Abstract
The oral cavity is a primary target for opportunistic infections, particularly oral candidiasis caused by Candida albicans. A commensal fungus commonly colonizing mucosal surfaces, under conditions of immune dysfunction C. albicans can become a pathogen causing recurrent infections. Yet, the role of host oral innate immunity in the development of candidiasis is not fully elucidated. Specifically, the host salivary antimicrobial peptide histatin-5 (Hst-5) has been proposed to play a protective role in the oral cavity against C. albicans. However, investigations demonstrating its efficacy oral tissue have been lacking. To that end in this study, an ex vivo murine model of infection was developed. Viable C. albicans counts and histopathological analyses demonstrated a significant protective effect for Hst-5 on mouse oral tissue against C. albicans. More importantly, host saliva exerted a comparable anti-candidal effect. However, this effect was neutralized upon treatment of saliva with proteases and C. albicans, previously shown to degrade Hst-5, indicating that Hst-5 is likely the salivary component responsible for the observed protection. Combined, the findings from this study demonstrate for the first time the efficacy of salivary Hst-5 in protecting host tissue against C. albicans infection, thereby affirming the therapeutic potential of this natural host peptide.
Keywords: Candida albicans, salivary histatin-5, antimicrobial peptide, innate immunity, murine model
INTRODUCTION
The human fungal species Candida albicans (C. albicans) is a commensal fungus commonly colonizing human mucosal surfaces (Calderone, 2002). However, under conditions of immune dysfunction, colonizing C. albicans can become an opportunistic pathogen causing recurrent mucosal infections, particularly in HIV+ individuals ( Klein, et al., 1984, de Repentigny, et al., 2004, Fidel, 2006). The increasing emergence of strains of C. albicans resistant to commonly used antifungal agents, has made clinical management of candidiasis increasingly difficult and the need for improved drug therapies crucial (Fidel, 2006, Perlroth, et al., 2007). Therefore, identifying the mechanisms and co-factors behind the enhanced susceptibility to oral candidiasis in vulnerable populations such as HIV+ individuals, would serve as a major breakthrough in our understanding of the pathogenesis of oral candidiasis.
The oral cavity is a unique environment and a primary target for opportunistic infections (Klein, et al., 1984, de Repentigny, et al., 2004, Fidel, 2006). However saliva, a complex mix of fluids from salivary glands, plays an important role in the protection of the oral mucosa against microbial infections (Edgar, 1992, Humphrey & Williamson, 2001, Kamagata-Kiyoura, et al., 2004). Specifically, saliva contains a set of peptides with broad spectrum antimicrobial properties produced by the host (Oppenheim, et al., 1988, Edgerton, et al., 1998). These peptides are considered to be an important part of the host’s innate immune system, contributing to maintaining the balance between health and disease in this complex environment (Edgerton, et al., 1998). Surprisingly, however, the important role of these natural antimicrobials in the protection of the oral cavity from candidiasis and particularly their potential as therapeutic agents is only just beginning to be appreciated.
Most notable among the antimicrobial peptides is histatin-5 (Hst-5), a 24-amino acid, histidine-rich, cationic protein produced and secreted by human parotid and submandibular-sublingual glands (Oppenheim, et al., 1988, Edgerton, et al., 1998, Nikawa, et al., 2002). Hst-5, reported to be present at concentrations ranging from 50 to 300μg/ml in the saliva of healthy adults, has exhibited potent activity against C. albicans, including strains resistant to commonly used antifungal agents (Tsai & Bobek, 1997, Helmerhorst, et al., 1999). In addition, Hst-5 was also shown to exert a synergistic effect with antifungal drugs, indicating a potential use for this peptide as a suitable candidate for combination therapy (Koshlukova, et al., 2000, Situ, et al., 2000 van ’t Hof, et al., 2000).
Although it has not been substantiated, Hst-5 has been proposed to play an important role in protecting the oral mucosa from C. albicans due to its potent antifungal properties (Oppenheim, et al., 1988, Helmerhorst, et al., 1997, Edgerton, et al., 1998, Gyurko, et al., 2001). These speculations were corroborated by a clinical study conducted at our institution, where HIV+ individuals highly colonized with C. albicans were shown to exhibit a significant decrease in Hst-5 levels in their saliva compared to healthy individuals (Torres, et al., 2008). These findings suggested an involvement for host innate immunity and specifically Hst-5 in the protection against C. albicans colonization.
