Abstract
Copper has been identified as a pollutant of concern by the Environmental Protection Agency (EPA) because of its widespread occurrence and toxic impact in the environment. Three nanoporous sorbents containing chelating diamine functionalities were evaluated for Cu2+ adsorption from natural waters -- ethylenediamine functionalized self-assembled monolayers on mesoporous supports (EDA-SAMMS®), ethylenediamine functionalized activated carbon (AC-CH2-EDA), and 1,10-Phenanthroline functionalized mesoporous carbon (Phen-FMC). The pH dependence of Cu2+ sorption, Cu2+ sorption capacities, rates, and selectivity of the sorbents were determined and compared with those of commercial sorbents (Chelex-100 ion exchange resin and Darco KB-B activated carbon). All three chelating diamine sorbents showed excellent Cu2+ removal (~95–99%) from river water and sea water over the pH range of 6.0–8.0. EDA-SAMMS and AC-CH2-EDA demonstrated rapid Cu2+ sorption kinetics (minutes) and good sorption capacities (26 and 17 mg Cu/g sorbent, respectively) in sea water, while Phen-FMC had excellent selectivity for Cu2+ over other metal ions (e.g. Ca2+, Fe2+, Ni2+, and Zn2+) and was able to achieve Cu levels below the EPA standards for river and sea waters.
Introduction
Copper has been identified as a pollutant of concern by the Environmental Protection Agency (EPA) because of its widespread occurrence and toxic impact in the environment. Copper is also one of the important trace transition metals found in human body and many living organisms and is involved in redox processes of a number of biomolecules [1, 2]. Copper is present in waste effluents generated by various industries (i.e. electroplating, wood, painting, textile, and paper industries), and can accumulate in environment and food chain, especially in fish. Excess copper in the human body has been reported to be linked to serious health threats such as cellular or organ damage and Wilson’s disease [1, 2]. Recent reports present evidence that copper is one of the transition metals (along with zinc and iron) that play a role in Alzheimer’s disease [3–6]. Copper is believed to be involved in both the aggregation of amyloid-β protein (Aβ) and generation of oxidative stress in the patient’s brain, which are key characteristics of Alzheimer’s disease [3–6]. Chelation therapy has been suggested as a promising therapeutic strategy for these diseases [4, 7, 8]. Although copper is necessary for growth and reproduction of species living in many aquatic environments, levels just slightly above the required level are toxic to various life stages of these organisms. For example, abnormal embryo development has been reported in the blue mussel after exposure to sea water containing 10 ppb of copper [9]. The dissolved cupric ion (Cu2+) is believed to be more toxic than complexed, precipitated, and adsorbed forms of Cu2+[10]. Although copper discharge limits have been set at ≤1 ppm to avoid causing environmental problems, accumulation in natural waters is still a problem [11, 12]. According to the 2000 Toxic Release Inventory (TRI), the release of total copper in the United Stated was over 690,000 tons/year [13], resulting in increased accumulation of Cu2+ in natural waters. In some areas, the concentration of Cu2+ has been reported to be as high as 50–80 ppb [9]. To avoid significant toxic effects on aquatic ecosystems, a cost-effective method to remove excess Cu2+ from natural waters is needed.
Adsorption technologies have proven to be effective methods for removing various metal ions from aqueous solutions. Various porous sorbents with natural and chemically modified surfaces have been investigated for copper adsorption, including mesoporous silica [14], synthetic polymers [15, 16], magnetite and biomolecule based materials [17–19], and low cost natural materials [20–22]. Among these sorbents, a number of sorbents have shown very high capacities (~50–330 mg/g sorbent) for the removal of Cu2+ from aqueous solutions. However, these Cu sorption capacities have been measured in deionized water, a simple matrix that fails to account for the effects of metal ion speciation, complexation, competing ions, and fouling of the sorbent materials by biomolecules often encountered in the real waters.
This manuscript is a systematic comparison of three new classes of nanoporous sorbents materials, designed around chelating diamine functionality, and tailored for Cu sorption in complex real waters. The materials were built upon a scaffold of mesoporous silica, mesoporous carbon, or activated carbon. They are ethylenediamine functionalized activated carbon (AC-CH2-EDA), ethylenediamine terminated self-assembled monolayers on mesoporous supports (EDA-SAMMS®, SAMMS is a registered trademark of Battelle Memorial Institute), and 1,10-Phenanthroline functionalized mesoporous carbon (Phen-FMC) for Cu2+ adsorption from river water and sea water. These materials are believed to be the best Cu binding materials among their classes. For a comparison purpose, a representative ion exchange resin (Chelex-100) and unmodified activated carbon (Darco KB-B) were also studied. Our goal is to make the recommendation on the best Cu sorbent materials for removal Cu from natural waters or other similar matrices.
Experimental procedures
Sorbent synthesis
Ethylenediamine self-assembled monolayers on mesoporous silica (EDA-SAMMS)
The EDA-SAMMS was prepared as described previously [23]. BET surface area analysis revealed a specific surface area of 105 m2/g, and an average pore size of about 38 Å. Thermogravimetric analysis (TGA) revealed a functional density of ~2.6 mmole of EDA per gram of sorbent.
Ethylenediamine modified activated carbon (AC-CH2-EDA)
The chloromethylated activated carbon (AC-CH2-Cl) was made as described previously [24]. BET surface area analysis revealed a specific surface area of 1200 m2/g. Elemental analysis (Galbraith Laboratories) revealed 4.33 weight percent N (which correlates to approximately 1.5 mmole EDA per gram of sorbent; indicating essentially quantitative displacement of the benzylic chloride by the EDA nucleophile).
