Abstract
Purpose
Inhibition of the anti-apoptotic BCL2 family is one of the most promising areas of anti-cancer drug development. However, ABT-737, a specific BCL2 inhibitor, is neither orally bioavailable nor metabolically stable. To overcome these problems, the structurally related molecule, ABT-263, was synthesized and recently entered clinical trials in hematological malignancies, including chronic lymphocytic leukemia (CLL). Almost all laboratory studies have been carried out with ABT-737 rather than ABT-263, the drug being used in clinical trials. Currently there are no published data on the comparative effects of these inhibitors. To gain insight into the potential value or limitations of ABT-263 in the clinic, we assess its ability to induce apoptosis in clinically relevant cellular models of CLL.
Experimental design
The susceptibility of freshly isolated primary CLL cells to these inhibitors was compared in standard culture conditions and to more closely mimic in vivo conditions in a whole blood assay system.
Results
ABT-737 was more potent than ABT-263 at inducing apoptosis in CLL cells. In whole blood, ~100-fold higher concentrations of both drugs were required to induce apoptosis. We found that ABT-263 was highly bound by albumin and that an increased albumin binding of ABT-263 as compared to ABT-737 accounted for the differential sensitivity of CLL cells.
Conclusions
Our data indicate that the exquisite in vitro sensitivity of CLL cells to BCL2-inhibitors may be lost in vivo due to high cell densities and the albumin binding of ABT-263. Modification of ABT-263 may yield a BCL2-inhibitor with greater bioavailability and more favorable pharmacokinetics.
Keywords: ABT-263, ABT-737, BCL2, apoptosis, CLL
Introduction
The induction of apoptosis by targeting of BCL2 proteins is one of the most promising therapeutic approaches in various malignancies including CLL. CLL is generally considered incurable, although chemotherapy, radiotherapy and antibody-therapy are all applied to slow down the progression of the disease in aggressive cases of CLL. Therefore, less toxic and more efficient therapies are urgently required for this very common leukemia. CLL cells display very high levels of BCL2 and are dependent on BCL2 expression for their survival. Anti-apoptotic BCL2 family proteins, including BCL2, BCL-XL, MCL1 and BCL2A1, inhibit apoptosis by sequestering pro-apoptotic BCL2-homology domain 3 (BH3) containing proteins, like BIM, PUMA or BAX/BAK. The interaction of anti-apoptotic BCL2 family members with these proteins occurs via a hydrophobic groove on the protein surface, into which BH3 domain-containing proteins can bind (1, 2). Dependent on the structure of the hydrophobic groove and of the BH3 domain, this binding can be very tight and very specific. Upon inhibition of BCL2 proteins, the pro-apoptotic binding partners are released and induce the release of cytochrome c from mitochondria into cytosol, resulting in caspase-dependent apoptosis.
Several small molecule BCL2-inhibitors have been developed that mimic BH3 peptides and target the hydrophobic groove on BCL2 proteins (3–5). Amongst these obatoclax, gossypol and ABT-263 are currently in early clinical trials e.g. for CLL and Non-Hodgkin’s lymphoma. However, more detailed mechanistic studies have highlighted that of all these potential BCL2-antagonists probably only ABT-737 and ABT-263 are specific BCL2 family antagonists (6, 7). Many other putative BCL2-antagonists seem to exert other major effects, which could lead to unwanted non-mechanism based toxicities (6, 7). Thus at the present time we propose that only ABT-737 or ABT-263 can be used in either the laboratory or clinic to evaluate both the therapeutic potential and mechanism based toxicity of specifically inhibiting anti-apoptotic BCL2 family members. ABT-737 was initially discovered in the Abbott laboratories using very elegant nuclear magnetic resonance-based screening, chemical synthesis and structure based-design (4). ABT-737 caused a rapid induction of apoptosis in many cell lines and exerted potent anti-cancer activity in various animal models either alone or more frequently in combination. However as ABT-737 was rapidly metabolized, had a short half-life and was not orally bioavailable, it was modified in three key positions, resulting in the synthesis of ABT-263, which is both more metabolically stable and orally bioavailable (8).