More recently, we demonstrated that C. albicans is capable of efficiently degrading Hst-5 via its secreted aspartic proteases (Saps), proteolytic enzymes considered to be important virulence determinants and shown to degrade a variety of host proteins (Meiller, et al., 2009, Naglik, et al., 1999, Villar, et al., 2007). Specifically, Sap9 was identified as the member of the family responsible for the degradation, thereby identifying Hst-5 as the first host-specific substrate for this isoenzyme (Meiller, et al., 2009).
Significantly, the level of peptide degradation was shown to be proportional to C. albicans cell density and inversely proportional to Hst-5 concentration. These parameters are important as they indicate that the transition of C. albicans from commensal to pathogen is finely balanced, with Hst-5 likely playing a key role in the process leading to the development of the infectious process. However, whether these host-pathogen interactions also occur in the oral cavity in a host, remain speculative. In fact, despite the extensive available studies on the antifungal properties of Hst-5 in vitro, explorations into its efficacy on host oral tissue have been lacking. Therefore, this current study aimed to assess the anti-candidal potency of Hst-5 in an environment more closely mimicking in vivo conditions. To that end, a murine ex vivo model of oral candidiasis was adopted to demonstrate the ability of Hst-5 and host saliva to protect oral tissue against C. albicans infection. More importantly, based on the available information from our previous studies, experiments were designed to identify Hst-5 as the salivary component responsible for the observed salivary protective effect.
The overall goal of this study is to contribute to our understanding of the host-pathogen factors and conditions that play a role in candidal infection. In addition, the findings generated will have important clinical implications as they will aid in the identification of novel therapeutic strategies aimed at the prevention and/or treatment of oral candidiasis.
EXPERIMENTAL PROCEDURES
Strains and growth conditions
C. albicans strain SC5314 was used in all experiments (Gillum, et al., 1984). C. albicans was grown in YPD broth (Difco Laboratories, Detroit, MI) overnight at 30°C with shaking and cells were equilibrated in fresh media to an optical density of 1.0 at OD600. Unless otherwise stated, for most experiments Hst-5 was used at a concentration of 150μg/ml (within the range of physiological salivary concentration) and C. albicans at a cell density of 1×107 cells/ml (arbitrary concentration chosen based on previous studies).
Reagents
Hst-5 peptide was synthesized by the Biopolymer Core Laboratory at the University of Maryland. The peptide was validated by mass spectrometry and purity checked by HPLC. Hst-5 was reconstituted in 1mM phosphate-buffered saline (PBS). The recombinant C. albicans proteases (Sap9 and Sap2) were produced in Pichia pastoris and purified as described previously (Albrecht, et al., 2006). All experiments were performed on at least three separate occasions.
Saliva samples
Approximately 5ml of unstimulated whole saliva was collected from 4 healthy adults. No patient identifiers were used and IRB approval and informed consent was obtained. Following collection, samples were immediately subjected to fungal culturing by plating 20μl of each sample on YPD agar plates in order to determine fungal carriage. In addition, oral swabs for fungal culturing were also obtained from all subjects by rubbing a sterile cotton swab across the tongue and buccal mucosa bilaterally to confirm the absence of candidal colonization. Only C. albicans non-colonized and non-infected subjects were included in order to eliminate interference by commensal strains. Saliva samples were clarified from cells and other debris by centrifugation for 10min at 16,000×g at 4°C and recovered supernatant was filter-sterilized.
Ex vivo model of infection
A murine ex vivo model of oral infection was developed in order to demonstrate the anti-candidal potency of Hst-5 and saliva on host oral tissue. For these experiments, tongues were excised from sacrificed eight-week old female CD-1 mice (Charles River Laboratories) (performed under approval of the University of Maryland--Baltimore Animal Care and Use Committee). Tongues were placed in the wells of 24-well tissue culture plates containing 1×107 cells/ml of C. albicans in 1ml PBS.
Effect of Hst-5 and saliva on C. albicans
In order to determine the dose-dependent effect of Hst-5 on C. albicans, tongues were infected in the presence of increasing concentrations of Hst-5 (50-500μg/ml) as described below. Similarly, to assess the susceptibility of C. albicans to Hst-5 (150μg/ml) at various cell densities, CFU counts from tongues infected with C. albicans at 1×106, 1×107, 2×108 or 2×109 cells/ml were determined and assessed as percent killing by Hst-5.
