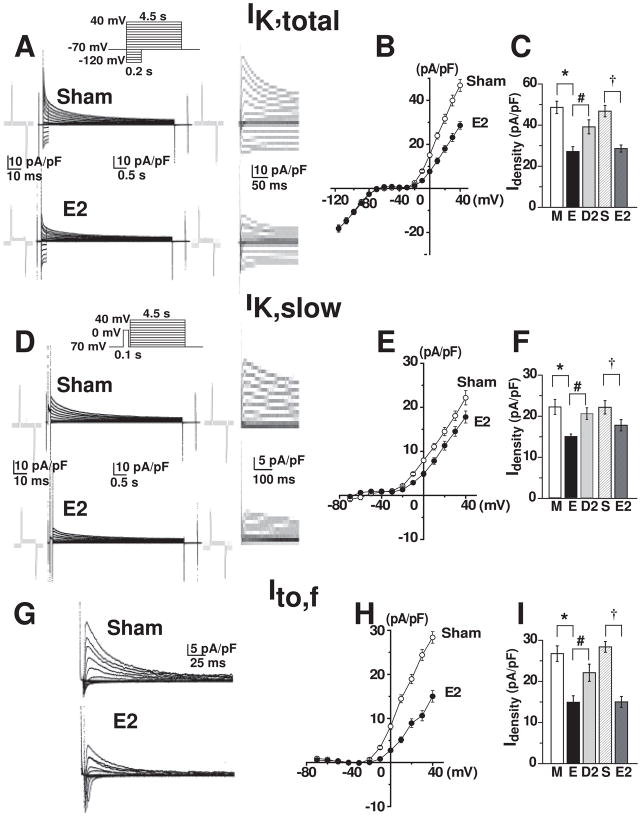
To test our hypothesis that estrogen levels determine outward K+ current densities, IK,total was recorded in right ventricular myocytes isolated from males, estrus and diestrus-2 (Fig. 2A). While inward rectifier K+ current (IKir) remained constant, IK,total at potentials higher than −10 mV in male mice and in diestrus-2 was significantly higher than in estrus (Fig. 2B). The steady-state current (Iss) at the end of the pulse is somewhat smaller in estrus than in males, but similar to diestrus-2 (Fig. 2C). For comparative purposes, the underlying components of IK,total were isolated by two methods: i) based on exponential fitting6 (Fig. 2D) and ii) based on short depolarizing prepulses as to inactivate Ito,f4 (Fig. 3). Both methods yielded virtually identical results (Table-1). The amplitudes and decay of IK,total are well described by the sum of fast (~100 ms) and slow (~1 s) exponential components (corresponding to Ito,f and IK,slow) and a steady-state current, Iss (Fig. 2D). Using this method, Fig. 2E demonstrates gender differences in Ito,f, IK,slow and Iss.
Figure 2. Gender differences in IK,total, Ito,f and IK,slow: current separation by exponential fitting.
A , IK,total recorded from isolated right ventricular myocytes. Inset: Pulse protocol with 10 mV increments. Traces at right are at higher time gain. B, Peak IK,total-voltage curve in male (○), estrus (●) and diestrus-2 (▲). C, IK,total, Iss and IKir for male (M), estrus (E), and diestrus-2 (D2). D, Separation of Ito,f and IK,slow with exponential fitting. Iss, baseline current 6, 8. E, Ito,f, IK,slow, and Iss for male (M), estrus (E), and diestrus-2 (D2). Itotal, Ito,f and IK,slow were measured at the peak at +40 mV; Iss was at steady-state at +40 mV; and IKir was at the end of a 100 ms pulse at −100 mV. Values are mean±SE. The number of mice and cells used in Figs 2–4,6 in each condition are given in Table-1. Significantly different (*) estrus vs. male, (#) estrus vs. diestrus-2, (&) diestrus-2 vs. male (Figs 2–4).
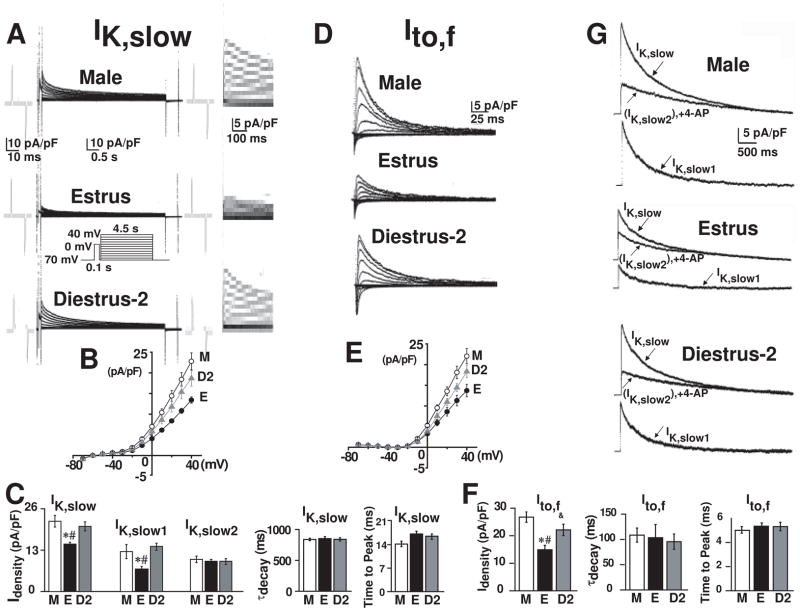
Figure 3. Higher Ito,f, and IK,slow in males than in females: prepulse separation.
