Abstract
Dasatinib, a dual Src family kinase and Abl inhibitor, is being tested clinically for the treatment of prostate cancer bone metastasis. Bidirectional interactions between osteoblasts and prostate cancer cells are critical in the progression of prostate cancer in bone, but the effect of dasatinib on osteoblasts is unknown. We found that dasatinib inhibited proliferation of primary mouse osteoblasts isolated from mouse calvaria and the immortalized MC3T3-E1 cell line. In calvarial osteoblasts from Col-luc transgenic mice carrying osteoblast-specific Collagen1α1 promoter-reporter, luciferase activity was inhibited. Dasatinib also inhibited FGF-2–induced osteoblast proliferation, but strongly promoted osteoblast differentiation, as reflected by stimulation of alkaline phosphatase activity, osteocalcin secretion, and osteoblast mineralization. To determine how dasatinib blocks proliferative signaling in osteoblasts, we analyzed the expression of a panel of tyrosine kinases, including Src, Lyn, Fyn, Yes, and Abl, in osteoblasts. In the Src family kinases, only Src was activated at a high level. Abl was expressed at a low level in osteoblasts. Phosphorylation of Src-Y419 or Abl-Y245 was inhibited by dasatinib treatment. Knockdown of either Src or Abl by lenti shRNA in osteoblasts enhances osteoblast differentiation, suggesting that dasatinib enhances osteoblast differentiation through inhibition of both Src and Abl.
Keywords: dasatinib, Src family kinase, osteoblast, prostate cancer, bone metastasis
Introduction
Prostate cancer metastasis to the bone is the major cause of mortality in afflicted patients (Ye et al., 2007). As a result, treatment strategies targeting bone metastasis have been one of the major focuses in prostate cancer therapy.
One of the unique characteristics of bone metastasis from prostate cancer is that the tumor lesions tend to be osteoblastic rather than osteolytic, and this phenomenon led to the hypothesis that bidirectional interactions between prostate cancer cells and osteoblasts play a critical role in the growth of metastatic cells in the bone (Logothetis and Lin, 2005). In support of this hypothesis, several investigations showed that prostate cancer cells secrete factors that stimulate osteoblast proliferation and/or differentiation. These factors include ET-1 (Nelson et al., 1995; Nelson et al., 1999), BMP-6 (Autzen et al., 1998; Dai et al., 2005), BMP-7 (Masuda et al., 2003), p45-sErbB3 (Chen et al., 2007; Lin et al., 2008; Vakar-Lopez et al., 2004), and fibroblast growth factor 9 (FGF-9) (Li et al., 2008). A number of observations also showed that increases in osteoblast proliferation and/or differentiation lead to secretion of factors, e.g., osteonectin (Chen et al., 2007; Jacob et al., 1999), that enhance the survival of prostate cancer cells in bone. Together, these observations suggest that therapy targeting both prostate cancer cells and osteoblasts will be effective in halting (or maybe retarding) prostate cancer progression in bone.
Dasatinib (BMS-354825) is a dual Src family kinase (SFK) and Abl kinase inhibitor that inhibits autophosphorylation of Src family nonreceptor protein tyrosine kinases, including Src, Lyn, Yes, and Lck (Lombardo et al., 2004), and Abl (Shah et al., 2004). The Src family protein tyrosine kinases are involved in several cancer types, including colorectal (Cartwright et al., 1989; Cartwright et al., 1990; Talamonti et al., 1993) and pancreatic (Ito et al., 2003; Summy and Gallick, 2003; Trevino et al., 2006). In prostate cancer, increased activity of Src has been observed in prostate cancer cells present in bone metastases (G. Gallick ans SH Lin, unpublished observations), and dasatinib effectively inhibits the proliferation and invasion of the bone metastasis–derived prostate cancer cell line PC-3 (Nam et al., 2005; Park et al., 2008), suggesting that Src family tyrosine kinases participate in the metastatic progression of prostate cancer in bone. It is interesting that Park et al. (2008) demonstrated that treating PC-3 cells with dasatinib led to the inhibition of proliferation and invasion as the result of decreased kinase activities of Lyn and Src activity, respectively. Thus, Src family members play different roles in prostate cancer progression.
Src activity is also critical to osteoclast function. Studies by Soriano et al. (Soriano et al., 1991) showed that mice with targeted disruption of the c-Src gene displayed a phenotype similar to that associated with osteopetrosis. The mechanism underlying osteopetrosis arising from a Src deficiency was determined to be mainly inhibition of osteoclast maturation (Boyce et al., 1992; Lowe et al., 1993). As expected, dasatinib inhibits osteoclast function (Brownlow et al., 2009). Whether Src has an effect on osteoblasts in c-Src–knockout mice was not known until Marzia et al. (2000) reported that reduction or deletion of Src expression enhances osteoblast differentiation. However, it is unclear whether other SFKs are expressed in osteoblasts, whether they participate in bone formation, and whether SFK/Abl inhibitors in clinical trial affect these functions.
Owing to its effectiveness in halting prostate cancer cell proliferation and invasion, dasatinib is being tested as an inhibitor of progression of prostate cancer bone metastasis in clinical trials (Araujo and Logothetis, 2009; Kopetz et al., 2007). Because osteoblasts are critical to the progression of prostate cancer in bone, it is important to examine the specific or overlapping roles of Src family members in osteoblast function. Dasatinib inhibits both SFKs and Abl, so its effects on osteoblasts may depend on the expression and activation of SFKs and Abl in those cells. Interestingly, Li et al. (Li et al., 2000) demonstrated that mice deficient in Abl are osteoporotic due to delayed maturation of osteoblasts, in contrast to the osteopetrotic phenotype resulting from a Src deficiency.
In this study, we examined the effect of dasatinib on osteoblast proliferation and differentiation. We found that dasatinib suppresses the proliferation of osteoblasts and enhances their maturation. Osteoblasts express a relatively high level of Src, Lyn, and Fyn) but a very low or undetectable level of Yes. Among Src, Lyn, and Fyn, only Src is phosphorylated in osteoblasts, and dasatinib inhibited its phosphorylation at Y419. Knockdown of either Src or Abl in osteoblasts enhances osteoblast differentiation. Together, our results suggest that dasatinib decreases osteoblast proliferation and enhances its differentiation by inhibiting both Src and Abl.
Materials and methods
Isolation of osteoblasts from mouse calvaria and proliferation assay
PMOs were prepared from mouse calvaria as described previously (Lin et al., 2008). The cells were then treated with various concentrations of dasatinib for 1–4 days. Cell numbers were counted using a hemacytometer.
Col-luc transgenic mice
Transgenic mice harboring a luciferase transgene under the control of the proximal 2.3-kb segments of the mouse Col1α1promoter were generated by injecting DNA construct containing the 2.3-kb pro-α1(I) promoter-luciferase (luc) gene into the pronuclei of mouse B6 X D2F2 fertilized ova as described elsewhere (Rossert et al., 1995). We named these transgenic mice Col-luc mice and bred them to be homozygous for the transgene to avoid the need for genotyping them.
Measurement of reporter protein activities in Col-luc mouse tissues
Tissues were dissected from 2- to 5-day-old Col-luc mice and homogenized, and the proteins were extracted with passive lysis buffer (Promega Corporation, Madison, WI). Luciferase activity in the tissues was assessed using a luciferase assay kit (Promega), and the luminescence was detected with a 20/20 luminometer (Turner Biosystems, Sunnyvale, CA). Results were expressed as relative luciferase units (RLU).
Bioluminescence imaging
For bioluminescence imaging, 4-day-old wild type or Col-luc pups were injected subcutaneously with d-luciferin solution (150 mg/kg body weight). Images were acquired and analyzed by using a Xenogen imaging system (Almeda, CA).
