Abstract
Serotonin is a physiological signal that translates both internal and external information about behavioral context into changes in sensory processing through a diverse array of receptors. The details of this process, particularly how receptors interact to shape sensory encoding, are poorly understood. In the inferior colliculus, a midbrain auditory nucleus, serotonin (5-HT) 1A receptors have suppressive and 5-HT1B receptors have facilitatory effects on evoked responses of neurons. We explored how these two receptor classes interact by testing three hypotheses: that they 1) affect separate neuron populations, 2) affect different response properties, or 3) have different endogenous patterns of activation. The first two hypotheses were tested by iontophoretic application of 5-HT1A and 5-HT1B receptor agonists individually and together to neurons in vivo. 5-HT1A and 5-HT1B agonists affected overlapping populations of neurons. During co-application, 5-HT1A and 5-HT1B agonists influenced spike rate and frequency bandwidth additively, with each moderating the effect of the other. In contrast, although both agonists individually influenced latencies and interspike intervals, the 5-HT1A agonist dominated these measurements during co-application. The third hypothesis was tested by applying antagonists of the 5-HT1A and 5-HT1B receptors. Blocking 5-HT1B receptors was complementary to activation of the receptor, but blocking 5-HT1A receptors was not, suggesting the endogenous activation of additional receptor types. These results suggest that cooperative interactions between 5-HT1A and 5-HT1B receptors shape auditory encoding in the IC, and that the effects of neuromodulators within sensory systems may depend nonlinearly on the specific profile of receptors that are activated.
Keywords: midbrain, auditory, serotonin, latency, interspike interval
Introduction
Neuromodulators like serotonin act as context-dependent filters within adult sensory systems (Hurley et al. 2004). Serotonin is released as a function of internal state or during specific behavioral contexts, and shapes ongoing sensory processing through heteroreceptors expressed by sensory neurons (Trulson and Jacobs 1979, 1981; Boutelle et al. 1990; Clement et al. 1998; Hurley et al. 2004). The serotonergic system is notable in the variety of receptors (Hoyer et al. 1994, 2002; Nichols and Nichols 2008) that affect different components within local circuits. For example, different receptors may influence the intrinsic excitability of neurons or presynaptically alter neurotransmitter release (Sari 2004, Monckton and McCormick 2002). Through such effects, the influence of multiple receptor types commonly converge on classes of neurons and on individual sensory neurons (Mooney et al. 1996, Xiang and Prince 2003). The hypothesis that multiple receptors interact cooperatively to shape responses to sensory stimuli is a logical outcome of studies in which receptors have been manipulated singly, but this concept has seldom been directly explored by concurrently manipulating multiple receptors in vivo.
In addition to its central role in auditory processing and aversive behavior/learning (Brunso-Bechtold et al. 1981; Huffman and Henson 1990; Heldt and Falls 2003; Castellan-Baldan 2006), the inferior colliculus (IC) is the best-understood auditory region in terms of how serotonin receptors influence the excitatory-inhibitory circuitry that determines responses to behaviorally relevant stimuli such as species-specific vocalizations or interaural cues for sound location (Klug et al 2002; Hurley et al. 2002, 2004; Pollak et al. 2003). Two metabotropic receptors of the 5-HT1 family, 5-HT1A and 5-HT1B receptors, are expressed widely by IC neurons and by neurons in other sensory regions (Waeber and Palacios 1990; Pompeiano et al. 1992; Aznar et al. 2003; Peruzzi and Dut 2004; Palchaudhuri and Flügge 2005; Watakabe et al. 2009). In the IC, 5-HT1A and 5-HT1B receptors have well-described effects on excitatory-inhibitory circuitry. Activation of the somatodendritic 5-HT1A receptor usually decreases stimulus-evoked spike rates and narrows frequency receptive fields (Hurley 2006, 2007), consistent with the reported link of this receptor to potassium channels (Lanfumey and Hamon 2004; Ögren et al. 2008). More rarely, activation of the 5-HT1A receptor increases stimulus-evoked spikes (Hurley 2006). Activation of the presynaptic 5-HT1B receptor increases evoked spike rates and widens frequency receptive fields through a decrease in GABA release (Hurley et al. 2008). How these opposing effects integrate to transform auditory responses during serotonin release is unknown.
We therefore investigated several hypotheses for how these two receptors could act cooperatively. These were that 5-HT1A and 5-HT1B receptors 1) affect separate populations of neurons, 2) have differential effects at the level of single neurons, so that they modify different response properties, or 3) show different patterns of endogenous activation. To test these hypotheses, we locally co-manipulated 5-HT1A and 5-HT1B receptors through the iontophoretic application of selective agonists and antagonists in vivo.
Materials and Methods
Animals and surgery
All procedures were approved by the Bloomington Institutional Animal Care and Use Committee and followed the NIH guidelines for the care and use of laboratory animals. Two hundred single neurons were recorded from 33 male CBA/J mice ranging from 5-10 weeks of age (Jackson Labs, Bar Harbor, ME). Mice were anesthetized by brief exposure to isoflurane fumes, followed by intraperitoneal injection of 120 mg/kg of ketamine and 5 mg/kg of xylazine. After the removal of hair on the top of the head with a depilatory cream, the skin along the midline of the head was incised along approximately 1.5 cm and the skin was reflected to each side. The surface of the skull cleared of adherent tissue and holes of approximately 1 mm in diameter were drilled in the skull above each IC. The dura was then incised with a sharpened tungsten probe, and the holes covered with silicon gel to prevent drying. A layer of glass beads and cyanoacrylate glue was applied to the skull anterior to lambda, and the mouse transferred to a sound attenuated chamber and placed in a custom stereotaxic device (Schuller et al. 1986), where body temperature was maintained between 36 and 37 °C with a temperature regulation system (FHC; Bowdoinham, ME). A post was affixed to the skull between bregma and lambda with dental cement. During the experiment, the level of anesthesia was maintained with supplemental doses of 1/5 of the pre-surgical doses of the anesthetic mixture or an equal amount of ketamine alone.
Electrodes and recording procedures
Extracellular recordings were made through high-resistance glass micropipettes (A-M Systems, Carlsborg, WA) connected by a silver-silver chloride wire to a Dagan 2400 amplifier (Minneapolis, MN). Attached to these single-barreled pipettes were either 3-barreled or 5-barreled pipettes that were used for the iontophoresis of drugs in a ‘piggy-back’ configuration (Havey and Caspary 1980). Multibarreled pipettes were pulled (A-M Systems; Stoelting 51210; Wood Dale, IL) and broken back to a tip diameter of 10- 15 μm, then attached to single-barreled recording pipettes so that the tip of the recording pipette protruded 10-20 μm from the tip of the tribarreled pipette (single electrode blanks: 6010, 3-barreled blanks: 6090; 5-barreled blanks: 6120; A-M Systems, Carlsborg, WA). These combination electrodes were positioned above the IC under visual control through a dissecting microscope and lowered with a piezoelectric microdrive (Burleigh/EXFO inchworm, Mississauga, Ontario) until action potentials were observed. Recordings were concentrated in the caudal and medial 2/3 of the IC based on landmarks including lambda and blood vessels (Paxinos and Franklin 2004; Hage and Ehret 2003). The serotonergic plexus in the IC shows a gradient of density from cortical regions extending smoothly through the central IC, with lowest densities in ventrolateral or ventromedial regions of the IC (Klepper and Herbert 1991; Kaiser and Covey 1997; Hurley et al. 2002). The resistance of the recording electrode (8-20 MΩ when filled with 1M NaCl) allowed the recording of single neurons. Recorded spikes had signal: noise ratios of 10 or more and could be ‘killed’ by the injection of small amounts of current at the end of recording. Spikes were fed through a spike signal enhancer (FHC; Bowdoinham, ME) before being digitized through a data acquisition processor board (Microstar; Bellevue, WA). Data was collected and stored for later analysis by the software package Batlab (Dr. Donald Gans, Kent State University).