In addition to Hst-5, experiments were also performed to determine whether host saliva exerts a protective effect on tissue against C. albicans. In these experiments, tongues were infected in purified saliva with no Hst-5 added. Controls of tongues infected in PBS (no saliva or Hst-5) were included. Plates were incubated at 35°C for 30min in the presence of Hst-5 or saliva with gentle rotation. Following washing 3 times with PBS, tongues were further incubated for 3h in fresh RPMI 1640 (supplemented with glutamine and HEPES) (Invitrogen, Grand Island, NY) to allow germination and hyphal production by adhering yeast cells. In order to determine the effect of longer exposure to Hst-5, experiments were also performed where additional Hst-5 (150μg/ml) was added to the RPMI media prior to the 3h incubation for a total exposure time to Hst-5 of 3.5h.
Identification of Hst-5 as the salivary mediator of protection
Based on previous information, experiments were designed in order to identify the key anti-candidal salivary component. In these experiments, saliva samples were pre-treated with 1μg of C. albicans purified Sap2 and Sap9 proteases (individually or in combination). As these proteases were shown to degrade and deactivate Hst-5, it was expected that treatment of saliva would neutralize its protective effect. In these experiments, samples were incubated with the proteases at 37°C for 1h with gentle shaking. Following incubation, protease-treated saliva was used in the ex vivo model as described above. The level of the protective effect of each protease-treated saliva sample on the tissue was assessed and compared to that exerted by saliva prior to protease treatment. Purified Saps have been shown be non-toxic to mammalian cells at the concentration used (personal communication with Dr. Hube).
Neutralization of salivary protection by C. albicans
In addition to Sap purified proteases, we have also previously shown that C. albicans cells degrade Hst-5. Therefore, experiments were performed to determine whether C. albicans exposure impacts the protective effect of saliva. In these experiments, purified saliva was incubated with 2×108 cells/ml C. albicans (cell density shown to result in 50% degradation and deactivation of Hst-5) for 2h at 37°C prior to application on the tissue. The protective effect of saliva pre-incubated with C. albicans was assessed and compared to that obtained prior to exposure to C. albicans.
CFU counts from infected tissue
Following incubation in RPMI, tongues were washed 3 times with PBS and cut in half longitudinally. One half of each tongue was weighed then homogenized in PBS and serial dilutions were plated in triplicate onto YPD agar containing ampicillin (20μg/ml). Plates were incubated at 35°C for 24h. Colonies were counted and results were expressed as CFUs/g tissue weight.
Tissue histopathology analyses
The other half of each tongue was fixed in paraformaldehyde, embedded in paraffin and sectioned. Sections were then stained with Periodic Acid Schiff (PAS). The whole periphery of each infected tongue section was examined by light microscopy and evaluated based on the presence and extent of adhering yeast cells and penetration of the epithelium by invasive hyphae. Alternatively, sections were also hybridized with a Cy3-conjugated C. albicans-specific probe for Peptide Nucleic Acid – Fluorescent in situ Hybridization (PNA-FISH), as per the manufacturer’s protocol (Advandx, Woburn, MA). Tissue sections were examined with a confocal scanning laser microscope (Zeiss LSM 510; Carl Zeiss, Thornwood, NY) using a DICII/Cy3 filter set. At least 3 sections from each tongue were examined.
Statistical Analysis
All experiments were performed on at least 3 separate occasions. Student’s t-test was used to assess the statistical significance of results along with standard error. A statistically significant difference was considered to be present at p < 0.05.
