Abstract
Recent advances in functional Magnetic Resonance Imaging (fMRI) have allowed for the possibility to help with dose ranging as well as potential drug efficacy and side-effect profiles. The problem that is being addressed in this article relates to the absence of any current guidelines or standards for fMRI that meet established standards of care. Given the potential use of fMRI in drug development, it would seem essential that guidelines are adopted to be used where patients are exposed to novel drugs in particular, but to any drug where immediate access to the patient is limited. When used in phase I trials or very soon after initial Phase I qualification, MRI mandates certain safety standards as subjects are laying positioned in the bore of a magnet with limited opportunity for direct observation or communication, or immediate access in an emergency; these limits can be more pronounced during fMRI acquisition. In developing novel approaches to testing new drugs using fMRI, we suggest that safety guidelines merit discussion and definition. This process could lead to the adoption of standards in the testing of novel agents. Some of these issues are well understood and can be adopted from experiences using sedation in the scanner, while others are unique to the application of the technology in early phase trials.
A. Background
A1. Human Subjects in Drug Evaluation: New Strategies
“Due to the continuous increase in time and cost of drug development and the considerable amount of resources required by the traditional approach, companies can no longer afford to continue to late phase 3 with drugs which are unlikely to be therapeutically effective.” 1. The failure in translation from preclinical to clinical success in drug development has led to a more aggressive approach in the use of human volunteers and patients in early stage of clinical evaluation of drugs, with a view to reducing subsequent compound attrition rates. Use of patients or surrogate models early in clinical phase central nervous system (CNS) drug development may offer more information than animal models on the likely benefits of a drug. Such an approach should also speed up the process if information such as targeted brain regions, drug dosing, CNS measures of side effects, etc., can be determined based on functional circuitry. Such studies yield additional information relevant to early go-no-go decisions. Indeed, the ability to ‘kill’ a drug candidate based on objective information in humans is invaluable. In addition, the use of objective endpoints may also include the integration of CNS based circuitry with pharmacokinetic and pharmacodynamic measures 2. This approach may also be useful in the reanalysis of approved drugs to identify and establish biomarkers. Such approaches can be used in pre- and post-therapy evaluation in the clinical setting.
“Taking responsibility for the link between research and development gives clinical pharmacology a major opportunity to assume a pivotal role in research and development of new drugs.” 1. Such responsibility, particularly when using new technologies such as functional imaging, obviously includes standards for safety, particularly in early phase clinical trials when the safety and tolerability profile of a new agent is less well-established. Currently the optimal use of fMRI in clinical CNS drug development in early phase trials may depend on the specific drug and the nature of the behavioral measures possible in the magnet if these are used.
A2. Deployment of New Technologies in Early Clinical Drug Development
In CNS drug development, the use of in vivo imaging has revolutionized the ability to visualize target engagement of novel drugs as a function of brain region through receptor binding using positron emission tomography (PET) 3 and, more recently, functional effects of drug action using functional magnetic resonance imaging (fMRI) 4, 2. As such, functional maps of a drug’s effect on the brain offer new approaches to develop paradigms of objective indices for ‘go-no go’ decisions based on a drug’s desired actions and/or undesired side effects. This may help in a more rapid development of drugs, and potentially limit the number of subjects required for early clinical assessment of drugs 4, 5. The potential of adopting new technologies, such as fMRI, for drug development is to improve the evaluation of agents in human subjects (volunteers, surrogate models or patients), providing insights into potential efficacy, CNS dosing, and side effects in early phase clinical trials – before, however, substantial ‘drug experience’ in large numbers of patients has accrued.
The potential use of functional imaging in drug development is its ability to evaluate the direct effects of a drug on specific functional CNS circuits that underlie the ‘behavioral’ effects (e.g., analgesia, anxiolytic, sedative etc.) of the pharmacological entity. These effects may be for primary CNS acting drugs, as well as for CNS side effects of a drug being developed for a different system. FMRI measures changes in the hemodynamic response in the brain and provides an indirect measure of neural activity 6, 7, 8. A number of recent reviews on functional imaging and drug development provide further details of the technology 9, 10. While there is great interest in the use of fMRI in drug development issues of sensitivity, specificity and reproducibility still require further investigation.