Drugs and iontophoresis
Agonists and antagonists of the 5-HT1A and 5-HT1B receptors were applied iontophoretically during neural recordings, and identical datasets were collected before, during, and after the application of drugs. Drugs included the 5-HT1A agonist (±)-8-Hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT), the two 5-HT1A antagonists 1-(2-Methoxyphenyl)-4-(4-succinimidobutyl) (MM 77) and (S)-N-tert-Butyl-3-(4-(2-methoxyphenyl)-piperazin-1-yl)-2-phenylpropanamide dihydrochloride (WAY-100135), the 5-HT1B agonist 1,4-Dihydro-3-(1,2,3,6-tetrahydro-4-pyridinyl)-5H-pyrro 1[3,2-b]pyridin-5-one dihydrochloride (CP 93129) and the 5-HT1B antagonist (2R)-2-[[[3-(4-Morpholinylmethyl)-2H-1-benzopyran-8-yl]oxy]methyl]morpholine dimethane-sulfonate (NAS-181). All drugs were obtained from Tocris Bioscience (Ellisville, MO). All drugs except MM 77 were dissolved at 10 mM in 200 mM NaCl at pH 4.5. MM 77 was dissolved in DMSO and diluted in 200 mM NaCl for a final concentration of 3% DMSO. Neither vehicle solution altered the responses of IC neurons when applied alone (for 200 mM NaCl, see Hurley and Pollak 1999, 2001; for DMSO plus 200 mM NaCl, p = .80, 2-tailed paired t-test, n = 12).
One barrel of the multibarreled pipette was filled with 1M NaCl and served as a sum channel, balancing the iontophoretic currents ejected through the other barrels. All other barrels were filled with drug solutions. The barrels were connected by silver-silver chloride wire to iontophoresis pump modules (Dagan ION-100; Minneapolis, MN or Medical Systems NeuroPhore: Harvard Apparatus, Holliston, MA). Drugs were retained in the multibarreled pipettes with a current of -10 to -20 nA, and ejected using currents that did not exceed +90 nA. After the collection of control data, drugs were ejected until a stable response was achieved or for 5-10 minutes, and a comparable dataset was then collected. The same procedure was repeated to measure the effect of a new drug treatment or the recovery of neurons from drugs. For a subset of neurons, the dose-dependence and saturability of 8-OH-DPAT and CP93129 were assessed by using a range of iontophoretic currents for the same drug. Multiple drugs were also applied sequentially or at the same time to most neurons. When multiple drugs were applied, the order of drug applications was varied to ensure that effects were not order-dependent.
Anesthetic state is a potential concern in these experiments for several reasons. One of the drugs in the anesthetic mix we used, ketamine, directly affects steps along the pathway of serotonin release and metabolism, including the transport of serotonin (Martin et al. 1990; Lindefors et al. 1997; Nishimura et al. 1999). Ketamine can also directly affect the NMDA receptor (Villars et al. 2004). Finally, mice under ketamine/xylazine anesthesia have decreased extracellular serotonin in the IC (Hall et al. 2010). A previous analysis of the effects of the selective serotonin receptor agonists used in this study, however, showed no difference in the effects of the agonists in awake animals versus in animals under ketamine/xylazine anesthesia (Hurley 2006).
Auditory stimuli
Tone bursts were generated with Batlab software and routed through a PA5 attenuator and FT-6 antialias filter (TDT; Alachua, FL). Stimuli were played through a midline freefield speaker (Infinity Emit B, Harman International Industries; Woodbury, NY). Calibration of the freefield speaker was accomplished by placing a measuring microphone (ACO Pacific PS9200 kit; Belmont, CA) in the position occupied by the mouse's head during experiments. The response of the speaker was flat within ± 6 dB from 13- 40 kHz, but produced a higher intensity of sound at lower frequencies.
Tones were 20- 30 ms in duration with .5 ms rise- and fall times and were presented at a rate of 4 Hz. Frequency tuning was measured by presenting tones across the frequency ranges of single neurons from 10 dB below threshold to 30-50 dB above threshold at the characteristic frequency (CF). The frequency intervals of the presented tones varied from .5 kHz to 5 kHz, depending on the bandwidth of the neuron recorded. Spontaneous activity was also recorded at the outset of each data file, so that multiple measurements of spontaneous rates were collected during the control and each drug treatment.
Analysis
Spike trains were recorded in Batlab. Spontaneous activity rates were generally low; for neurons in which spontaneous spikes occurred, spikes were only counted in the temporal window defined by clear onsets and offsets in peristimulus time histograms (PSTHs). Spike rates for evoked responses were expressed as the number of spikes per stimulus during the presentation of a set of 32 repetitions of a given stimulus and were measured at the frequency evoking the largest response at 10-20 dB above threshold at the CF. Spike rates for spontaneous activity were expressed as the number of spikes per second measured during 32 repetitions of a 200 ms window with no stimulus. Proportional changes in spontaneous and evoked activity were compared by transforming all values (log[drug/control+.1]), so that all values were nonzero. Frequency bandwidth was measured from isointensity frequency-response functions at intensities of 10-30 dB above the threshold at the CF. The frequency bandwidth was defined as the width of the frequency-response function at a level of half of the maximum spike rate, in octaves. High- and low-frequency borders were determined by a linear interpolation between the frequencies evoking spike counts above and below the criterion values. Response latencies were measured as the median time elapsed between the onset of the stimulus and the first spike fired by the neuron, in response to 32 stimulus repetitions, or the median first-spike latency. The interspike interval was measured between the first and second spikes for neurons consistently producing more than one spike per stimulus. Drug-evoked changes in evoked spike rate and bandwidth were expressed as the proportional difference between drug and control values, and changes in latency and interspike interval were expressed as the absolute difference between drug and control values, in ms.
Statistical comparisons were made using SPSS (Chicago, IL). Because drug effects across the neuron population did not appear to be distributed normally, nonparametric Spearman's correlations were used to compare the effects of receptor agonists with other response properties. Wilcoxon signed rank tests were used to determine whether drugs altered response properties relative to predrug values, and Mann-Whitney U tests (Wilcoxon rank-sum tests) were used to determine whether drugs had equivalent effects in different populations of neurons. Multiple regressions were used to compare the effects of different agonists with their co-application. The chi squared test was used to assess whether multiple 5-HT1A antagonists had similar distributions of effects.
Results
5-HT1A and 5-HT1B agonists target overlapping neuron populations
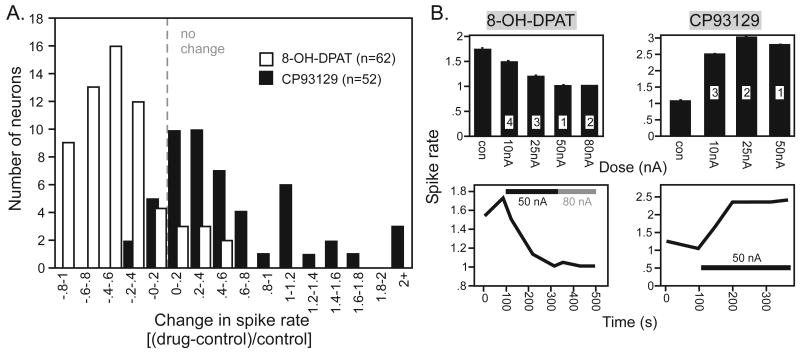
To test the effects of co-manipulating the 5-HT1A and 5-HT1B receptors on the evoked responses of IC neurons, 200 neurons were recorded in 33 male CBA/J mice of 5-10 weeks of age. Neurons were recorded extracellularly before, during, and after the iontophoretic application of selective 5-HT1A and 5-HT1B agonists and antagonists. When applied alone, selective agonists of the 5-HT1A and 5-HT1B receptors modulated evoked responses in ways similar to those reported previously (Hurley 2006, 2007; Hurley et al. 2008). The 5-HT1A agonist 8-OH-DPAT generally decreased, and the 5-HT1B agonist CP93129 generally increased, the spike rate in response to a CF tone presented 10-20 dB above threshold (Fig 1A). The population distribution of spike rate decreases in response to the application of 8-OH-DPAT were shifted to more extreme values than in previous reports (Hurley 2006, 2007). To determine whether the larger proportional change could have been due to a higher baseline of evoked activity caused by the application of CP93129, we compared experiments in which 8-OH-DPAT was applied before versus after CP93219. The order of application did not alter the effects of 8-OH-DPAT, but when CP93129 was present in another pipette barrel, 8-OH-DPAT had a proportionally larger effect, causing a median proportional decrease in spikes of .55 as opposed to .35 when CP93129 was not present (Mann-Whitney U test; U=533.5, p=.048). This suggests that, although neurons recovered from iontophoretic application of drugs before subsequent drug applications were made, the leak of CP93129 increased baseline spike rates slightly, but that the iontophoresis of CP93129 had a much larger effect.