Col-luc osteoblast proliferation assays
Calvarial PMOs were plated on a 48-well cell culture dish (5 × 104 cells/well) in 0.5 mL of complete medium (α-MEM medium with 10% FBS and antibiotics) overnight. Cells were then treated with the test compounds for 72 h, lysed in 1× passive lysis buffer (Promega), and the luciferase activities in the cell extracts measured.
Osteoblast differentiation assays
PMOs and MC3T3-E1 cells were grown to confluence. The medium was then changed to differentiation medium (with 100 µg/mL ascorbic acid, 5 mM β-glycerol phosphate, and 10% FBS) with and without dasatinib. The medium was changed every 3 days. Alkaline phosphatase activity in the cell extracts was measured using p-nitrophenyl phosphate liquid substrate system (Sigma) and the absorbance was determined at 405 nm. Osteocalcin in the conditioned medium was detected by performing a mouse osteocalcin ELISA (Biomedical Technologies, Stoughton, MA). Von Kossa’s staining for mineralized bone matrix was carried out as described elsewhere (Bhargava et al., 1988). Osteonectin in the conditioned medium was determined by western blot as described elsewhere (Chen et al., 2007).
Osteoclast assays
PMOs or RAW264.7 cells were plated 10, 000 cells per well in 96-well plate. The following day, cells were treated with RANKL (10–100 ng/ml). After incubation at 37°C for 96 hrs, tartrate-resistant alkaline phosphatase (TRAP) activity in the culture supernatants was measured and the cells were processed for TRAP staining using TRAP Staining kit (Kamiya Biomedical Company).
Immunoprecipitation and Western blot analyses
Proteins from PMOs and MC3T3-E1 cells untreated or treated with dasatinib were subjected to 4–12% SDS-PAGE. Proteins in the gel were transferred onto nitrocellulose membranes and blotted with antibody against anti-p-SFK (rabbit anti–p-Src-416) (Calbiochem, San Diego, CA). Tubulin and actin levels, detected by anti–α-tubulin or anti-actin antibody (both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA), were used as loading control. The expression of Src, Lyn, Yes, and Fyn in MC3T3-E1 cells was determined by immunoprecipitation of total cell lysate with antibodies against Src (Santa Cruz), Lyn (Santa Cruz), Yes (Wako, Richmond, VA), and Fyn (Alexis, San Diego, CA), respectively, followed by Western blotting with anti-p-SFK (Santa Cruz) antibody. Blots were further immunoblotted with antibodies against each tyrosine kinase to confirm the efficacy of immunoprecipitation. The expression of Abl in PMOs or MC3T3-E1 cells was determined by Western blotting of total cell lysate with anti–Abl antibody 8E9 (Upstate, Charlottesville, VA) or by immunoprecipitation of cell lysate with anti–Abl antibody K12 (Santa Cruz) followed by Western blotting with anti p-Y245–c-Abl antibody (Cell Signaling, MA). All antibodies used in this study have been shown to react with the corresponding mouse and human proteins.
Knockdown of Src or Abl in PMOs
PMOs were plated at 50,000 cells per well in a 24-well plate. After overnight incubation, the cells were infected with lentiviral particles containing shRNAs for Src, Abl, or control non-targeting shRNA (Santa Cruz) at a ratio of 2.5 infectious units per cell. PMOs infected with copGFP lentil particles (Santa Cruz) were used as a control for viral infection. After 24 hrs, the virus-containing medium was removed and replaced with α-MEM medium containing 10% FBS. The cells were allowed to grow to confluence for three days and the medium was changed to differentiation medium. The conditioned medium was collected every three days and the cell lysates collected at days 9 and 18. Von-Kossa staining of differentiated PMOs was done on day 18.
Protein concentration determination
Protein concentrations were measured by testing 10 µL of each sample in 200 µL of Coomassie Plus™ Protein Assay Reagent (Thermo Scientific, Waltham, MA), using BSA as the protein standard. The reaction was performed in a 96-well microtiter plate, and the color was read at 595 nm.
Statistical analyses
Statistical analysis of the data was performed by using Student’s t test (two-tailed, paired). A P value of less than 0.05 was considered significant. Data are expressed as the means ± SD.
Results
Dasatinib treatment inhibits proliferation of PMOs
The process of bone formation includes initial osteoblast proliferation followed by increased alkaline phosphatase activity, deposition of extracellular matrix, and mineralization (Aubin, 1998; Stein and Lian, 1993). We first examined the effects of dasatinib on osteoblast proliferation. PMOs isolated from calvaria of 2- to 5-day-old mice were treated with various concentrations of dasatinib. As shown in Fig. 1, at a concentration as low as 50 nM, dasatinib caused an approximate 50% reduction of osteoblast cell numbers when the osteoblasts were cultured in the absence (0.1% BSA) or presence of serum (10% FBS)-containing medium for 72 h (Fig. 1A and B). We further examined the time course of dasatinib on PMOs proliferation. As shown in Fig. 1C, dasatinib treatment led to a time-dependent inhibition of PMOs proliferation.
Figure 1.
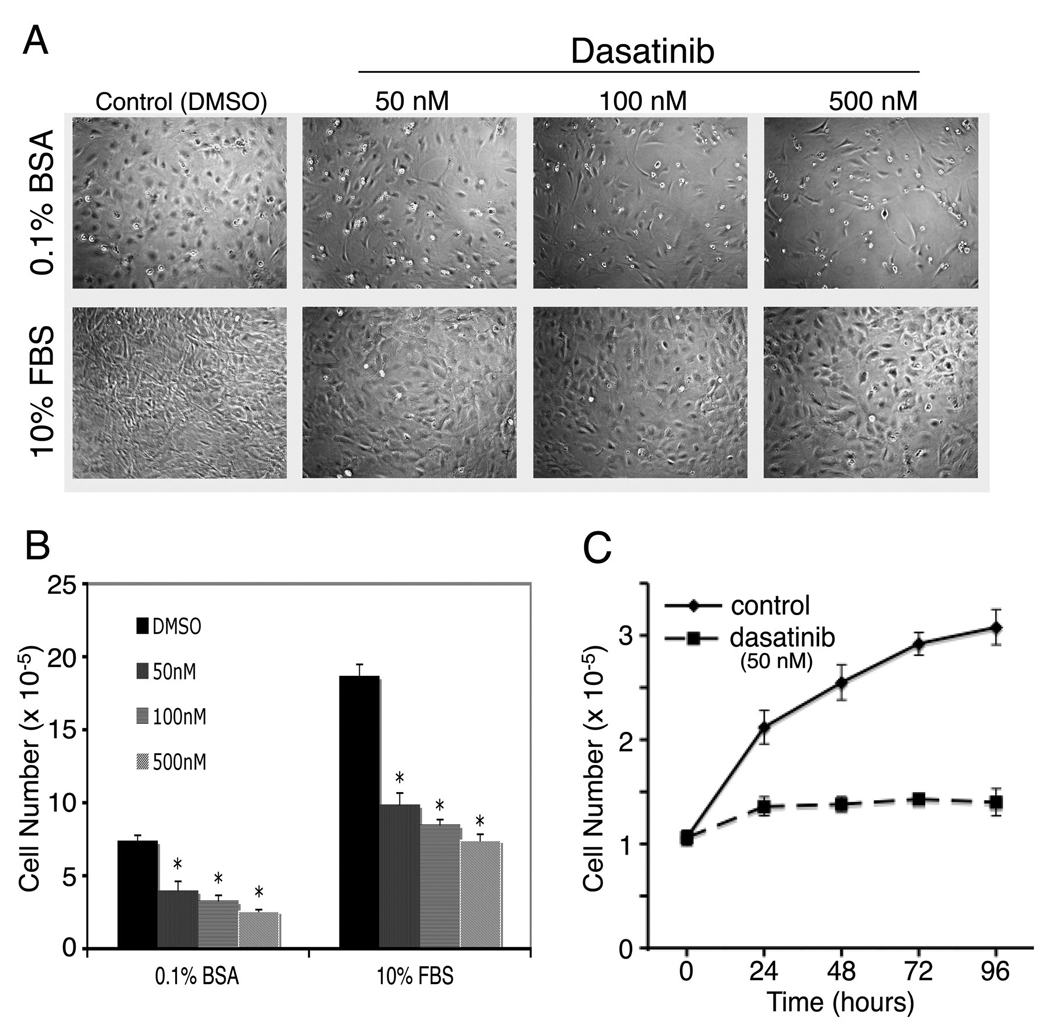
Dasatinib inhibits calvarial osteoblast (PMOs) proliferation. A, cell density and morphologic characteristics after treatment of PMOs with various concentrations of dasatinib for 72 h. PMOs were cultured in 10% FBS or in 0.1% BSA as indicated. B, quantification of cell numbers. C. Time course of effect of dasatinib on PMOs proliferation. The PMOs were cultured in 10% FBS and treated without or with 50 nM dasatinib. Data are means ± SD. *P < 0.05 vs. control (DMSO). The experiment was repeated three times with similar results.