The pharmaceutical industry has invested significant resources in imaging for drug development. The types of trials involving imaging include functional magnetic resonance imaging (fMRI), magnetic resonance spectroscopy (MRS) and anatomical imaging (AI). Both fMRI and MRS can evaluate the ‘de-novo’ immediate and early effects of a drug following acute administration, as well as the effects of chronic administration (days to weeks).
fMRI offers the ability to detect significant changes in CNS circuits in response to the direct effects of drugs in a relatively small number of subjects 11. This, coupled with the accelerated approval of therapeutically novel pharmacotherapies by the FDA, presents additional risks to volunteers participating in research/clinical studies in the development of these agents 12. Consequently, those utilizing imaging as a technique in the evaluation of new drugs must have effective standards to address any side effects during imaging trials; particularly when the subject is ‘in the scanner’. In the following we describe approaches that will increase safety of our patients or healthy volunteers.
A3. Early Phase Clinical Trials
Early phase clinical trials evaluate safety (i.e., how well a new drug is tolerated), pharmacokinetics and initial side effect profile. Administration of drugs in Phase I have specific risks associated with the process and these are usually conducted in facilities with specialized services (e.g., emergency medical services including critical care). As noted in a recent review, Phase I trials (or early Phase trials) may be considered as opportunities for more than safety evaluation of a drug 13. The Food and Drug Administration published (Federal Register on December 17, 1997) new guidelines on the design and terminology used in clinical trials, including early phase clinical trials (http://www.fda.gov/cder/guidance/1857fnl.pdf). In this early-phase context, imaging methods can serve as informative biomarkers, providing an early signal of target engagement or central functional activity (including functional connectivity) potentially informative on likely efficacious dose. Moreover, imaging studies may require fewer subjects to obtain a clear signal of effect from novel drugs. Thus, the use of fMRI in early phase clinical trials would at this time seem to be most obvious in the transition between phase I (first in man) and phase II trials.
B. Clinical Issues
B1. Healthy vs. Patient Trials: Differences and Difficulties
A clinical drug trial is a carefully controlled study in which healthy volunteers or patients take a drug to determine whether it is safe and effective, as well as and the optimal dosage. While the criteria for enrollment differ, healthy subjects or patients entering into a trial that uses imaging concomitant with drug administration are subjected to different issues that may impact patient safety. This depends on the phase of the clinical trial: phase I trials aim to establish safety and tolerability margins in single dose and sub-chronic dosing while phase II trials usually seek to provide some proof of concept or efficacy signal. Biomarker studies concomitant with, or closely following the phase I trials seek primarily to assess target engagement, brain penetration or functional effect to (a) provide confidence that the drug is inducing an expected pharmacodynamic signal and (b) help define dose selection for efficacy (phase II) trials. If new drugs are evaluated in phase I studies of patients (as opposed to healthy volunteers), patients volunteering for a study will most likely be on or have been taking medications that can potentially interact with the drug being tested, or they may stop taking their medication possibly resulting in changes in their condition.
B2. Need for Standards for Human Protection
In addition to the normal issues involved in an informed consent for phase I trials, particularly in patients, additional factors relate to early trails in terms of informed consent. A particular concern relates to the possible expectations of benefit that the subjects may have when enrolling for such studies 14.
Institutional Review Boards (IRB) vary but have specific federally mandated guidelines to follow 15 (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm). It is only relatively recently that IRB’s have addressed requests to perform evaluation of drugs, including anesthetics, in the scanner environment. As such informed consents must be able to appropriately apprise participants of possible dangers to the novel treatment, as well as the nature of the study in that the study takes place in a restricted and relatively closed environment. While the nature of the environment in the scanner is clearly obvious, in reality there is relatively little data on the safety of such trials. As such it may be appropriate to consider an FDA type program for Imaging Drug Trials to document the cumulative experience as interest/utilization increases. If imaging is going to be used in early clinical trials, then the level of uncertainty relating to the use of imaging for novel drug evaluation needs to be defined and provided to volunteers participating in the study.
B3. Patient Monitoring during Scanning
Based on the above, it is apparent that protocols for safety monitoring in the scanner environment need to be clearly defined and made operational. The latter is probably the most applicable in terms of the composition of teams that work together performing such studies. Medical coverage consistent with the nature of the study should be supported. For any novel (i.e., first in man) drug we would recommend a minimum of one physician present with access to supporting medical personnel. In most cases an emergency would probably relate to an allergic reaction that may require interventions by a medical team. Thus, based on medical assessment, the number of individuals in the medical team could vary. For high-risk drugs, the inclusion of a critical care physician, appropriate emergency equipment and high-level patient support equipment immediately outside the magnet, should be considered in any protocol involving drugs with potential cardiopulmonary or serious CNS side effects.