RESULTS
Assessment of tissue protection by Hst-5 and host saliva
C. albicans viable counts recovered from Hst-5-treated tongues
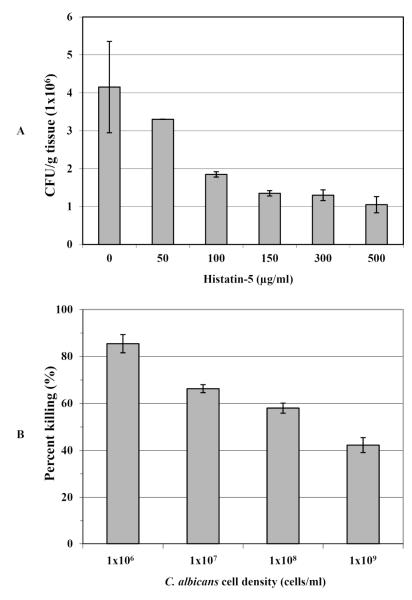
In order to assess Hst-5 treatment on the viability of C. albicans under various conditions, CFU counts from tongue homogenates were determined. Results demonstrated a dose-dependent killing potency for Hst-5 based on decreasing number of CFU counts proportional to Hst-5 concentration (Fig.1A). Interestingly, no significant difference in anti-candidal effect was observed when the tongues were exposed to Hst-5 for the duration of the experiment (3.5h) compared to those tongues exposed to Hst-5 for only 30min (data not shown). In contrast to the proportional Hst-5 concentration-dependent effect, the susceptibility of C. albicans to Hst-5 was inversely proportional to its cell density, where based on percent of killing, higher C. albicans cell densities demonstrated decreasing susceptibility to Hst-5 (Fig. 1B).
Figure 1. Protective effect of Hst-5 on C. albicans viability (CFU/g tissue) using an ex vivo model of oral infection.
(A) Significant decrease in C. albicans viable counts recovered from tongues infected in the presence of increasing Hst-5 concentrations demonstrating a dose-dependent inhibitory effect for Hst-5 on C. albicans (B) In contrast, the anti-candidial effect of Hst-5 was inversely proportional to C. albicans cell density where based on percentage of Hst-5 killing, the susceptibility of C. albicans to Hst-5 decreased with increasing cell density. Error bars indicate the standard errors of the means. No significant difference is seen between 0-50μg/ml Hst-5 concentration (p>0.05). For all other values p<0.05.
C. albicans viable counts recovered from saliva-treated tongues
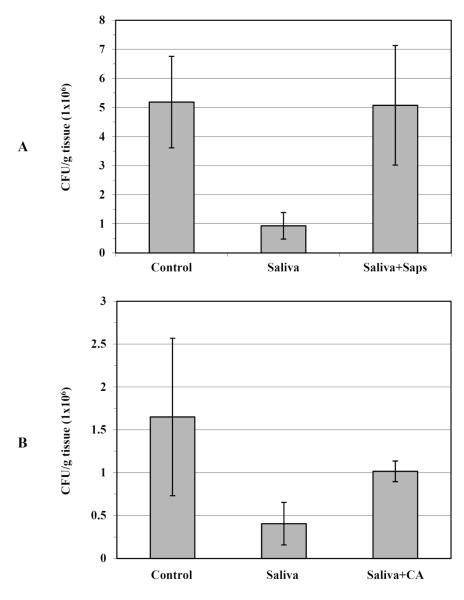
As Hst-5 is a salivary peptide, experiments were performed to assess the effect of saliva on infected tissue. Saliva samples from healthy individuals were collected and tested in the ex vivo infection model. Based on clinical evaluation and fungal culturing of swabs from oral cavities and saliva samples, subjects were determined to be non-Candida colonized or infected. Results from the experiments with human saliva were comparable to those obtained from Hst-5 treatment, where a drastic reduction was observed in the viable numbers (CFUs) of C. albicans from tongues treated with saliva (Saliva) compared to control tongues (Control) (infected in PBS) (Fig. 2A).
Figure 2. Salivary protection of oral tissue and neutralization of protection.
(A) A significant decrease in C. albicans CFU counts recovered from tongues infected in saliva (Saliva) compared to control tongues (Control) with PBS. However, no significant effect on CFU counts was seen when saliva was pre-treated with purified proteases (Saliva+Saps). (B) Similarly, the anti-candidal effect of saliva was compromised following its pre-incubation with C. albicans cells (Saliva+CA), suggesting proteolysis and deactivation of a protective salivary component by treatment of saliva with Saps and C. albicans cells. Results represent the averages of data from 4 subjects tested on several occasions. Error bars indicate the standard errors of the means. No significant difference is seen between control and treated saliva (p>0.05). For all other values p<0.05.