Effects of dasatinib on proliferation of PMOs from Col-luc mice
Although calvarial osteoblasts isolated from 2-to 5-day-old mice are frequently used for measuring osteoblast activity, the digested calvaria usually contain other cell types as well (Bellows and Aubin, 1989). Because SFKs are expressed in most cell types, including fibroblasts, the decrease we found in cell numbers might be due to the inhibition of osteoblasts, fibroblasts, or both. To further confirm that dasatinib has an effect on cells of osteoblast lineage, we prepared PMOs from transgenic mice (Col-luc mice) carrying an osteoblast-specific reporter construct.
Col-luc transgenic mice were previously generated by injecting DNA construct containing the 2.3kb Col1α1 promoter–luciferase gene into the pronuclei of fertilized mouse ova (Rossert et al., 1995) (Fig. 2A). Previous studies showed that luciferase activity is specifically expressed in osteoblasts because the highest specific activity was detected in the bones (Rossert et al., 1995). To avoid the need to genotype the mice when preparing the PMOs, we bred the Col-luc transgenic mice to homozygosity, which we confirmed by the observation that 100% of the offspring were positive for luciferase activity in their tail protein lysates when they were bred to wild-type mice (data not shown). To confirm the tissue specificity of the reporter in the Col-luc mice, the luciferase activities in 4-day-old Col-luc tissues were measured. Expression of the luciferase gene was highly tissue specific, with tibia, tooth, calvaria, and tail expressing high levels of luciferase activity, whereas other tissues, including liver, lung, heart, kidney, and skin, had low or undetectable levels of luciferase activity (Fig. 2B). The bone-specific expression of the luciferase reporter gene was further confirmed by using bioluminescence imaging to detect the luciferase activity in vivo. Strong bioluminescence signals were detected in the skull and hind limbs (Fig. 2B). These data indicate that the proximal 2.3-kb Col1α1 promoter directs an osteoblast-specific expression of luciferase in the mice homozygote with Col1α1 transgene.
Figure 2.
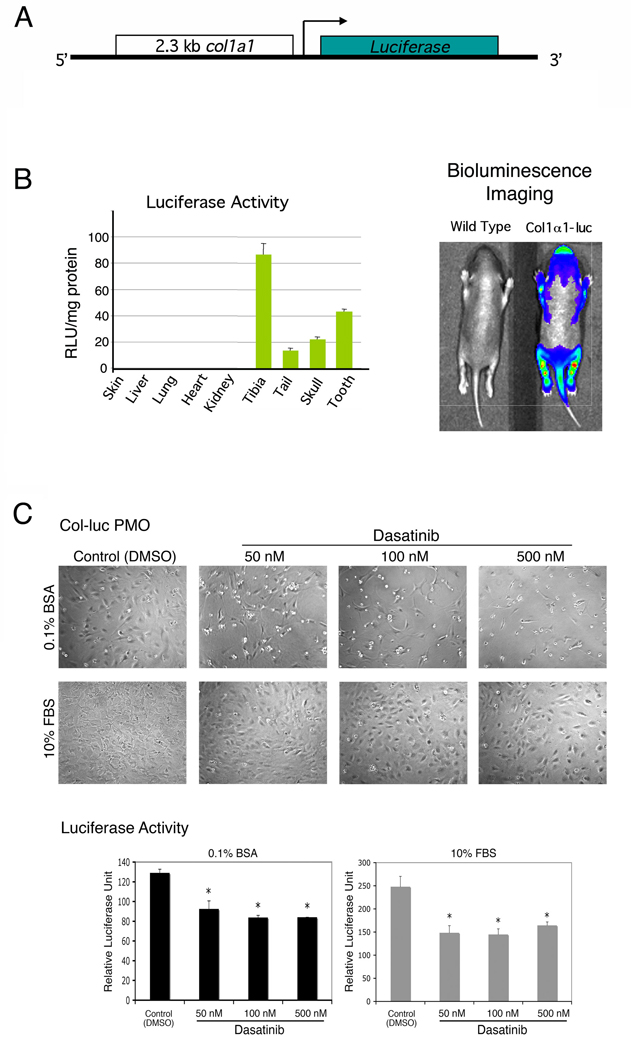
Dasatinib treatment inhibits the proliferation of PMOs isolated from calvaria of Col-luc transgenic mice. A, DNA construct used for the generation of Col-luc mouse. The proximal 2.3-kb col1α1 promoter was used to drive the luciferase gene expression. B, measurement of luciferase activity in various organs and bioluminescence detection of luciferase expression in 4-day-old Col-luc mice. C, the cell density and luciferase activity of Col-luc PMOs after treatment with various concentrations of dasatinib for 72 h. The Col-luc PMOs were cultured in 10% FBS or in 0.1% BSA as indicated. Data are means ± SD. *P < 0.05 vs. control. The experiment was repeated at least three times with similar results; the data shown are representative of all the findings.
We examined the effect of dasatinib on the PMOs isolated from Col-luc mice. As shown in Fig. 2C, treatment of Col-luc osteoblasts with dasatinib led to inhibition (~40%) of both cell numbers and luciferase activity when cultured in medium containing either 0.1% BSA or 10% FBS. We further assessed the effect of dasatinib on the proliferation of MC3T3-E1, an immortalized osteoblasts that have the characteristics of pre-osteoblasts (Franceschi and Iyer, 1992), and found that dasatinib inhibited MC3T3-E1 proliferation in a time-dependent manner (Fig. 3A). Together, these results demonstrated that dasatinib suppresses osteoblast proliferation.
Figure 3.