B4. General MRI Safety
A recent review on standards for medical devices in MRI summarizes the issues around use of these devices as defined by two working groups that focus on MR equipment as well as active implants with leads such as stimulators 16. Known safety issues now have standard protection processes in place in MR imaging 17. Issues include: (i) biological effects of magnetic fields (i.e., potential deleterious effects to a fetus), (ii) risk of metal objects brought into the field (i.e., projectiles); (iii) auditory effects (i.e., noise of the magnet); and (iv) psychological effects (i.e., anxiety). These and other guidelines are provided at the following websites: http://www.fda.gov/cdrh/ode/primerf6.html and http://www.ansi.org/. The addition of pharmacological interventions adds a level of complexity to safety in the magnet and could limit the type of behavioral paradigms. However, most paradigms (sensory, visual, cognitive etc) currently used are of relatively short duration (<10 min of scanning time).
B5. Standard Monitoring in the Scanner
In most modern MR systems, monitoring equipment for end tidal carbon dioxide (ETCO2), electrocardiogram (EKG), pulse oximetry, respiratory rate (RR) and blood pressure (BP) are easy to use and can be viewed via a monitor outside the magnet. All data are collected on-line for subsequent evaluation of physiological changes occurring as a result of the drug. A number of systems offer methods for monitoring patients. However not all measures (e.g., EKG) are detected with the usual standard outside the magnet or when the magnet is not operating. Other options are also available including MRI video that allows observers in the control room to view the subjects directly. Maintaining verbal contact with the subject during breaks in scanning acquisitions is essential to being able to help define the clinical status of the subject. Basic questions relating to “How are you doing?” should be routine (part of a defined protocol) at every break in scanning.
C. Proposed Guidelines for Patient Safety in MRI Evaluation of Novel Drugs
In any study involving human subject aside from specific regulatory processes, good clinical practice (GCP) should apply. Such guidelines have been defined by the FDA “Good clinical practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording, and reporting trials that involve the participation of human subjects. Compliance with this standard provides public assurance that the rights, safety, and well-being of trial subjects are protected, consistent with the principles that have their origin in the Declaration of Helsinki, and that the clinical trial data are credible.” (http://www.fda.gov/cder/guidance/959fnl.pdf). International Conference on harmonization for Good Clinical Practice (ICH-GCP) Guidelines (http://www.ich.org/cache/compo/276-254-1.html) make recommendations to achieve unified standards for the conduction of investigations in human subjects for the purpose of registration. The idea is to limit the need for duplication of tests involved in development of new drugs. However, in the case of the use of functional imaging in drug development, this is a new domain that has not yet achieved consensus in terms of applications in drug development. Nevertheless, studies are being conducted with greater frequency without pragmatic standardization of safety guidelines. As an initial step towards the establishment of specific guidelines/GCP related to the use of fMRI, we would like to offer the following suggestions for standard operating procedures in the following domains (1) Medical, (2) Teamwork and training, (3) Equipment and (4) Error Reporting and Quality Improvement.
C1. Medical Issues
Physician Training
There are a number of standards that should be applied depending on the nature of the drug infusion. When working with novel agents it is recommended that two Acute Life Support (ACLS) certified physicians should be present in or near the scanner. At least one physician should be proficient in the use of airway management and preferably trained in critical care (for major side effects such as anaphylactic shock).
Co-ordination with Pharmacy
Working with a research pharmacy has a number of advantages including special packaging of the drug (e.g., over-encapsulating) and protocol process (e.g., blinding). In the event of an adverse event it is critical that access to un-blinding the drug can be done immediately. Thus a research pharmacy with 24-hour coverage is essential.
Emergency Plans, Oversight and Practice Sessions
From a safety point of view the importance of this issue cannot be overstated and details addressing plans, checklists, rehearsals and physician oversight are detailed below.
Emergency Drugs for Common or Expected Side Effects
A code cart with most of the emergency medications should be available and have certification of routinely being checked for content and drug expiration dates. A summary of the medications, dosage, and use is noted in Table 1.