1,10-Phenanthroline functionalized mesoporous carbon (Phen FMC)
Phen-FMC was made as previously described [25]. It had a specific surface area of 870 m2/g, an average pore size of 35Å, and 8.2 weight percent N (which correlates to approximately 2.9 mmoles Phen per gram of sorbent).
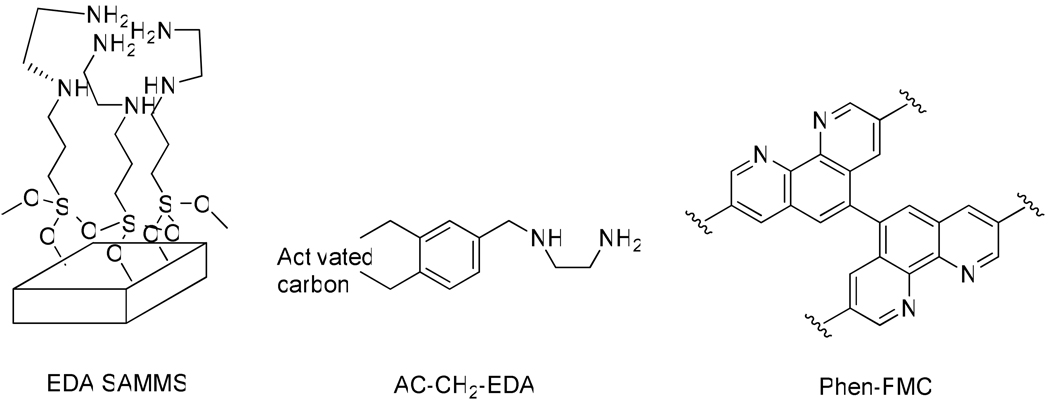
The chemical structures of the three sorbents are presented in Figure 1.
Figure 1.
Solid phase sorbents built around chelating diamine ligands, designed for Cu capture -- EDA-SAMMS, AC-CH2-EDA and Phen-FMC.
Batch sorption experiments
Batch metal sorption experiments were performed with river water (Columbia River, Richland, WA) and sea water (Sequim Bay, WA). Seawater normally contains (approximately) Ca2+ (400 ppm) and Mg2+ (1,300 ppm) as major divalent cations; Na+ (10,000 ppm) and K+ ( 400 ppm) as major monovalent cations; and Cl− (20,000 ppm), SO42− (3,000 ppm), and HCO3− (150 ppm) as major anions. River water contains (approximately) Ca2+ (20 ppm) and Mg2+ (5 ppm) as major divalent cations; Na+ (10 ppm) and K+ (1 ppm) as major monovalent cations; and HCO3− (70 ppm), SO42− (20 ppm), Cl− (10 ppm), and NO3− (1 ppm) as major anions. Both waters had the starting pH of 7.7 to 8.0. The waters were used after filtering through a 0.45 µm cellulose membrane (MF-Millipore™). The metal ion solutions were prepared from ICP standard solutions, purchased from Aldrich. After adding the metal ion stock to natural waters, the solutions were incubated for 30 min. Then the sorbent was added to achieve desired liquid per solid ratio (L/S in mL/g). The sample was then shaken for 2 h (to insure complete equilibration based on the kinetics data) at 200 rpm on an orbital shaker. After 2 h, the suspension was filtered with 0.45-µm Nylon-membrane syringe filters. The filtrate was kept in 2 vol. % HNO3 prior to metal analysis using inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7500ce, Agilent Technologies, CA). The metal concentrations in the control (no sorbent) with and without filtration were tested in order to check for precipitation of metal ions; reduced Cu concentrations in the filtrates from those in the non-filtered solutions would indicate that Cu forms insoluble species that are collected on the filters. No precipitation of Cu was observed within the reported data. For studying the pH effect, the waters were adjusted with HNO3 and/or NH4OH to the desired pH (in our previous work added NH3 was found to increase the solubility of transition metals in natural waters [26]). The solution pH was measured once prior to adding sorbents and again after the batch sorption was completed; the latter values were reported along with the sorption data. No significant pH change occurred, owing to the buffer properties of the natural waters and the very low solid per solution ratio (e.g., 0.2 g/L). More details of each batch study can be found in Supplement Information. All batch experiments were performed in triplicates and the average values reported.
Results and discussion
EDA-SAMMS, AC-CH2-EDA, and Phen-FMC were selected for Cu capture because they all contained chelating diamine, known to form strong complex with Cu2+[27]. Although the synthesis and initial evaluation of these sorbents have recently been reported by our research group (e.g., [23, 24, 26]), none of the work has truly focused on copper capture. This is also the first time that the three sorbents are systemically compared in the same matrices with the goal of recommending best sorbent materials for various Cu capture needs. The Cu2+ complexes formed with both EDA and Phen consist of 5-membered rings and are particularly stable. Both complexes involve σ–donation of the N lone pairs to the Cu2+, and in the case of Phen, additional bond strength is obtained through π back-bonding from the populated d orbitals on Cu2+ to the π* orbitals of the Phen aromatic ring system.
Effect of solution pH and matrices
The pH and ionic strength of a solution are known to play important roles in the binding of metal ions to the surface functional groups of sorbents. The solution pH affects the surface charges of functionalized sorbents and the speciation of metal ions, and hence the interactions between metal ions and sorbent surfaces. At pH below 6, cupric ion (Cu2+) is a dominant and soluble form. In natural waters, Cu2+ may form complexes with common anions, including SO42−, OH−, PO43−, HCO3−, NO3−, and CO32−. In general, the precipitate formation depends on the Cu2+ concentration, other cation concentrations, types and concentrations of anions, temperature, and time to reach equilibrium. The most frequent Cu precipitates in natural waters are Cu(OH)2 (Ksp of 10−19.3) and Cu2(OH)2(CO3), the latter forms at high bicarbonate content and high pH (e.g., in ground water).