In both early clinical trials and animal studies the major dose-limiting mechanism-based toxicity of ABT-263 is a transient thrombocytopenia due to apoptosis of platelets, whose survival is dependent on BCL-XL (9). Owing to their similar structure and binding affinities, ABT-737 and ABT-263 are often used interchangeably, and both display very high binding affinities to BCL2, BCL-w and BCL-XL, but only weak binding to MCL1 or BCL2A1 (4, 8). Therefore, high expression of MCL1 or BCL2A1 has been found to confer resistance to ABT-737 (6, 10–12). Previous studies have shown that ABT-737 rapidly induces apoptosis in purified CLL cells at nanomolar concentrations in vitro (4, 13, 14). Although separate studies on both ABT-737 and ABT-263 have been carried out, to our knowledge there are no published studies directly comparing ABT-263 and ABT-737. In this study, to mimic the clinical situation, CLL cells were incubated with ABT-737 and ABT-263 in a whole blood assay. Under these conditions, the sensitivity of CLL cells to both compounds was reduced by ~100-fold due to a combination of higher cell densities in blood and significant albumin binding.
Material and Methods
Reagents
ABT-737 was provided by S. Rosenberg (Abbott Laboratories, Abbott Park, IL) and ABT-263 was provided by G. Shore (GeminX, Montreal, Canada). ABT-263 was synthesized by published methods (8, 15) and its purity was 95.1% as assessed by HPLC and a correct mass of m/z=975. After the start of this study a commercial source of ABT-263 also became available (Selleck Chemicals Co., Shanghai, China). Essentially identical results were obtained with both sources of ABT-263 (data not shown). Bovine serum albumin (BSA) was from Sigma (Sigma Aldrich, Poole, United Kingdom), CD5-PE and CD19-FITC antibodies from Dako Cytomation (Dako Cytomation, Ely, United Kingdom), rabbit anti-BAK antibody was from Upstate (Upstate Biotechnology, Lake Placid, NY), Annexin-APC and tetramethylrhodamine ethyl ester (TMRE) were from Invitrogen (Invitrogen, Paisley, United Kingdom). Caspase-3 antiserum was provided by Dr. Sun (MRC Toxicology Unit).
Cell culture
Peripheral blood samples from CLL patients were obtained with patient consent and local ethical committee approval. Unless otherwise indicated, lymphocytes were purified and cultured in RPMI 1640 medium supplemented with 10% FCS and 2 mM L-glutamine (all from Life Technologies, Inc, Paisley, United Kingdom) at 1 x 106 cells ml−1. CLL cells were incubated with ABT-737 or ABT-263 at 37°C and apoptosis assessed as previously described (14). Alternatively, blood from patients was incubated with ABT-737 or ABT-263 at 37°C in 48-well plates as described previously (16). Murine embryonic fibroblasts were cultured in DMEM medium (Life Technologies, Inc) supplemented with 10% FCS and 2 mM L-glutamine.
Release of cytochrome c
10 x106 CLL cells were washed and permeabilized with 0.05 % digitonin in mitochondrial isolation buffer (250 mM sucrose, 20 mM Hepes pH7.4, 5 mM MgCl2, 10 mM KCl) for 10 min on ice. The cytosol was removed by centrifugation at 13 000 rpm for 3 min. The permeabilized cells were washed three times in mitochondrial isolation buffer without digitonin and incubated with different concentrations of ABT-737 or ABT-263 for 1 h at 37°C. Upon incubation, the supernatant containing the released cytochrome c was isolated by centrifugation at 13 000 rpm for 3 min and the supernatant and pellet were analyzed by western blotting.
F-Dextran release assay
The release of fluorescein-dextrans (F-dextrans) from liposomes was measured as described previously (17). Briefly, liposomes containing 7% cardiolipin were generated by the extrusion method and internally loaded with fluorescein-dextran (10 kD) (Invitrogen). They were incubated with recombinant proteins (human origin); BAX, N/C-BID (cleaved form of BID), BCL-XL with and without ABT-737 or ABT-263 (0.04–5 μM). After incubation, the assay mix was filtered to collect released dextrans in the filtrate and the fluorescence was measured against a detergent solubilized sample, which gave 100 % release.
Immunoprecipitation
For immunoprecipitation (IP), 5 x 108 CLL cells were treated with ABT-737 or ABT-263 for 2 h before lysis in buffer containing 1 % CHAPS, 20 mM TrisHCl (pH 8), 150 mM NaCl, and Protease Inhibitor Cocktail (Roche Diagnostics, Basel, Switzerland). Hamster anti-BCL2 Ab (BD Bioscience, San Diego, CA) was crosslinked with ProtA-dynabeads using 20 mM dimethylpimelinediimidate (Fluka Biochemika, Switzerland). Crosslinked antibody or ProtA-dynabeads were incubated with 500 μg protein for 2 h at 4°C. Beads were washed with lysis buffer before elution in SDS-loading dye and western blotting.