Anesthesia Machine
The American Society for Anesthesia (ASA) has established specific guidelines for anesthesia outside of operating room (http://www.asahq.org/publicationsAndServices/standards). Ideally, an MRI compatible anesthesia machine should be immediately available. Although not necessary, there is an added safety factor in having an MRI compatible machine as it can be brought directly into the magnet room in urgent/acute situations.
Location of Magnet/Study
This is a serious consideration if magnets are located off-site (i.e., not on the clinical premises) or at institutions that do not have specialized emergency care facilities (e.g., code teams). In such cases it is essential to ensure that the appropriate equipment (including an anesthesia machine or ventilator) is present at the imaging site. In addition a process to call emergency services (e.g., 911 for Emergency Medical Service (EMS)) are in place.
Institutional Review Board Approval
All human studies require IRB approval. Given that the risks for new drugs are greater than drugs where a greater clinical experience has been accrued, it is important to define clear processes that relate to subject protection. For example, it is essential that the principal investigator (PI) or designated physician on the study discusses the protocol with the subject and that appropriate consent process is implemented.
Response to a Medical Emergency in the Scanner (Figure 2)
Figure 2.
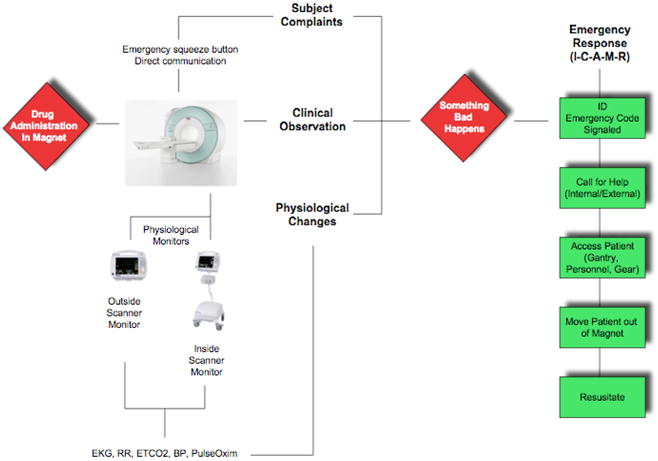
Emergency Deployment
Convention requires that any clinical emergency arising in the scanner room may necessitate removing the patient from the magnet and out of the magnet room prior to initiating any required resuscitation or care. Typical issues arising can involve respiratory or cardiac compromise and may often be as a result of an allergic reaction to the medication. Anaphylactic consequences may come on within minutes of exposure to the drug. Critical to any emergency situation, is reliance upon a standardized approach to medical emergencies (viz., Advanced Cardiovascular Life Support – ACLS). In preparing the scanning-team it is imperative that procedures and roles are understood by all (see teamwork above) and regular rehearsals are conducted in and around the scanning facility. Figure 2 demonstrates a standardized approach to managing resuscitation. It is also vital that appropriate emergency equipment and medications (to include oxygen), usually mandated by hospital policies be verified as immediately available prior to initiating any scan. Thus appropriate ‘good clinical practice’ (GCP) that applies to MRI operating and safety procedures should be implemented (see above).
As noted in Figure 2 an adverse clinical event is noted. This can be demonstrated as an unexpected change in vital signs, or initiated by a subject’s complaint. Priorities include patient rescue, call for assistance and initiation of resuscitation. It is imperative that while ascertaining the nature of the issue, the subject is extracted from the magnet room, placed on an appropriate (magnet compatible) stretcher, and brought to a pre-designated area where further emergency treatment can be provided.
Adverse Drug Event (ADE) documentation
For new drugs, the FDA has specific methods for documentation of ADE’s. This issue is discussed in detail in FDA issued documents (see http://www.fda.gov/oc/ohrt/irbs/). Events are categorized as major or minor, with specific reporting and review criteria for each.
C2. Teamwork
Medical Team Management (MTM) training formally recognizes poor communications skills and ineffective teamwork as the primary source of many adverse medical outcomes. Teamwork is a relatively new concept in medical history 18 and has been applied in operating rooms and critical care settings in a similar manner to non-medical processes. The importance is to limit preventable errors. It is critical that the team involved in these procedures understand the technology employed, establish and rehearse functional interpersonal processes, utilize proper communication procedures and practiced emergency response (e.g., appropriate removal of subject form magnet etc.) commonly encountered in acute clinical scenarios. Thus, the importance of standard operating procedures (SOP’s) that are well-defined, understood and implemented are critical to patient safety.
