Abstract
Glutamate transporter subtype 1 (GLT-1) activation is a promising – and understudied - approach for managing aspects of morphine tolerance caused by increased glutamatergic transmission. Identification of beta-lactam antibiotics as pharmaceuticals which activate GLT-1 transporters prompted us to hypothesize that repeated beta-lactam antibiotic (ceftriaxone) administration blocks development of tolerance to morphine antinociception through GLT-1 activation. Here, we injected rats with morphine (10 mg/kg, s.c.) twice daily for 7 days to induce tolerance and used the hot-plate assay to determine antinociception on days 1, 4 and 7 of repeated morphine administration. Ceftriaxone and a selective GLT-1 transporter inhibitor dihydrokainate (DHK) were co-administered with morphine to determine if GLT-1 activation mediated the ceftriaxone effect. Tolerance was present on days 4 and 7 of repeated morphine administration. Ceftriaxone (50, 100 or 200 mg/kg, i.p.) administration dose-dependently blocked development of morphine tolerance. DHK (10 mg/kg, s.c.), administered 15 min before each morphine injection, prevented inhibition of morphine tolerance by ceftriaxone (200 mg/kg, i.p.). These results identify an interaction between ceftriaxone and morphine in opioid-tolerant rats and suggest beta-lactam antibiotics preserve analgesic efficacy during chronic morphine exposure.
Keywords: beta-lactam antibiotic, GLT-1, ceftriaxone, glutamate, morphine, opioid, tolerance
1. Introduction
GLT-1 transporters, expressed by rat and human (excitatory amino-acid transporter-2, EAAT2) astrocytes, mediate 90% of extracellular glutamate uptake. GLT-1 activation is a promising therapeutic approach for glutamate-related pathologies but is understudied because of a lack of compounds which activate the transporter. Beta-lactam antibiotics, identified recently as drugs that increase glutamate uptake through GLT-1 activation, are being used to explore GLT-1 pharmacology (Rothstein et al., 2005; Lipski et al., 2007; Rawls et al., 2007). Morphine tolerance remains a clinical problem because the progressively higher morphine doses, which are required to relieve pain, limit safety and exacerbate morphine dependence and withdrawal. In animal models, antinociceptive tolerance produced by chronic morphine exposure requires increased glutamatergic transmission at NMDA, AMPA and mGluR type-I (subtype mGluR5) receptors (Trujillo and Akil, 1991). Because beta-lactam antibiotics activate GLT-1, we tested the hypothesis that that ceftriaxone blocks development of morphine tolerance in rats.
2. Methods
2.1. Animals and drug preparation
Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (Ace Laboratories, Boyertown, PA) weighing 275–300 g were housed 2 per cage for 5 days before experimental use and maintained on a 12-hr light/dark cycle and fed rat chow and water ad libitum. Morphine sulfate (NIDA, Rockville, MD, USA); ceftriaxone hydrochloride (Apotex Corporation, Weston, FL, USA); and dihydrokainate (DHK) (Tocris Bioscience, St. Louis, MO, USA) were dissolved in saline. Ceftriaxone and DHK were administered intraperitoneally (i.p.), and morphine was injected subcutaneously (s.c.).
2.2. Hot-plate assay of thermal nociception
Antinociceptive responses were determined at 54 °C and defined by the animal licking its back paw as previously described (Wang et al., 2008). Rats were placed onto a hot-plate maintained at 37 °C for 1 min. Thirty min later, the same rat was placed back onto the hot-plate, maintained at 50 °C, until a nociceptive response was observed. Thirty min later, and prior to morphine administration, baseline latency was measured at 54 °C. Immediately following baseline determination, morphine was injected, and response latency was recorded 60 min post-injection. A 45-s cut-off time was used.
