Abstract
Aim
To examine efficacy and predictors of response to a lifestyle intervention for obese youth.
Methods
Retrospective chart review of 214 children and adolescents aged 8-19 years. Linear regression identified baseline predictors of response (Δ BMI z-score) at first and ultimate follow-up visits.
Results
Mean Δ BMI z-score from baseline was -0.04 (p <0.001) at first follow-up and -0.09 (p <0.001) at ultimate follow-up (median time 10 mo) among 156 children and adolescents. Higher baseline BMI z-score predicted poor response at first and ultimate follow-up, explaining 10% of variance in response. Fasting insulin explained 6% of response variance at first follow-up. Δ BMI z-score at the first visit along with baseline BMI z-score explained up to 50% of variance in response at ultimate visit.
Conclusion
Clinic-based interventions improve weight status. Baseline variables predict only a small proportion of response; response at the first visit is a more meaningful tool to guide clinical decisions.
Keywords: obesity, insulin, lifestyle counseling, adolescents, weight loss
INTRODUCTION
The development of obesity in childhood and adolescence is influenced by a complex interplay of behavioral, socio-economic, genetic, and biochemical factors1,2. Behaviors that promote weight gain, including physical inactivity3, television viewing4, and soda consumption5, also contribute to insulin resistance6. Adolescence, a period of relative insulin resistance7, is also accompanied by rapid changes that affect eating behavior8. These include social-environmental influences, such as eating out with peers and keeping up with status foods; access to new physical environments, such as open campus and fast food outlets; and increasing exposure to societal influences, such as mass media and advertising9.
Improving weight status in children and adolescents reduces insulin resistance and other markers of cardiovascular risk10. Recent meta-analyses suggest that lifestyle interventions including counseling and education aimed at reducing obesity in children and adolescents have limited short term efficacy, and the authors recommend identifying mediators and moderators of response to treatment11,12.
To date, efforts to identify predictors of positive response to lifestyle interventions to reduce pediatric overweight have been inconclusive. Despite the disproportionate prevalence of obesity among minority youth, the role of race as a predictor of response has not been examined in most pediatric studies to date, many of which have been limited by ethnically homogeneous populations. Additionally, while fasting insulin has recently been implicated in poor response to pediatric obesity interventions13,14, results have not been consistent15. Finally, most investigators have dichotomized subjects into responders and non-responders when attempting to identify significant predictors, which does not characterize the wide range of responses to obesity interventions. We therefore sought to examine the efficacy of a multidisciplinary lifestyle intervention and to determine the proportion of variance in response that could be explained by baseline characteristics among a diverse group of children and adolescents seen in a pediatric obesity clinic.
PATIENTS AND METHODS
Patient population
Patients aged 8-19 years were seen in the UCSF Weight Assessment for Teen and Child Health (WATCH) Clinic with an initial visit between August 2003 and February 2006. Data from patients receiving pharmacotherapy for weight loss were excluded. Follow-up data were extracted through June 2006. This retrospective chart review was approved by the UCSF Committee on Human Research.
Clinic intervention
The WATCH Clinic takes place in a hospital-associated outpatient clinic with an interdisciplinary team including a pediatric endocrinologist, gastroenterologist, preventative cardiologist, general pediatricians, dietitians, and a psychologist. Primary care physicians refer patients who are less than 21 years old, with body mass index (BMI) greater than the 95th percentile for age and sex. Approximately 10 new patients are seen monthly. Fasting laboratory studies include glucose, insulin, hemoglobin Alc, liver and thyroid function tests, and a lipid profile.