Setting up the Imaging Team
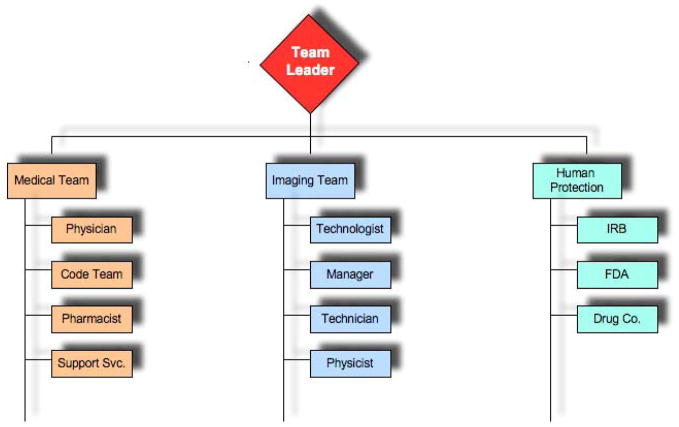
An appropriate team needs to be in place for MRI studies. Such a team should encompass the necessary skill sets (e.g., MRI technologist; Research Assistants, Physicists etc) that have a working knowledge of the protocol and the magnet environment. Teams that work together on a regular basis are clearly preferable in this type of study. Leadership can optimize the performance of team members by ensuring good communications, risk management, delegation of assignments and fostering working relationships. The essential feature is to optimize high performance management of the team in a manner similar to that implemented in more traditional medical settings such as operating rooms and/or trauma teams 19. See Figure 1.
Figure 1.
Team Responsibilities
Team Training
It is essential to conduct training sessions prior to placing any subject into the magnet for drug evaluation. Such training sessions should include (1) standard operating procedures for the protocol that include checklists; (2) individual assignment of responsibilities; (3) rehearsal of emergency procedures for extracting subjects from the magnet, and movement outside the magnet room to a room with appropriate monitoring and emergency equipment; (4) appropriate knowledge of location and use of emergency carts support equipment.
Communications
In the setting of drug MR studies, some of the team will usually be inside the magnet room and communications may rely either on non-verbal means or the use of a writing board. Specific signals for starting, stopping and emergencies should be defined and understood by all team members. In these studies there needs to be someone who is responsible for all aspects of the study, most notably for subject safety and protection.
Checklists
Checklists allow for a number of important processes to take place including (1) standardization of procedure; (2) optimization of safety (e.g., checking the infusion system; drug volume or dose; (3) helping to define roles; (4) defining appropriate sign off for specific processes (e.g., drug administration etc); and (5) providing a detailed log of events for each session. A number of studies have indicated improved clinical safety and quality improvement with appropriate checklists 20.
Test Protocols
Given that imaging procedures are demanding, even in the absence of drug administration, it is imperative to have a program of ‘sham runs’ of all aspects of a protocol prior to implementation in subjects; even to consider one or two ‘placebo’ tests. This allows individuals to implement their specific roles for the team, to function appropriately, and to define areas that may need improvement.
C3. Monitoring, Infusion Equipment
Standard oversight of equipment service and use is normally carried out by the imaging center under the guidance of the Biomedical Engineering group. Thus, monitoring equipment or infusion equipment should be regularly checked and serviced for optimal operation. Standard operating procedures (SOP’s) should be implemented that include such things as a maintenance schedule to assure proper function of the equipment.
C4. Errors and Safety Reports
The importance of defining and reporting errors via the appropriate channels (e.g., the IRB or FDA or QA) is essential. Analysis of medical errors in this new environment must be collated at a central repository that should provide, upon analysis, an opportunity to enhance the practice of drug evaluation employing this technology. Such systems have been deployed for medication errors 21, 22, 23. Thus, critical event monitoring and reporting to the appropriate agencies is very important for improving the program of MR in drug development.
Conclusions
As new technologies offer novel and improved approaches to understanding drug affects on CNS systems, developing appropriate safety procedures to ensure subject care in the event of an emergency remains a challenge. In the case of fMRI, the magnet environment offers unique challenges, and systems (e.g., GCP, SOP’s) should be in place so as not to compromise appropriate and proper safety oversight and emergency procedures. As this new approach is adopted into clinical trials there is no doubt that this oversight will need to improve to an agreed upon set of standards. We hope that this ‘white paper’ puts forward an initial approach that can be built upon by further data experience and additional specialist input. While the Federal Drug Administration (FDA) may be interested in the evaluation of smarter approaches to drug development, using smaller populations and therefore diminishing the overall risk, the need to apply and adapt human protection standards to meet the challenges of this new technology is obvious. This will become even more important if these approaches are properly validated and perhaps adopted by regulatory agencies. This paper hopes to encourage consensus statements on the issue that should help with IRB standards for safety in testing novel drugs using fMRI.