A , IK,slow with Ito,f inactivating prepulse (inset). Capacity transients and higher display are as in Fig. 2A. B, Peak IK,slow-voltage curves. C, Bar graphs at +40 mV of IK,slow (male 37 cells from 7 mice, estrus 33 cells from 10 mice, diestrus 34 cells from 10 mice), IK,slow1 (male:12.5±2.2 16 cells from 4 mice; estrus:7.1±0.7 13 cells from 3 mice and diestrus-2:14.1±1.1, 14 cells from 3 mice) and IK,slow2 (male:10.1±1 16 cells from 4 mice; estrus:9.5±0.6 13 cells from 3 mice and diestrus-2:9.4±0.8, 14 cells from 3 mice), τdecay and time to peak. D, Ito,f from Itotal-IK,slow. E, Peak Ito,f-voltage curves. F, Bar graphs at +40 mV of Ito,f density, τdecay and time to peak. *, #, & are as in Fig. 2. G, IK,slow at +40 mV (Ito,f inactivated by prepulse) before (IK,slow) and after application of 50 μM 4-AP (IK,slow2). The traces are Iss baseline subtracted. IK,slow1 = IK,slow−IK,slow2.
Table 1.
Gender differences and estrogen regulation of K+ currents, APD and QTc interval
| Male | Estrus | Diestrus-2 | Sham | E2-treated | |
|---|---|---|---|---|---|
| 21–37 cells 7–11 mice |
20–33 cells 7–10 mice |
20–34 cells 7–10 mice |
8 cells 3 mice |
6 cells 3 mice |
|
| Itotal (pA/pF)a | 48.6±3.0 | 27.2±2.3** # | 39.1±3.4& | 46.4±3.0 | 28.4+1.6†† |
| Iss (pA/pF)b | 4.4±0.2 | 3.6±0.3* | 3.8±0.2 | 5.2±1.1 | 4.1±0.4 |
| IKir (pA/pF)c | −11.5±0.7 | −10.6±0.5 | −11.8±0.6 | −10.5±1.0 | −10.8±1.1 |
| Ibase (pA/pF)c | 4.5±0.2 | 3.6±0.3* | 3.9±0.2 | 5.4±1.1 | 4.1±0.3 |
| Ifast (pA/pF) | 26.2±1.9 | 14.3±1.6** # | 20.3±1.6& | 25.3±2.0 | 11.8±0.9†† |
| Ifast-τ (ms) | 80±7 | 99±11* # | 72±4 | 64±5 | 115±15†† |
| IK,slow (pA/pF)c | 14.9±1.3 | 7.6±0.7** # | 12.4±1.7 | 12.8±1.3 | 11.1±1.0 |
| IK,slow-τ (ms)d | 1128±34 | 1351±75** ## | 1089±23 | 1088±73 | 1344±82 |
| Ito,f (pA/pF) | 26.8±1.9 | 14.9±1.6** # | 22.1±2.1& | 26.6±1.6 | 12.8±1.0†† |
| Ito,f-τ (ms)d | 108±14 | 103±26 | 95±15 | 80±11 | 103±25 |
| Ito,f-time to Peak(ms)d | 4.9±0.2 | 5.2±0.2 | 5.3±0.4 | 4.3±0.2 | 4.6±0.1 |
| IK,slow (pA/pF) | 21.9±1.8 | 14.6±0.6** # | 20.3±1.4 | 22.2±1.6 | 17.2±1.4† |
| IK,slow-τ (ms)d | 838±20 | 852±30 | 840±26 | 767±37 | 971±48† |
| IK,Slow-time to Peak(ms) | 14.02±1.40 | 17.10±1.09 | 16.84±1.39 | 16.83±1.69 | 21.92±1.78 |
| 8 cells, 3 mice | 14 cells, 5 mice | 8 cells, 4 mice | 8 cells, 3mice | 15 cells, 4 mice | |
| APD50 (ms) | 3.11±0.21 | 5.21±0.83* # | 2.88±0.3 | 2.42±0.26 | 5.11±0.86†† |
| APD75 (ms) | 5.75±0.59 | 10.63±2.0* # | 5.62±0.58 | 3.65±0.41 | 11.44±1.96†† |
| APD90 (ms) | 12.52±1.24 | 29.53±6.21* | 11.96±1.19 | 9.28±1.74 | 34.01±4.2†† |
| 10 mice | 14 mice | 11 mice | 12 mice | 9 mice | |
| RR (ms) | 124.4±3.0 | 123.9±4.0# | 119±4.8 | 122.7±2.46 | 125.22±3.9 |
| QRS (ms) | 9.60±0.22 | 9.38±0.25 | 9.36±0.20 | 9.58±0.28 | 9.55±0.17 |
| QT (ms) | 52.0±1.2 | 57.0±3.7## | 44.6±1.2& | 44.1±0.8 | 52.22±1.5†† |
| QTc (ms) | 46.7±1.0 | 50.9±2.6## | 41.05±0.96& | 39.8±0.8 | 46.7±1.4†† |
| HR | 492±10 | 498±13 | 510±18 | 491±10 | 483±15 |
Outward current values are at +40 mV.