2.3. Experimental Design
Ceftriaxone effects on development of morphine tolerance
Rats were injected with ceftriaxone (200 mg/kg) or saline once daily (7 AM) for 10 days. Beginning on the fourth day, rats from both groups were injected with morphine (10 mg/kg) or saline twice daily (10 AM and 10 PM) for 7 days except for day 7, when only the morning injection of morphine (10 mg/kg) or saline was administered. Hot-plate experiments were conducted following the morning injection of morphine on days 1, 4 and 7 of repeated morphine administration. Following baseline response latency determination, morphine (10 mg/kg) or saline was injected and response latency was determined 30 min post-administration. The experiment was repeated to investigate dose-related effects of ceftriaxone (25, 50 and 100 mg/kg).
DHK and ceftriaxone effects on development of morphine tolerance
Rats were injected with ceftriaxone (200 mg/kg) or saline once daily (7 AM) for 10 days. Beginning on the fourth day, rats from both groups were injected with a combination of saline/morphine (10 mg/kg) and DHK (10 mg/kg)/morphine (10 mg/kg) twice daily (10 AM and 10 PM) for 7 days except for the last day, in which case the combination was given only in the morning. DHK (or saline) was administered 30 min before morphine. Hot-plate experiments were conducted following the morning morphine injection on days 1, 4 and 7 of repeated morphine administration. Following determination of baseline latency, DHK (10 mg/kg) or saline was administered, followed 30 min later by morphine (10 mg/kg). Response latency was determined 30 min post-morphine injection.
2.4. Data analysis
Response-latency values were expressed as percentage of maximum possible effect (%MPE):
Two-way analysis of variance (ANOVA) with repeated measures on day followed by a Bonferroni's post-hoc analysis identified differences between individual treatment groups. Values of P < 0.05 were considered significant.
3. Results
3.1. Ceftriaxone inhibits morphine tolerance
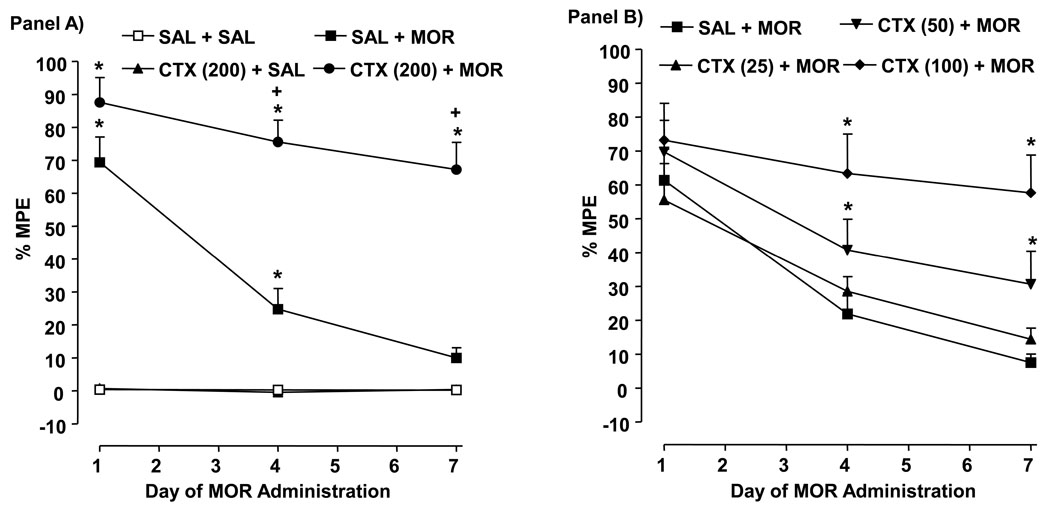
Drug [F (3, 24) = 172.7, P < 0.0001] and time [F (2, 48) = 18.65, P < 0.0001] effects were present (Fig. 1, Panel A). In rats injected with saline/morphine, antinociceptive tolerance developed by day 4, and by day 7, response latency was not different from the saline/saline group (P > 0.05). Rats treated with ceftriaxone (200 mg/kg)/morphine displayed greater antinociception on days 4 (P < 0.05) and 7 (P < 0.05) than rats injected with saline/morphine (10 mg/kg) and greater response latency on days 1, 4 and 7 than rats exposed to saline/saline (P < 0.05).