Figure 1.
Effects of two selective 5-HT1 agonists on evoked responses of IC neurons. A. Population histogram of proportional changes in spike rate [(drug-control)/control] at the characteristic frequency in response to application of the 5-HT1A agonist 8-OH-DPAT (n = 62) and the 5-HT1B agonist CP93129 (n = 52). B. Current-response plots (top) and timecourses (bottom) for each agonist. Numbers in the current-response plots refer to the order of application of different iontophoretic currents. Error bars represent the standard deviations of spike rates in different treatments. For a given agonist, current-response functions and timecourses were measured from the same neuron.
The absolute concentrations of drugs are not controllable with iontophoresis, but we performed several control experiments to assess whether the effects of the 5-HT agonists were dose-dependent and could saturate. We did this by applying a range of iontophoretic currents in a subset of neurons. Figure 1B shows two such experiments in single neurons. For the neuron exposed to 8-OH-DPAT, increasing iontophoretic currents caused incrementally decreasing spike rates, an effect that saturated at 50 nA (Fig. 1B, top left). For the iontophoretic current at 50 nA, the first level of current that was applied to this neuron, it took 2.2 minutes for 8-OH-DPAT to reach its maximum effect (Fig. 1B, lower left). For the neuron tested with CP93129, the increase in spike rate saturated at the lowest level of iontophoretic current of 10 nA (Fig. 1B, upper right), and the time to the maximum effect at 50 nA was 3.6 minutes (Fig. 1B, lower right). The dose-dependence and saturation of the effects of both 8-OH-DPAT and CP93129 using iontophoresis in the IC was reported previously (Hurley 2006). In most neurons in this study, we used relatively high iontophoretic currents of 50-75 nA to ensure a maximal drug effect.
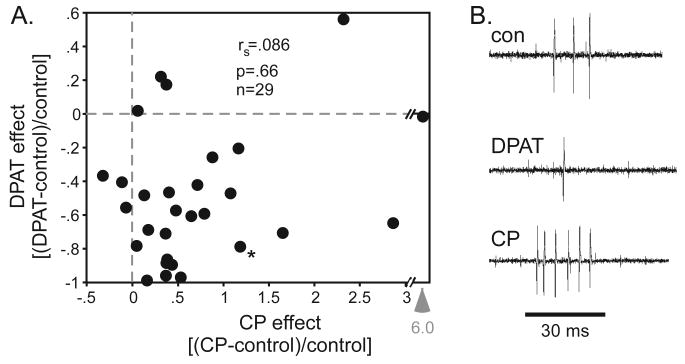
The hypothesis that 5-HT1A and 5-HT1B receptors act on different neuron populations was tested by applying both 8-OH-DPAT and CP93129 to the same neurons (Fig 2A), an experiment that has not previously been performed. Although 5-HT1A and 5-HT1B receptors are thought to be localized to post- and pre-synaptic neurons, respectively, they could potentially both be expressed by the same local circuit, by either a presynaptic or a postsynaptic neuron, so that both would influence the responses of the neurons we recorded. If the effects of the receptors did not converge in this way, it would be consistent with the two receptors acting on separate neuron populations. Some neurons did show nonoverlapping effects of the two agonists. For example, a cluster of neurons that exhibited large decreases in spike rate during 8-OH-DPAT application showed comparatively small CP93129-induced increases. The overall correlation in the effects of these two agonists was not significant (Spearman's correlation, rs=.086, p=.66). Fig 2B shows single voltage traces of a single neuron that responded to both 8-OH-DPAT and CP93129 with changes in spike count in the opposite direction. The proportional changes in spike count for this neuron are represented in figure 2A (data point with asterisk). Thus, although some neurons responded more to the application of the 5-HT1A or -1B agonists, many neurons responded to both.
Figure 2.
Co-application of agonists. A. Proportional changes in spike rate for the application of 8-OH-DPAT versus CP93129 to the same neurons. Arrow indicates an outlying value for CP93129. B. Representative voltage traces of neuron marked with an asterisk in figure 2A. The stimulus is marked by the horizontal line and consisted of a tone at 19 kHz.
Because some serotonin receptor agonists differentially affect neurons with particular physiological characteristics such as long latencies or high CFs (Hurley 2007; Bohorquez and Hurley 2009), we also tested whether the two agonists affect populations of neurons with significantly different latencies, CFs, or depths. These three characteristics can define functional domains of serotonergic effects within the tonotopic map of the IC (Hall and Hurley 2007). Neither the effects of 8-OH-DPAT nor those of CP93129 were significantly correlated with CF, recording depth, or median first spike latency (Spearman's correlations; rs=-.047 and p=.71, rs=.053 and p=.68, and rs=-.056 and p=.67 for 8-OH-DPAT versus CF, depth, and latency respectively; rs=-.052 and p=.71, rs=-.18 and p=.22, and ; rs=-.021 and p=.88 for CP93129 versus CF, depth, and latency respectively). We additionally examined whether neurons that responded to both agonists by changing their spike rates ≥ 20% (n = 19) versus neurons that responded to only one or to neither agonist (n = 10) had different mean values of latency, CF, or depth. There were no significant differences between these groups of neurons (Mann-Whitney U tests; U=75 and p=.36 for CF, U=61 and p=.12 for depth, and U=94 and p=.96 for latency).
5-HT1A and 5-HT1B agonists modulate both evoked and spontaneous activity
According to our model of 5-HT1A and 5-HT 1B receptors, both receptors have the potential to influence spontaneous activity. The 5-HT1A receptor postsynaptically decreases excitability, and the 5-HT1B receptor decreases GABA release, a neurotransmitter that reduces spontaneous as well as evoked activity in the IC (Faingold et al., 1989). We compared the effects of 8-OH-DPAT and CP93129 on evoked and spontaneous activity in the same neurons. Drug effects on evoked activity were measured as in figures 1 and 2, as the proportional change in spike rate per stimulus, and drug effects on spontaneous activity were measured as proportional changes in spike rates per second. Neurons showed generally low rates of spontaneous activity, as has been previously reported in our preparations (Hurley and Pollak 1999, 2001). Of 84 neurons tested with 8-OH-DPAT or CP93129, only 29 showed spontaneous rates of over 1.5 spikes/sec in the pre-drug condition (Fig 3A). We analyzed the effects of the two agonists on any neuron showing spontaneous rates of over 1.5 spikes/sec in either the pre-drug condition or during drug application. The effects of 8-OH-DPAT and CP93129 on spontaneous and evoked activity were generally similar (Fig. 3B; Spearman's correlations: rs=.63, p=.002, n=22 for 8-OH-DPAT, outlier marked by * excluded; rs =.72, p<.001, n=22 for CP93129). The largest effects for both agonists were seen for spontaneous changes, but this may be due to the fact that the low rates of spontaneous activity created large proportional changes. Nevertheless, it is interesting to note that although 8-OH-DPAT could reduce spontaneous activity to very low levels, including totally abolishing spontaneous activity, it did not reduce evoked activity to this extent in this subset of neurons.
Figure 3.
Comparison of effects of 5-HT1A and 5-HT1B agonists on spontaneous and evoked activity. A. Histogram of spontaneous spike rates in neurons tested with the agonists. Most neurons had low rates of spontaneous activity. B. Proportional changes in evoked versus spontaneous spike rates induced by 8-OH-DPAT and CP93129 for neurons with spike rates over 1.5 spikes/sec. Changes in spontaneous and evoked activity are represented as transformed values (log[drug/control+.1]), so that all values are nonzero.