The WATCH clinic lifestyle intervention is modeled after a low glycemic load diet as developed by Ludwig and colleagues16, which has been shown in a randomized trial to reduce BMI and fat mass among adolescents over a 1-year period17. A registered dietitian delivers a 1-hour group teaching breakfast at the intake visit that conveys the following specific recommendations: 1) Eliminate all sugared beverages, including soda and juice. 2) Reduce refined carbohydrates, substituting high-fiber whole grains instead. 3) Increase fruits and vegetables. 4) Include a lean protein or low-fat dairy product with meals and snacks. A visual plate model reinforces this lesson in which one quarter of the plate depicts whole grains, one quarter depicts lean protein, and one half of the plate depicts fruits and vegetables. Other key messages include waiting 20 minutes for second portions, in order to allow satiety cues to develop; and reducing television time to no more than 2 hours per day and removing televisions from children's rooms. Attention is also given to reduced portion sizes and increased physical activity. Parents are encouraged to allow children and adolescents to earn TV time, minute-for-minute, with moderate to vigorous physical activity. Based on the youth's physical activity preferences, an exercise prescription is provided individually during the physician segment of the intake visit. Written educational materials reinforcing the plate model and the dietary and physical activity recommendations are provided.
Patients and families are seen individually by members of the interdisciplinary team at follow-up visits, which are scheduled at 3-month intervals unless additional visits are necessary to manage serious co-morbidities, or for behavioral reinforcement. The lifestyle intervention is continually reinforced, individualized nutrition and physical activity goals are refined and monitored, and barriers to implementation are addressed.
Statistical analysis
All analyses were performed using STATA version 9.2 (Statacorp LP, College Station, TX).
Primary dependent variable
Our primary outcome was change in BMI z-score, used because BMI z-score standardizes an individual's size, adjusting for age and sex. We examined change in BMI z-score at the first follow-up visit (approximately 3 months from baseline) and the ultimate follow-up visit during the period covered by chart review.
Predictor variables
Based on a thorough review of the literature, the following potential predictors were included in the model: sex, race (African American, Asian, Caucasian, Latino, and Other), age, BMI z-score, fasting insulin, insulin resistance (HOMA-IR calculated as: fasting insulin [μU/ml]*fasting glucose [mmol/1]/22.5), and a surrogate measure of socio-economic status (median income for zip code). Self-reported variables included parental BMI (parent-reported); average daily calories from sugared beverages (estimated from ounces of sodas and juice consumed per day, assuming 12.5 kcal/oz); number of times breakfast is eaten per week; number of days per week patient exercises (estimated from a single question); average hours of TV viewing per day (estimated from a single question); and quality of life from PedsQL18 (possible range is from 0-100; higher scores reflect better quality of life). Change in BMI z-score at first follow-up was included as a predictor of response at ultimate follow-up.
Univariate and multivariate regression models examined the association between predictor variables and change in BMI z-score at first and ultimate follow-up visits. In order to easily compare the predictive ability of variables of different units, the correlations presented are standardized beta coefficients (β). In univariate models, βs are identical to Pearson's R; the square of a predictor variable's β is directly interpreted as the proportion of variance in response (change in BMI z-score) it explains. This interpretation is similar in multivariate models. We used ANOVA to examine differences by race in the dependent variables and included interaction terms to explore race (both categorical and dichotomous) as a potential mediator of the effect of other baseline variables. ‘Response’ to the intervention was defined as a decrease in BMI z-score at follow-up.
RESULTS
A total of 214 patients between 8 and 19 years of age presented for an initial WATCH clinic visit during the study period (excluding 11 patients receiving pharmacotherapy), 156 (73%) of whom returned for at least one follow-up visit. The group was ethnically diverse (28% Non-Hispanic White, 26% Hispanic, l3% Non-Hispanic Black, 14% Asian/Pacific Islander, 10% mixed race, and 8% declined to state) and severely overweight and insulin resistant (Table 1). Baseline age, weight status, and fasting laboratory values did not differ by race/ethnicity. However, African-American youth reported consuming 379 kcal/day in sugared beverages compared to 199 kcal/day among other patients (p <0.01), and families of Caucasian youth reported lower quality oflife (65 vs 81 on PedsQL, p <0.01).
TABLE 1.