Current density at the end of 4.5 s pulse.
Current density at the end of 100 ms pulse at −100 mV.
Measurements after current separation by 100 ms prepulse to 0 mV4.
p<0.05 Estrus vs. Male;
p<0.01 Estrus vs. Male;
p<0.05 Estrus vs. Diestrus-2;
p<0.01 Estrus vs. Diestrus-2;
p<0.05 Diestrus-2 vs. Male;
p<0.05 E2 treated vs. Sham;
p<0.01 E2 treated vs. Sham.
Figure 3A shows IK,slow (in the same cells as in Fig. 2A), elicited after inactivation of Ito,f with a 100 ms prepulse to 0 mV. Ito,f (Fig. 3D) was thus obtained from IK,total (Fig. 2A) minus IK,slow (Fig. 3A). While there were no gender differences in the decay time constant (τdecay) of IK,slow (3C) and Ito,f (3F), their densities showed exactly the same tendency as in gender differences where the currents were separated by exponential fitting6 (Figs. 3C, 3F vs. Fig. 2E). The kinetics of activation was similar for both IK,slow and Ito,f in males, estrus and diestrus-2 (Fig. 3C, F). These results indicate that the gender differences found in current densities are independent of the method used.
To identify which component(s) of IK,slow underlies lower IK,slow in estrus, we used 4-AP (50 μm) to isolate IK,slow1 (Kv1.5) from IK,slow2 (Kv2.1)9,10. We first recorded IK,slow using a pre-pulse to inactivate Ito,f, then IKslow2 in the presence of 50 μM 4-AP which blocks IK,slow1 and IKslow1=IKslow−IKslow2 (Fig. 3G). We validated this approach by directly recording IKslow1 after blocking IKslow2 with TEA (25 mM) and calculated IKslow2=IKslow−IKslow1 (not shown). In agreement with quantitative Real Time PCR data (See Fig. 5D,E), IKslow1, but not IKslow2 were significantly lower in estrus (under the influence of the high estrogen peak at proestrus) when compared to diestrus-2 and male (Fig. 3C,G). This data demonstrates that gender differences in IK,slow is mediated by the differences only in IK,slow1, but not IK,slow2.
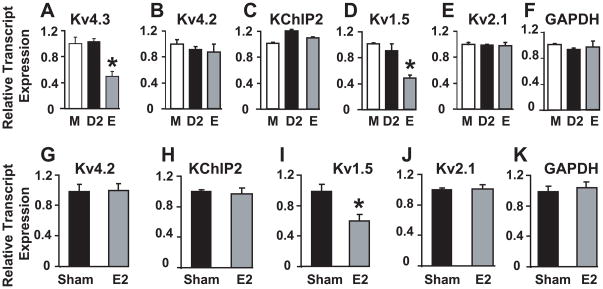
Figure 5. Cardiac Kv4.3 and Kv1.5 transcripts levels are lower under the influence of high estrogen.
Mean±SE relative transcripts in males (M), estrus (E) and diestrus-2 (D) normalized to male for Kv4.3 (A), Kv4.2 (B), KChIP2 (C), Kv1.5 (D), Kv2.1 (E) and GAPDH (F) (N=4–6 mice, *p<0.01 vs. male). Mean±SE relative transcripts in OVX sham (S), and E2-treated normalized to sham for Kv4.2 (G), KChIP2 (H), Kv1.5 (I), Kv2.1 (J) and GAPDH (K) (N=4–6 mice*p<0.01 vs. sham).
Inhibition of outward K+ currents by E2 treatment in OVX animals
To further test the hypothesis of E2-induced inhibition of outward K+ currents as being one mechanism underlying gender differences, we directly tested the effect of E2 on OVX mice. E2 treatment significantly reduced IK,total at potentials more depolarized than −10 mV, without any change in IKir (Fig. 4A,B). IK,total densities in OVX sham were similar to males and diestrus-2, while IK,total densities in E2-treated OVX mice were similar to estrus (Fig. 4A–C). Similar results were obtained for IK,slow and Ito,f, and the inhibitory action of E2 was even more pronounced for Ito,f than IK,slow (Fig. 4D–Fvs. Fig. 4G–I).
Figure 4. Estrogen-induced inhibition of IK,total, IK,slow and Ito,fA, D, G.
IK,total, IKir, IK,slow and Ito,f were obtained as in Fig. 2A and Fig. 3 for sham- and E2-treated OVX mice. A, Capacity transients and higher display are as in Fig. 2A. B, E, H, IK,total- IK,slow- Ito,f –voltage curves for sham (○) and E2-treated (●). C, F, I, Bar graphs for IK,total, IK,slow and Ito,f comparing male (M), estrus (E) and diestrus-2 (D2) (from Figs. 2–3) with OVX-sham (S) and E2-treated (E2). Significantly different (†) OVX-sham vs. OVX-E2-treated.