Fig. 1.
Repeated ceftriaxone (CTX) inhibits antinociceptive tolerance to morphine (MOR). Data are expressed as mean % MPE ± S.E.M. determined 60 min following MOR administration on days 1, 4 and 7 of repeated MOR exposure. N = 6–8 rats/group. Panel A) *P < 0.05 compared to saline (SAL) + SAL. Panel B) +P < 0.05 compared to SAL + MOR.
3.2. Ceftriaxone dose-dependently inhibits morphine tolerance
Drug [F (3, 36) = 9.886, P < 0.0001] and time [F (2, 72) = 19.4, P < 0.0001] effects were present (Fig. 1, Panel B). Rats injected with ceftriaxone (25 mg/kg)/morphine did not display antinociception on days 1, 4, and 7 that differed from rats exposed to saline/morphine (P > 0.05). Higher ceftriaxone doses did reduce morphine tolerance, such that on days 4 and 7 the response latency of rats treated with ceftriaxone (50 mg/kg)/morphine (P < 0.05) or ceftriaxone (100 mg/kg)/morphine (P < 0.05) was enhanced compared to saline/morphine-exposed rats.
3.3. GLT-1 transporter inhibition prevents blockade of morphine tolerance by ceftriaxone
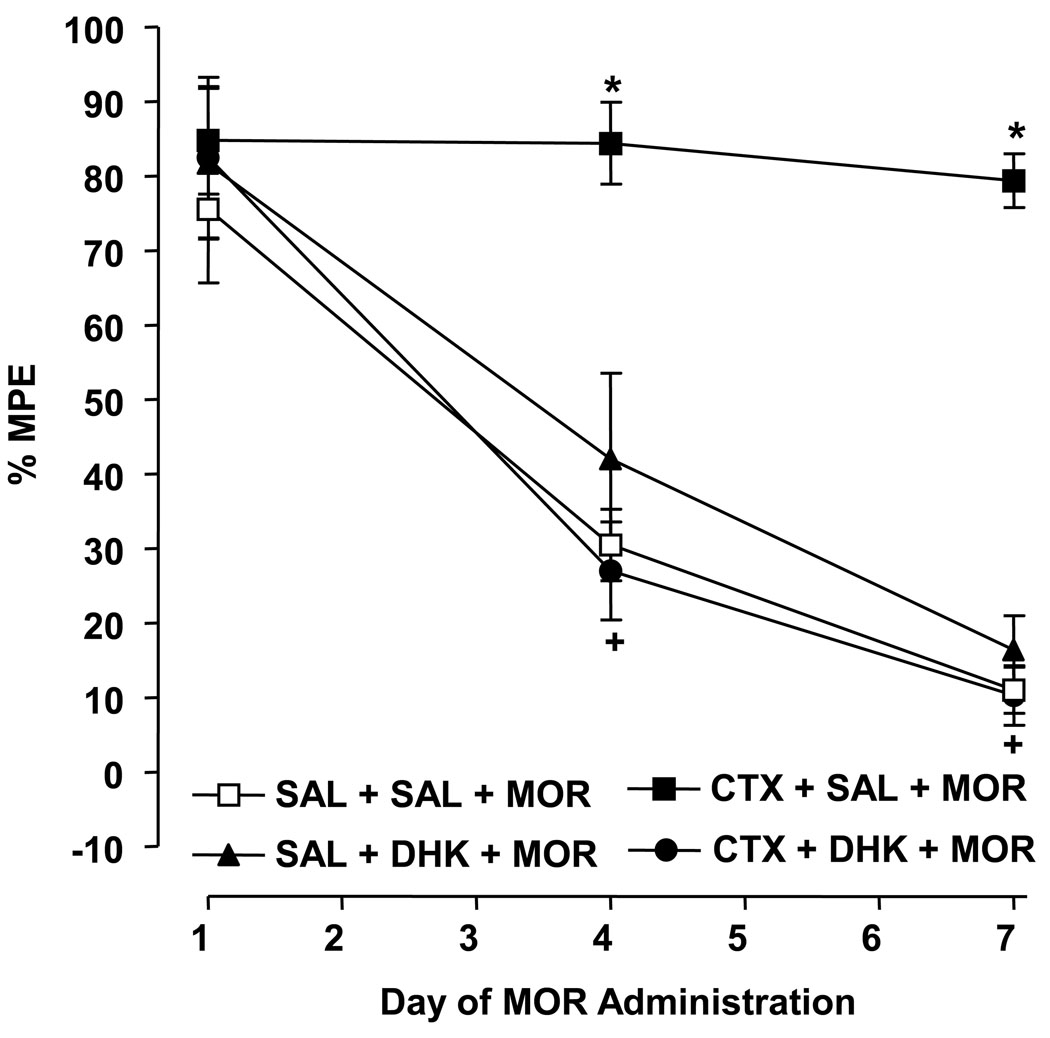
Drug [F (3, 24) = 23.55, P < 0.0001] and time [F (2, 48) = 51.02, P < 0.0001] effect was present (Fig. 2). In morphine-exposed rats, concurrent treatment with ceftriaxone (200 mg/kg)/saline produced greater antinociception on days 4 and 7 than concomitant administration of either saline/saline (P < 0.05) or ceftriaxone (200 mg/kg)/DHK (10 mg/kg) (P < 0.05), indicating that DHK prevented the effect of ceftriaxone. In ceftriaxone-naïve rats exposed to morphine, concomitant treatment with DHK (10 mg/kg)/saline did not result in antinociception on days 1, 4, and 7 that differed from concurrent saline/saline treatment (P > 0.05).
Fig. 2.
DHK (10 mg/kg) blocks inhibition of morphine (MOR) tolerance by repeated ceftriaxone (CTX) (200 mg/kg). Data are expressed as mean % MPE ± S.E.M. determined 60 min following MOR administration on days 1, 4 and 7 of repeated MOR exposure. N = 6–8 rats/group. *P < 0.05 compared to saline (SAL) + SAL + MOR and +P < 0.05 compared to CTX + SAL + MOR.