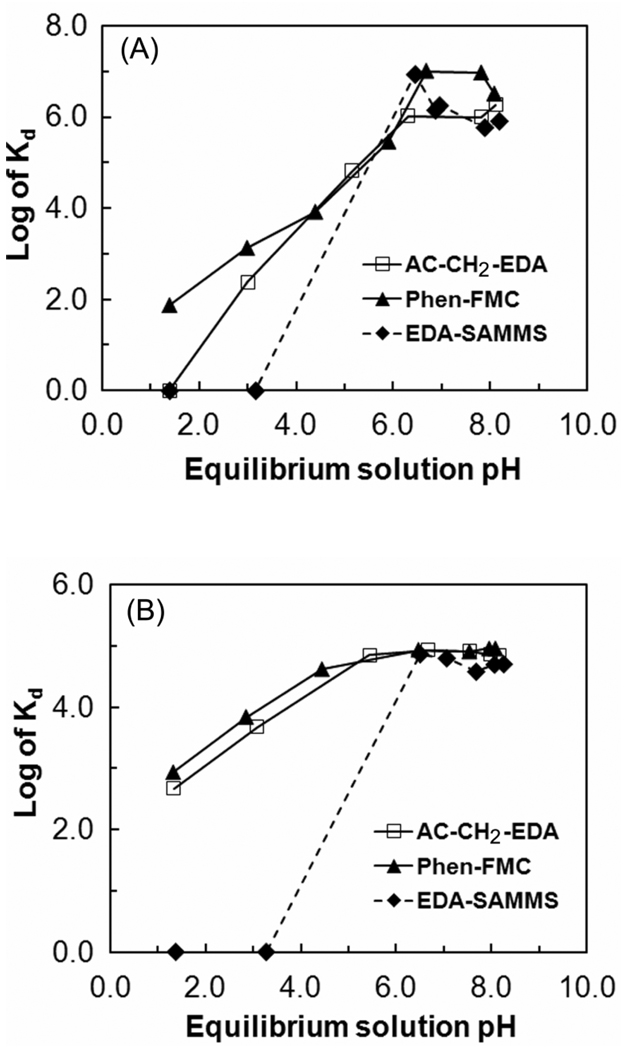
The effect of solution pH on Cu2+ binding affinity of a sorbent is presented by the distribution coefficient, Kd (mL/g), which is simply mass-weighted partition between Cu on the sorbent phase and Cu in bulk solution (see Supporting Information). The Kd values of Cu on the three sorbents measured in pH-adjusted river water and sea water (the latter inherently has much higher ionic strength) are shown in Figures 2(A) and 2(B), respectively. The initial pH of the test solutions was varied from 1 to 8, and the solutions were monitored to ensure no significant precipitation of Cu2+ at high pH. Ground water was not tested in this study, but had been tested in our other work and shown to be a more similar matrix to the river water than the sea water in term of the matrix effect on the metal binding affinity of sorbent materials [26, 28].
Figure 2.
Effects of solution pH on Cu2+ adsorption by EDA-SAMMS, AC-CH2-EDA, and Phen-FMC; initial Cu2+ concentration of ~100 ppb, L/S of 5000 mL/g in (A) pH-adjusted filtered river water and (B) pH-adjusted filtered sea water.
Solution pH had a significant impact on the binding of Cu2+ to these chelating diamine sorbents in both river water and seawater. In river water, the Kd values of the three sorbent materials displayed similar trends, with sharply increased Cu2+ binding as the pH increased from pH 1.4 to 6.5 for AC-CH2-EDA and Phen-FMC and from pH 3.0 to 6.5 for EDA-SAMMS. No Cu2+ adsorption was found for AC-CH2-EDA at pH < 1.4 and for EDA-SAMMS at pH < 3.2, while moderate adsorption (Kd ~ 100) was found for Phen-FMC at pH of 1.4. About 100% of Cu removal was found for the three materials from pH 6.5 to 8.2 (which nicely encompasses natural waters and many wastes). At this pH range, the Kd values of the three materials in river water were as high as 106 (and 107 for Phen-FMC).
Similar Kd trend for Cu2+ adsorption was also found in sea water. In sea water, the AC-CH2-EDA behaved very similar to the Phen-FMC; Kd of ~ 103 was found at pH as low as 1.4, increased as the pH increased to 6.0, and peaked between pH 6.0 – 8.2. Again, there was no Cu adsorption on EDA-SAMMS at pH < 3.2. The maximum Kd values in sea water (105) for the three sorbent materials were about one order of magnitude lower than those in river water (106–107). Sea water contains a variety of metals ions that may be competing for the binding sites on the sorbent interface, making it more difficult for Cu2+ to undergo chelation at the binding sites. Sea water also contains high concentrations of many counter-ions, which likely changes the speciation of the Cu ions, potentially hindering the chelation process. However, all three sorbents still offered excellent Kd values in the range of 105. Interestingly, in sea water, the maximum Cu2+ adsorptions by all three sorbents remained relatively constant over a wider pH range (pH 6.5–8.3 for EDA-SAMMS, and pH 4.5–8.2 for AC-CH2-EDA and Phen-FMC), compared to those in river water.
Neutral amine groups (particularly chelating diamines) are more readily able to coordinate/chelate the Cu2+ than the ammonium salts [29]. As a result, amine-based sorbents such as EDA-SAMMS and AC-CH2-EDA tend to display lower Cu2+ sorption under acidic conditions than they do at higher pH (similar to a sorbent functionalized by diethylenetriamine (DETA) [16]). The precipitation of Cu2+ at high pH (> 6.5 to 8.2) reported by others [17, 22, 30] was not observed in this work. This was presumably due to the much lower Cu2+ concentrations (~0.1 ppm) used in this work than those in the previous studies (~25–500 ppm), the higher chloride ions in seawater, and NH3 ions used to increase the solution pH.