Neutralization 230 of protection
Treatment of saliva with purified proteases
In order to identify Hst-5 as the salivary component responsible for the observed anti-candidal effect of saliva, experiments were simultaneously performed where saliva was pre-treated with purified proteases. The C. albicans purified Saps were previously shown to degrade and deactivate Hst-5 in vitro (Meiller, et al., 2009). Therefore, this property was utilized to neutralize salivary activity that may be due to Hst-5. The results from the Saps-neutralization experiments demonstrated that pre-treatment of the saliva samples with proteases (Saliva+Saps) resulted in almost complete loss of the protection exerted by the saliva prior to treatment with Saps (Saliva) (Fig. 2A). The loss of protective effect was more drastic when a combination of Sap2 and Sap9 proteases was used. Individually, at the same concentration, Sap2 was more effective in neutralizing the saliva (data not shown).
Pre-incubation of saliva with C. albicans cells
In addition to proteolysis of Hst-5 by purified Saps, Sap-producing C. albicans cells were are also shown to degrade Hst-5 in vitro. Therefore, similar neutralization experiments were performed where saliva was pre-incubated with C. albicans cells prior to application on the tissue. Results from these experiments demonstrated that pre-incubation of saliva for 2h with 2×108cells/ml C. albicans cells resulted in 35-40% (p<0.05) loss of saliva’s initial anti-candidal capability (Fig. 2B).
Histopathology analyses of treated and untreated infected tissue
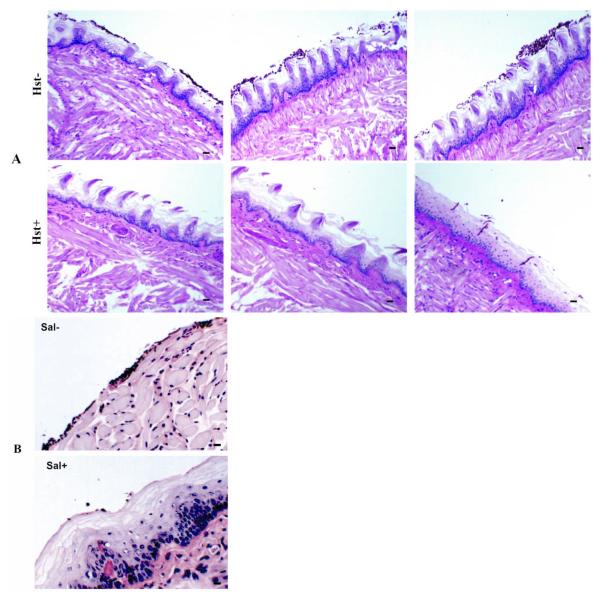
In addition to viability assays to assess the protective capabilities of Hst-5 and saliva, infected tissue was also subjected to histopathology analysis in order to visualize the interaction of C. albicans with the oral epithelium. Following PAS-staining or PNA-FISH, tissue sections were analyzed by light or confocal microscopy, respectively. Consistent with the results from the CFU counts, images from Hst-5-treated tissue revealed significantly decreased presence of adhering yeast cells and penetration of the epithelium by the hyphae in the tongues treated with Hst-5 (Hst+) compared to untreated (Hst−) tongues (Fig.3A).
Figure 3. Histopathology analysis of mouse tongues infected with C. albicans in the absence and presence of Hst-5 and saliva.
Representative microscopic images (20x) from PAS-stained tissue demonstrating significantly decreased levels of C. albicans adherence and invasion of the tongue tissue treated with (A) Hst-5 (Hst+) compared to the untreated (Hst−) tissue. (B) A similar protective effect on the oral tissue against C. albicans infection was seen in the saliva-treated (Sal+) compared to the untreated (Sal−) tissue. Bar represents 20μm.
Similarly, results from analysis of tissue sections from tongues treated with saliva were comparable to those from Hst-5 treatment, where significantly diminished numbers of C. albicans were seen adhering to and invading the saliva-treated (Sal+) tongues compared to control tongues infected in PBS (Sal−) (Fig. 3B).
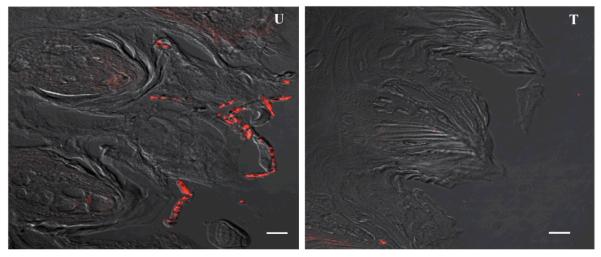
PNA-FISH image results were consistent with those for the PAS-stained sections in all experiments performed. A sample representative PNA-FISH image is shown in Fig. 4 demonstrating the typical picture seen with Hst-5 or saliva-treated (T) tissue compared to untreated (U) tissue. Similarly, Fig. 5 is a representative magnified image from a tongue infected with C. albicans demonstrating the typical extensive adherence and hyphal invasion of the epithelium occurring in the absence of Hst-5 or saliva.