Fluorescence Polarization Assay
The binding affinity of ABT-737 and ABT-263 to human serum albumin (HSA) was measured using fluorescence polarization assay (FPA) as described previously (18). Briefly, 500 nM dansyl sarcosine or 1 μM dansyl L-glutamate (both from Sigma Aldrich) were mixed with 5 or 10 μM HSA (CSL Behring, Marburg, Germany), respectively, and different concentrations of ABT-737 or ABT-263. Polarized light (Exc: 340 nm, Em: 535 nm) was measured using EnVision 2102 Multilabel Reader (Perkin Elmer, Waltham, MA).
Results
ABT-263 is less potent than ABT-737 in inducing apoptosis in CLL cells
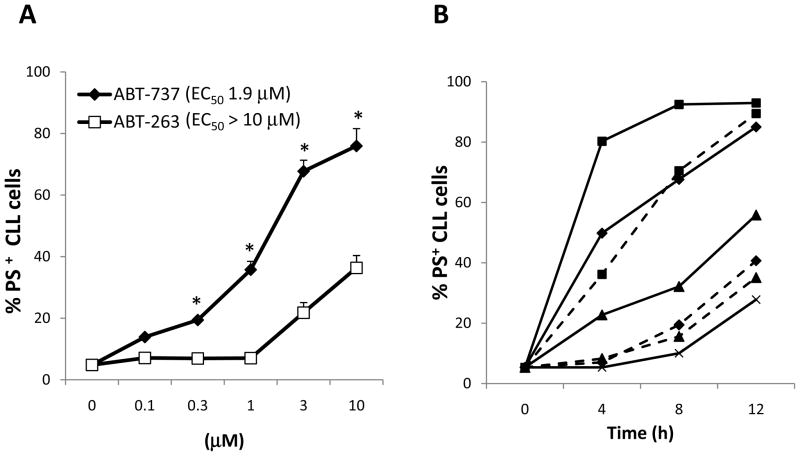
In this study we compared the in vitro efficacy of two very closely related BCL2-antagonists, ABT-737 and ABT-263. A direct comparison of the susceptibility of freshly isolated CLL cells to ABT-737 and ABT-263 in RPMI supplemented with 10% FCS revealed that both compounds induced efficient apoptosis but ABT-737 was ~4-fold more potent (Fig. 1A). This higher susceptibility of CLL cells to ABT-737 was observed in all 21 samples examined (Fig. 1B). These initial findings indicate that despite their apparent structural similarity (Fig. 1C) the two compounds possess different properties.
Fig. 1. Apoptosis induced by ABT-737 and ABT-263 in purified CLL cells.
A, CLL cells freshly isolated from peripheral blood of CLL patients were incubated in RPMI + 10% FCS with different concentrations of ABT-737 or ABT-263 for 4 h before apoptosis was assessed by externalization of phosphatidylserine (PS) and staining with AnnexinV-FITC (n=21, *=P<0.05). B, the half maximal effective concentration (EC50) values for ABT-737 and ABT-263 were calculated with GraphPad Prism and compared for each patient. C, the chemical structures of ABT-737 and ABT-263 are shown. Position 1, 2, and 3 indicate the 4-chlorobiphenyl, arylnitro and dimethylamino groups of ABT-737, respectively.
One possible explanation of the reduced potency of ABT-263 compared with ABT-737 could be due to it being inherently less potent. To investigate this possibility we compared their activities in a model biochemical system, using liposomes loaded with fluorescein conjugated 10 kD dextran (F-dextran) (17). Addition of a combination of BAX and N/C-BID resulted in permeabilization of the liposomes, as assessed by release of F-dextrans, and this permeabilization was inhibited by BCL-XL in agreement with previous findings (17). The BCL-XL mediated inhibition of liposome permeabilization was reversed in an almost identical concentration dependent manner by both ABT-263 and ABT-737 (Fig. 2A). These results demonstrated that both ABT-263 and ABT-737 target anti-apoptotic BCL-XL with similar efficiency in this model liposome system containing only BCL2 family members but devoid of extraneous proteins. Another explanation of the lower potency of ABT-263 could be due to a lower plasma membrane permeability, which would result in less drug reaching its intracellular target(s), the anti-apoptotic BCL2 family members. To address this possibility, we investigated the potential of ABT-737 and ABT-263 to induce cytochrome c release from permeabilized CLL cells (containing nuclei, mitochondria and endoplasmic reticulum) (Fig. 2B). ABT-737 was clearly more potent in inducing cytochrome c release from permeabilized cells. Taken together these results indicate that the reduced potential of ABT-263 to induce apoptosis cannot be explained solely by either differential plasma membrane permeability or by a lower potency of ABT-263 compared to ABT-737 to inhibit anti-apoptotic BCL2 family members.