4. Discussion
The current study identified an interaction between beta-lactam antibiotics and morphine in opioid-tolerant rats and demonstrated that ceftriaxone blocked antinociceptive tolerance to morphine. GLT-1 transporter inhibition by DHK prevented the ceftriaxone effect, suggesting that ceftriaxone efficacy was dependent on GLT-1 activation (Rothstein et al., 2005; Lipski et al., 2007; Rawls et al., 2007). Prior work indicates that morphine tolerance is accompanied by a reduction in GLT-1 transporter activity (Lim et al., 2005; Wu et al., 2008) and inhibited by MS-153, a diverse agent which enhances glutamate uptake in COS-7 cells but also exerts glutamate transporter-independent, anti-glutamatergic effects that oppose opioid tolerance, including inhibition of high voltage-gated calcium channels (Nakagawa et al., 2001; Uenishi et al., 1999). The mechanism by which GLT-1 transporter activation attenuated development of morphine tolerance in our experiments is unclear. The most plausible explanation is that rats co-treated with ceftriaxone and morphine displayed enhanced cellular glutamate uptake which prevented extracellular glutamate levels from increasing during repeated morphine exposure, thereby inhibiting the normal enhancement in ionotropic and metabotropic glutamate receptor activity upon which the development of morphine tolerance is highly dependent (Trujillo and Akil, 1991). One specific possibility is that reduced mGluR5 receptor activity decreased the internalization and desensitization of mu opioid receptors produced by chronic morphine exposure, thus preserving the analgesic efficacy of morphine (Schröder et al., 2009). Considering the evidence that morphine tolerance is inhibited by directly acting GABA agonists and GABA-transaminase inhibitors, it is equally possible that GLT-1 transporter activation prevented tolerance by enhancing GABA transmission, perhaps by increasing GABA synthesis via the glial glutamate-GABA shunt (McKenna, 2007; Sivam and Ho, 1985; Chavooshi et al., 2009).