Sorption kinetics
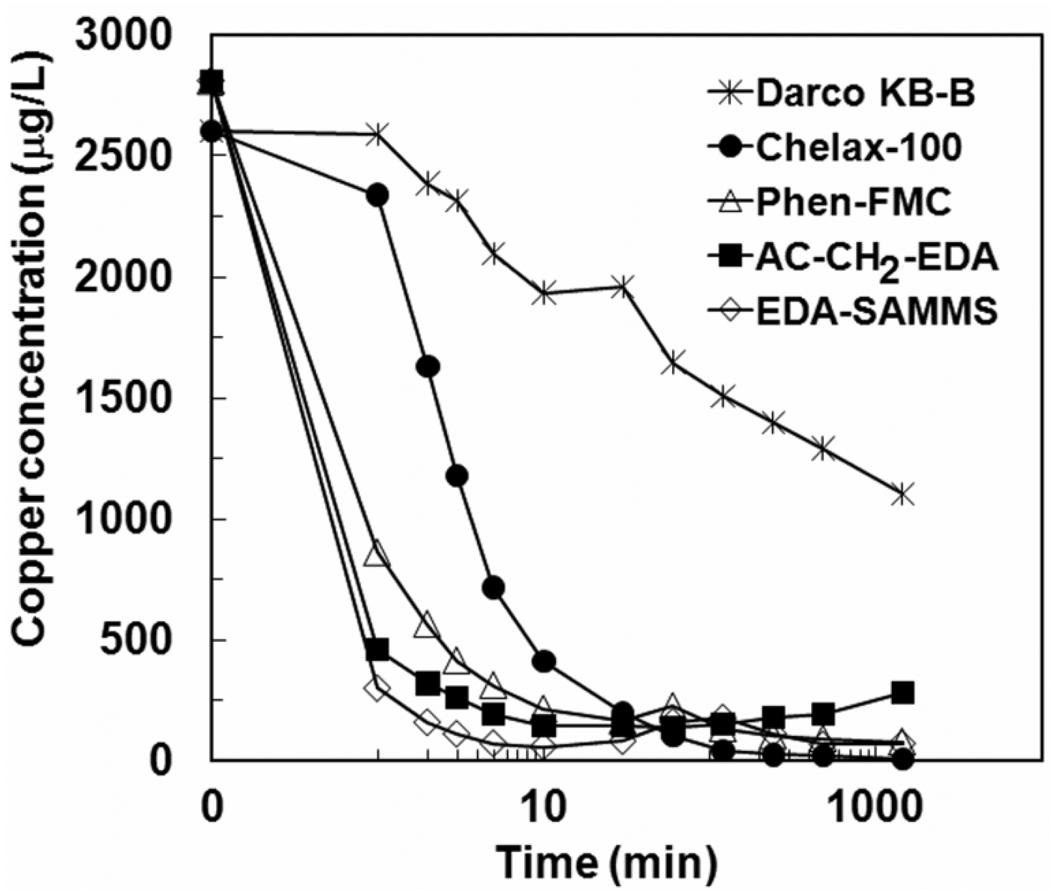
It is important that a sorbent material offers rapid sorption to minimize the time required to remove metal ions, particularly in natural waters where the Cu content is low, which means that a sorbent must overcome higher diffusion limitation for the transport of Cu2+ from the bulk solution to the sorbent interface, compared to wastes with high Cu content. Adsorption kinetics of the three nanoporous sorbents and two commercial sorbents were measured in seawater (pH ~ 7.0) with initial Cu conc ~ 2.8 ppm and L/S of 1000 mL/g. Figure 3 shows the residual Cu2+ concentration in seawater as a function of time that it was in contact with each sorbent (e.g., from 0 to 24 hrs, with several time points within the first 10 min in which equilibrium normally is reached). The adsorption kinetics of divalent metal ions on sorbent materials assuming chemisorption has often been explained by pseudo-second order [31]. Herein, such model could describe the Cu kinetics very well (R2 > 0.999). Table 1 shows the pseudo-second order kinetic rate constants of the sorbents, which are in the decreasing order of EDA-SAMMS > AC-CH2-EDA > Phen-FMC > Chelex-100 > Darco KB-B.
Figure 3.
Adsorption kinetics of Cu2+ on EDA-SAMMS, AC-CH2-EDA, Phen-FMC, Chelex-100 and Darco KB-B in sea water (pH ~7), L/S of 1,000 mL/g.
Table 1.
Pseudo-second order kinetics and Langmuir adsorption parameters of Cu(II) measured in seawater.