Baseline characteristics
| Children with follow-up n = 156 | |
|---|---|
| BMI z-score | 2.43 ± 0.37 |
| Age (yr) | 13.0 ± 2.7 |
| BMI (kg/m2) | 36.4 ± 10.1 |
| Fasting Insulin (μU/ml) | 25.9 ± 18.3 |
| HOMA-IR | 5.7 ± 4.3 |
| Triglycerides (mg/dl) | 108 ± 70 |
| HDL-cholesterol (mg/dl) | 42.3 ± 9.2 |
| AST (IU/l) | 27.6 ± 13.6 |
| ALT (IU/l) | 33.5 ± 29.3 |
| Maternal BMI (kg/m2) | 29.8 ± 7.9 |
| Paternal BMI (kg/m2) | 29.5 ± 5.8 |
| Sugared beverages (kcal/d) | 219 ± 234 |
| Breakfast (days/wk) | 4.8 ± 2.7 |
| Physical activity (days/wk) | 3.2 ± 2.3 |
| TV viewing (h/day) | 3.0 ± 1.9 |
| PedsQL total | 75 ± 18 |
| Average income ($000s) | 58 ± 16 |
| Female | 80 (51%) |
| Race | |
| Non-Hispanic Black | 17 (11%) |
| Asian or Asian/PI | 25 (16%) |
| Non-Hispanic White | 49 (31%) |
| Hispanic | 37 (24%) |
| Mixed/Unknown | 28 (18%) |
Fasting insulin and glucose were available for 126 children with follow-up.
Follow-up duration and changes in weight status are shown in Table 2. Other than having slightly higher HDL-cholesterol (42 vs 39 mg/dl), children who returned for follow-up were not significantly different from those who did not return. However, patients reporting lower quality of life tended to be less likely to return (p = 0.07) as did those reporting greater soda consumption at baseline. Median time to first follow-up was 3.2 months (interquartile range 3.2-4.1 months); while 90% of children with a follow-up visit returned within 6 months, five children returned one year or more after their initial visit (maximum 20 months).
TABLE 2.
Follow-up and response to intervention
| First follow-up visit |
Ultimate follow-up visit |
|||||
|---|---|---|---|---|---|---|
| All | Responders | Non-responders | All | Responders | Non-responders | |
| n | 156 | 110 | 46 | 94 | 64 | 30 |
| Months elapsed from baseline [range] | 4.1 ± 2.6 [0.5, 19.7] | 4.0 ± 2.8 [0.5, 19.7] | 4.1 ± 2.6 [0.5, 13.7] | 12.1 ± 6.3 [3.6, 29.5] | 12.3 ± 6.3 [3.6, 29.4] | 11.6 ± 6.3 [4.1, 29.5] |
| Total follow-up visits [range] | 1 | 1 | 1 | 3.2 ± 1.6 [2, 8] | 3.2 ± 1.6 [2, 8] | 3.2 ± 1.7 [2, 8] |
| Mean Δ BMI z-score [95% CI] | -0.04* [-0.06, -0.03] | -0.08 [-0.09, -0.06] | 0.05 [0.03, 0.06] | -0.11* [-0.16, -0.07] | -0.19 [-0.25, -0.14] | 0.06 [0.04, 0.08] |
| Mean Δ BMI (kg/m2) [95% CI] | -0.2* [-0.4, 0.0] | -0.8 [-1.0, -0.5] | 1.1 [0.9, 1.5] | -0.4* [-0.8, 0.1] | -1.3 [-1.8, -0.8] | 1.6 [1.1, 2.1] |
Significantly different (p <0.05) between responders and non-responders.
At the first follow-up visit, 110 patients (71%) decreased their BMI z-score an average of 0.08 units (responders) while 46 increased their BMI z-score by 0.05 units (non-responders). Among patients with two or more follow-up visits, 64 patients had decreased their BMI z-score at the ultimate visit (mean -0.19 units), which corresponded to a 1.3 kg/m2 decrease in BMI, and 30 increased their BMI z-score by a mean of 0.06 units (Table 2).