5-HT1A and 5-HT1B agonists differentially influence evoked response properties
We next assessed whether the 5-HT1A and 5-HT1B receptors interacted with each other to influence the auditory response properties of single neurons. To do this, we not only applied 8-OH-DPATand CP93129 alone, but also applied both of them together to a group of neurons. For 29 of these neurons, all three drug treatments were completed before losing the single-neuron recordings. For 7 neurons, the drug co-application plus only one of the agonists alone were applied. The effects of the agonists alone versus during co-application on 5 different evoked response properties were compared: 1) response rate, 2) bandwidth of frequency response, 3) latency, 4) interspike interval, and 5) response variability.
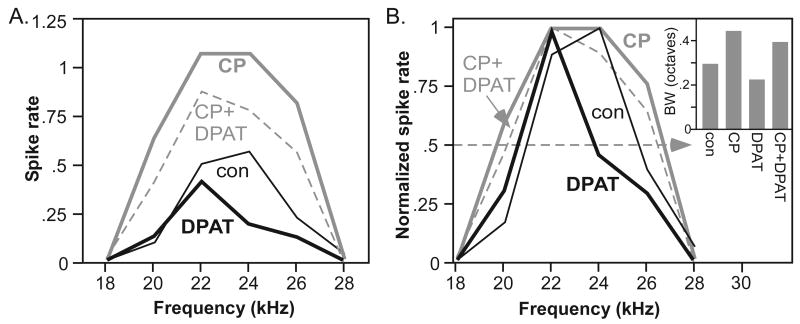
The first 2 of these properties are illustrated in figure 4, plots of spike rate versus frequency for individual neurons (frequency-response functions). The effects of agonists on spike rate were measured the same way as in figure 1, as the change in rate in response to tone bursts at the CF during drug application relative to the control. Frequency bandwidth was measured from frequency-response functions consisting of tones across the excitatory ranges of neurons, presented at 10-20 dB above the threshold at CF. Bandwidth was measured as the width of the frequency-response function at a level of half of the maximum spike rate. The frequencies corresponding to the upper and lower borders of the bandwidth were determined by linear interpolation. When iontophoresed alone, either 8-OH-DPAT or CP93129 could change both spike rate (Fig. 4A) and the half-maximum bandwidth (Fig. 4B, normalized spike rates).
Figure 4.
Effects of 8-OH-DPAT and CP93129 on frequency bandwidth. A. Frequency response plot (frequency vs. spike rate) of a single neuron comparing the effects of 8-OH-DPAT alone (DPAT), CP93129 alone (CP), and the combination of drugs (CP+DPAT). B. Normalized plots of spike rate for the same neuron to facilitate comparisons of frequency bandwidth: dashed line marks the 50% spike rate for all plots. Inset depicts the bandwidths in each of the 4 treatments, in octaves.
When applied together, the effects of each agonist on spike rate and bandwidth were apparent in their joint effect. 8-OH-DPAT decreased, and CP93129 increased, the spike rate when these agonists were applied alone (Fig 4A, CP+DPAT). When the agonists were co-applied, the spike rate at CF was intermediate to the spike rates in each agonist alone, suggesting that the 8-OH-DPAT-evoked decrease and the CP93129-evoked increase balanced one another to some extent. Changes in half-maximum bandwidth followed a similar pattern, with 8-OH-DPAT decreasing bandwidth by .08 octaves, CP93129 increasing bandwidth by .15 octaves, and the drug combination increasing bandwidth by .10 octaves (Fig. 4B). Across the population of neurons, the effects of the two agonists alone were compared with their co-application using multiple regressions. If the effect of a single agonist was positively correlated with the effect of drug co-application, then the effect of the agonist was maintained against the background of multiple receptor activation. The effects of both 8-OH-DPAT and of CP93129 on spike rate were positively correlated with the effects of drug co-application (multiple regression, F=38.213, dF 2,25, p<.001 for the overall model, adjusted r2=.734), indicating that both of the agonists influenced spike rate (Fig 5A). A similar pattern was observed for the change in bandwidth, with the effects of each individual drug significantly correlated with the effect of drug co-application (Fig. 5B; multiple regression, F=58.34, dF 2,19, p<.001 for the overall model, adjusted r2=.845). This means that the effect of each of the receptor agonists was maintained in the presence of the other.
Figure 5.
5-HT1A and 5-HT1B agonists interact additively to influence spike rate and bandwidth. Effects of single agonists (y-axis) versus the co-application of both agonists (x-axis) on measurements of A. spike rate at CF [(drug-control)/control], and B. bandwidth (drug-control, octaves), across the neuron population.
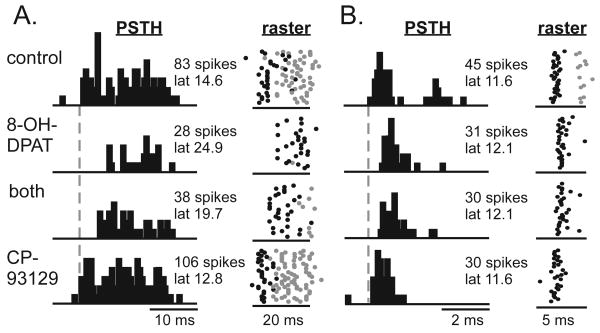
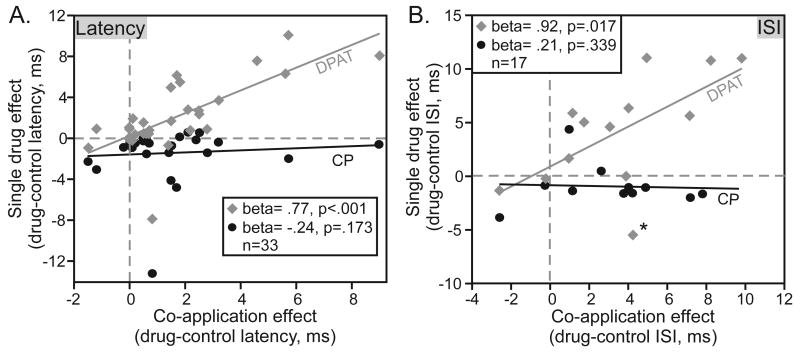
Similar to the case for spike rate and bandwidth, both the 5-HT1A and the 5-HT1B agonist altered the initial spike latency and interspike interval when applied alone (Wilcoxon signed rank tests, for 8-OH-DPAT Z=-3.69 and p<.001 for latency and Z=-2.61 and p=.009 for interspike interval; for CP93129 Z=-3.85 and p<.001 for latency and Z=-2.2 and p=.028 for interspike interval). Despite this, when the agonists were co-applied, their effects together were correlated with the effects of 8-OH-DPAT, but not with the effects of CP93129 alone. Figure 6 illustrates this pattern in the PSTHs of two individual neurons. For the first neuron (Fig. 6A), the median first spike latency increased when 8-OH-DPAT caused a reduction in spikes, achieved an intermediate value when the spike number was intermediate in the presence of both agonists, and was decreased when the spike number increased in the presence of CP93129 alone. Thus, for this neuron, the median latency matched the effect of the drug on spike number. For the second neuron (Fig. 6B), the spike number was decreased in the presence of 8-OH-DPAT and did not recover when 8-OH-DPAT iontophoresis was stopped. Nevertheless, the median latency increased in the presence of 8-OH-DPAT and returned to the control value when CP93129 alone was applied. Across the neuron population, the median latencies during the co-application of both selective agonists were significantly correlated with the effects of 8-OH-DPAT but not with those of CP93129 in the same neurons (Fig. 7A; multiple regression, F=10.548, dF 2,21, p=.001 for the overall model, adjusted r2=.526), even though CP93129 decreased latencies substantially in some neurons. The pattern of drug effects for interspike interval is similar in appearance (Fig. 7B), although fewer neurons consistently produced both first and second spikes. The multiple regression for interspike interval was not significant, because of one outlying value (asterisk; multiple regression, F=.44, dF 2,4, p=.672 for the overall model, adjusted r2=-.23; p=.035 and adjusted r2=.82 without outlier).
Figure 6.
Effects of 8-OH-DPAT and CP93129 on spike timing. A. PSTHs for one neuron in response to 8-OH-DPAT, CP93129, or drug co-application (‘both’). Values for latency are the median latencies of the first spikes in each condition, in ms. The bin size is 500 μs and the total duration of the PSTH is 30 ms. PSTHs are accompanied by raster plots showing the spike timing for each stimulus repetition. Black dots represent first spikes, and gray dots represent subsequent spikes. B. PSTHs and rasters for a second neuron. The bin size is 100 μs and the total duration of the PSTH is 6 ms. For both neurons, the order of drug treatments represents the sequence of drug application.