Fig. 2. The reduced biological activity of ABT-263 is not due to a reduced potency or plasma membrane permeability.
A, F-dextran loaded liposomes were incubated with BAX, N/C-BID and BCL-XL and the indicated concentrations of ABT-737 or ABT-263 (0.04, 0.2, 1 or 5 μM) for 2.5 h at room temperature. Both ABT-737 and ABT-263 reversed BCL-XL mediated inhibition of BAX and N/C-BID liposome permeabilization. B, Purified CLL cells were treated with 0.05% digitonin to permeabilize the plasma membrane and the cells were washed and pelleted by centrifugation at 13000 rpm. The permeabilized cells were incubated with different concentrations of ABT-737 and ABT-263 for 1 h at 37°C. The release of cytochrome c (Cyt c) from the cell pellet into the supernatant (SN) was assessed after centrifugation by western blotting.
To gain insight into the mechanism of ABT-263 induced cell death, we asked whether ABT-263 induced activation of apoptotic signalling pathways. ABT-263 induced a rapid cleavage of caspase-3 and loss of mitochondrial membrane potential, but was again less potent than ABT-737 (Fig. 3A and B). Since unstimulated CLL cells express very little or no BCL-XL, the main target of ABT-263 and ABT-737 in CLL cells is BCL2, where they act by displacing pro-apoptotic BH3 domain-containing proteins. To investigate the activity of both compounds at the level of BCL2-inhibition, we immunoprecipitated BCL2 upon drug treatment and measured the levels of BAK displaced by ABT-737 and ABT-263. Previously, we had shown that small amounts of BAK but not BAX were sequestered by BCL2 (12). In agreement with our earlier study, BAK was again shown to be associated with BCL2 (Fig. 3C). ABT-737 (10 or 100 nM) efficiently displaced BAK from BCL2, whereas higher concentrations of ABT-263 (100 nM) were required to induce release of BAK (Fig. 3C). These data demonstrate that while both compounds have the ability to displace BAK from BCL2, ABT-263 is less efficient than ABT-737. In addition, we recently described that ABT-737 induces a novel paradigm of apoptosis in CLL cells involving all the normal characteristics of apoptosis accompanied by a rupture of the outer mitochondrial membrane (14). Here we show that ABT-263 (100 nM) induced similar ultrastructural changes to ABT-737 (10 nM), including condensed chromatin, rupture of the outer mitochondrial membrane and loss of mitochondrial matrix density (Fig. 3D and E). Finally, ABT-263 induced apoptosis was completely inhibited in murine embryonic fibroblasts deficient for Bax and Bak (Fig. 3F), suggesting that ABT-263, like ABT-737 (6, 7), is a specific inhibitor of BCL2 proteins. Taken together, our data indicate that both compounds induce cell death by a similar mechanism involving displacement of BAK from BCL2 and activation of the intrinsic apoptotic pathway accompanied by rupture of the outer mitochondrial membrane.
Fig. 3. Mechanism of cell death induced by ABT-737 and ABT-263.
A, purified CLL cells were incubated with 10 or 100 nM ABT-737 or ABT-263 for 2 or 4 h before analysis of caspase-3 cleavage by western blotting. α–Tubulin was used as a loading control. B, CLL cells were incubated with 10 or 100 nM ABT-737 or ABT-263 for 4 h before staining with 50 nM TMRE and analysis of the loss of mitochondrial membrane potential (n=3, * P<0.05). C, CLL cells were incubated with 10 or 100 nM ABT-737 or ABT-263 before immunoprecipitation (IP) of BCL2. Binding of BAK was detected by Western blotting. D-E, CLL cells were exposed to 100 nM ABT-263 for 4 h before electron microscopy. D, low power magnification shows apoptotic nuclear morphology, with condensed chromatin and disintegration of nucleoli, in CLL cells exposed to ABT-263 (bar=2 μm). E, high power magnification shows swollen mitochondria and breaks in the outer mitochondrial membrane (bar=100 nm). The morphology resembles that previously described for ABT-737. F, murine embryonic fibroblasts (wt or Bax/Bak double knockout) were exposed to different concentrations of ABT-263 for 48 h and apoptosis was assessed by externalization of phosphatidylserine (PS) and staining with AnnexinV-FITC.