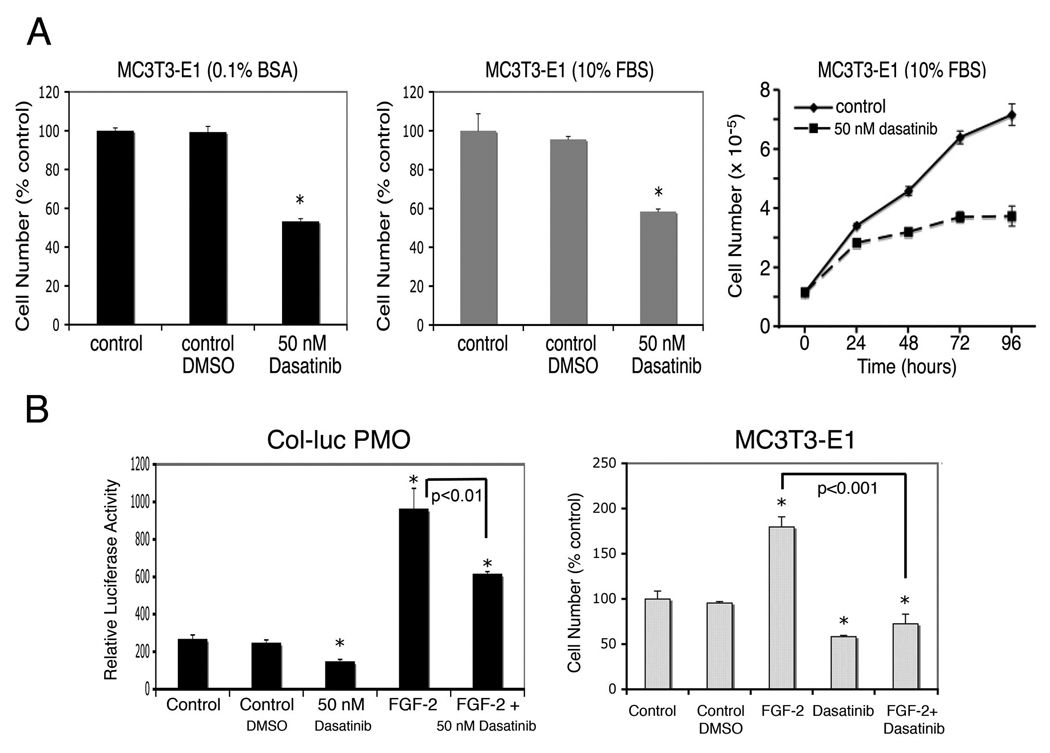
Dasatinib inhibits FGF-2–induced osteoblast proliferation. A, cell density after the MC3T3-E1 cells were treated with or without 50 nM dasatinib in either 0.1% BSA (left panel) or 10% FBS (middle panel). Right panel, time course of effect of dasatinib on MC3T3-E1 cell proliferation. B, PMOs isolated from Col-luc transgenic mice or MC3T3-E1 cells were untreated or treated with 10 ng/mL of FGF-2 in the presence or absence of 50 nM dasatinib. DMSO was used as the vehicle control. Data are means ± SD. *P < 0.05 vs. control. The experiment was repeated twice with similar results; the data shown are representative of all the findings.
Dasatinib inhibits FGF-2–induced osteoblast proliferation
FGFs reportedly play key roles in prostate tumor progression (Dorkin et al., 1999a; Dorkin et al., 1999b) and stimulation of bone formation (Canalis et al., 1988; Fakhry et al., 2005 ; Globus et al., 1988; Mayahara et al., 1993; McCarthy et al., 1989). In prostate cancer bone metastasis, FGFs secreted by metastatic prostate cancer cells may contribute to the osteoblastic phenotype of the prostate cancer bone lesion (Li et al., 2008). Debiais et al. (2001) showed that several signaling molecules, including Src, were activated by treating immortalized human neonatal calvaria cells with FGF-2. We thus examined the effect of dasatinib on FGF-2–induced osteoblast proliferation. As shown in Fig. 3B, treatment of Col-luc osteoblasts with dasatinib resulted in partial inhibition of FGF-2–induced osteoblast proliferation (FGF + dasatinib vs. FGF; P < 0.05). Dasatinib also inhibited FGF-2–induced proliferation of MC3T3-E1 cells (Fig. 3B). These results suggest that dasatinib is effective in inhibiting FGF-2–induced osteogenesis.
Effect of dasatinib on differentiation of osteoblasts
Because osteoblast maturation is a balance between proliferation and differentiation, suppression of osteoblast proliferation by dasatinib may enhance differentiation. To determine the effect of dasatinib on osteoblast differentiation and/or maturation, PMOs cultured in osteoblast differentiation medium were untreated or treated with 50 nM dasatinib. The expression of osteoblast differentiation markers, i.e., alkaline phosphatase, osteocalcin, and mineral deposition, during the time course of differentiation was measured.
As shown in Fig. 4A, treatment of PMOs with dasatinib led to stimulation of alkaline phosphatase activity, which could be detected as early as 3 days, and the stimulation reached 19 times that of the control at the late stage of differentiation (Fig. 4A). The alkaline phosphatase activities of the control samples are also shown in the insert in Fig. 4A. Similar effects were seen with MC3T3-E1 cells (Fig. 4A). However, in MC3T3-E1 cells, the increase in alkaline phosphatase activity was only about twice that of the control, possibly because MC3T3-E1 is an immortalized calvarial cell line. Treatment of PMOs with dasatinib also stimulated the expression of osteocalcin relative to the control samples (Fig. 4B), and enhanced osteoblast mineralization, as reflected in greater von Kossa staining than that in the control (Fig. 4C). Together, these observations suggest that dasatinib treatment increases the differentiation of osteoblasts.
Figure 4.
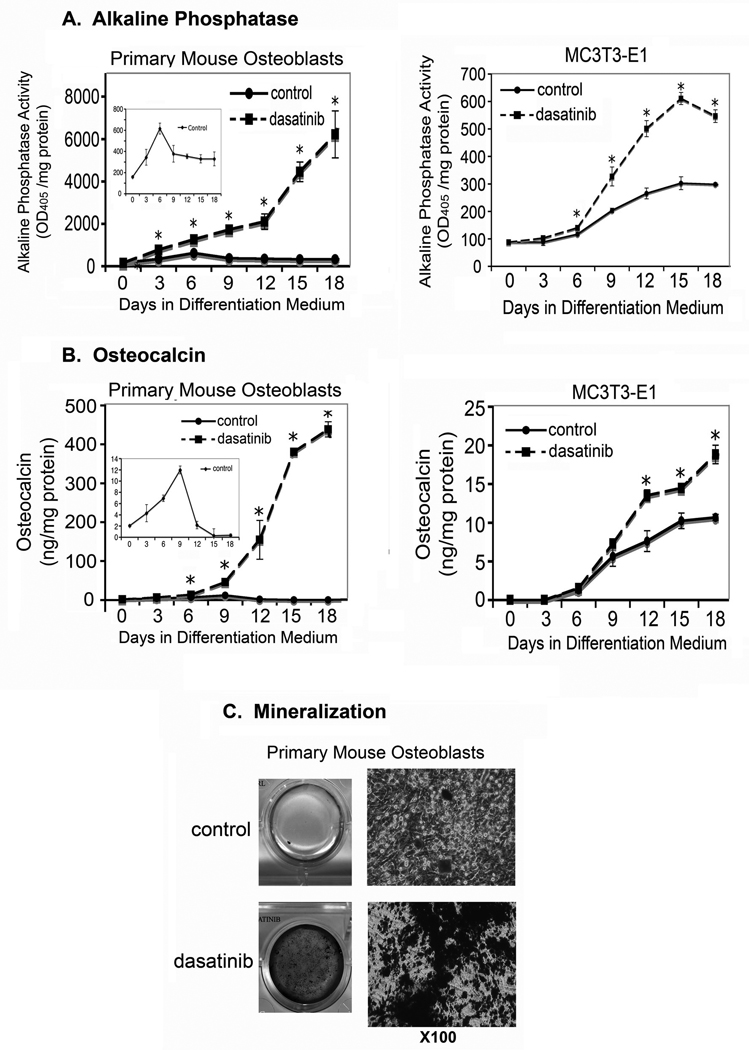
Effects of dasatinib on the differentiation of osteoblasts. PMOs and MC3T3-E1 cells were cultured in osteoblast differentiation medium for the times indicated. A, alkaline phosphatase activity in cell lysates. The alkaline phosphatase activities of the control samples are also shown in the insert in Fig. 4A. Data are means ± SD. *P < 0.05 vs. control. B, osteocalcin concentration in the conditioned medium, as measured by using ELISA. The osteocalcin levels of the control samples are also shown in the insert in Fig. 4B. Data are means ± SD. *P < 0.05 vs. control. C, mineralized nodules detected on von Kossa staining. Right panel, higher magnification of mineralized nodules.
Effect of dasatinib on SFKs in osteoblasts
We next determined the expression of dasatinib targets in osteoblasts. The kinase activity of both the SFKs and Abl is reported to be inhibited by dasatinib (Lombardo et al., 2004; Shah et al., 2004). Previous studies showed that deletion of Src or Abl in mice led to changes in bone phenotype (Li et al., 2000; Soriano et al., 1991), suggesting that both kinases are involved in bone formation.