Figure 7.
Effects of single agonists versus the co-application of both agonists on median first spike latency (A) and interspike interval (B) across the neuron population. Both are expressed as drug- control values, in ms. Asterisk marks an extreme value. Beta and p values in (B) indicate values for a regression excluding this outlier.
The presence of 8-OH-DPAT also altered the variation in spike timing for some neurons (see raster plot for Neuron 1 in Fig. 6), but the effects of 8-OH-DPAT on the standard deviation of the initial latency and the interspike interval were not correlated with the effects of drug co-application on these values (multiple regressions; F=1.546, dF 2,12, p=.253 for the overall model, adjusted r2=.072 for the standard deviation of the initial latency; F=1.232, dF 2,2, p=.448 for the overall model, adjusted r2=.104 for the standard deviation of the interspike interval).
Thus, both selective agonists could alter spike rate, bandwidth, initial spike latency, and interspike interval when applied alone. In comparison, they had different effects on these response properties when they were co-applied. During co-application, the agonists both significantly contributed to changes in the response magnitude including spike rate and bandwidth, indicating an additive interaction. In contrast, during co-application, 8-OH-DPAT significantly influenced spike timing but CP93129 did not, suggesting that 8-OH-DPAT has the dominant influence on spike timing when both receptors are activated.
5-HT1A and 5-HT1B antagonists have different patterns of effects
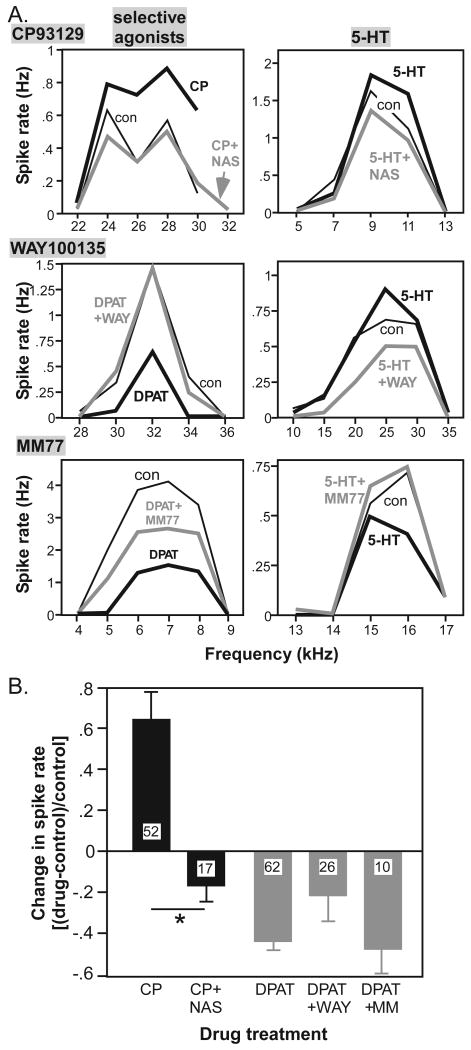
To determine whether the 5-HT1A and 5-HT1B receptors are both activated by endogenous sources of serotonin in our preparation, we used selective antagonists to block activation of the two receptors. We first assessed the ability of antagonists to block the effects of the selective agonists 8-OH-DPAT, CP93129, and of exogenously applied serotonin itself (Fig. 8). Antagonists of the two receptor types had different patterns of effects. The simplest pattern was exhibited by the 5-HT1B antagonist, NAS-181. This antagonist reversed increases in spike rate evoked by CP93129 (Fig. 8A, top row, left) an effect that was significant across the neuron population (for CP vs CP+NAS, Z=-3.62, p<.001, n=17, Wilcoxon signed rank tests). NAS-181 also reversed increases in spike rate evoked by serotonin itself in some neurons (Fig. 8A, top row, right).
Figure 8.
Selective 5-HT1A and 5-HT1B antagonists block the effects of agonists. A. Frequency response plots for 6 different neurons illustrating that the selective antagonists NAS-181 (5-HT1B receptor; top plots), WAY-100135 (5-HT1A; middle plots) and MM 77 (5-HT1A; lower plots) partially or totally reverse the effects of selective agonists (left plots) and of serotonin (right plots). B. Mean effects of different 5-HT1B (black bars) and 5-HT1A (gray bars) receptor antagonists in combination with agonists on changes in the spike rate [(drug-control)/control].
Two 5-HT1A antagonists, WAY-100135 and MM 77, could each block or reduce decreases in spike rate evoked by 8-OH-DPAT in some neurons (Fig. 8A, middle and lower rows, left). Both antagonists could also reverse the effects of iontophoresed serotonin (Fig. 8A, middle and lower rows, right), even when serotonin increased rather than decreased spike count in some cases (Fig. 8A, upper row, right). The effects of these two antagonists were quite variable. Across the neuron population, neither of the 5-HT1A antagonists significantly reduced the effect of 8-OH-DPAT (Fig. 8B; for DPAT vs. DPAT+WAY, Z=-1.16, p=.248, n=23; for DPAT vs. DPAT+MM, Z=-.56, p=.58 n = 10; Wilcoxon signed rank tests).
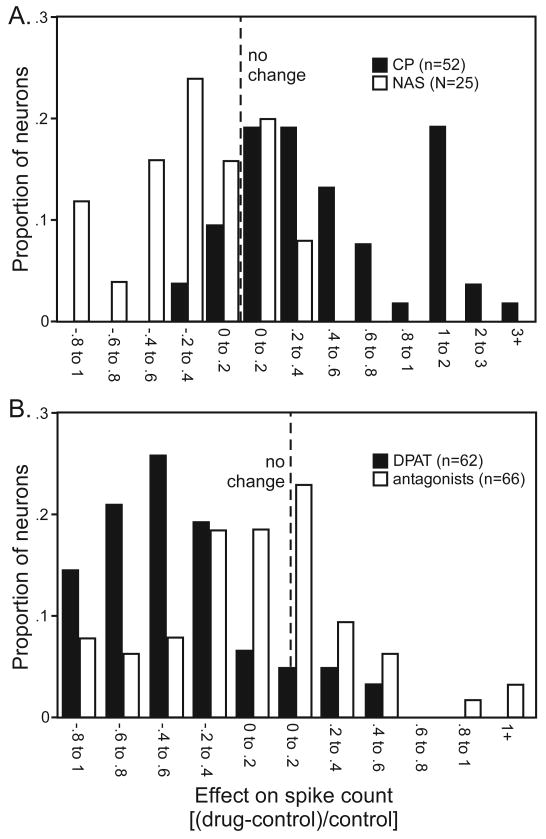
5-HT1A and 5-HT1B antagonists both influenced the spike rate when applied alone as well as when applied in combination with their respective agonists. This suggests the 5-HT1A and 5-HT1B receptors are activated endogenously in our preparation, even though serotonin levels in the IC are lower during ketamine/xylazine anesthesia than in awake animals (Hall et al. 2010). The effects of antagonists alone reflected their effects during the application of complementary agonists. That is, the distribution of the effects of NAS-181 alone mirrored the distribution of CP93129, with CP93129 increasing, and NAS-181 decreasing, spike rates across the population (Fig. 9A). In contrast, the distribution of the effects of the 5-HT1A antagonists alone on spike rate did not mirror the effect of 8-OH-DPAT alone, despite the ability of antagonists to block the effect of the agonist. 8-OH-DPAT predominantly decreased the spike rate, but WAY-100135 and MM 77 could decrease or increase the spike rate when applied alone (Fig. 9B; p = .79 for the distributions of WAY-100135 vs MM 77, chi squared test). Thus, for the 5-HT1A receptor, the effects of the selective antagonists on spike rate were qualitatively different than the effects of the selective agonist.
Figure 9.
Population histograms comparing the effects of selective agonists (black bars) and antagonists (white bars; WAY-100135 and MM 77) of A. the 5-HT1B receptor and B. the 5-HT1A receptor when these drugs were applied alone.