The efficiency of ABT-263 and ABT-737 decreased in whole blood
In order to more closely mimic the in vivo situation, we compared the efficacy of the two compounds to induce apoptosis using whole blood from CLL patients rather than the standard cell culture medium. At 4 h, ~100-fold higher concentrations of both BCL2-antagonists were required to induce apoptosis in CLL cells in blood compared to cell culture medium (compare Figs. 1A and 4A). The reduced potency of ABT-263 as compared to ABT-737 was even more pronounced in whole blood than in purified CLL cells. To investigate whether longer exposure times increased apoptosis, we incubated blood with different drug concentrations for up to 12 h. Whereas lower concentrations of ABT-737 (0.1 – 1 μM) induced apoptosis at longer exposure times, ABT-263 only induced apoptosis at concentrations > 1 μM even when incubated for up to 12 h (Fig. 4B).
Fig. 4. Apoptosis induced by ABT-737 and ABT-263 is inhibited in whole blood of CLL patients.
A, heparinised whole blood from patients with CLL was incubated at 37°C for 4 h with different concentrations of ABT-737 or ABT-263 (n=22). B, whole blood from patients with CLL was incubated for 4, 8, or 12 h without (x-x) or with 0.1 μM (▲-▲), 1 μM (◆-◆), or 10 μM (■-■) of ABT-737 (solid lines) or ABT-263 (dotted lines) (n=7). A-B, after incubation, 20 μl of blood were stained with CD5-FITC/CD19-RPE antibody for 20 min, washed with Annexin-buffer (10 mM Hepes, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, pH7.4) and the pellet was resuspended in 500 μl Annexin-buffer + 1 μl Annexin-APC before analysis on FACScalibur with a gate on CD5+CD19+ CLL cells.
Binding to albumin confers resistance to ABT-737 and ABT-263
As these data clearly show that CLL cells lose their striking sensitivity towards these inhibitors in the presence of whole blood, we next investigated which components of whole blood caused the loss of sensitivity to ABT-737 and ABT-263. In CLL, the white blood cell count in peripheral blood is regularly > 1 x 108/ml, much higher than the concentration of cells typically used in vitro (1 x 106/ml). To examine the influence of cell density on the sensitivity to BCL2-antagonists, CLL cells were incubated at different densities in culture medium. Although a 10-fold higher cell density than used in our standard culture did not significantly affect sensitivity, higher cell densities similar to those found in the blood of CLL patients (1–5 x 108 cells/ml) required higher concentrations of ABT-737 and ABT-263 to induce cell death (Fig. 5A and B). Furthermore, we investigated whether the high serum content in blood compared to the 10% serum routinely used in cell culture medium affected susceptibility to BCL2-antagonists. Higher serum concentrations (50% FCS) induced resistance to ABT-737 and ABT-263, while lower serum concentrations (1% FCS) markedly sensitized CLL cells to apoptosis induced by either drug (Fig. 5C and D). Similar responses to the presence of serum were found in cell lines, where higher serum concentrations resulted in decreased sensitivity to ABT-737 and ABT-263 (data shown to referees). During the initial chemical synthesis of ABT-737, it was recognized that the lead compounds were largely inactivated by the presence of serum and the major inactivating component was due to binding to domain III on human serum albumin (HSA) (19). Consequently ABT-737 was designed, in part, to overcome this high binding to albumin, which adversely affected the binding to BCL-XL (4, 19). To investigate whether the inhibitory effect of serum on apoptosis induced by ABT-737 or ABT-263 was due to the presence of albumin, 3% bovine serum albumin (BSA), corresponding to the concentration of albumin in 50% FCS, was added. Notably, addition of BSA induced a similar shift in the concentration-response to ABT-737 or ABT-263 as 50% FCS (Fig. 5C and D). Since the sensitization by low FCS concentrations (1%) could be completely reversed by addition of albumin, lack of growth factors or cytokines following serum withdrawal cannot account for the sensitization to BCL2-antagonists. These results strongly suggest that albumin was the predominant factor in serum responsible for resistance to ABT-263 or ABT-737.
Fig. 5. Cell density and albumin binding confer resistance to ABT-737 and ABT-263.
A-B, purified CLL cells were incubated at different cell densities in RPMI + 10% FCS with different concentrations of ABT-737 (A) or ABT-263 (B) for 4 h. Apoptosis was assessed by phosphatidylserine (PS)-externalisation and staining with AnnexinV-FITC (n=8). C-D, CLL cells were incubated at 1x106 cells/ml in RPMI with different concentrations of FCS and ABT-737 (C) or ABT-263 (D) with or without bovine serum albumin (3%) for 4 h. Apoptosis was assessed by PS-externalisation and AnnexinV-FITC binding (n=8).