Figure 4. PNA-FISH magnified images (100x) of tongue tissue sections hybridized with a Cy3-conjugated C. albicans-specific probe.
Representative images demonstrating C. albicans presence on the tissue of untreated (U) tongues compared to presence on tongues treated (T) with Hst-5 or saliva. Bar represents 20μm.
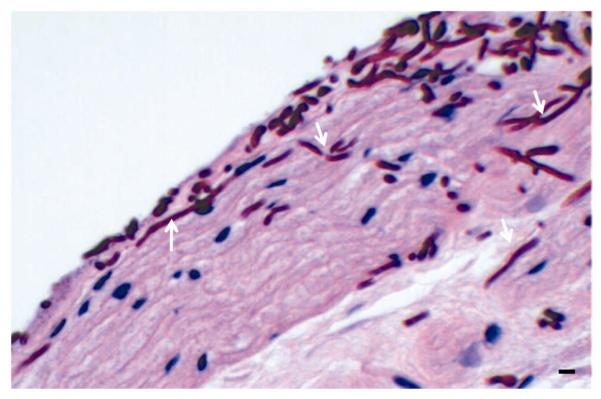
Figure 5. A magnified typical image of PAS-stained section of untreated tongue tissue infected with C. albicans.
Representative image demonstrating extensive adherence and hyphal invasion (arrows) of the parakeratin and spinous layers of the epithelium. Bar represents 20μm.
DISCUSSION
It is well-established that the process of development and course of microbial infections are regarded as an encounter between the virulence of a microorganism and the ability of the host to prevent microbial colonization or invasion. This is particularly evident in the oral cavity, a unique environment constantly bombarded with microbial challenges. C. albicans is perhaps the most successful oral opportunistic pathogen and its success greatly depends on the aptitude of the host factors responsible for maintaining it in a commensal, non-harmful state. In the oral cavity, host innate immune factors such as antimicrobial peptides, are considered to play a crucial role in this process. In the case of oral candidiasis, Hst-5 has gained considerable attention due to its potent antifungal properties. However, although the anti-candidal potency of Hst-5 has been clearly established in vitro, studies exploring its efficacy in protecting host tissue against candidal infection have been lacking.
Models of mucosal candidiasis that closely parallel in vivo dynamics are highly desirable as they are central to assessing fungal pathogenicity and host defenses. More importantly, such models are useful in assessing the efficacy of various agents for the treatment of oral candidiasis. The murine model has been the animal model of choice for studying mucosal candidiasis due to the demonstrated similarities of specific host processes. Therefore, we adopted a murine ex vivo model of oral infection in order to feasibly evaluate the anti-candidal potency of Hst-5 on host oral tissue. Furthermore, although human saliva has been shown to exhibit a protective anti-candidal effect on oral tissue, the effect was not attributed to any specific salivary component (Kamagata-Kiyoura, et al., 2004). To that end, experiments were also designed to evaluate the protective effect of saliva on oral tissue and attribute a role for Hst-5 in the process.
Based on viable C. albicans counts recovered from untreated and treated infected tongues, the findings demonstrated a decreasing number of CFUs proportional to Hst-5 concentration used (Fig. 1A). These findings were analogous to those obtained from in vitro killing assays from previous studies (Meiller, et al., 2009). Surprisingly no significant difference in the level of anti-candidal protection was observed when tongues were exposed to Hst-5 for 30min or 3.5h, indicating that Hst-5 exerts its anti-candidal effect very rapidly.