Better initial weight status, defined as lower baseline BMI and BMI z-score, significantly predicted greater reductions in BMI z-score at first and ultimate follow-up visits (Table 3) in univariate analyses and after adjusting for age, sex, and time elapsed from intake visit; weight status explained 4-10% of variance in response. Higher fasting insulin at baseline explained approximately 6% of the increase in BMI z-score at first follow-up, but did not predict change in BMI z-score at the ultimate visit. Including both baseline BMI z-score and insulin in the model explained only 7% of the variance in response at first follow-up. Similarly, higher triglycerides (which, when accumulated in muscle, have been shown to be correlated with insulin resistance 19,20) significantly predicted poorer response at the first but not ultimate follow-up visit. Females showed greater response at ultimate follow-up than did males. In univariate analyses only, higher sugared beverage consumption at baseline showed a trend to predict poorer response at first follow-up, and eating breakfast more often significantly predicted better response at ultimate follow-up.
TABLE 3.
Univariate and adjusted correlation between baseline variables and change in BMI z-score at first and ultimate visits
| Baseline variables | Correlation with Δ BMI z-score at first follow-up |
Correlation with Δ BMI z-score at ultimate follow-up (if ≥2 follow-up visits) |
||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||
| β | p | β | p | β | p | β | p | |
| BMI z-score | 0.21 | <0.01 | 0.19 | 0.02 | 0.32 | <0.01 | 0.27 | 0.01 |
| Age (yr) | 0.03 | 0.75 | 0.02 | 0.81 | 0.17 | 0.10 | 0.14 | 0.17 |
| BMI (kg/m2) | 0.21 | <0.01 | 0.28 | <0.01 | 0.31 | <0.01 | 0.29 | 0.01 |
| Female | -0.07 | 0.39 | -0.01 | 0.89 | -0.23 | 0.03 | -0.19 | 0.06 |
| Fasting insulin (μU/ml) | 0.25 | <0.01 | 0.21 | 0.03 | 0.22 | 0.06 | 0.09 | 0.51 |
| HOMA-IR | 0.22 | 0.02 | 0.16 | 0.09 | 0.19 | 0.12 | 0.04 | 0.74 |
| Triglycerides (mg/dl) | 0.22 | 0.01 | 0.21 | 0.02 | 0.07 | 0.52 | 0.05 | 0.62 |
| HDL-cholesterol (mg/dl) | -0.02 | 0.84 | -0.01 | 0.89 | -0.11 | 0.32 | -0.04 | 0.75 |
| AST (IU/l) | 0.04 | 0.66 | 0.02 | 0.86 | 0.04 | 0.75 | 0.00 | 0.98 |
| ALT (IU/l) | 0.08 | 0.36 | 0.04 | 0.63 | 0.08 | 0.48 | 0.01 | 0.89 |
| Maternal BMI (kg/m2) | 0.10 | 0.25 | 0.03 | 0.75 | 0.14 | 0.23 | 0.07 | 0.56 |
| Paternal BMI (kg/m2) | 0.08 | 0.39 | 0.01 | 0.93 | 0.11 | 0.40 | 0.06 | 0.64 |
| Sugared beverage intake (kcal/d) | 0.15 | 0.09 | 0.07 | 0.44 | 0.14 | 0.22 | -0.09 | 0.47 |
| Breakfast (days/wk) | -0.05 | 0.61 | 0.03 | 0.75 | -0.23 | 0.05 | -0.10 | 0.42 |
| Physical activity (days/wk) | -0.06 | 0.49 | -0.06 | 0.55 | 0.03 | 0.78 | 0.07 | 0.57 |
| TV viewing (h/day) | 0.02 | 0.79 | 0.00 | 0.99 | 0.07 | 0.54 | -0.06 | 0.63 |
| PedsQL total | -0.16 | 0.18 | -0.09 | 0.53 | 0.05 | 0.78 | 0.15 | 0.37 |
| Average income ($000s) | -0.09 | 0.40 | 0.03 | 0.78 | -0.12 | 0.40 | -0.08 | 0.57 |
| Time to follow-up (mo) | -0.03 | 0.75 | 0.00 | 0.96 | -0.14 | 0.18 | -0.08 | 0.44 |
| Δ BMI z-score at 1st follow-up | 0.43 | <0.001 | 0.37 | <0.001 | ||||
Correlations shown are standardized beta coefficients, equivalent to Pearson's R for unadjusted analyses. A negative correlation suggests that children with higher levels of baseline variables showed greater improvements in BMI z-score. Laboratory values obtained fasting.