ABT-263 binds more tightly than ABT-737 to albumin
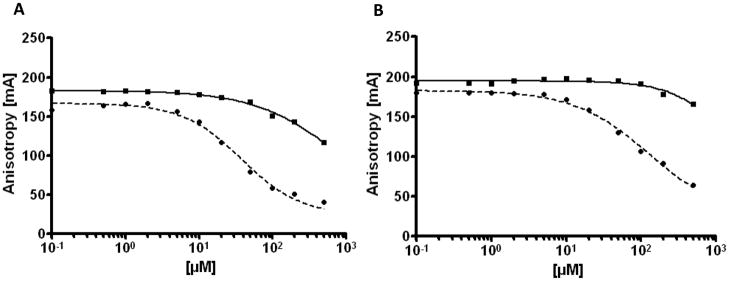
To further characterize the nature of the binding of ABT-737 and ABT-263 to albumin, we used a fluorescence polarization assay. There are two main drug binding sites on HSA, site 1 on subdomain IIA and site 2 on subdomain IIIA (20, 21). Using two different probes, dansyl sarcosine, which binds to site 2, and dansyl L-glutamate, which binds to site 1, we were able to distinguish binding of ABT-737 and ABT-263 to the different HSA subdomains. Interestingly, ABT-263 displayed a markedly higher binding affinity to site 2 on HSA-IIIA than ABT-737 (Fig. 6A). Notably, the half maximal inhibitory concentration (IC50) of ABT-263 in this assay was 37 μM, which was lower than that displayed by the positive control naproxen (51 μM) which binds strongly to that site (18), indicating a very tight interaction between HSA and ABT-263. While ABT-737 showed no binding to site 1, ABT-263 also bound to site 1 on HSA-IIA with an IC50 of 145 μM (positive control phenylbutazone: 49 μM) (18) (Fig. 6B). These data demonstrate that ABT-263 has a higher albumin binding capacity than ABT-737, thus limiting the amount of free drug that is available to interact with the intended target, BCL2.
Fig. 6. ABT-263 has a higher albumin binding affinity than ABT-737.
The binding capacity of ABT-737 (solid lines) and ABT-263 (dotted lines) to human serum albumin (HSA) was investigated using a fluorescence polarization assay. A, to measure binding to site 2 on HSA-IIIA, dansyl sarcosine was used as a probe. In this assay the IC50 was 711 and 37 μM for ABT-737 and ABT-263, respectively. B, to measure binding to site 1 on HSA-IIA, dansyl L-glutamate was used as a probe. The IC50 was >1000 and 145 μM for ABT-737 and ABT-263, respectively.
Discussion
Due to their ability to directly induce apoptosis, BCL2-inhibitors offer a great potential for cancer therapy, especially for malignancies with high BCL2 expression and a dependency on BCL2 expression for survival (22). CLL cells were previously reported to be highly sensitive to BCL2 inhibition and treatment with ABT-737 (4, 13, 14, 23). To our knowledge we now show for the first time that freshly isolated CLL cells display a similar, albeit somewhat lower, nanomolar sensitivity to ABT-263 (Fig. 1). ABT-263 induced a rapid activation of the intrinsic pathway of apoptosis that was absolutely dependent on Bax and Bak. Induction of apoptosis in CLL cells by ABT-263 was accompanied by chromatin condensation together with rupture of the outer mitochondrial membrane and a decreased mitochondrial matrix density (Fig. 3), ultrastructural features virtually identical to those we had previously observed with ABT-737 and described as a novel paradigm of apoptosis (14). These results further emphasize the similar activities of ABT-737 and ABT-263 in agreement with recent studies (15, 24) and strongly support the hypothesis that they both act to induce apoptosis in CLL cells by an identical mechanism. We emphasize the importance of studying these effects with ABT-263, as this is the compound, not ABT-737, currently being used in clinical trials.
Most importantly our data demonstrate that isolated CLL cells lose their striking nanomolar sensitivity to both ABT-737 and ABT-263 in the presence of whole blood. The EC50 of CLL cells to ABT-263 in whole blood is >10 μM at 4 h of treatment (Fig. 4A). Notably, the maximal plasma concentrations of ABT-263 achieved in clinical trials with a 250 mg daily dosing schedule are around 5 μM (25), indicating that the concentrations in whole blood might not be optimal in the current phase I and phase II clinical trials for ABT-263. In our study we identify two factors that affect the efficacy of these BCL2-inhibitors: high cell density and plasma protein binding. In leukemic patients, the high circulating cell densities might contribute to the resistance of CLL cells to ABT-737 and ABT-263 that we observed in whole blood as compared to standard cell culture (Fig. 5AB). The impact of cell density on drug sensitivity may be a particular problem in certain microenvironments, such as lymph nodes, where drugs encounter even higher cell densities than in the peripheral blood. We also describe that these BCL2-inhibitors are extensively bound to albumin and that in the presence of albumin higher drug concentrations are required for apoptosis induction (Fig. 5C-D). Albumin is the most abundant protein in human plasma and is well known to bind a wide range of drugs as well as endogenous molecules (18, 20, 21). ABT-263 bound to albumin much more strongly than ABT-737 (Fig. 6) and it bound to site 2 on subdomain IIIA more strongly than naproxen (18), indicating a very tight interaction between albumin and ABT-263. Extensive binding to albumin may have profound effects on the absorption, distribution, metabolism and excretion of molecules, often meaning that higher doses need to be administered in vivo (26, 27). The high albumin binding of ABT-263 may act a reservoir of the drug, resulting in a long half life. However, the high albumin-binding might also be a source of potential drug interactions as ABT-263 may be displaced by other drugs that bind extensively to albumin, thus increasing the potential efficacy and toxicity of ABT-263. Additionally, our data indicate that patients with a hypoalbuminemia might react differently to ABT-263 and conditions where albumin levels are affected need to be closely monitored for toxicities.