In line with previous findings, the potency of Hst-5 on the tissue was inversely proportional to C. albicans cell density (Fig. 1B). These results are significant in terms of the ability of C. albicans to degrade Hst-5; based on densitometric analysis of intact Hst-5 vs. degradation products following electrophoretic separation of fragmented Hst-5, the level of degradation was shown to be proportional to C. albicans cell density (Meiller, et al., 2009). In this current study, at a C. albicans density unable to significantly degrade Hst-5 (1×106 cells/ml), 85% of the cells were killed (Fig. 1B). Conversely, a cell density of 1×109 cells/ml causing 45% loss of Hst-5 integrity, approximately 58% of C. albicans cells were killed (p<0.05). Collectively, these findings strongly suggest that the diminishment of Hst-5 potency as C. albicans numbers increase is likely due to proteolysis of the peptide by proliferating C. albicans.
More importantly however, was the demonstration of a comparable protective effect for saliva to that exhibited by Hst-5 alone (Fig. 2). Interestingly, the level of salivary protection (based on C. albicans CFU counts) was equivalent to that for Hst-5 concentrations within the salivary physiological range (150-300μg/ml), as determined from the Hst-5 concentration-dependent killing curve (Fig. 1A). Although this observed salivary effect may not necessarily be due to Hst-5, the results from subsequent neutralization assays were highly suggestive of Hst-5 being the key salivary component. Based on available information demonstrating fragmentation and deactivation of Hst-5 by Saps, saliva was pre-treated with the proteases prior to application on the oral tissue (Meiller, et al., 2009). As expected, results demonstrated an almost complete loss of the initially observed protective effect of the saliva (Fig. 2A). Individually, Sap2 treatment proved to be more effective in neutralizing Hst-5 than Sap9, at the same concentrations. These observations were interesting as they are consistent with previous results where, based on RP-HPLC and MALDI-TOF/TOF MS analysis of Hst-5 degradation products, Sap2 was shown to result in enhanced degradation of Hst-5 (Meiller, et al., 2009).
More relevant to the clinical in vivo picture, incubating the saliva with C. albicans at a cell density predetermined to significantly compromise Hst-5 integrity, resulted in 35-40% loss of saliva’s initial protective capability (p<0.05) (Fig. 2B). Nevertheless, to conclusively identify Hst-5 as the key salivary element would require neutralization of salivary protection using a Hst-5-specific antibody. Although a monoclonal antibody specific to Hst-5 is currently under production in our laboratories, none are currently available. However, it is important to note that Hst-5 is the only identified host-specific target for Sap9. Therefore, neutralization of saliva upon Sap9-treatment indicates that most likely, Hst-5 is the component responsible for the salivary protection.
Furthermore, by previously testing an array of SAP null mutant C. albicans strains for their ability to degrade Hst-5, no inhibition of Hst-5 degradation was seen with the C. albicans sap2 mutant strain, whereas with the sap9 mutant, 100% inhibition of degradation was observed (Meiller, et al., 2009). Combined, these findings indicate that although the Sap2 purified protease efficiently degrades Hst-5, this protease is not the C. albicans cell-associated factor responsible for Hst-5 and saliva neutralization following incubation with C. albicans yeast cells.
In summary, this study identifies Hst-5 as a salivary mediator involved in the protection of the oral cavity against C. albicans, thereby affirming the therapeutic potential of this peptide. Based on the combined findings, it is reasonable to speculate that Hst-5 plays a key role in preventing the transition of C. albicans from commensal to pathogen. Specifically, disruption of the fine balance between host and pathogen factors dictates the dynamics of the infectious process in the oral cavity. However, clinical studies are necessary to fully elucidate the role of host innate immunity in the enhanced predisposition to oral candidiasis, within the context of HIV, Hst-5 salivary levels and candidal prevalence.
With the limited arsenal of antifungals available, coupled with the increasing emergence of resistant C. albicans strains, the prospect of precluding candidiaisis by using innate antimicrobial peptides as alternative drug therapies is becoming increasingly attractive (Helmerhorst, et al., 1999, Situ & Bobek, 2000, van ’t Hof, et al., 2001). Specifically, its potent anti-candidal properties combined with a lack of toxicity to human cells makes Hst-5 a promising therapeutic agent for the prevention and/or treatment of oral candidiasis, particularly in HIV-infected individuals. However, to fully explore the therapeutic potential of Hst-5 in a host, an in vivo model is warranted. These experiments are currently in preparation in our laboratories.
Acknowledgments
This work was supported by NIH grants DE14424 and DE016257.
We would like to thank Dr. Bernhard Hube for his assistance and for providing us with the Sap purified proteases.