Adjusted models include baseline BMI z-score, age, sex and duration of follow-up (BMI is not adjusted for BMI z-score).
While race/ethnicity did not significantly predict response to the intervention using ANOVA (p = 0.28), because of the exploratory nature of this study, we examined differences between races using t-tests and found that African-American patients showed smaller improvements in BMI z-score at first follow-up visit than did all other patients combined (mean -0.003 vs -0.046, p = 0.07). Nonetheless, race did not modify the relationship between baseline BMI z-score (or insulin) and response to the intervention.
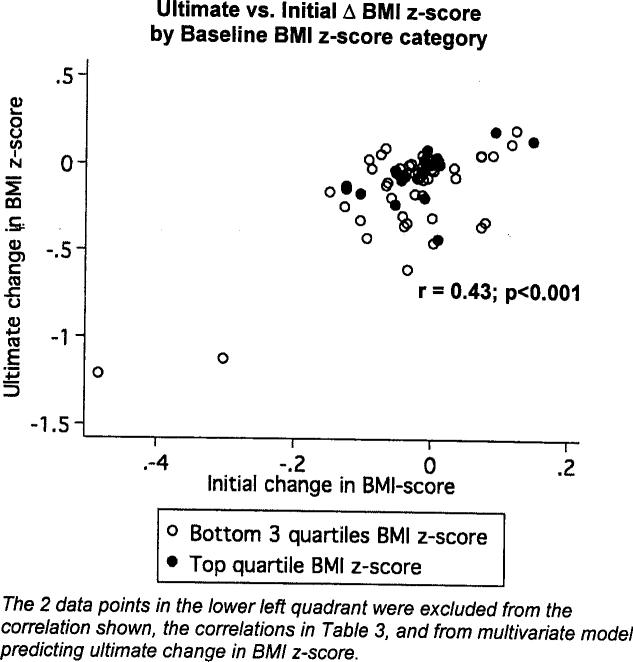
Change in BMI z-score at the first follow-up visit explained 18% of the ultimate change in BMI z-score for patients with ≥2 follow-up visits. A model with baseline BMI z-score and change in BMI z-score at first follow-up explained 24% of the variance in ultimate response. Figure 1 shows this relationship and highlights two patients who showed significant decreases in BMI z-score at first and ultimate follow-up visits. These two data points were highly influential and, therefore, were not included in the above model predicting ultimate response, nor were they included in Table 3 correlations. Including these patients in the above model would increase the variance explained to almost 50%. Adding insulin to the model did not improve its predictive ability.
Fig. 1.
Ultimate versus initial change in BMI z-score, by quartile of baseline BMI z-score.
Because of varying lengths of follow-up, all multivariate models predicting change in BMI z-score included time as a predictor. Duration of follow-up did not correlate with response. Sensitivity analyses excluding patients with first follow-up later than 10 months from intake did not alter these findings.
DISCUSSION
There is a growing body of literature on the efficacy of clinical interventions to address child and adolescent obesity. Consistent with what has been reported, we demonstrate an improvement in weight status in response to a multidisciplinary intervention aimed at changing diet and physical activity behaviors. Recent studies suggest that clinic-based interventions such as the WATCH clinic that focus on lifestyle modification can decrease BMI z-score by 0.03-0.05 over approximately 1 year of follow-up13,21.