It is interesting to consider the higher albumin binding of ABT-263 compared to ABT-737 in relation to their structures. During the development of ABT-263, the 4-chlorobiphenyl, arylnitro and dimethylamino groups of ABT-737 (Fig. 1C, positions 1, 2 and 3, respectively) were modified primarily to decrease metabolism and increase oral bioavailability without losing cellular activity. However, the dimethylamino group in ABT-737, which had been specifically designed to reduce binding to albumin (19), is replaced by a morpholino group in ABT-263 (8). In this regard the introduction of a morpholino group at this position in the development of ABT-737 (compound 77R in (19)) was much less effective than a dimethylamino group in reducing the deactivating effects of serum on the binding affinity to BCL-XL and it was concluded that a charged species in this position was particularly effective in reducing serum binding (19). In the absence of serum, the lead compounds in the ABT-737 series with the dimethylamino or morpholino group showed similar binding affinities to BCL2 proteins, whereas in the presence of serum, the morpholino group resulted in a complete loss of binding to BCL-XL. These results indicate the potential deleterious effects of a morpholino group at this position and indicate that although ABT-263 clearly shows important biological activity the possibility that substitution of another group in this position may yield a BCL2 family inhibitor with more favorable pharmacokinetics.
To exclude any effects of serum or albumin, we wished to test the susceptibility of CLL cells to ABT-737 and ABT-263 in a serum-free system. However, culture of CLL cells in the complete absence of any serum is not feasible because it is too toxic and cells underwent spontaneous apoptosis (data not shown). Using a biochemical F-dextran release assay in liposomes, we found that both compounds have the same capacity to target BCL2 proteins, in line with previously published data reporting a similar affinity of both compounds for BCL2 and BCL-XL (4, 28). However, the reduced activity of ABT-263 was also observed in permeabilized cells in the absence of any serum or albumin (Fig. 2B). These data indicate that besides the binding to albumin, other factors determine the different biological activities of ABT-737 and ABT-263. One explanation could be that besides binding to albumin, ABT-263 is also sequestered by other cellular proteins and therefore less drug reaches BCL2 even in the absence of serum. Some support for this hypothesis is provided by our finding that in contrast to ABT-737, ABT-263 also binds to site 1 on HSA-IIA, indicating that it binds in a more promiscuous manner than ABT-737. Another explanation for the reduced activity of ABT-263, even in permeabilized cells in the absence of serum, might lie in as yet undescribed differences in their affinity to anti-apoptotic BCL2 family proteins or in their potential to displace BH3 domain-containing proteins. In CLL cells obtained from blood, the main target of ABT-737 and ABT-263 is BCL2, since BCL-XL and BCL-w expression in circulating CLL cells is very low. However, the published data on the binding affinities of ABT-737 and ABT-263 to BCL2 (< 1 nM) or BCL-XL (< 0.5 nM) display no difference (4, 28), possibly due to insufficient assay sensitivity.
Interestingly, published data indicate that there might be cell-type specific differences in the sensitivity to ABT-737 and ABT-263. In agreement with this suggestion, small cell lung cancer cell lines, H889 and H1417, showed a higher sensitivity to ABT-737 compared with ABT-263, whereas others, including H146 and H82, demonstrated a similar sensitivity (28, 29). Our finding of a differential nonspecific binding of ABT-737 and ABT-263 to proteins, such as albumin, provides the first mechanistic explanation for a differential efficacy of ABT-737 and ABT-263. Whether a differential expression profile of BCL2 proteins also contributes to their differential sensitivity is not known.