We first examined which SFK(s) play a role in osteoblast function. Treatment of PMOs and MC3T3-E1 cells with dasatinib led to inhibition of the phosphorylation of SFKs, as detected by anti–p-SFK antibody, which detects SFK proteins phosphorylated at their autophosphorylation site, indicative of their activation (Fig. 5A). The effect of dasatinib on individual SFKs was further examined by immunoprecipitation using antibodies specific to each kinase. Cell lysates from PC-3, L3.6pl, K562, and HT29 cells were used as positive controls for Src, Lyn, Fyn, and Yes, respectively. All the positive controls for immunoprecipitations were run on the same gel with the immunoprecipitation samples. As shown in Fig. 5B, Src was expressed and activated in MC3T3-E1 cells as determined by reactivity with the anti-p-SFK antibody. Its phosphorylation was significantly inhibited by the treatment of osteoblasts with 50 nM dasatinib. Although both Lyn and Fyn were expressed in MC3T3-E1 cells, they were not phosphorylated (Fig. 5B). The kinase Yes was not expressed at detectable levels in MC3T3-E1 cells (Fig. 5B). These results suggest that among several SFKs, only Src is expressed and present in its activated form in osteoblasts.
Figure 5.
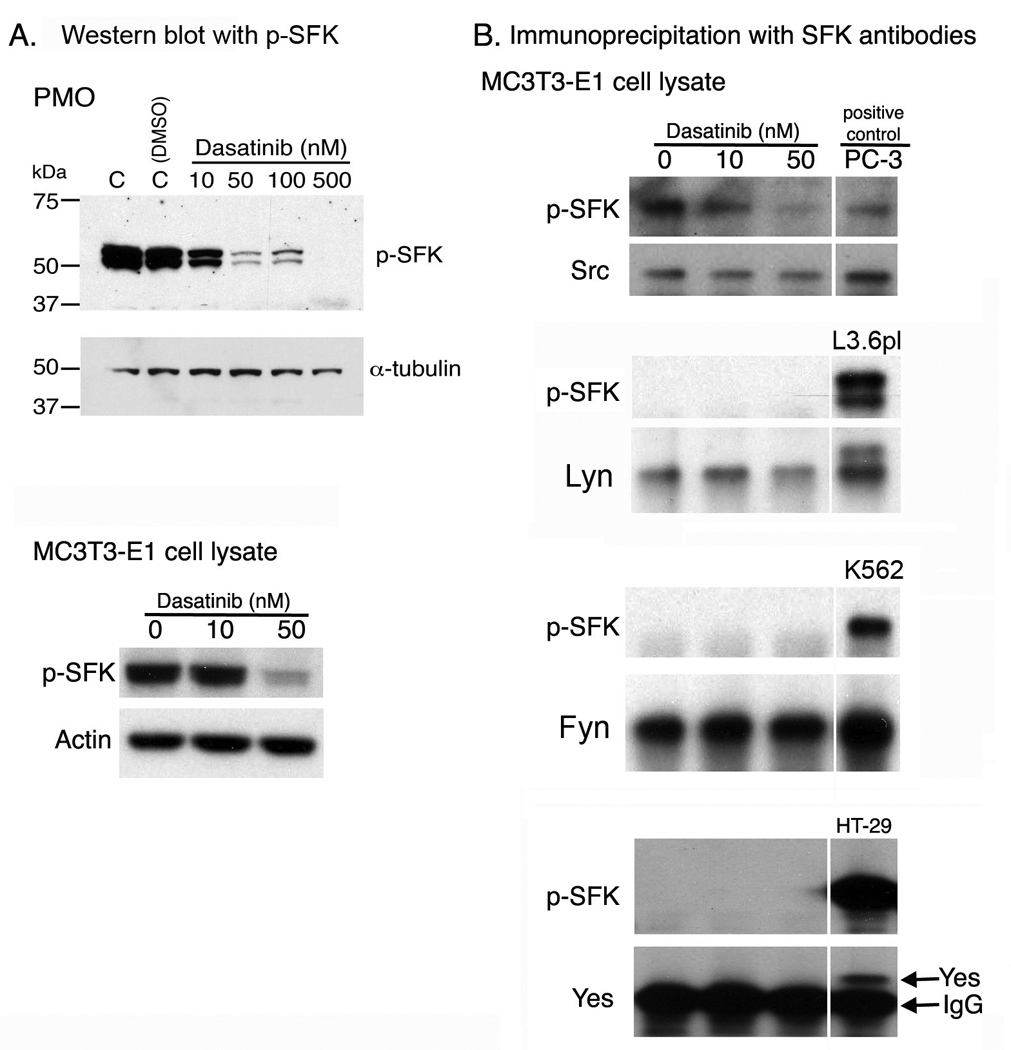
Expression of SFKs in osteoblasts. A, Western blot of osteoblast cell lysates with anti–p-SFK antibody. B, immunoprecipitation of Src, Lyn, Fyn, and Yes from MC3T3-E1 cell lysates followed by Western blotting with anti–p-SFK antibody. All the positive cell controls for immunoprecipitation were run on the same gel with MC3T3-E1 immunoprecipitation samples, however, the positive control lanes were separated for clarity purpose.
Effect of Src knock down on osteoblast differentiation
To test the role of Src in osteoblasts, Src-specific lenti-shRNA was used to knockdown Src on PMOs. Lentivirus containing non-targeting shRNA was used as control. PMOs infected with copGFP lenti virus showed that PMOs could be transduced by lentivirus effectively (Fig. S1). For the effect of Src knockdown on osteoblast differentiation, PMOs were infected with Src lenti-shRNA and cultured in differentiation medium for 9 or 18 days (Fig. 6A). The level of Src was reduced by about 50% in the PMOs infected with Src-specific lenti-shRNA compared to that in control shRNA infected cells (Fig. 6B). Knockdown of Src led to an increase in alkaline phosphatase activity (Fig. 6C), osteocalcin (Fig. 6D), and mineralization (Fig. 6E). These observations suggest that downregulation of Src in PMOs stimulates osteoblast differentiation.
Figure 6.