Discussion
Insight into how particular receptors interact with each other is essential to understanding the role that neuromodulators play in fine-tuning sensory processing. The IC provides an opportunity to assess such interactions between specific receptor types. 5-HT1A and 5-HT1B heteroreceptors are commonly co-expressed within sensory regions (Mooney et al 1996, Butt et al. 2002; Peruzzi and Dut 2004). In the IC these receptors have opposite effects on responses to auditory stimuli (Hurley 2006). We tested 3 hypotheses for how these receptors could act in complementary or cooperative ways. These were that the receptors affect different neurons, that when activated at the same time they influence response properties in different or synergistic ways, and that they have different patterns of endogenous activation. We found that the two receptor types interacted to some extent at each of these levels.
5-HT1A and 5-HT1B receptors do not target exclusive sets of neurons
The hypothesis that the 5-HT1A and 5-HT1B receptors always affect separate neuron populations was not supported by applying selective agonists of each of the two receptors to the same neurons. There was no inverse relationship between the effects of the agonists, and a substantial subpopulation of neurons responded to both. This pattern is consistent with previous reports that 5-HT1A and 5-HT1B receptors are widely expressed by neurons in the IC (Thompson et al. 1994; Peruzzi and Dut 2004). That many neurons responded to agonists of both receptor types also suggests that the iontophoretic application of drugs in this study effectively reached both types of receptors.
Some neurons were responsive to the agonist for one receptor but not the other, suggesting that the effects of the presynaptic 5-HT1B receptor and the postsynaptic 5-HT1A receptor do not always converge. Although the populations of 5-HT1A and 5-HT1B-responding neurons overlapped in our study, the overlapping effects of different serotonin receptors can still distinguish among classes of neurons in other brain regions such as visual cortex (Xiang and Prince 2003). The effects of activating serotonin receptors has corresponded to whether neurons share other characteristics such as depth, CF, or latency, in several previous studies (Hurley 2007; Bohorquez and Hurley 2009).
5-HT1A and 5-HT1B receptors differentially influence rate versus timing
One of the most intriguing and unexpected findings of this study was that the effects of concurrently activating the 5-HT1A and 5-HT1B receptors differentially influence evoked response properties. Agonists of the 5-HT1A and 5-HT1B receptors interacted additively to influence properties related to response magnitude, including the spike rate and measurements of the bandwidth of frequency responses. In contrast, the effect of co-application closely matched that of activating the 5-HT1A receptor, but not the 5-HT1B receptor, across the neuron population. Thus, even when both receptors were activated, as would occur during increasing levels of endogenous serotonin, the 5-HT1A agonist dominated the timing of responses to auditory stimuli. This finding could be due partly to limits on the amount that latency can be shortened as opposed to lengthened, but the selective 5-HT1B agonist is capable of shortening latency in IC neurons.
We obtained these findings using iontophoresis to locally apply drugs with minimal damage to tissue. The absolute concentrations of drugs are not controllable with this technique, so we are not certain that we reached all of the 5-HT1A and 5-HT1B receptors that are capable of influencing a particular neural circuit in the IC. However, we demonstrated in this study and in previous studies that increasing levels of iontophoretic current create effects that are dose-dependent and saturate (Hurley and Pollak 2001; Hurley 2006). In addition, affecting all available receptors is not crucial to the argument that the 5-HT1A and 5-HT1B receptors interact additively to influence spike rate but nonadditively to influence spike timing. This is because the same level of drug co-application differentially influenced these response properties in the same neurons and at the same time.
Our findings with 5-HT1A and 5-HT1B agonists are mechanistically consistent with previous models of the 5-HT1A and 5-HT1B receptors in the IC. The 5-HT1A receptor is usually somatodendritically located, triggering the opening of potassium channels (Lanfumey and Hamon 2004; Ögren et al. 2008). Such an effect could increase membrane conductance, an intrinsic property that is important in determining spike latency in the IC (Sun and Wu 2008). Potassium channels activated through the 5-HT1A receptor also contribute to regulating the level of spontaneous activity (Russo et al. 2008, Saenz del Burgo et al. 2008). 5-HT1B receptors regulate GABAergic inputs to IC neurons (Hurley et al. 2008). GABAergic inputs also regulate both spontaneous and evoked activity in the IC (Faingold 1989), and influence latencies by triggering rebound responses or by delaying spikes (Park and Pollak 1993; Peruzzi et al 2000; Xu-Friedman and Regehr 2005; Voytenko and Galazyuk 2008; Sun and Wu 2008). Many studies point to GABA as playing an important role in the encoding of different features of acoustic stimuli, including sound source location (Park and Pollak 1993), and GABA is also implicated in the generation and elaboration of defensive behavior in response to threatening stimuli (Brandão et al. 1988; Castellan-Baldan et al. 2006, 2007). Activation of the 5-HT1B receptor did indeed change spike latencies and interspike interval in some neurons in this study, as well as in a previous study (Fig. 5 in the current study; also see Fig. 7 from Hurley et al. 2008). However, when the two receptors were co-activated, the 5-HT1A receptor dominated the modulation of first-spike latency across the neuron population, potentially because its effects are more proximal to the spike initiation site (Amargos-Bosch et al 2004).
5-HT1A and 5-HT1B receptors may have different patterns of endogenous activation
The application of 5-HT1A and 5-HT1B antagonists alone created qualitatively different patterns of effects from each other, and from the application of agonists. The 5-HT1B antagonist not only reversed the effects of the 5-HT1B agonist, but also had an effect opposite to that of the agonist when applied alone. This is consistent with the endogenous activation of 5-HT receptors in our preparation by the relatively low level of endogenous serotonin (Hall et al. 2010). In contrast, the effects of two separate 5-HT1A antagonists were unexpectedly similar to those of the agonist in many neurons. For one of these, MM 77, its lower aqueous solubility could have made it a less effective antagonist in our study. One possibility that would account for this finding is that the two 5-HT1A antagonists used in this study have pharmacological targets other than the 5-HT1A receptor, particularly since the absolute concentrations of the antagonists during iontophoresis are not known (Mokrosz et al. 1994). The observed pattern of effects is also consistent with the block of 5-HT1A receptors located on inhibitory neurons presynaptic to the ones recorded in this study, a possibility considered in a previous model of 5-HT1A receptors in the IC (Hurley 2006). This hypothesis is testable by examining the influence of 5-HT1A agonists and antagonists on inhibitory inputs to IC neurons, as it has been done previously for the 5-HT1B receptor (Hurley et al. 2008).
In the current study we focused on 5-HT1A and 5-HT1B receptors because we have a relatively well-substantiated model for how each of these receptors acts in the IC (Hurley 2006, 2007, Hurley et al. 2008), which suggests an interesting interplay between pre-and postsynaptic modulation. Additional 5-HT receptor types could also have potentially contributed to our findings. 5-HT1A and 5-HT1B receptors are only 2 of the many types of serotonin receptors that are expressed by IC neurons (Hurley et al. 2002, 2004). Endogenous serotonin may be present at low levels in our in vivo preparation, so our agonists and antagonists were likely to be acting in a background of endogenously activated receptor types. Indeed, the fact that the application of antagonists alone affected neural responses supports this hypothesis. The activation of at least two additional receptor types, 5-HT2C and 5-HT3 receptors, alter the evoked responses of IC neurons (Hurley 2006, Bohorquez and Hurley 2009). Blocking the 5-HT1A or 5-HT1B receptors could therefore have unmasked the effects of other receptors, or disrupted cooperative receptor effects. Thus, our findings serve as an illustration of one of multiple types of 5-HT receptor interactions that are possible.
Model of receptor interaction
The fact that activation of the 5-HT1A and 5-HT1B receptors differentially alters the rate and timing of responses in the IC raises the possibility that interaction of receptors could have highly specific effects on stimulus encoding. Within multiple sensory systems, spike rates, first-spike latencies, and the timing of spike trains may all encode relevant stimulus properties (for example, Panzeri et al. 2001; Reich et al. 2001; Foffani et al. 2008). In different regions of the auditory system, including the IC, these properties include frequency and spatial location (for example, Phillips 1998; Sanderson and Simmons 2000; Furukawa and Middlebrooks 2002; Stecker and Middlebrooks 2003; Nelken et al. 2005; Chechik et al. 2006; Qiu et al. 2007; Chase and Young 2006, 2007, 2008).