Improvements in weight status, such as those demonstrated herein, have been associated with improvements in lipid profiles21. However, larger decreases in BMI z-score may be necessary for additional cardiovascular benefit, particularly improved insulin sensitivity. In a follow-up study among 7-15 year-old obese children. and adolescents, Reinehr et al. found that only patients who decreased their BMI z-score by 0.5 units improved insulin sensitivity22. Interventions that have achieved this magnitude of improvement in weight status have involved more frequent visits, structured family programs, and delivery of a group exercise component10,23,24. These programs represent best-practice for the treatment of severe obesity in youth and are consistent with the current guidelines, which recommend weekly visits for a minimum of 8-12 weeks with subsequent monthly visits25. However, they have required NIH funding for enactment10,23,24 and are difficult to replicate in the clinical setting. Interventions such as the WATCH clinic would be more easily replicated but, because they are constrained by clinical revenues that limit follow-up to every 1-3 months13,15,21, are likely to show more modest improvements in weight status. Further research will be needed to assess the impact of lower-intensity clinics on measures of cardiovascular risk.
Given what can be accomplished in ‘real-life’ clinic-based obesity programs, it is important to determine which patients will require more intensive treatment. While many studies have attempted to identify baseline characteristics that predict response, most have dichotomized patients into responders versus non-responders (generating odds ratios for predictors). Instead, we used a linear regression model for two reasons. First, a continuous outcome variable better represents the spectrum of response seen in pediatric obesity clinics, in which many patients make small changes; dichotomizing is more appropriate when dealing with a cohort of patients with extreme responses. Second, when both predictor and outcome are naturally continuous, correlations are the preferred method for determining the amount of variance in the outcome that can be explained by the predictor. This is particularly important when examining predictors from a clinical practice perspective. A variable may significantly predict response, but still explain only a small portion of it.
We found that response at the first visit was the strongest predictor of response at the ultimate visit, accounting for approximately 20% of the variance. This is similar to studies with follow-up ranging from 1 to 10 years23,26-28, demonstrating that initial response was a strong predictor of long-term success. Initial success may generate some self-confidence and beget greater self-efficacy for adhering to clinic recommendations, as was seen by Braet in an in-patient treatment program27. Alternatively, environmental, familial, or behavioral factors may preclude some patients from adhering to recommendations at the first visit, and such barriers are likely to continue to exert an effect at subsequent visits. Our data suggest that the first follow-up visit is an important visit at which to re-examine strategy and consider moving to more aggressive therapy, such as pharmacotherapy or compUlsory physical activity, particularly for adolescents who were severely overweight at baseline.
Unlike information available at the first follow-up, baseline characteristics are much less consistent in the literature in their ability to predict response. We found that greater obesity at baseline was associated with poorer response at first and ultimate follow-up, explaining up to 10% of response to the intervention. While Eliakim et al.29 and Pinhas-Hameil et al.13 also found greater baseline obesity predicted poor response to a lifestyle intervention, other investigators found the opposite23,27. Among these studies, no single feature related to study participants (such as age, race, or obesity severity) or the intervention itself (such as visit frequency or intervention components) appears unique to either those showing a positive or negative impact of baseline weight status on response. Therefore, it is unclear what factor might modify the effect of baseline weight status on response, and thereby explain the inconsistency of these results.
Our study also implicates insulin resistance in poor initial response to the lifestyle intervention. While insulin resistance was no longer predictive at ultimate follow-up, two other studies have suggested that greater insulin resistance may predict poorer response13,14. Conversely, a recent study found greater insulin resistance predicted better response; however, this study was short (12 weeks) and overall, patients tended to increase their BMI z-score15. While further research may confirm the relationship between greater insulin resistance and poor response, insulin alone explained only 6% of the variance in response in our diverse cohort, similar to results from another clinic-based intervention13. From the clinician's perspective, knowing a patient's insulin resistance is unlikely to change recommended treatment at the outset. As the new Endocrine Society guidelines suggest, although obtaining fasting insulin is optional, it is an expensive test that is not necessary to establish the need for weight loss30. Further, while insulin resistance has been proposed as a means of identifying children at high-risk for diabetes mellitus and cardiovascular disease, its use as a screening tool for therapy will require the development of effective options specific to insulin resistant youth.