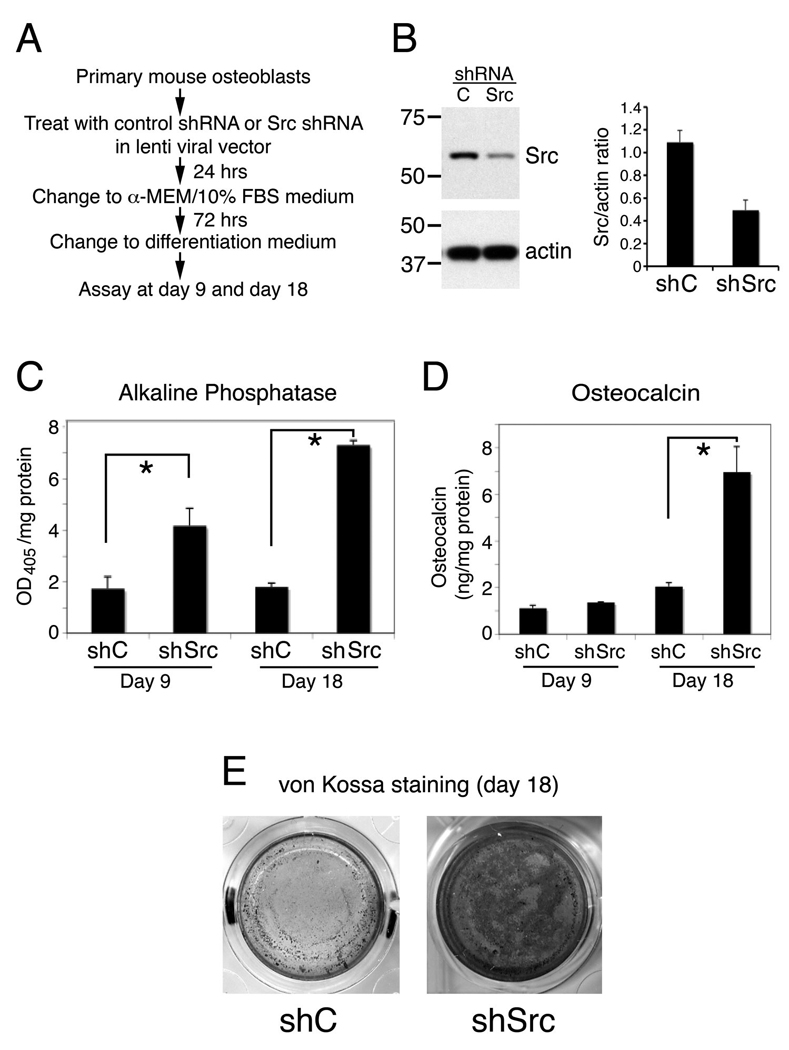
Effect of Src knock down on osteoblast differentiation. A, Experimental plan for knocking down Src in PMOs and examining its effect on osteoblast differentiation. B, The levels of Src in PMOs treated with lentiviral control shRNA (shC) or lentiviral Src shRNA (shSrc). C, alkaline phosphatase activity in cell lysates. Data are means ± SD. *P < 0.05 vs. control. D, osteocalcin concentration in the conditioned medium, as measured by using ELISA. Data are means ± SD. *P < 0.05 vs. control. E, mineralized nodules detected on von Kossa staining. The experiment was repeated twice with similar results.
Effect of Abl knock down on osteoblast differentiation
Next, we examined the expression of Abl in osteoblasts. The levels of Abl in both PMOs and MC3T3-E1 cells were low and PMOs have much lower level of Abl compared to that in MC3T3-E1 cells (Fig. 7A). Treatment of PMOs and MC3T3-E1 cells with dasatinib led to inhibition of the phosphorylation of Abl at tyrosine-245, which was detected by immunoprecipitation followed with anti–p-Y245-Abl antibody (Fig. 7A). These results indicate that dasatinib inhibits Abl kinase activity.
Figure 7.
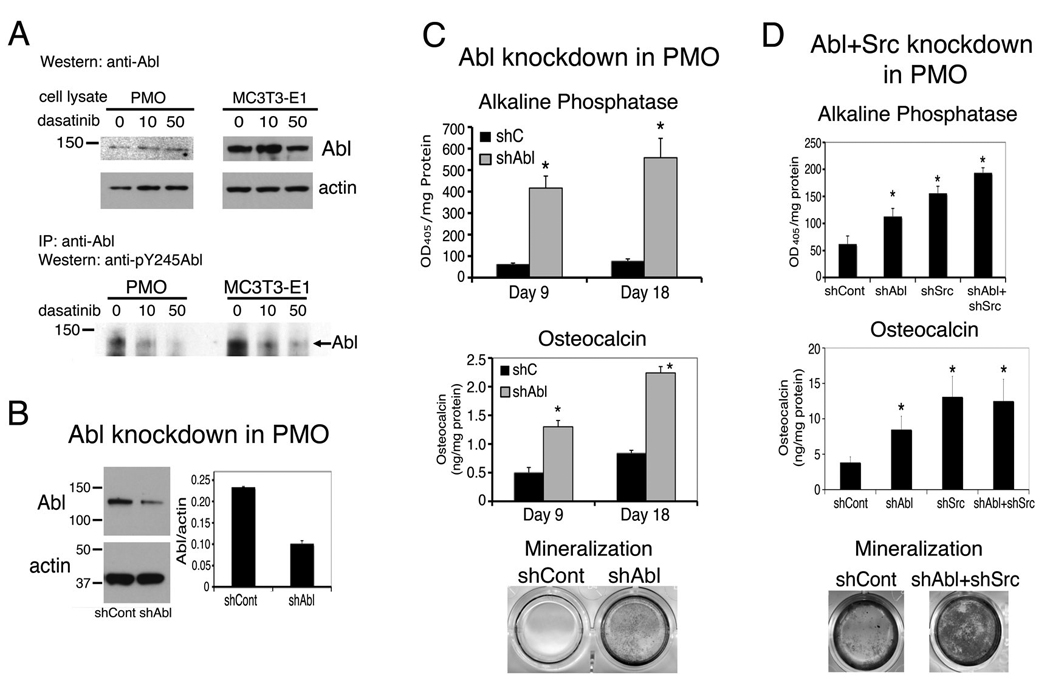
Expression of Abl in osteoblasts and effect of Abl knockdown on osteoblast differentiation. A, Immunoblot of PMO or MC3T3-E1 cell lysate with anti–Abl antibody (upper panel). Immunoprecipitation of PMO or MC3T3-E1 cell lysates with anti–Abl and immunoblot with anti–pY245–Abl antibody (lower panel). B, The levels of Abl in PMOs treated with lentiviral control shRNA (shCont) or Abl shRNA (shAbl). C, alkaline phosphatase activity, osteocalcin concentration in the conditioned medium, and von kossa staining of PMOs treated with control shRNA (shCont) or Abl shRNA (shAbl) lentivirus. D, PMOs were treated with lentiviral Src shRNA (shSrc), or Abl shRNA (shAbl), or both. Data are means ± SD. *P < 0.05 vs. control.
To test the role of Abl in osteoblasts, Abl-specific lenti-shRNA was used to knockdown Abl on PMOs. Treatment with Abl-specific lenti-shRNA reduced Abl level to 50% compared to that in control shRNA infected cells (Fig. 7B). Knockdown of Abl led to an increase of alkaline phosphatase activity and osteocalcin, and a modest increase in mineralization (Fig. 7C). These observations suggest that downregulation of Abl in PMOs stimulates osteoblast differentiation. However, Abl seems to affect osteoblast differentiation to a much lesser extent compared to those from Src knockdown when the knockdown of Abl or Src was done in the same experiment (Fig. 7D). When the PMOs were treated with both Src shRNA and Abl shRNA, the combined effects from knockdown of Src and Abl are similar to those from Src knockdown alone (Fig. 7D). Together, these observations suggest that the effect of dasatinib on osteoblast differentiation is due to the inhibition of both Src and Abl activity.
Effect of dasatinib on osteonectin expression
Previous studies have shown that osteonectin is implicated as one of the osteoblast-derived factors that stimulate prostate cancer cells (Chen et al., 2007; Jacob et al., 1999). We thus examined whether dasatinib has an effect on osteonectin expression. We found that osteonectin expression was decreased during PMO differentiation and dasatinib treatment increased osteonectin production in the conditioned medium (Fig. 8). We also analyzed the levels of osteonectin in the media from PMOs that had been infected with Src- or Abl- or both Src and Abl lenti shRNAs. Western blots of osteonectin showed that knockdown of Src slightly increased osteonectin expression in the early time points (Fig. 8). In contrast, knockdown of Abl greatly increased the production of osteonectin throughout the entire time course of differentiation. Knockdown of both Src and Abl led to increase of osteonectin levels similar to that observed by Abl knockdown alone. Thus, the stimulation of osteonectin production by dasatinib is mainly through inhibition of Abl. These studies also showed that Src and Abl have different effects on osteoblasts, suggesting that Src-specific inhibitors will have different effects from dasatinib.
Figure 8.