REFERENCES
- Albrecht A, Felk A, Pichova I, et al. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J Biol Chem. 2006;281:688–694. doi: 10.1074/jbc.M509297200. [DOI] [PubMed] [Google Scholar]
- Calderone RA. Candida and Candidiasis. ASM Press; Washington: 2002. [Google Scholar]
- de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729–759. doi: 10.1128/CMR.17.4.729-759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar WM. Saliva: its secretion, composition and functions. Brit Dent J. 1992;172:305–312. doi: 10.1038/sj.bdj.4807861. [DOI] [PubMed] [Google Scholar]
- Edgerton M, Koshlukova SE, Lo TE, Chrzan BG, Straubinger RM, Raj PA. Candidacidal activity of salivary histatins. J Biol Chem. 1998;272:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- Fidel PL., Jr. Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res. 2006;19:80–84. doi: 10.1177/154407370601900116. [DOI] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine‘5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Gyurko C, Lendenmann U, Helmerhorst EJ, Troxler RF, Oppenheim FG. Killing of Candida albicans by histatin 5: cellular uptake and energy requirement. Antonie van Leeuwenhoek. 2001;79:297–309. doi: 10.1023/a:1012070600340. [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, van’t Hof W, Veerman ECI, Simoons-Smit I, Amerongen AV Nieuw. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem J. 1997;326:39–45. doi: 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst EJ, Reijnders IM, van’t Hof W, Simoons-Smit I, Veerman ECI, Amerongen AV Nieuw. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob Agents Chemother. 1999;43:702–704. doi: 10.1128/aac.43.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst EJ, Hodgson R, van ’t Hof W, Veerman EC, Allison C, Amerongen AV Nieuw. The effects of histatin-derived basic antimicrobial peptides on oral biofilms. J Dent Res. 1999;78:1245–1250. doi: 10.1177/00220345990780060801. [DOI] [PubMed] [Google Scholar]
- Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthetic Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- Kamagata-Kiyoura Y, Abe S, Yamaguchi H, Nitta T. Protective effects of human saliva on experimental murine oral candidiasis. J Infect Chemother. 2004;10:253–255. doi: 10.1007/s10156-004-0330-6. [DOI] [PubMed] [Google Scholar]
- Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. New Eng J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Koshlukova SE, Araujo MWB, Baev D, Edgerton M. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect Immun. 2000;68:6848–6856. doi: 10.1128/iai.68.12.6848-6856.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiller TF, Hube B, Schild L, et al. A novel immune evasion strategy of Candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS ONE. 2009;4:e5039. doi: 10.1371/journal.pone.0005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Infect Immun. 1999;67:2740–2745. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa H, Jin C, Makihira S, Hamada T, Samaranayake LP. Susceptibility of Candida albicans isolates from the oral cavities of HIV-positive patients to histatin-5. J Prosthetic Dent. 2002;88:263–267. doi: 10.1067/mpr.2002.127907. [DOI] [PubMed] [Google Scholar]
- Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. Histatins, a novel family of histidine-rich proteins in human parotid secretion. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- Situ H, Bobek LA. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob Agents Chemother. 2000;44:1485–1493. doi: 10.1128/aac.44.6.1485-1493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SR, Garzino-Demo A, Meeks V, Meiller TF, Jabra-Rizk MA. Salivary Histatin-5 and oral fungal colonization in HIV+ individuals. Mycoses. 2008;52:11–15. doi: 10.1111/j.1439-0507.2008.01602.x. [DOI] [PubMed] [Google Scholar]
- Tsai H, Bobek LA. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Animicrob Agents Chemother. 1997;41:2224–2228. doi: 10.1128/aac.41.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Hof W, Veerman ECI, Helmerhorst EJ, Amerongen AV Nieuw. Antimicrobial peptides: properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- van ’t Hof W, Reijnders IM, Helmerhorst EJ, Walgreen-Weterings E, Simoons-Smit IM, Veerman EC, Amerongen AV. Synergistic effects of low doses of histatin 5 and its analogues on amphotericin B anti-mycotic activity. Antonie Van Leeuwenhoek. 2000;78:163–169. doi: 10.1023/a:1026572128004. [DOI] [PubMed] [Google Scholar]
- Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor rim 101p and protease Sap5p. Infect Immun. 2007;75:2126–2135. doi: 10.1128/IAI.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]