Our diverse population allowed us to explore differences in response by race. While each subgroup was relatively small, African-American patients tended to show a lesser response to the intervention at both initial and ultimate follow-up than did their peers. This may suggest that our intervention lacks cultural relevance for African-Americans, or that these adolescents face greater barriers to adherence. Alternatively, the differential response by race may reflect underlying physiological differences, as some research suggests31-34. Further work should be done to design and evaluate culturally appropriate therapies to ensure that children and adolescents of all ethnic backgrounds receive the best possible care.
We report modest correlations between behavioral variables, such as sugared beverage and breakfast consumption, and response to this intervention. Actual correlations might well be higher; behaviors were generally assessed using single questions at the intake clinic, which likely decreases response accuracy and underestimates the relationship between behaviors and response. Unfortunately, behavioral variables are difficult to assess in the clinical setting, as most measures of behavior are self-reported and prone to social desirability bias35.
We recognize additional limitations of our study. Poor follow-up is a common problem in weight management programs36, and while duration of follow-up did not predict response, it is likely that patients with no follow-up are different from those who return for follow-up. Additionally, our average follow-up time of 10 months is inadequate to describe the persistence of short-term treatment effects. Some data exist on the long-term impact of high-intensity obesity treatment programs23,26, but to our knowledge, none currently exist for ‘real-life’ obesity clinics. Longer follow-up will be essential to better elucidate the comparative efficacy of different approaches to obesity treatment.
The current study may have lacked the statistical power to identify other predictors of response, such as interactions between race and other potential predictors of therapeutic response. Further, we did not directly assess socio-economic status, nor did we examine other potential predictors, such as degree or compartment of adiposity, or leptin level.
Our findings demonstrate that response at first follow-up visit is likely to be a valuable means of identifying children in need of more intensive therapy. Severely overweight youth who did not respond to the initial lifestyle counseling in the present study were unlikely to change their trajectory. While evidence is mounting to describe the most efficacious interventions, identifying funding mechanisms for these strategies in the real-world will be critical to addressing the problem of pediatric obesity.
ACKNOWLEDGEMENTS
Funding was provided by NICHD K23HD054 470-01A1 and American Heart Association Beginning Grant-in-Aid 0865005F.
REFERENCES
- 1.Lustig RH. Autonomic dysfunction of the beta-cell and the pathogenesis of obesity. Rev Endocr Metab Disord. 2003;4:23–32. doi: 10.1023/a:1021819318484. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie L, Ivey S, Masch M, Woodward-Lopez G, Ikeda J, Crawford PB. Pediatric Overweight: A Review of the Literature. The Center for Weight and Health; Berkeley, CA: 2001. [Google Scholar]
- 3.Berkey CS, Rockett HR, Gillman MW, Colditz GA. One-year changes in activity and in inactivity among 10- to 15-year-old boys and girls: relationship to change in body mass index. Pediatrics. 2003;111:836–843. doi: 10.1542/peds.111.4.836. [DOI] [PubMed] [Google Scholar]
- 4.Robinson TN. Reducing children's television viewing to prevent obesity: a randomized controlled trial. JAMA. 1999;282:1561–1567. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 6.McGavock JM, Anderson TJ, Lewanczuk RZ. Sedentary lifestyle and antecedents of cardiovascular disease in young adults. Am J Hypertens. 2006;19:701–707. doi: 10.1016/j.amjhyper.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Moran A, Jacobs DR, Jr, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 8.Jasik CB, Lustig RH. Adolescent obesity and puberty: the “perfect storm”. Ann NY Acad Sci. 2008;1135:265–279. doi: 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- 9.Story M, Neumark-Sztainer D, French S. Individual and environmental influences on adolescent eating behaviors. J Am Diet Assoc. 2002;102(Suppl):S40–51. doi: 10.1016/s0002-8223(02)90421-9. [DOI] [PubMed] [Google Scholar]