Taken together our data suggest that although structurally similar and exhibiting similar binding affinities to anti-apoptotic BCL2 family proteins, ABT-263 is less potent than ABT-737 at inducing apoptosis in CLL cells. Furthermore, our data indicate that binding of ABT-263 to albumin will markedly increase the concentration of drug required to induce apoptosis and clear CLL cells from the blood in vivo. We suggest that modification of the albumin binding of ABT-263 either by alteration of its structure or by other strategies may restore the inherent susceptibility of CLL cells to these targeted BCL2-inhibitors thereby increasing their therapeutic potential.
Translational Relevance.
Targeting the anti-apoptotic BCL-2 family is an exciting area for novel anti-cancer drug development. One such inhibitor, ABT-263 has recently entered clinical trials. Almost all mechanistic and animal studies have been carried out with the structurally related, ABT-737. Although such inhibitors will undoubtedly have greatest value in combination chemotherapy, ABT-737 has shown promising potent single agent activity against certain primary tumor cells, including chronic lymphocytic leukemia (CLL) cells. We now show that although somewhat less active than ABT-737, ABT-263 is also a potent inducer of apoptosis in CLL cells by a similar mechanism. However when tested in whole blood to mimic the in vivo situation, the activity of both inhibitors decreased ~100-fold largely due to binding to albumin, resulting in a loss of the potency and thus the selectivity of these inhibitors. The high binding of ABT-263 to albumin highlights the necessity to monitor carefully patients for potential drug interactions.
Acknowledgments
We thank Drs. S. Rosenberg and S. Elmore, Abbott Laboratories for the supply of ABT-737 and Drs. G. Shore and L. Belec, GeminX for ABT-263. We thank Dr. D. Dinsdale for the EM studies and J. Wolf for technical assistance. MEFs (wt and Bax/Bak double knockout) were kindly provided by A. Strasser (WEHI, Melbourne, Australia) and G. Haecker (University of Freiburg, Freiburg, Germany).
References
- 1.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 3.Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321–6. doi: 10.1038/nrc2615. [DOI] [PubMed] [Google Scholar]
- 4.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 5.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–7. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 6.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogler M, Weber K, Dinsdale D, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 8.Park CM, Bruncko M, Adickes J, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem. 2008;51:6902–15. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- 9.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Morgan-Lappe S, Huang X, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-X(L) inhibitor ABT-737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 12.Vogler M, Butterworth M, Majid A, et al. Concurrent upregulation of BCL-XL and BCL2A1 induces ~1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–13. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 13.Del Gaizo Moore V, Brown JR, Certo M, et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogler M, Dinsdale D, Sun XM, et al. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 2008;15:820–30. doi: 10.1038/cdd.2008.25. [DOI] [PubMed] [Google Scholar]
- 15.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 16.Wheat LM, Kohlhaas SL, Monbaliu J, et al. Inhibition of bortezomib-induced apoptosis by red blood cell uptake. Leukemia. 2006;20:1646–9. doi: 10.1038/sj.leu.2404290. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana T, Mackey MR, Perkins G, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 18.Mathias U, Jung M. Determination of drug-serum protein interactions via fluorescence polarization measurements. Anal Bioanal Chem. 2007;388:1147–56. doi: 10.1007/s00216-007-1351-7. [DOI] [PubMed] [Google Scholar]
- 19.Wendt MD, Shen W, Kunzer A, et al. Discovery and structure-activity relationship of antagonists of B-cell lymphoma 2 family proteins with chemopotentiation activity in vitro and in vivo. J Med Chem. 2006;49:1165–81. doi: 10.1021/jm050754u. [DOI] [PubMed] [Google Scholar]
- 20.Ghuman J, Zunszain PA, Petitpas I, et al. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol. 2005;353:38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 21.Sudlow G, Birkett DJ, Wade DN. The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol. 1975;11:824–32. [PubMed] [Google Scholar]
- 22.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27 (Suppl 1):S149–57. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason KD, Khaw SL, Rayeroux KC, et al. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23:2034–41. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- 24.Ackler S, Xiao Y, Mitten MJ, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008;7:3265–74. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 25.Wierda G, Roberts P, Brown R, et al. Pharmacokinetics, safety and anti-tumor activity of ABT-263 in patients with relapsed or refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Hematologica. 2009;94(suppl 2):138. abs 0350. [Google Scholar]
- 26.Curry S. Lessons from the crystallographic analysis of small molecule binding to human serum albumin. Drug Metab Pharmacokinet. 2009;24:342–57. doi: 10.2133/dmpk.24.342. [DOI] [PubMed] [Google Scholar]
- 27.He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–15. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- 28.Shoemaker AR, Mitten MJ, Adickes J, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 29.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]