Although few IC neurons displayed large amounts of suprathreshold spontaneous activity in this study, consistent with previous studies in our and other labs (Pollak and Bodenhamer 1981; Hurley and Pollak 1999; Basta and Ernest 2004), it is instructive that both 5-HT1A and 5-HT1B receptors altered spontaneous and evoked activity in similar directions. These effects on spontaneous activity suggests that 5-HT1A and 5-HT1B receptors contribute to the regulation of the excitatory-inhibitory ‘tone’ of neurons and that they could therefore play a role in regulating the information conveyed by spontaneous spikes, including synchronization to the hippocampal theta rhythm (Pedemonte et al. 1996). In neurons displaying spontaneous activity, 5-HT1A receptors could abolish spontaneous activity but not evoked activity, suggesting further that the signal-to-noise ratio of evoked activity could be favorably influenced by this receptor type.
Our results contribute to a model of how serotonin modulates auditory processing in accordance with behavioral state. Levels of serotonin in many regions of the brain, including the IC, vary not only across the sleep-wake cycle, but also relatively rapidly (within minutes) in response to sensory stimuli, stressors, or social challenges (Boutelle et al. 1990; Clement et al. 1998; Mas et al. 1995; Hall et al. 2010). Serotonin is therefore a physiological signal that can translate information about behavioral context into changes in auditory processing. The details of this translation process, however, are rather poorly understood. Here, we have demonstrated that multiple serotonin receptors can interact in nonlinear ways to differentially shape the rates and latencies of evoked responses. The effects of serotonin are therefore multifaceted, even for the simple tonal stimuli we used in this study. We have previously proposed that serotonin promotes the selective encoding of acoustic signals such as species-specific vocalizations in the IC (Hurley et al. 2004; Hurley and Pollak 2005a). Determining whether the characteristics of the serotonergic receptor machinery that we have identified modify the encoding of natural stimuli in a way that matches the behavioral significance of such signals will be a good test of this hypothesis.
Acknowledgments
These experiments were funded by National Institute of Deafness and Other Communication Disorders grant DC-008963.
References
- Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- Basta D, Ernest A. Noise-induced changes of neuronal spontaneous activity in mice inferior colliculus brain slices. Neurosci Lett. 2004;368:297–302. doi: 10.1016/j.neulet.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Bohorquez A, Hurley LM. Activation of serotonin 3 receptors changes in vivo auditory responses in the mouse inferior colliculus. Hear Res. 2009;251:29–38. doi: 10.1016/j.heares.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle MG, Zetterstrom T, Pei Q, Svensson L, Fillenz M. In vivo neurochemical effects of tail pinch. J Neurosci Methods. 1990;34:151–157. doi: 10.1016/0165-0270(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Tomaz C, Leão Borges PC, Coimbra NC, Bagri A. Defense reaction induced by microinjections of bicuculline into the inferior colliculus. Physiol Behav. 1998;44:361–365. doi: 10.1016/0031-9384(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol. 1981;197:705–722. doi: 10.1002/cne.901970410. [DOI] [PubMed] [Google Scholar]
- Butt CM, Zhao B, Duncan MJ, Debski EA. Sculpting the visual map: the distribution and function of serotonin-1A and serotonin-1B receptors in the optic tectum of the frog. Brain Res. 2002;931:21–31. doi: 10.1016/s0006-8993(01)03370-4. [DOI] [PubMed] [Google Scholar]
- Castellan-Baldan L, da Costa Kawasaki M, Ribeiro SJ, Calvo F, Correa VM, Coimbra NC. Topographic and functional neuroanatomical study of GABAergic disinhibitory striatum-nigral inputs and inhibitory nigrocollicular pathways: neural hodology recruiting the substantia nigra, pars reticulata, for the modulation of the neural activity in the inferior colliculus involved with panic-like emotions. J Chem Neuroanat. 2006;32:1–27. doi: 10.1016/j.jchemneu.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Castellan-Baldan L, da Costa Kawasaki M, Ribeiro SJ, Calvo F, Correa VM, Coimbra NC. Erratum to “Topographic and functional neuroanatomical study of GABAergic disinhibitory striatum–nigral inputs and inhibitory nigrocollicular pathways: Neural hodology recruiting the substantia nigra, pars reticulata, for the modulation of the neural activity in the inferior colliculus involved with panic-like emotions”. J Chem Neuroanat. 2007;33:65–66. doi: 10.1016/j.jchemneu.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Chase SM, Young ED. Spike-timing codes enhance the representation of multiple simultaneous sound-localization cues in the inferior colliculus. J Neurosci. 2006;26:3889–3898. doi: 10.1523/JNEUROSCI.4986-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase SM, Young ED. First-spike latency information in single neurons increases when referenced to population onset. Proc Natl Acad Sci U S A. 2007;104:5175–5180. doi: 10.1073/pnas.0610368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase SM, Young ED. Cues for sound localization are encoded in multiple aspects of spike trains in the inferior colliculus. J Neurophysiol. 2008;99:1672–1682. doi: 10.1152/jn.00644.2007. [DOI] [PubMed] [Google Scholar]
- Chechik G, Anderson MJ, Bar-Yosef O, Young ED, Tishby N, Nelken I. Reduction of information redundancy in the ascending auditory pathway. Neuron. 2006;51:359–368. doi: 10.1016/j.neuron.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Clement HW, Kirsch M, Hasse C, Opper C, Gemsa D, Wesemann W. Effect of repeated immobilization on serotonin metabolism in different rat brain areas and on serum corticosterone. J Neural Transm. 1998;105:1155–1170. doi: 10.1007/s007020050119. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Gehlbach G, Caspary DM. On the role of GABA as an inhibitory transmitter in inferior colliculus neurons: iontophoretic studies. Brain Res. 1989;500:302–312. doi: 10.1016/0006-8993(89)90326-0. [DOI] [PubMed] [Google Scholar]
- Foffani G, Chapin JK, Moxon KA. Computational role of large receptive fields in the primary somatosensory cortex. J Neurophysiol. 2008;100:268–280. doi: 10.1152/jn.01015.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Middlebrooks JC. Cortical representation of auditory space: information-bearing features of spike patterns. J Neurophysiol. 2002;87:1749–1762. doi: 10.1152/jn.00491.2001. [DOI] [PubMed] [Google Scholar]
- Hage SR, Ehret G. Mapping responses to frequency sweeps and tones in the inferior colliculus of house mice. Eur J Neurosci. 2003;18:2301–2312. doi: 10.1046/j.1460-9568.2003.02945.x. [DOI] [PubMed] [Google Scholar]
- Hall IC, Hurley LM. The serotonin releaser fenfluramine alters the auditory responses of inferior colliculus neurons. Hear Res. 2007;228:82–94. doi: 10.1016/j.heares.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Rebec GV, Hurley LM. Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. JEB. 2010 doi: 10.1242/jeb.035956. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havey DC, Caspary DM. A simple technique for constructing ‘piggy-back’ multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol. 1980;48:249–251. doi: 10.1016/0013-4694(80)90313-2. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Falls WA. Destruction of the inferior colliculus disrupts the production and inhibition of fear conditioned to an acoustic stimulus. Behav Brain Res. 2003;144:175–185. doi: 10.1016/s0166-4328(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huffman RF, Henson OW., Jr The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Res Brain Res Rev. 1990;15:295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- Hurley LM. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J Neurophysiol. 2006;96:2177–2188. doi: 10.1152/jn.00046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res. 2007;1181:21–29. doi: 10.1016/j.brainres.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci. 1999;19:8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. J Neurophysiol. 2001;85:828–842. doi: 10.1152/jn.2001.85.2.828. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. J Comp Physiol A. 2005a;191:535–546. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM, Pollak GD. Serotonin in the inferior colliculus. Hear Res. 2002;168:1–11. doi: 10.1016/s0378-5955(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Tracy JA, Bohorquez A. Serotonin 1B receptor modulates frequency response curves and spectral integration in the inferior colliculus by reducing GABAergic inhibition. J Neurophysiol. 2008;100:1656–1667. doi: 10.1152/jn.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Covey E. 5-HT innervation of the auditory pathway in birds and bats. In: Syka JL, editor. Acoustical Signal Processing in the Central Auditory System. Plenum; New York: 1997. pp. 71–78. [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol. 2002;88:1941–1954. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Barati S, O'Connor WT. Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res. 1997;759:205–212. doi: 10.1016/s0006-8993(97)00255-2. [DOI] [PubMed] [Google Scholar]