Kv4.3 and Kv1.5 transcript levels are lower in estrus and are downregulated by E2 treatment in OVX mice
To identify the molecular correlates underlying the lower Ito,f and IK,slow in estrus compared to male and diestrus-2, we used real-time PCR to quantify relative transcript levels of Kv4.3, Kv4.2, and KChIP2 (molecular correlates of Ito,f) and of Kv1.5 and Kv2.1 (molecular correlates of IK,slow). Kv4.3 transcript levels were ~2 fold lower in estrus compared to male and diestrus-2, while Kv4.2 and KChIP2 transcript levels were similar in the three conditions (Fig. 5A–C), thus making Kv4.3 as a strong candidate to contribute to the gender differences of Ito,f. Regarding the molecular correlates of IK,slow, Kv1.5 (Fig 5D) but not Kv2.1 (Fig. 5E) transcript levels were ~2 fold lower in estrus compared to male and diestrus-2, which is in agreement with our electrophysiological data showing that gender differences in IK,slow are mediated by the differences in IK,slow1 (encoded by Kv1.5), but not IK,slow2 (encoded by Kv2.1).
We have previously shown that cardiac Kv4.3 transcript levels were downregulated by E28. We show now that cardiac Kv1.5 transcript levels are also downregulated by E2 treatment (Fig. 5I). Kv4.2, KChIP2 and Kv2.1 (Fig. 5G–J) transcripts were not affected by E2 treatment. These results support the view that only Kv4.3 and Kv1.5 are regulated by E2.
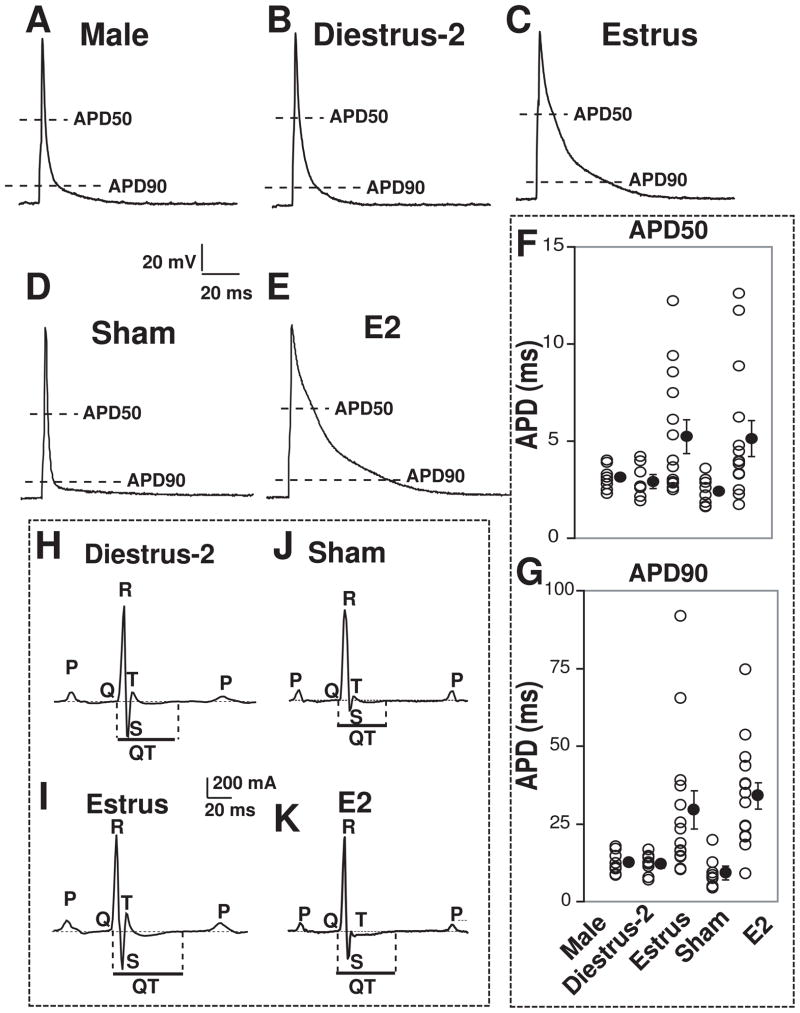
Action potential duration and QTc interval are longer in estrus compared to diestrus-2 and in E2 treated OVX mice
We further investigated whether changes in K+ currents in estrus vs. male and diestrus-2 are associated with altered action potential duration and QTc interval. Fig. 6 shows that action potential durations (APD) at 50% (APD50) and 90% (APD90) of the depolarization were similar in males and in diestrus-2 (~3.1 ms and 12 ms respectively), while they were significantly longer in estrus (~5 ms and 30 ms, respectively). E2 treatment of OVX mice increased both APD50 and APD90 (from 2.4 ms to 5.1 ms and from 9.28 ms to 34 ms, respectively) to values similar to estrus (Table-1). This data supports the role of E2 in gender related differences of cardiac repolarization.
Figure 6. Action potential duration and QT interval are longer in estrus compared to male and diestrus-2; and E2 treatment increased both the action potential duration and QT interval.