- 10.Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 11.Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26:521–532. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93:4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 13.Pinhas-Hamiel O, Lerner-Geva L, Copperman N, Jacobson MS. Insulin resistance and parental obesity as predictors to response to therapeutic life style change in obese children and adolescents 10-18 years old. J Adolesc Health. 2008;43:437–443. doi: 10.1016/j.jadohealth.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents according to degree of weight loss. Pediatrics. 2004;114:1569–1573. doi: 10.1542/peds.2003-0649-F. [DOI] [PubMed] [Google Scholar]
- 15.Cummings DM, Henes S, Kolasa KM, Olsson J, Collier D. Insulin resistanee status: predicting weight response in overweight children. Arch Pediatr Adolesc Med. 2008;162:764–768. doi: 10.1001/archpedi.162.8.764. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:e26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 17.Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157:773–779. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Seid M, Rode C. The PedsQL(TM): measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Manco M, Mingrone G, Greco AV, Capristo E, Gniuli D, De Gaetano A, et al. Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism. 2000;49:220–224. doi: 10.1016/s0026-0495(00)91377-5. [DOI] [PubMed] [Google Scholar]
- 20.Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, et al. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–950. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 21.Skelton JA, DeMattia LG, Flores G. A pediatric weight management program for high-risk populations: a preliminary analysis. Obesity (Silver Spring) 2008;16:1698–1701. doi: 10.1038/oby.2008.243. [DOI] [PubMed] [Google Scholar]
- 22.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419–422. doi: 10.1136/adc.2003.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13:373–383. doi: 10.1037//0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- 24.Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Huerta M, Silverstein JH, et al. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 26.Reinehr T, Temmesfeld M, Kersting M, de Sousa G, Toschke AM. Four-year follow-up of children and adolescents participating in an obesity intervention program. Int J Obes (Lond) 2007;31:1074–1077. doi: 10.1038/sj.ijo.0803637. [DOI] [PubMed] [Google Scholar]
- 27.Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity (Silver Spring) 2006;14:148–155. doi: 10.1038/oby.2006.18. [DOI] [PubMed] [Google Scholar]
- 28.Boden G, Chen X, Kolaczynski JW, Polansky M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest. 1997;100:1107–1113. doi: 10.1172/JCI119621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eliakim A, Friedland O, Kowen G, Wolach B, Nemet D. Parental obesity and higher pre-intervention BMI reduce the likelihood of a multidisciplinary childhood obesity program to succeed–a clinical observation. J Pediatr Endocrinol Metab. 2004;17:1055–1061. doi: 10.1515/jpem.2004.17.8.1055. [DOI] [PubMed] [Google Scholar]
- 30.August GP, Caprio S, Fennoy I, Freemark M, Kaufman FR, Lustig RH, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93:4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson MS, Figueroa-Colon R, Huang TT, Dwyer JH, Goran MI. Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. J Clin Endocrinol Metab. 2001;86:3182–3187. doi: 10.1210/jcem.86.7.7665. [DOI] [PubMed] [Google Scholar]
- 32.McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Frazer TE, et al. Efficacy of orlistat as an adjunct to behavioral treatment in overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr Endocrinol Metab. 2004;17:307–319. doi: 10.1515/jpem.2004.17.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preeyasombat C, Bacchetti P, Lazar AA, Lustig RH. Racial and etiopathologic dichotomies in insulin hyper-secretion and resistance in obese children. J Pediatr. 2005;146:474–481. doi: 10.1016/j.jpeds.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyper-insulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care. 2008;31:1445–1447. doi: 10.2337/dc08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewitt J. Ethical components of researcher researched relationships in qualitative interviewing. Qual Health Res. 2007;17:1149–1159. doi: 10.1177/1049732307308305. [DOI] [PubMed] [Google Scholar]
- 36.Parra-Medina D, D'Antonio A, Smith SM, Levin S, Kirkner G, Mayer-Davis E. Successful recruitment and retention strategies for a randomized weight management trial for people with diabetes living in rural, medically underserved counties of South Carolina: the POWER study. J Am Diet Assoc. 2004;104:70–75. doi: 10.1016/j.jada.2003.10.014. [DOI] [PubMed] [Google Scholar]