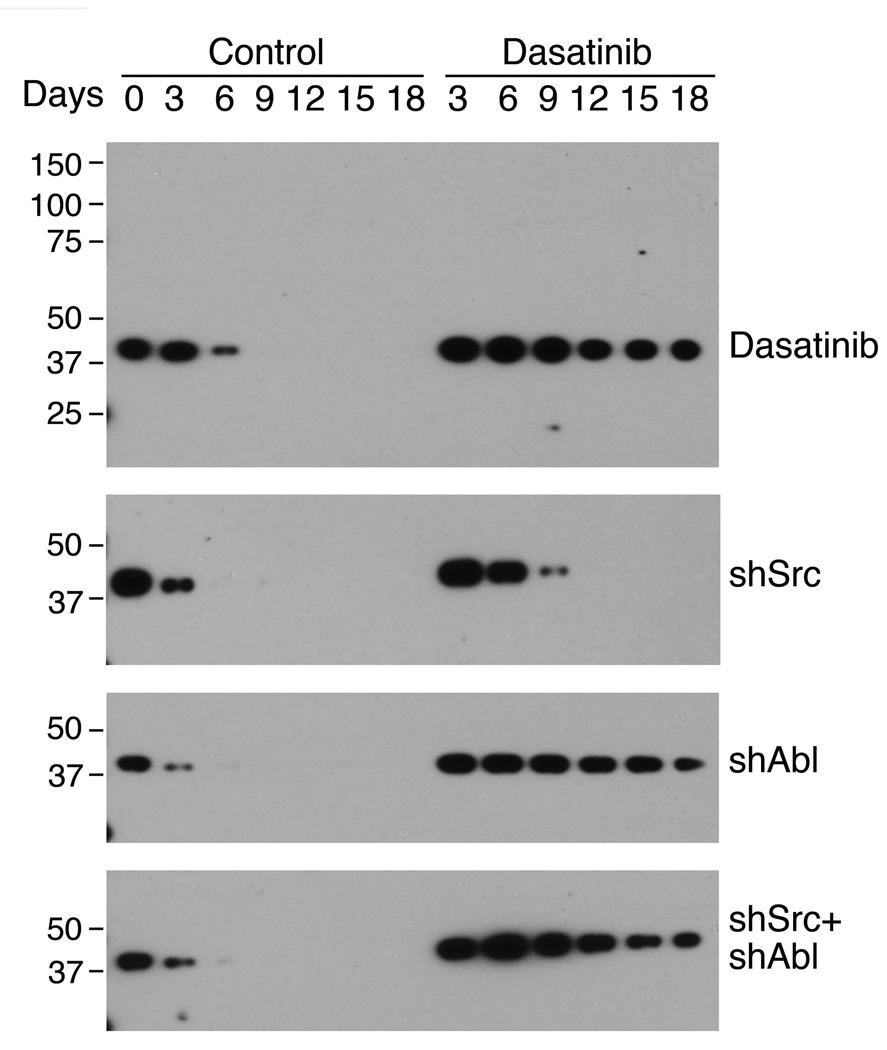
Effects of dasatinib on osteonectin expression. Proteins in the conditioned medium from PMOs treated with dasatinib, Src shRNA, Abl shRNA, or both Src shRNA and Abl shRNA were separated on SDS-PAGE and immunoblotted with anti-osteonectin antibody.
Discussion
Protein tyrosine kinases play important roles in bone homeostasis (Li et al., 2000; Soriano et al., 1991). Therapies that inhibit tyrosine kinase activities in the treatment of various cancer types may also have effects on bone. Such an unintended effect on bone has been reported with the use of imatinib for the treatment of chronic myelogenous leukemia or gastrointestinal stromal tumors (Berman et al., 2006). The use of tyrosine kinase inhibitors for the treatment of prostate cancer bone metastases is particularly important because prostate cancer cells are in direct interaction with the other cells present in the bone microenvironment, especially osteoblasts. Because various non–receptor protein tyrosine kinases (e.g., Abl and Src) reportedly have different roles in the proliferation and maturation of osteoblasts, it is important both to determine which protein tyrosine kinases play direct roles in these processes, and to examine the effect of dasatinib, which targets multiple tyrosine kinases, on osteoblast functions. Our studies demonstrated that dasatinib suppresses the proliferation and enhances the differentiation of osteoblasts and that this effect occurs mainly through the inhibition of Src and Abl activities.
Our results also indicate that among the several SFKs expressed in osteoblasts, Src plays a dominant role in regulating osteoblast activity. We observed that both Lyn and Fyn are expressed in osteoblasts but are not activated (Fig. 5B), whereas Yes was not detectable (Fig. 5B). In contrast, Src is highly expressed in osteoblasts and present in the active state. Our observations are consistent with the results reported by Marzia et al. (Marzia et al., 2000), who showed that decreased Src expression in osteoblasts using Src antisense RNA led to decreased osteoblast proliferation concomitant with increased osteoblast differentiation. Similarly, Shor et al. (Shor et al., 2007) demonstrated that Src is critical in various types of osteosarcoma and that dasatinib was effective in inducing apoptosis and inhibiting the migration and invasion of osteosarcoma cells (Shor et al., 2007). Together, these observations suggest that Src plays a central role in regulating osteoblast function.
Li et al. (Li et al., 2000) demonstrated that mice deficient in Abl are osteoporotic due to delayed maturation of osteoblasts, in contrast to the osteopetrotic phenotype that results from Src deficiency. Our studies using shRNA to knockdown Abl in vitro showed that decrease in the level of Abl stimulates osteoblast maturation as measured by expression of alkaline phosphatase and osteocalcin. The differences in the effect of Abl on osteoblast maturation may be due to compensation of the effect of Abl deletion by other mechanisms in vivo, which may not occur in the in vitro studies.
Src has been shown to play critical role in osteoclast function. To address the possibility that the effect dasatinib on osteoblast may be indirectly due to inhibition of osteoclasts contaminated in the osteoclast culture, we stained the PMOs culture for the presence of osteoclasts. We did not detect TRAP positive osteoclasts by quantitative TRAP assay (Fig. S2A) or by TRAP staining (Fig. S2B) in the PMOs. We also did not detect any osteoclast in the PMOs treated with various concentrations of RANKL (Fig. S2). The positive controls are RAW264.7 cells induced by various concentrations of RANKL (Fig. S2). Thus, the presence of osteoclasts, if any, is minimal in our culture condition.
Our observation that dasatinib inhibited collagen type I promoter driven luciferase activity in Col-luc calvarial osteoblasts raised the issue that dasatinib may inhibit osteoblast proliferation and/or collagen expression. We examined the levels of collagen type I in the osteoblasts treated with or without dasatinib by Western blot and found that dasatinib does not affect the levels of collagen type1 expression in the PMOs (data not shown). Thus, dasatinib inhibition of luciferase activity in Col-luc osteoblasts is due to its effect on osteoblast proliferation, not collagen expression. Consistent with this finding, dasatinib also did not affect collagen type I expression in PC3 and LNCaP prostate cancer cell lines (data not shown).
The results presented in this study demonstrate not only novel roles for Src in osteoblast function, but have implications for use of Src inhibitors in metastatic bone disease. Studies of the SFK inhibitor AZD0530 in normal volunteers demonstrated the predicted effect of bone thickening, due to decreased osteoclast function (Hannon et al., 2009). Koreckij et al. (Koreckij et al., 2009) demonstrated that following intratibial injection of C4-2B cells in SCID mice, dasatinib’s antitumor effect correlated with a 25% increase in mineral density in tibia-bearing tumors. These studies do not consider the ability of tumor cells to secrete factors affecting osteoblasts when tumors are growing in the bone. Early results from Phase 1/2 clinical trials in prostate cancer patients with metastases in bone using the combination of dasatinib and docetaxel have suggested markers of bone turnover are inhibited when PSA is decreased (Araujo, J. et al., unpublished results), however, which effects are due to dasatinib alone cannot be determined from this trial. Collectively, these results demonstrate conclusively that bone remodeling is affected by Src inhibition. However, our results challenge the idea that the ability of Src inhibitors to affect bone remodeling is solely due to the inhibitor’s effects on tumor and osteoclast functions. Rather, our data suggest that the effect of dasatinib on osteoblasts also plays a critical role in these processes. Because the quantitative and qualitative release of soluble modifiers from the tumor is likely to govern osteoblastic or osteolytic lesions, we predict that if dasatinib blocks tumor growth, secreted factors that promote osteoblast differentiation will also be inhibited. However, if the tumors themselves are not sensitive to dasatinib treatment, dasatinib may enhance osteoblastic lesions.