Action potentials were elicited by 1 ms pulses in male (A), diestrus-2 (B), estrus (C), OVX sham (D) and E2-treated (E). Individual action potential durations (○) and their averages (●) at 50% (APD50, F) and 90% (APD90, G) of depolarization. Typical ECG recordings in dietrus-2 (H), estrus (I), OVX sham (J) and E2 treated (K). ECG parameters are given in Table-1.
Analyses of ECG recordings revealed no differences in the shape of P waves or the QRS complexes in estrus vs. diestrus-2 and in sham vs. E2-treated OVX mice (Fig 6H–K). The heart rate, RR and QRS durations were also similar between estrus, diestrus-2, sham and E2-treated mice (Table-1). Interestingly QTc interval was significantly longer in estrus (51 ms) vs. diestrus-2 (41 ms) and in E2-treated OVX (47 ms) vs. sham (40 ms), indicating that changes in action potential durations in females are associated with altered ventricular repolarization. In male mice however, although the APD was similar to diestrus-2, the QTc interval was significantly longer than diestrus-2, possibly due to the higher body weight and larger cardiac size compared to same-aged female mice (Table-1).
In summary, in female mice, changes in outward K+ current densities were associated with changes in action potential duration and ventricular repolarization.
DISCUSSION
Previous studies have shown conflicting data regarding gender related differences in K+ current densities and ventricular repolarization in mice4–6. This conflicting data may arise from the use of female mice at random without establishing their estral stage. Taking into consideration that estrogen levels vary during the female estral cycle (Fig. 1), we revisited gender-related differences in K+ current densities and cardiac repolarization in male and female mice at estrus and diestrus-2, and in E2-treated OVX mice. We show for the first time that plasma E2 concentration is one of the key players in determining outward K+ current densities as well as affecting ventricular repolarization in mouse heart.
Higher estrogen levels correlate with reduced outward K+ current densities
In agreement with the role of E2 in downregulating K+ current densities, IK,total densities were lower in animals under the influence of high plasma E2 (estrus and E2-treated OVX) compared with low plasma E2 levels (diestrus-2, males and OVX; Figs. 2, 4A). The lower IK,total densities in animals under the influence of high plasma E2 were due to lower densities of both IK,slow and Ito,f (Figs. 3, 4). Supporting a role of E2 in downregulating Ito,f, we found that males, which have lower levels of [E2] compared to females (Fig. 1), have significantly higher levels of Ito,f densities than females. We also show that Ito,f density in females is regulated during the estral cycle and by E2 treatment. These findings complement our previous work which shows that Ito,f is downregulated at the end of pregnancy8, where levels of plasma E2 are high.
Table-2 summarizes previously reported IK,total, IK,slow and Ito,f current densities, as well as our present work, in male and female mice. Lower IK,total and IK,slow but not Ito,f densities in female vs. male were reported by Trepanier-Boulay et al. using the CD-1 strain 4. In contrast to our finding and to those of Trepanier-Boulay et al. for IK,total and IK,slow 4, Brunet et al.6 using C57BL/6 mice reported no gender differences in IK,total, IK,slow and Ito,f densities, thus differences in strain is not a suitable explanation for the inconsistency in the results. Similar to our results, and opposite to Trepanier-Boulay et al. 4 and Brunet et al.6, higher Ito,f densities in males vs. females was previously reported by Wu & Anderson5. How can we explain the differences in these findings? One explanation is that if one uses animals at random, there is a lower probability to sample animals in estrus, where the changes are more pronounced. In addition one can argue that the lack of significant differences in the Ito,f densities reported by Trepanier-Boulay et al.4 in males and females could be due to recording currents from cells isolated from the whole ventricle and that the regional heterogeneity in the expression levels of Ito,f could have masked gender differences. In this work, we maximized homogeneity of our myocyte population by comparing only currents from cells isolated from the right ventricle, which also have the highest density of Ito,f6. However, the regional differences can not explain the discrepancy between our data and Brunet et al6, who demonstrated no significant differences in the IK,total, Ito,f and IK,slow densities in right ventricular myocytes between male and female mice2. The contradiction between our present findings and Brunet et al.6 in comparing Ito,f from the same ventricular regions could lie in the experimental method. Brunet et al.6 used cardiomyocytes maintained in serum-free medium for up to 24 hrs, in which time they can undergo remodeling. In our study, we only used freshly dissociated cardiomyocytes within 4–6 hr after isolation.
Table 2.