| Sorbent | Kinetics(a) | Langmuir isotherm(b) | ||
|---|---|---|---|---|
| k (g/mg/min) | qe (mg Cu/g) | Qmax (mg/g) | KL (L/mg) | |
| EDA-SAMMS | 11.52 | 2.74 | 26.9 | 26.6 |
| AC-CH2-EDA | 2.92 | 2.68 | 17.1 | 7.7 |
| Phen-FMC | 1.03 | 2.67 | 10.3 | 22.7 |
| Chelex-100 | 0.10 | 2.65 | NA | NA |
| Darco KB-B | 0.02 | 1.53 | NA | NA |
(a) by fitting data in Figure 3 to the linearized pseudo second order kinetics model, given by , where qt is the adsorption capacity at a given time, t is the contact time (min), qe is the estimated steady state adsorption capacity (mg/g), and k is the kinetic constant (mg/g/min)
By fitting data in Figure 4 to the linearized Langmuir model given by , where Qe (mg/g) and Ce (mg/L) is the equilibrium concentration of Cu2+ in solution and on the sorbent, respectively, Qmax is the estimated maximum adsorption capacity (mg/g), and KL (L/mg) is the Langmuir constant
The sorption rates of Cu2+ on all three sorbents were very rapid, and reached equilibrium within ~5 minutes. This rapid adsorption rate is directly owed to the rigid, open pore structure of these sorbents and the ready accessibility of the chelating diamines inside the pores walls, making it easy for the Cu2+ to undergo chelation. On the other hand, the adsorption rates of Cu2+ on commercial sorbents were much slower, with Chelex-100 reaching equilibrium after 30 min, and Darco KB-B not reaching equilibrium even after 24 h. To achieve 95% of Cu2+ removal, it took EDA-SAMMS 3 min, AC-CH2-EDA 10 min, and Phen-FMC 30 min, while it took Chelex-100 about 1 h. Darco KB-B was not able to achieve 95% Cu2+ removal even after 24 h. In addition to the suitable structure of the three nanoporous sorbents, their faster kinetics may also be due to the more basic ligands of EDA and Phen over the carboxylate anions of the Chelex-100 and Darco KB-B. Among the ligands of the three nanoporous materials, EDA is more basic than Phen ligand, hence the EDA-SAMMS and AC-CH2-EDA yielded more rapid Cu2+ removal rate than the Phen-FMC. Even though the kinetics were measured in sea water (i.e. a rather challenging matrix due to the high ionic strength), the adsorption rates of all three chelating diamine sorbents were still faster than those reported for other natural and chemically modified sorbents studied in DI water or buffer solutions [18–22]. A noteworthy work is on the removal of Cu using polyethyleneimine nanoclusters immobilized on macroporous cation exchange resin [32], which indicates 2 hours for the sorbent to reach equilibrium Cu sorption even at the starting Cu2+ of 100 mg/L. Hence it is remarkable that the three nanoporous sorbents reached equilibrium within minutes even in seawater and at much lower starting concentration of Cu2+ (~ 2.8 mg/L).
The data in Figure 3 also suggest stability of the materials; one might conclude that during the 24 hr contact with seawater containing Cu, only AC-CH2-EDA, and not EDA-SAMMS and Phen-FMC, appeared to somewhat lose its material stability resulting in slightly decrease of Cu uptake starting after 8 hrs of contact time (which may be due to fouling of the binding sites by seawater constituents). Nevertheless, the excellent stability of the three sorbents is derived from the strong covalent bonding between the organic functional groups and the substrates of the sorbents
Sorption isotherms
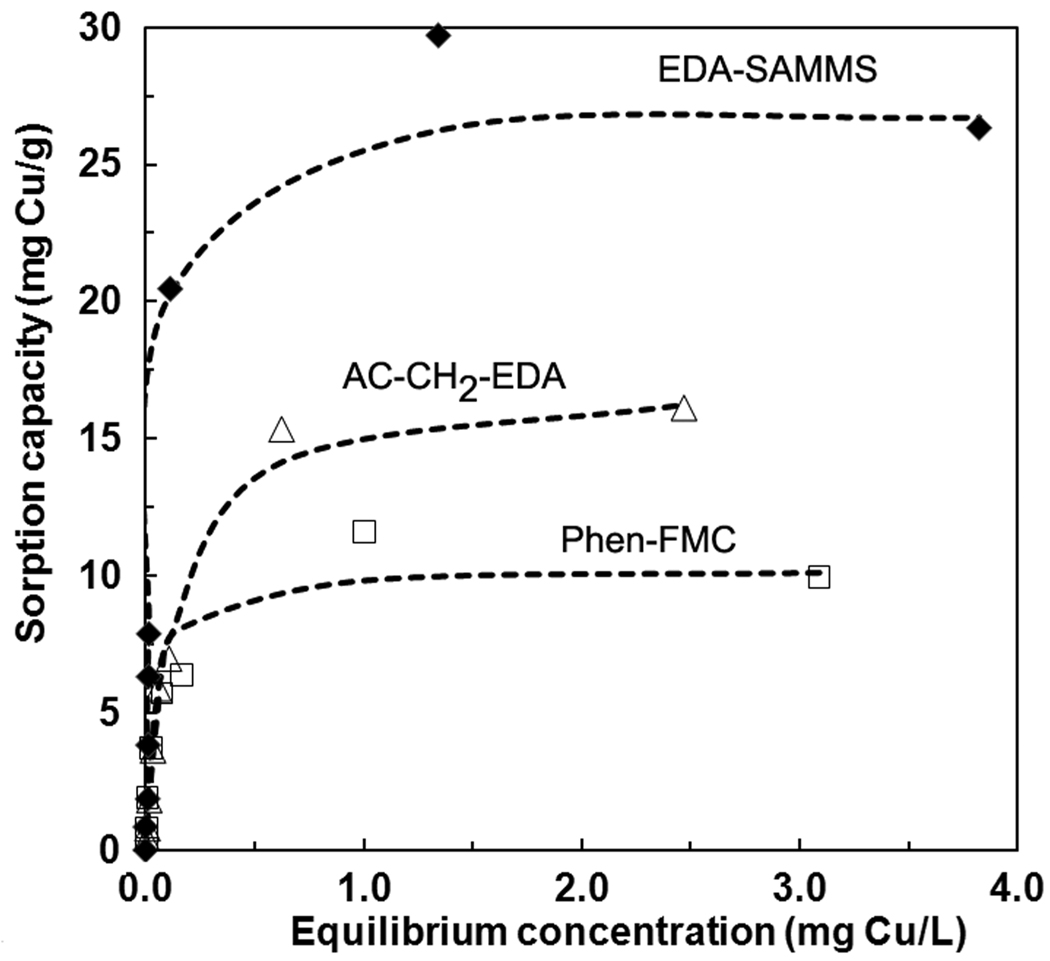
Adsorption isotherms of Cu on three nanoporous sorbents were measured in sea water at the initial Cu conc of 0.05– 4 mg/L and L/S of 10,000 mL/g. Two hrs of contact time was selected for the contact time to ensure that equilibrium was reached (based on the kinetics study). As shown in Figure 4 (data in low conc range can be seen in Figure S1 of Supporting Information), the isotherm data of all sorbents were best described by the Langmuir model (all with R2 > 0.995) compared to the Freundlich model [33] (which does not predict saturation adsorption) and the Redlich-Peterson model [34] (having the exponent of ~ 1, reducing it to Langmuir model [35]). The Langmuir constants are summarized in Table 1. Langmuirian adsorption is indicative of a