Ceftriaxone doses of 50 and 100 mg/kg, as well as the 200 mg/kg dose routinely used in animal models of glutamate-related pathologies, inhibited morphine tolerance. Intraperitoneal administration of 200 mg/kg yields CNS ceftriaxone concentrations comparable to those CNS levels required to increase GLT-1 expression (3.5 µM) and attained with meningitis therapy (0.3–6 µM), but this dose given to rats is equivalent to a dose of 13 g/d for a typical adult patient, significantly greater than the maximal dose (2 g/d) administered to patients as an antibiotic (Rothstein et al., 2005; Chandrasekar et al., 1984). Hence, the current demonstration that lower ceftriaxone doses are effective is important, and future studies will determine if the ceftriaxone effect is mimicked by potent beta-lactam antibiotics (e.g. meropenem) and beta-lactamase inhibitors (e.g. clavulanic acid), which are structurally similar to beta-lactam antibiotics but lack anti-bacterial efficacy.
In summary, the antinociceptive efficacy of morphine was preserved in rats exposed repeatedly to a combination of ceftriaxone and morphine. Adverse effects produced by beta-lactam antibiotics may limit their usefulness in the clinical management of morphine tolerance, but their ability to inhibit tolerance in a preclinical model through GLT-1 activation might stimulate development of a non-antibiotic, beta-lactam containing compound displaying enhanced clinical versatility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chandrasekar PH, Rolston KV, Smith BR, LeFrock JL. Diffusion of ceftriaxone into the cerebrospinal fluid of adults. J. Antimicrob. Chemother. 1984;14:427–430. doi: 10.1093/jac/14.4.427. [DOI] [PubMed] [Google Scholar]
- Chavooshi B, Saberi M, Pournaghash Tehrani S, Bakhtiarian A, Ahmadiani A, Haghparast A. Vigabatrin attenuates the development and expression of tolerance to morphine-induced antinociception in mice. Pharmacol. Biochem. Behav. 2009;93:155–159. doi: 10.1016/j.pbb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Lim G, Wang S, Mao J. cAMP and protein kinase A contribute to the downregulation of spinal glutamate transporters after chronic morphine. Neurosci. Lett. 2005;376:9–13. doi: 10.1016/j.neulet.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J. Neurosci. Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur. J. Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Tallarida RJ, Robinson W, Amin M. The beta-lactam antibiotic, ceftriaxone, attenuates morphine-evoked hyperthermia in rats. Br. J. Pharmacol. 2007;151:1095–1102. doi: 10.1038/sj.bjp.0707309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Schröder H, Wu DF, Seifert A, Rankovic M, Schulz S, Höllt V, Koch T. Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the micro-opioid receptor. Neuropharmacology. 2009;56:768–778. doi: 10.1016/j.neuropharm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Sivam SP, Ho IK. GABA in morphine analgesia and tolerance. Life Sci. 1985;37:199–208. doi: 10.1016/0024-3205(85)90645-9. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Uenishi H, Huang CS, Song JH, Marszalec W, Narahashi T. Ion channel modulation as the basis for neuroprotective action of MS-153. Ann. N. Y. Acad. Sci. 1999;890:385–399. doi: 10.1111/j.1749-6632.1999.tb08018.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen Y, Xu W, Lee DY, Ma Z, Rawls SM, Cowan A, Liu-Chen LY. 2-Methoxymethyl-salvinorin B is a potent kappa opioid receptor agonist with longer lasting action in vivo than salvinorin A. J. Pharmacol. Exp. Ther. 2008;324:1073–1083. doi: 10.1124/jpet.107.132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Lu XQ, Yan HT, Su RB, Wang JF, Liu Y, Hu G, Li J. Aquaporin 4 deficiency modulates morphine pharmacological actions. Neurosci. Lett. 2008;448:221–225. doi: 10.1016/j.neulet.2008.10.065. [DOI] [PubMed] [Google Scholar]