IK,total, IK,slow and Ito,f current densities in male and female mice
| Currents | Gender dependency | Strain | Region | Fresh vs. Culture | Estral cycle evaluation | Ref |
|---|---|---|---|---|---|---|
| IK,total | ||||||
| M>F | CD-1 | WV | Fresh | Unknown | 4 | |
| No-diff. | C57Bl/6 | LV | Up-24 h | Unknown | 6 | |
| No-diff. | C57Bl/6 | RV | Up-24 h | Unknown | 6 | |
| M>E D2>E |
C57Bl/6 | RV | Fresh | Yes | Present work | |
| No-diff. | db/+ | RV | Fresh | Unknown | 26 | |
| M>F | C57Bl/6 | Septum | Up-24 h | Unknown | 6 | |
| IK,Slow | ||||||
| M>F | CD-1 | WV | Fresh | Unknown | 4 | |
| No-diff. | C57Bl/6 | LV | Up-24 h | Unknown | 6 | |
| No-diff. | C57Bl/6 | RV | Up-24 h | Unknown | 6 | |
| M>E D2>E |
C57Bl/6 | RV | Fresh | Yes | Present work | |
| M>F | C57Bl/6 | Septum | Up-24 h | Unknown | 6 | |
| Ito,f | ||||||
| No-diff. | CD-1 | WV | Fresh | Unknown | 4 | |
| M>F | C57Bl/6 129SVE |
LV | Fresh | Unknown | 5 | |
| No-diff. | C57Bl/6 | LV | Up-24 h | Unknown | 6 | |
| M>E D2>E |
C57Bl/6 | RV | Fresh | Yes | Present work | |
| No-diff. | C57Bl/6 | RV | Up-24 h | Unknown | 6 | |
| M>F | C57Bl/6 | Septum | Up-24 h | Unknown | 6 | |
M indicates male, F female, WV whole ventricle, RV right ventricle, LV left ventricle, Fresh (within 4–6 hr after cell isolation), E female at estrus, D2 female at diestrus-2 and No-diff. indicate no difference.
Higher estrogen levels correlate with prolongation of action potential duration and QTc interval
Here we show that estrogen plays a key role in regulating the ventricular repolarization in mouse heart. Lower Ito,f and IK,slow densities in female mice in estrus compared to male and dietrus-2 were associated with longer action potential duration in estrus (Fig. 6). E2 treatment of OVX mice resulted also in the prolongation of action potential duration and QTc interval to a comparable level as in estrus, underscoring the role of E2 in the regulation of action potential duration and ventricular repolarization. Supporting a role of E2, in larger animals E2 treatment can downregulate cardiac Kv1.5 (HK2) and KCNE1 transcript levels of rabbit hearts, which is correlated with a prolonged QTc interval11. In addition, E2 can also influence the expression as well as conductance of the L-type calcium channel (ICa,L) which plays an important role in the cardiac repolarization phase and in arrhythmogenesis. ICa,L channels of cardiomyocytes differ significantly between day 0 (peak of plasma estrogen, proestrus) and day 4 (peak of progesterone-to-estrogen ratio, diestrus-2) of the estral cycle of female guinea pigs, suggesting that ICa,L density varies according to estral hormonal changes (See review1). Moreover, E2 treatment reduced ICa,L density of OVX rat12, and ICa,L density was increased in estrogen receptor α (ERα)-deficient mice (See review1), thus demonstrating that the inhibitory action of E2 was mediated mainly via the ERα. Here we speculate that the large variability in the duration of APD50 and APD90 in estrus and in E2-treated OVX mice (Fig. 6) is due to different expression levels of ERα in different cardiomyocytes, and the variability is more evident under the influence of high estrogen (Fig. 6). Supporting the role of sex hormones in regulating APD and cardiac repolarization, testosterone has been previously proposed to play a role in gender-based cardiac repolarization differences, as androgen deficiency in castrated mice produced a longer APD and prolongation of QTc interval (See review1). Similar to mice, ventricular repolarization was significantly altered by testosterone treatment in dogs13 and rabbits (See review1).
Mechanism of estrogen action: Regulation of Kv4.3 and Kv1.5 transcript levels
Several reports suggest that the expression of Kv4.3 and Kv1.5 are regulated by sex hormones8, 14. Our data demonstrate that Kv4.3 was the only molecular correlate of Ito,f which its transcript levels were lower under the influence of high estrogen (estrus and E2-primed OVX) compared to low estrogen (diestrus-2, male and OVX sham). E2-induced decreased expression of Kv4.3 was not accompanied with significant alteration in Kv4.2 and KChIP2 transcripts (Fig. 5). The lower Kv4.3 transcripts in estrus vs male and diestrus-2 and in E2-treated OVX mice vs sham coincided with reduced Ito,f densities. Regarding the molecular correlates of IK,slow, Kv1.5 but not Kv2.1 transcript levels were lower in estrus compared to male and diestrus-2 and were downregulated by E2 treatment. The fact that IK,slow2 peak current densities were similar (encoded by Kv2.1) in male, estrus and dietrus-2 (Fig. 3C,D), whereas IK,slow1 (encoded by Kv1.5) peak current densities were significantly lower in estrus compared to male and diestrus-2, further support that the transcriptional changes in Kv1.5 underlie changes in IK,slow ruling out the possibility of estrogen-induced posttranslational modification of Kv1.5 protein. Higher Kv1.5 transcripts in males than in females has been reported, and testosterone has been suggested to be the main player in this regulation14. Our data suggest that transcriptional regulation of Kv4.3 and Kv1.5 by E2 is at least one of the mechanisms responsible for the action of estrogen in remodeling cardiac repolarization.