single monolayer adsorption of Cu that was uniformly distributed across the sorbent surface, not nucleating or precipitating out of solution [35, 36]. The estimated maximum Cu2+ sorption capacities (Qmax) of EDA-SAMMS, AC-CH2-EDA, and Phen-FMC were 26.9, 17.1, and 10.3 mg Cu/g sorbent, respectively (i.e. 0.16–0.42 mmole Cu/g sorbent), while their ligand loading capacities were determined to be 2.6 mmole of EDA/g, 1.5 mmole EDA/g and 2.9 mmole Phen/g, respectively. This indicates that not all of the binding sites are utilized. Since these chelating diamines are all neutral ligands, the binding of metal cations results in charge accumulation at the sorbent interface. Since metal ion binding is an equilibrium process, this charge accumulation is likely to be playing a role in limiting the Cu2+ capacity of these sorbents. Once again, the more basic EDA ligand is seen to out-perform the less basic Phen ligand. What’s more, the ordered array of EDA ligands in the EDA-SAMMS (which can allow for multiple metal-ligand interactions) is seen to provide a higher Cu2+ capacity than the AC-CH2-EDA (where the EDA ligands are more disordered, making single metal-ligand interaction more likely). Since the sea water was used for evaluating the capacities of these three sorbents, it is not surprising that the maximum capacities these sorbents were lower than those measured in buffer solutions [16, 18, 19, 22, 35]. The Cu sorption capacities of the sorbents in less complex matrices like buffer solutions, river water, and ground water are projected to be higher than these values reported for seawater.
Figure 4.
Adsorption isotherm of Cu2+ on EDA-SAMMS, AC-CH2-EDA, and Phen-FMC in sea water (pH ~7.5–8.0), L/S of 10,000 mL/g, symbols represent data, and dash-line represent Langmuir isotherm fitting.
Competing divalent cations
Natural waters and wastewaters may contain a number of other metals that may compete with Cu2+ for the binding sites of sorbent materials. To evaluate the impact of other metals on Cu2+ removal, competition experiments were carried out in sea water with ~100 ppb (each) of Cu2+ and other common transition metals (Fe2+, Zn2+, and Ni2+, all of which may have good affinity for chelating diamine) and ~ 3 ppm of Ca2+ (the latter is inherent in the sea water). The % removal of each metal is summarized in Table 2. Under these conditions, all sorbents could remove > 97% of Cu2+ (with the exception of Darco KB-B, which removed only 80%). However, Phen-FMC had the greatest selectivity toward Cu2+; it removed 99% of Cu2+, with small competition from Ca2+, Fe2+, Ni2+, and Zn2+ (only 0, 2, 14, and 28% removal, respectively). In contrast, EDA-SAMMS and Chelex-100 both showed significant competition from Ni2+and Zn2+ (at 91–97% removal). On AC-CH2-EDA, the competition from Ni2+ and Zn2+ was moderate (~ 40% removal) and that from Fe and Cu was negligible
Table 2.
The selectivity of sorbents toward the Cu2+ in sea water (pH ~7.5); initial metal concentration of 100 ppb each and L/S ratio of 5 g/L.
| Metal ions |
% Removal | ||||
|---|---|---|---|---|---|
| EDA-SAMMS | AC-CH2-EDA | Phen-FMC | Chelex-100 | Darco KB-B | |
| Ca2+ | 1 | 0 | 0 | 7 | 0 |
| Fe2+/Fe3+ | 5 | 0 | 2 | 0 | 0 |
| Ni2+ | 91 | 41 | 14 | 91 | 0 |
| Zn2+ | 97 | 37 | 28 | 96 | 7 |
| Cu2+ | 97 | 97 | 99 | 97 | 80 |
In river and sea waters containing ~1 ppm of Cu2+ (an allowable discharge limit of Cu in waste effluents), at 1 g per liter, all three chelating diamine sorbents were able to remove from 97–100% of Cu2+ (see Table 3). The most notable one is Phen-FMC, which could reduce Cu content down to 0.1 ppb in river water and 4.6 ppb in seawater, meeting the EPA’s recommendation for preventing chronic and acute toxic effects of Cu on aquatic species in both fresh water (9 ppb for chronic, 13 ppb for acute) and salt waters (3.1 ppb for chronic and 4.8 ppb for acute) [11, 12]. Due to the fact that the materials are built based on a strong covalent bonding of organic functional groups and biologically benign silica or carbon substrates, they can potentially be used for purifying river and ground waters into drinking water.
Table 3.