Acknowledgments
Supported by the L Herlands fund for pain research (DB and LB) and K24 NINDS NS064050 (DB)
Footnotes
Conflict of Interest: None of the authors have any conflicts to declare.
References
- 1.Kuhlmann J. Alternative strategies in drug development: clinical pharmacological aspects. Int J Clin Pharmacol Ther. 1999;37:575–83. [PubMed] [Google Scholar]
- 2.Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage. 2002;16:999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- 3.Lee CM, Farde L. Using positron emission tomography to facilitate CNS drug development. Trends Pharmacol Sci. 2006;27:310–6. doi: 10.1016/j.tips.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5:411–24. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- 5.Borsook D, Bleakman D, Hargreaves R, Upadhyay J, Schmidt KF, Becerra L. A ‘BOLD’ experiment in defining the utility of fMRI in drug development. Neuroimage. 2008;42:461–6. doi: 10.1016/j.neuroimage.2008.04.268. [DOI] [PubMed] [Google Scholar]
- 6.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–5. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 7.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–69. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 8.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 9.Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging. 2007;25:978–88. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–64. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 11.Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118:115–28. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- 12.Olson MK. Are novel drugs more risky for patients than less novel drugs? J Health Econ. 2004;23:1135–58. doi: 10.1016/j.jhealeco.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Khandekar J, Khandekar M. Phase 1 clinical trials: not just for safety anymore? Arch Intern Med. 2006;166:1440–1. doi: 10.1001/archinte.166.14.1440. [DOI] [PubMed] [Google Scholar]
- 14.Weinfurt KP, Seils DM, Tzeng JP, Compton KL, Sulmasy DP, Astrow AB, Solarino NA, Schulman KA, Meropol NJ. Expectations of benefit in early-phase clinical trials: implications for assessing the adequacy of informed consent. Med Decis Making. 2008;28:575–81. doi: 10.1177/0272989X08315242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendler D, Belsky L, Thompson KM, Emanuel EJ. Quantifying the federal minimal risk standard: implications for pediatric research without a prospect of direct benefit. Jama. 2005;294:826–32. doi: 10.1001/jama.294.7.826. [DOI] [PubMed] [Google Scholar]
- 16.Woods TO. Standards for medical devices in MRI: present and future. J Magn Reson Imaging. 2007;26:1186–9. doi: 10.1002/jmri.21140. [DOI] [PubMed] [Google Scholar]
- 17.Kanal E, Shellock FG, Talagala L. Safety considerations in MR imaging. Radiology. 1990;176:593–606. doi: 10.1148/radiology.176.3.2202008. [DOI] [PubMed] [Google Scholar]
- 18.Cooter R. Teamwork. Lancet. 2004;363:1245. doi: 10.1016/s0140-6736(04)15977-1. [DOI] [PubMed] [Google Scholar]
- 19.Jain AK, Thompson JM, Chaudry J, McKenzie S, Schwartz RW. High-performance teams for current and future physician leaders: an introduction. J Surg Educ. 2008;65:145–50. doi: 10.1016/j.jsurg.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Hewson KM, Burrell AR. A pilot study to test the use of a checklist in a tertiary intensive care unit as a method of ensuring quality processes of care. Anaesth Intensive Care. 2006;34:322–8. doi: 10.1177/0310057X0603400222. [DOI] [PubMed] [Google Scholar]
- 21.Edgar TA, Lee DS, Cousins DD. Experience with a national medication error reporting program. Am J Hosp Pharm. 1994;51:1335–8. [PubMed] [Google Scholar]
- 22.Santell JP, Hicks RW, McMeekin J, Cousins DD. Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharmacol. 2003;43:760–7. [PubMed] [Google Scholar]
- 23.Catalano K. Proposed regulations for enforcement of the Patient Safety and Quality Improvement Act of 2005. Plast Surg Nurs. 2008;28:96–8. doi: 10.1097/01.PSN.0000324783.26332.91. [DOI] [PubMed] [Google Scholar]