How can estrogen regulate the transcriptional activity of Kv4.3 and Kv1.5 genes? Estrogen can regulate the transcriptional activity of a gene via a direct genomic classical pathway, which requires the presence of Estrogen Response Elements (ERE) in the promoter of the gene. Alternatively, estrogen can stimulate the transcription through the DNA-associated transcription factors known as indirect genomic pathway via estrogen receptors15. Analysis of 10 kb upstream of Kv4.3 and Kv1.5 transcription start sites failed to identify an ERE, but showed several half-EREs as well as Sp1 and Ap1 elements, that could be involved in reducing expression of Kv4.3 and Kv1.5 by E2. There is also an increasing body of evidence that direct application of estrogens can acutely regulate different types of K+ and ICa,L currents, affecting action potential durations through non-genomic pathways1, 16. For example, direct application of raloxifene (a selective estrogen receptor modulator) to human atrial myocytes inhibited Ito,f and IK,slow within minutes in a concentration-dependent manner, as well as prolonged APD16. Although our Real Time PCR data supports a genomic action of E2, a non-genomic contribution cannot be excluded.
Influence of estrogen on QTc interval and arrhythmia susceptibility during the menstrual cycle and estrogen replacement therapy in women
Here we showed that in female mice, the changes in plasma E2 levels during the estral cycle, as well as by E2 treatment, affect the QTc interval. The QTc is significantly longer in high estrogenic conditions (estrus and E2-treated OVX) in comparison to low estrogenic conditions (diestrus-2 and OVX). In premenopausal women, circulating levels of estrogen and progesterone change dramatically during the menstrual cycle. The early follicular phase (menses) is characterized by a low level of estrogen (~100pmol/l). Estrogen levels steadily increase after onset of menses and reach a peak (~750 pmol/l) at about day 14 of the ovulatory phase17. Estrogen levels fall during the luteal phase, when progesterone levels begin to increase. Changes in QTc interval during the menstrual cycle has been cited with conflicting results. Several investigators found no change in the QTc interval at different times of the menstrual cycle17–19, whereas Nakagawa et al. reported a shorter QTc interval during the luteal phase compared to the follicular phase20. The shorter QTc interval in the luteal phase has been attributed to higher levels of plasma progesterone concentration (~5 fold) compared to the follicular phase, as plasma estrogen and testosterone concentrations were similar in both phases20. It is well accepted that women are at a higher risk of developing drug-induced torsades de pointes than men. How the changes in QTc interval during the menstrual cycle might influence the arrythmogenesis in female patients with ventricular arrhythmia or paroxysmal supraventricular tachycardia (SVT) has been of great interest. The prolongation of QTc duration induced by ibutilide, an antiarrhythmic agent, was significantly greater at menses and the ovulatory phase than in the luteal phase19. The drug-induced prolongation of QTc was inversely correlated with higher levels of progesterone and progesterone-to-estrogen ratio, rather than estrogen alone19. The occurrence and the duration of SVT episodes in women has been suggested to be affected by the menstrual cycle, being higher in day 28 when progesterone levels are higher, as opposed to day 7 of the menstrual cycle, when estrogen predominates21. The incidence of arrhythmia in SVT patients was found to be slowed down by estrogen and facilitated by progesterone21.
The effect of estrogen and progesterone replacement therapy on ventricular repolarization has also been investigated in healthy postmenopausal women. Hormone replacement therapy with estrogen alone significantly prolonged QTc interval22,23, whereas combined estrogen and progestin therapy had no effect24 or reduced the QTc interval23, 25. These findings suggest that sex hormones may directly modulate ventricular repolarization; estrogen is reported to account for the prolongation of QTc interval similar to our results in mice whereas progesterone tends to reduce QTc interval. A detailed study to correlate plasma estrogen and progesterone to the QTc interval during the menstrual cycle and hormone replacement therapy should provide key information to set the appropriate timing of the treatment or electrophysiological procedure in premenopausal women more prone to arrhythmias.
Supplementary Material
Acknowledgments
Sources of Funding:
Supported by NIH grants HL089876 (ME), HL080111, HL088640 (ES) and HL54970 (LT).
Non-standard Abbreviations and Acronyms
- Ito,f
fast-transient outward K+ current
- IKur or IK,slow
ultra-rapid delayed rectifier K+ current
- Iss
steady-state current
- IKir
inward rectifier K+ current
- ICa,L
L-type calcium channel
- E2
estrogen
- ERE
Estrogen Response Elements
- ERα
estrogen receptorα
- OVX
ovariectomized
- APD
action potential duration
- QTc
corrected QT
- TEA
tetraethylammonium
- 4-AP
4-aminopyridine
- SVT
paroxysmal supraventricular tachycardia
Footnotes
Disclosures
None.