The Cu2+ removal from natural waters, initial Cu2+ concentration of ~ 0.8 ppm and L/S ratio of 1 g/L.
| Sorbents | River water (pH ~ 7.9) | Seawater (pH ~ 7.9) | ||||
|---|---|---|---|---|---|---|
| Initial Cu (ppm) |
Final Cu (ppb) |
% Removal |
Initial Cu (ppm) |
Final Cu (ppb) |
% Removal |
|
| EDA-SAMMS | 0.75 | 6.3 | 99 | 0.79 | 15.3 | 98 |
| AC-CH2-EDA | 0.75 | 10.7 | 99 | 0.79 | 25.1 | 97 |
| Phen-FMC | 0.75 | 0.1 | 100 | 0.79 | 4.6 | 99 |
Nanoporous sorbents built around a chelating diamine (e.g. EDA-SAMMS, AC-CH2-EDA, and Phen-FMC) are highly efficient for Cu2+ removal from natural waters. The metal capture ability of a sorbent depends on both functionality (chemistry and loading of the organic groups) and substrates. Overall, their Cu2+ binding affinity was found to be Phen-FMC ≈ EDA-SAMMS > AC-CH2-EDA > Chelex 100 >> Darco KB-B, while the selectivity toward Cu2+ was found to be Phen-FMC > AC-CH2-EDA > EDA-SAMMS ≈ Chelex-100. Therefore, the three nanoporous sorbents can be chosen to best suit Cu capture needs; e.g., EDA-SAMMS is best if large capacity is needed; Phen-FMC is best if selective capture of Cu among other transition cations or if reducing low ppm level of Cu to a level meeting EPA standard is needed; Phen-FMC and AC-CH2-EDA are best if large working pH window is needed (from pH 1.0 and above).
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences (NIEHS), grant# R21 ES015620, and PNNL’s Laboratory Directed Research and Development. A portion of research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at PNNL. PNNL is operated by Battelle for the U.S. Department of Energy under contract DE-AC06-67RLO 1830.
Footnotes
Supporting Information Available
Detailed experimental approaches for material synthesis and batch contact studies (e.g., adsorption kinetics, isotherms and competition); equations for distribution coefficient and percent metal removal; and adsorption isotherm data for low Cu concentration range of Figure 4.
Synopsis
Three classes of nanoporous sorbents designed around chelating diamine and two commercial sorbents are evaluated for copper removal in natural waters.
Contributor Information
Wilaiwan Chouyyok, Pacific Northwest National Laboratory Richland, WA 99352.
Yongsoon Shin, Pacific Northwest National Laboratory Richland, WA 99352.
Joseph Davidson, Pacific Northwest National Laboratory Richland, WA 99352.
William D. Samuels, Pacific Northwest National Laboratory Richland, WA 99352
Nikki H. LaFemina, Pacific Northwest National Laboratory Richland, WA 99352
Ryan D. Rutledge, Pacific Northwest National Laboratory Richland, WA 99352
Glen E. Fryxell, Pacific Northwest National Laboratory Richland, WA 99352.
Thanapon Sangvanich, Biomedical Engineering, Oregon Health & Science University (OHSU), Portland, OR 97239.
Wassana Yantasee, Biomedical Engineering, Oregon Health & Science University (OHSU), Portland, OR 97239.
Cited Literature
- 1.Aaseth J, Norseth T. Handbook on the Toxicity of Metals. Amsterdam: Elsevier; 1986. [Google Scholar]
- 2.Tisato F, Marzano C, Porchia M, Pellei M, Santini C. Copper in diseases and treatments, and copper-based anticancer strategies. Med Res Rev. 2010;30(4):708–749. doi: 10.1002/med.20174. [DOI] [PubMed] [Google Scholar]
- 3.Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. Metal Binding and Oxidation of Amyloid-β within Isolated Senile Plaque Cores: Raman Microscopic Evidence. Biochemistry. 2003;42(10):2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 4.Finefrock AE, Bush AI, Doraiswamy PM. Current Status of Metals as Therapeutic Targets in Alzheimer's Disease. J Am Geriatr Soc. 2003;51(8):1143–1148. doi: 10.1046/j.1532-5415.2003.51368.x. [DOI] [PubMed] [Google Scholar]
- 5.Hindo SS, Mancino AM, Braymer JJ, Liu Y, Vivekanandan S, Ramamoorthy A, Lim MH. Small Molecule Modulators of Copper-Induced Aβ Aggregation. J Am Chem Soc. 2009;131(46):16663–16665. doi: 10.1021/ja907045h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn JF, Crane S, Harris C, Wadsworth TL. Copper in Alzheimer's disease: too much or too little? Expert Rev of Neurother. 2009;9(5):631–637. doi: 10.1586/ern.09.27. [DOI] [PubMed] [Google Scholar]
- 7.Storr T, Merkel M, Song-Zhao GX, Scott LE, Green DE, Bowen ML, Thompson KH, Patrick BO, Schugar HJ, Orvig C. Synthesis, Characterization, and Metal Coordinating Ability of Multifunctional Carbohydrate-Containing Compounds for Alzheimer's Therapy. J Am Chem Soc. 2007;129(23):7453–7463. doi: 10.1021/ja068965r. [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Garrett MR, Men P, Zhu X, Perry G, Smith MA. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochim Biophys Acta. 2005;1741(3):246–252. doi: 10.1016/j.bbadis.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick JL, Nadella S, Bucking C, Balshine S, Wood CM. The relative sensitivity of sperm, eggs and embryos to copper in the blue mussel (Mytilus trossulus) Comp Biochem Physiol C Toxicol Pharmacol. 2008;147(4):441–449. doi: 10.1016/j.cbpc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Washington, DC: Office of Water, Office of Science and Technology, U.S. EPA; 2003 draft update of ambient water quality criteria for copper. 2003 November; EPA 822-R-03-26.
- 11.Office of wastewater management 4203, U.S.EPA; Local limits developments guidance. 2004 July; EPA 833-R-04-002A.
- 12.Office of wastewater management 4203, U.S.EPA; Local limits developments guidance appendices. 2004 July; EPA 833-R-04-002B.
- 13.Washington, DC: Office of Environmental Information (2810A), U.S. EPA; 2000 Toxics Release Inventory (TRI) Public Data Release Report. 2002 May; EPA-260-R-02-003 20460.