- Martin DC, Introna RP, Aronstam RS. Inhibition of neuronal 5-HT uptake by ketamine, but not halothane, involves disruption of substrate recognition by the transporter. Neurosci Lett. 1990;112:99–103. doi: 10.1016/0304-3940(90)90329-8. [DOI] [PubMed] [Google Scholar]
- Mas M, Fumero B, Gonzalez-Mora JL. Voltammetric and microdialysis monitoring of brain monoamine neurotransmitter release during sociosexual interactions. Behav Brain Res. 1995;71:69–79. doi: 10.1016/0166-4328(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Monckton JE, McCormick DA. Neuromodulatory role of serotonin in the ferret thalamus. J Neurophysiol. 2002;87:2124–2136. doi: 10.1152/jn.00650.2001. [DOI] [PubMed] [Google Scholar]
- Mokrosz MJ, Chojnacka-Wójcik E, Tatarczyńska E, Kłodzińska A, Filip M, Boksa J, Charakchieva-Minol S, Mokrosz J. 1-(2-Methoxyphenyl)-4-(4-succinimido)butyl)piperazine (MM-77): A new, potent, postsynaptic antagonist of 5-HT1A receptors. Med Chem Res. 1994;4:161–169. [Google Scholar]
- Mooney RD, Huang X, Shi MY, Bennett-Clarke CA, Rhoades RW. Serotonin modulates retinotectal and corticotectal convergence in the superior colliculus. Prog Brain Res. 1996;112:57–69. doi: 10.1016/s0079-6123(08)63320-8. [DOI] [PubMed] [Google Scholar]
- Nelken I, Chechik G, Mrsic-Flogel TD, King AJ, Schnupp JW. Encoding stimulus information by spike numbers and mean response time in primary auditory cortex. J Comput Neurosci. 2005;19:199–221. doi: 10.1007/s10827-005-1739-3. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K. Ketamine stereoselectively inhibits rat dopamine transporter. Neurosci Lett. 1999;274:131–134. doi: 10.1016/s0304-3940(99)00688-6. [DOI] [PubMed] [Google Scholar]
- Ögren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekstrom JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri M, Flugge G. 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res. 2005;321:159–172. doi: 10.1007/s00441-005-1112-x. [DOI] [PubMed] [Google Scholar]
- Panzeri S, Petersen RS, Schultz SR, Lebedev M, Diamond ME. The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron. 2001;29:769–777. doi: 10.1016/s0896-6273(01)00251-3. [DOI] [PubMed] [Google Scholar]
- Park TJ, Pollak GD. GABA shapes a topographic organization of response latency in the mustache bat's inferior colliculus. J Neurosci. 1993;13:5172–5187. doi: 10.1523/JNEUROSCI.13-12-05172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Elsevier Science; Oxford: 2004. [Google Scholar]
- Pedemonte M, Pena JL, Velluti RA. Firing of inferior colliculus auditory neurons is phase-locked to the hippocampus theta rhythm during paradoxical sleep and waking. Exp Brain Res. 1996;112:41–46. doi: 10.1007/BF00227176. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Dut A. GABA, serotonin and serotonin receptors in the rat inferior colliculus. Brain Res. 2004;998:247–250. doi: 10.1016/j.brainres.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Sivaramakrishnan S, Oliver DL. Identification of cell types in brain slices of the inferior colliculus. Neuroscience. 2000;101:403–416. doi: 10.1016/s0306-4522(00)00382-1. [DOI] [PubMed] [Google Scholar]
- Phillips DP. Factors shaping the response latencies of neurons in the cat's auditory cortex. Behav Brain Res. 1998;93:33–41. doi: 10.1016/s0166-4328(97)00139-3. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Bodenhamer RD. Specialized characteristics of single units in inferior colliculus of mustache bat: frequency representation, tuning, and discharge patterns. J Neurophysiol. 1981;46:605–620. doi: 10.1152/jn.1981.46.3.605. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends Neurosci. 2003;26:33–39. doi: 10.1016/s0166-2236(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Mechler F, Victor JD. Temporal coding of contrast in primary visual cortex: when, what, and why. J Neurophysiol. 2001;85:1039–50. doi: 10.1152/jn.2001.85.3.1039. [DOI] [PubMed] [Google Scholar]
- Russo MJ, Yau HJ, Nunzi MG, Mugnaini E, Martina M. Dynamic metabotropic control of intrinsic firing in cerebellar unipolar brush cells. J Neurophysiol. 2008;100:3351–3360. doi: 10.1152/jn.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q, Tang J, Yu Z, Zhang J, Zhou Y, Xiao Z, Shen J. Latency represents sound frequency in mouse IC. Sci China C Life Sci. 2007;50:258–264. doi: 10.1007/s11427-007-0020-6. [DOI] [PubMed] [Google Scholar]
- Saenz del Burgo L, Cortes R, Mengod G, Zarate J, Echevarria E, Salles J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J Comp Neurol. 2008;510:581–606. doi: 10.1002/cne.21810. [DOI] [PubMed] [Google Scholar]
- Sanderson MI, Simmons JA. Neural responses to overlapping FM sounds in the inferior colliculus of echolocating bats. J Neurophysiol. 2000;83:1840–1855. doi: 10.1152/jn.2000.83.4.1840. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods. 1986;18:339–350. doi: 10.1016/0165-0270(86)90022-1. [DOI] [PubMed] [Google Scholar]
- Stecker GC, Middlebrooks JC. Distributed coding of sound locations in the auditory cortex. Biol Cybern. 2003;89:341–349. doi: 10.1007/s00422-003-0439-1. [DOI] [PubMed] [Google Scholar]
- Sun H, Wu SH. Modification of membrane excitability of neurons in the rat's dorsal cortex of the inferior colliculus by preceding hyperpolarization. Neuroscience. 2008;154:257–272. doi: 10.1016/j.neuroscience.2007.10.055. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngol Head Neck Surg. 1994;110:93–102. doi: 10.1177/019459989411000111. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Activity of serotonin-containing neurons in freely moving cats. In: J BL, G A, editors. Serotonin Neurotransmission and Behavior. The MIT Press; Cambridge, MA: 1981. pp. 360–363. [Google Scholar]
- Villars PS, Kanusky JT, Dougherty TB. Stunning the neural nexus: mechanisms of general anesthesia. AANA J. 2004;72:197–205. [PubMed] [Google Scholar]
- Voytenko SV, Galazyuk AV. Timing of sound-evoked potentials and spike responses in the inferior colliculus of awake bats. Neuroscience. 2008;155:923–936. doi: 10.1016/j.neuroscience.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C, Palacios JM. 5-HT1 receptor binding sites in the guinea pig superior colliculus are predominantly of the 5-HT1D class and are presynaptically located on primary retinal afferents. Brain Res. 1990;528:207–211. doi: 10.1016/0006-8993(90)91659-5. [DOI] [PubMed] [Google Scholar]
- Watakabe A, Komatsu Y, Sadakane O, Shimegi S, Takahata T, Higo N, Tochitani S, Hashikawa T, Naito T, Osaki H, Sakamoto H, Okamoto M, Ishikawa A, Hara S, Akasaki T, Sato H, Yamamori T. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their bidirectional modulatory effects on neuronal responses. Cereb Cortex. 2009;19:1915–1928. doi: 10.1093/cercor/bhn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Prince DA. Heterogeneous actions of serotonin on interneurons in rat visual cortex. J Neurophysiol. 2003;89:1278–1287. doi: 10.1152/jn.00533.2002. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Dynamic-clamp analysis of the effects of convergence on spike timing. I. Many synaptic inputs. J Neurophysiol. 2005;94:2512–2525. doi: 10.1152/jn.01307.2004. [DOI] [PubMed] [Google Scholar]