References
- 1.James AF, Choisy SC, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol. 2007 July;94(3):265–319. doi: 10.1016/j.pbiomolbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol. 1999 May;113(5):661–78. doi: 10.1085/jgp.113.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouillette J, Clark RB, Giles WR, Fiset C. Functional properties of K+ currents in adult mouse ventricular myocytes. J Physiol. 2004 September 15;559(Pt 3):777–98. doi: 10.1113/jphysiol.2004.063446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trepanier-Boulay V, St Michel C, Tremblay A, Fiset C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ Res. 2001 August 31;89(5):437–44. doi: 10.1161/hh1701.095644. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Anderson ME. Reduced repolarization reserve in ventricular myocytes from female mice. Cardiovasc Res. 2002 February 15;53(3):763–9. doi: 10.1016/s0008-6363(01)00387-x. [DOI] [PubMed] [Google Scholar]
- 6.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol. 2004 August 15;559(Pt 1):103–20. doi: 10.1113/jphysiol.2004.063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaPolt PS, Matt DW, Judd HL, Lu JK. The relation of ovarian steroid levels in young female rats to subsequent estrous cyclicity and reproductive function during aging. Biol Reprod. 1986 December;35(5):1131–9. doi: 10.1095/biolreprod35.5.1131. [DOI] [PubMed] [Google Scholar]
- 8.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005 June 10;96(11):1208–16. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res. 1999 October 1;85(7):623–33. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]
- 10.London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, Koren G. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci U S A. 1998 March 17;95(6):2926–31. doi: 10.1073/pnas.95.6.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996 September 15;94(6):1471–4. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 12.Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium-handling proteins, beta-adrenergic receptors, and function in rat heart. Life Sci. 2006 August 22;79(13):1257–67. doi: 10.1016/j.lfs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Fulop L, Banyasz T, Szabo G, Toth IB, Biro T, Lorincz I, Balogh A, Peto K, Miko I, Nanasi PP. Effects of sex hormones on ECG parameters and expression of cardiac ion channels in dogs. Acta Physiol (Oxf) 2006 November;188(3–4):163–71. doi: 10.1111/j.1748-1716.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 14.Brouillette J, Trepanier-Boulay V, Fiset C. Effect of androgen deficiency on mouse ventricular repolarization. J Physiol. 2003 January 15;546(Pt 2):403–13. doi: 10.1113/jphysiol.2002.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001 October;81(4):1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Jin MW, Xiang JZ, Huang Y, Sun HY, Chiu SW, Lau CP, Li GR. Raloxifene inhibits transient outward and ultra-rapid delayed rectifier potassium currents in human atrial myocytes. Eur J Pharmacol. 2007 June 1;563(1–3):61–8. doi: 10.1016/j.ejphar.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 17.Hulot JS, Demolis JL, Riviere R, Strabach S, Christin-Maitre S, Funck-Brentano C. Influence of endogenous oestrogens on QT interval duration. Eur Heart J. 2003 September;24(18):1663–7. doi: 10.1016/s0195-668x(03)00436-6. [DOI] [PubMed] [Google Scholar]
- 18.Burke JH, Ehlert FA, Kruse JT, Parker MA, Goldberger JJ, Kadish AH. Gender-specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol. 1997 January 15;79(2):178–81. doi: 10.1016/s0002-9149(96)00707-2. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001 March 14;285(10):1322–6. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa M, Ooie T, Takahashi N, Taniguchi Y, Anan F, Yonemochi H, Saikawa T. Influence of menstrual cycle on QT interval dynamics. Pacing Clin Electrophysiol. 2006 June;29(6):607–13. doi: 10.1111/j.1540-8159.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosano GM, Leonardo F, Sarrel PM, Beale CM, De LF, Collins P. Cyclical variation in paroxysmal supraventricular tachycardia in women. Lancet. 1996 March 23;347(9004):786–8. doi: 10.1016/s0140-6736(96)90867-3. [DOI] [PubMed] [Google Scholar]
- 22.Carnethon MR, Anthony MS, Cascio WE, Folsom AR, Rautaharju PM, Liao D, Evans GW, Heiss G. A prospective evaluation of the risk of QT prolongation with hormone replacement therapy: the atherosclerosis risk in communities study. Ann Epidemiol. 2003 August;13(7):530–6. doi: 10.1016/s1047-2797(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 23.Haseroth K, Seyffart K, Wehling M, Christ M. Effects of progestin-estrogen replacement therapy on QT-dispersion in postmenopausal women. Int J Cardiol. 2000 September 15;75(2–3):161–5. doi: 10.1016/s0167-5273(00)00317-x. [DOI] [PubMed] [Google Scholar]
- 24.Altunkeser BB, Ozdemir K, Icli A, Celik C, Akyurek C, Gok H. Effects of long-term hormone replacement therapy on QT and corrected QT dispersion during resting and peak exercise electrocardiography in post-menopausal women. Jpn Heart J. 2002 January;43(1):1–7. doi: 10.1536/jhj.43.1. [DOI] [PubMed] [Google Scholar]
- 25.Yildirir A, Aybar F, Kabakci MG, Yarali H, Akgul E, Bukulmez O, Tokgozoglu SL, Gurgan T, Oto A. Hormone replacement therapy shortens QT dispersion in healthy postmenopausal women. Ann Noninvasive Electrocardiol. 2001 July;6(3):193–7. doi: 10.1111/j.1542-474X.2001.tb00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimoni Y, Chuang M, Abel ED, Severson DL. Gender-dependent attenuation of cardiac potassium currents in type 2 diabetic db/db mice. J Physiol. 2004 March 1;555(Pt 2):345–54. doi: 10.1113/jphysiol.2003.055590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.