- 14.Aguado J, Arsuaga JM, Arencibia A, Lindo M, Gascón V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater. 2009;163(1):213–221. doi: 10.1016/j.jhazmat.2008.06.080. [DOI] [PubMed] [Google Scholar]
- 15.Deng S, Bai, Chen JP. Aminated Polyacrylonitrile Fibers for Lead and Copper Removal. Langmuir. 2003;19(12):5058–5064. [Google Scholar]
- 16.Liu C, Bai R, Hong L. Diethylenetriamine-grafted poly(glycidyl methacrylate) adsorbent for effective copper ion adsorption. J Colloid Interface Sci. 2006;303(1):99–108. doi: 10.1016/j.jcis.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 17.Wong KS, Wong KH, Ng S, Chung WK, Wong PK. Adsorption of copper ion on magnetite-immobilised chitin. Water Sci Technol. 2007;65(7):135–143. doi: 10.2166/wst.2007.672. [DOI] [PubMed] [Google Scholar]
- 18.Ely A, Baudu M, Basly J-P, Kankou MOSAO. Copper and nitrophenol pollutants removal by Na-montmorillonite/alginate microcapsules. J Hazard Mater. 2009;171(1–3):405–409. doi: 10.1016/j.jhazmat.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Zheng Y, Wang A. Fast removal of copper ions from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite composites. J Hazard Mater. 2009;168(2–3):970–977. doi: 10.1016/j.jhazmat.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 20.Qian Q, Mochidzuki K, Fujii T, Sakoda A. Removal of copper from aqueous solution using iron-containing adsorbents derived from methane fermentation sludge. J Hazard Mater. 2009;172(2–3):1137–1144. doi: 10.1016/j.jhazmat.2009.07.107. [DOI] [PubMed] [Google Scholar]
- 21.Özçimen D, Ersoy-Meriçboyu A. Removal of copper from aqueous solutions by adsorption onto chestnut shell and grapeseed activated carbons. J Hazard Mater. 2009;168(2–3):1118–1125. doi: 10.1016/j.jhazmat.2009.02.148. [DOI] [PubMed] [Google Scholar]
- 22.Bouzid J, Elouear Z, Ksibi M, Feki M, Montiel A. A study on removal characteristics of copper from aqueous solution by sewage sludge and pomace ashes. J Hazard Mater. 2008;152(2):838–845. doi: 10.1016/j.jhazmat.2007.07.092. [DOI] [PubMed] [Google Scholar]
- 23.Fryxell GE, Liu J, Hauser TA, Nie Z, Ferris KF, Mattigod S, Gong M, Hallen RT. Design and Synthesis of Selective Mesoporous Anion Traps. Chem Mater. 1999;11(8):2148–2154. [Google Scholar]
- 24.Samuels WD, LaFemina NH, Sukwarotwat V, Yantasee W, Li XS, Fryxell GE. Chloromethylated Activated Carbon: A Useful New Synthon for Making a Novel Class of Sorbents for Heavy Metal Separations. Sep Sci Technol. 2010;45(2):228–235. doi: 10.1080/01496390903423550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin Y, Fryxell GE, Engelhard MH, Exarhos GJ. Functional mesoporous carbon built from the 1,10-phenanthroline building block: A new class of catalyst support. Inorg Chem Commun. 2007;10(12):1541–1544. [Google Scholar]
- 26.Chouyyok W, Yantasee W, Shin Y, Grudzien RM, Fryxell GE. Transition metal ion capture using functional mesoporous carbon made with 1,10-phenanthroline. Inorg Chem Commun. 2009;12(11):1099–1103. doi: 10.1016/j.inoche.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotton FA, Wilkinson G, Murillo C, Bochmann M. Advanced Inorganic Chemistry. 6th ed. New York: Wiley Interscience; 1999. [Google Scholar]
- 28.Yantasee W, Fryxell GE, Addleman RS, Wiacek RJ, Koonsiripaiboon V, Pattamakomsan K, Sukwarotwat V, Xu J, Raymond KN. Selective removal of lanthanides from natural waters, acidic streams and dialysate. J Hazard Mater. 2009;168(2–3):1233–1238. doi: 10.1016/j.jhazmat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Sun C, Xu J, Li Y. The use of raw and acid-pretreated bivalve mollusk shells to remove metals from aqueous solutions. J Hazard Mater. 2009;168(1):156–162. doi: 10.1016/j.jhazmat.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Aydin H, Bulut Y, Yerlikaya ç. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manage. 2008;87(1):37–45. doi: 10.1016/j.jenvman.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Ho YS, McKay G. The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Research. 2000;34(3):735–742. [Google Scholar]
- 32.Chen Y, Pan B, Li H, Zhang W, Lv L, Wu J. Selective Removal of Cu(II) Ions by Using Cation-exchange Resin-Supported Polyethyleneimine (PEI) Nanoclusters. Environ Sci Technol. 2010;44:3508–3513. doi: 10.1021/es100341x. [DOI] [PubMed] [Google Scholar]
- 33.Freundlich H. Adsorption in solution. Z. Phys. Chem. 1906;57:385. [Google Scholar]
- 34.Redlich O, Peterson DL. A useful adsorption isotherm. J. Phys. Chem. 1959;63:1024. [Google Scholar]
- 35.Yantasee W, Lin Y, Fryxell GE, Alford KL, Busche BJ, Johnson CD. Selective Removal of Copper(II) from Aqueous Solutions Using Fine-Grained Activated Carbon Functionalized with Amine. Ind Eng Chem Res. 2004;43(11):2759–2764. [Google Scholar]
- 36.Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Timchalk C, Warner MG. Removal of Heavy Metals from Aqueous Systems with Thiol Functionalized Superparamagnetic Nanoparticles. Environ Sci Technol. 2007;41(14):5114–5119. doi: 10.1021/es0705238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.