Our studies also showed that inhibition of Abl but not Src expression by shRNA knockdown in osteoblasts led to an increase in osteonectin expression, suggesting that Src and Abl have different effects on osteoblasts. Thus, if Src-specific inhibitors are developed, they may have different outcomes from dasatinib. Because osteonectin has been implicated as the osteoblast-derived factor that stimulates the invasion of prostate cancer cells (Chen et al., 2007), these data imply that the effects of dasatinib on osteoblasts may promote prostate cancer progression. A phase 3 clinical trial that test dasatinib in combination with docetaxel on prostate cancer bone metastasis is in progress. Understanding the changes in bone biomarkers and osteonectin and correlating them with the clinical outcomes will be important in future trial designs with SFK/Abl inhibitors.
Supplementary Material
PMOs (50,000 cells) were infected with copGFP lenti viral particles for 24 hrs. (A) Expression of copGFP after the PMOs were incubated for five days in growth medium. ×200. (B) Expression of copGFP after the PMOs were incubated in the growth medium for 3days followed in differentiation medium for 2 days. ×200. The PMOs in the differentiation medium assumed a flattened and extended morphology and appeared bigger in their sizes compared to those PMOs in growth medium.
Absence of osteoclasts in the PMOs. PMOs or RAW264.7 cells were treated without or with various concentrations of RANKL as indicated for 96 hrs. The presence of osteoclasts was detected by TRAP staining. (A) Quantitative TRAP assay. (B) TRAP staining.
Acknowledgements
We thank Karen Philips for editing the manuscript.
Financial support: Supported by National Institutes of Health grants CA111479 (SH Lin), P50 CA140388 (SH Lin, GE Gallick), and DK53176 (LY Yu-Lee), and by the Prostate Cancer Foundation (SH Lin).
Footnotes
Conflict of interest statement
There is no conflict of interest from all authors.
References
- Araujo J, Logothetis C. Targeting Src signaling in metastatic bone disease. Int J Cancer. 2009;124:1–6. doi: 10.1002/ijc.23998. [DOI] [PubMed] [Google Scholar]
- Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899–910. [PubMed] [Google Scholar]
- Autzen P, Robson CN, Bjartell A, Malcolm AJ, Johnson MI, Neal DE, et al. Bone morphogenetic protein 6 in skeletal metastases from prostate cancer and other common human malignancies. Br J Cancer. 1998;78:1219–1223. doi: 10.1038/bjc.1998.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows CG, Aubin JE. Determination of numbers of osteoprogenitors present in isolated fetal rat calvaria cells in vitro. Dev Biol. 1989;133:8–13. doi: 10.1016/0012-1606(89)90291-1. [DOI] [PubMed] [Google Scholar]
- Berman E, Nicolaides M, Maki RG, Fleisher M, Chanel S, Scheu K, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354:2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- Bhargava U, Bar-Lev M, Bellows CG, Aubin JE. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone. 1988;9:155–163. doi: 10.1016/8756-3282(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow N, Mol C, Hayford C, Ghaem-Maghami S, Dibb NJ. Dasatinib is a potent inhibitor of tumour-associated macrophages, osteoclasts and the FMS receptor. Leukemia. 2009;23:590–594. doi: 10.1038/leu.2008.237. [DOI] [PubMed] [Google Scholar]
- Canalis E, Centrella M, McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J. Clin. Invest. 1988;81:1572–1577. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989;83:2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright CA, Meisler AI, Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci U S A. 1990;87:558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Ye XC, Chu K, Navone NM, Sage EH, Yu-Lee LY, et al. A secreted isoform of ErbB3 promotes osteonectin expression in bone and enhances the invasiveness of prostate cancer cells. Cancer Res. 2007;67:6544–6548. doi: 10.1158/0008-5472.CAN-07-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005;65:8274–8285. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- Debiais F, Lemonnier J, Hay E, Delannoy P, Caverzasio J, Marie PJ. Fibroblast growth factor-2 (FGF-2) increases N-cadherin expression through protein kinase C and Src-kinase pathways in human calvaria osteoblasts. J Cell Biochem. 2001;81:68–81. doi: 10.1002/1097-4644(20010401)81:1<68::aid-jcb1024>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Dorkin TJ, Robinson MC, Marsh C, Bjartell A, Neal DE, Leung HY. FGF8 overexpression in prostate cancer is associated with decreased patient survival and persists in androgen independent disease. Oncogene. 1999a;18:2755–2761. doi: 10.1038/sj.onc.1202624. [DOI] [PubMed] [Google Scholar]
- Dorkin TJ, Robinson MC, Marsh C, Neal DE, Leung HY. aFGF immunoreactivity in prostate cancer and its co-localization with bFGF and FGF8. J Pathol. 1999b;189:564–569. doi: 10.1002/(SICI)1096-9896(199912)189:4<564::AID-PATH480>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Fakhry A, Ratisoontorn C, Vedhachalam C, Salhab I, Koyama E, Leboy P, et al. Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone. 2005;36:254–266. doi: 10.1016/j.bone.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- Globus RK, Patterson-Buckendahl P, Gospodarowicz D. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor beta. Endocrinology. 1988;123:98–105. doi: 10.1210/endo-123-1-98. [DOI] [PubMed] [Google Scholar]
- Hannon RA, Clack G, Rimmer M, Swaisland A, Lockton JA, Finkelman RD, et al. Effects of the Src Kinase Inhibitor Saracatinib (AZD0530) on Bone Turnover in Healthy Men: A Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose Phase I Trial. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090830. [DOI] [PubMed] [Google Scholar]
- Ito H, Gardner-Thorpe J, Zinner MJ, Ashley SW, Whang EE. Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery. 2003;134:221–226. doi: 10.1067/msy.2003.224. [DOI] [PubMed] [Google Scholar]
- Jacob K, Webber M, Benayahu D, Kleinman HK. Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res. 1999;59:4453–4457. [PubMed] [Google Scholar]
- Kopetz S, Shah AN, Gallick GE. Src continues aging: current and future clinical directions. Clin Cancer Res. 2007;13:7232–7236. doi: 10.1158/1078-0432.CCR-07-1902. [DOI] [PubMed] [Google Scholar]
- Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101:263–268. doi: 10.1038/sj.bjc.6605178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Boast S, de los Santos K, Schieren I, Quiroz M, Teitelbaum SL, et al. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat Genet. 2000;24:304–308. doi: 10.1038/73542. [DOI] [PubMed] [Google Scholar]
- Li Z, Mathew P, Yang J, Starbuck M-W, Zurita AJ, Liu J, et al. Androgen receptor–negative human prostate cancer cells induce osteogenesis through FGF9-mediated mechanisms. J. Clin. Invest. 2008;118:2697–2710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-H, Cheng CJ, Lee Y-C, Ye X, Tsai W-W, Kim J, et al. A 45-kDa ErbB3 secreted by prostate cancer cells promotes bone formation. Oncogene. 2008;27:5195–5203. doi: 10.1038/onc.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis C, Lin S-H. Osteoblasts in prostate cancer metastasis to bone. Nature Reviews Cancer. 2005;5:21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Lowe C, Yoneda T, Boyce BF, Chen H, Mundy GR, Soriano P. Osteopetrosis in Srcdeficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci U S A. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, Bernardini S, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000;151:311–320. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Fukabori Y, Nakano K, Takezawa Y, CSuzuki T, Yamanaka H. Increased expression of bone morphogenetic protein-7 in bone metastatic prostate cancer. Prostate. 2003;54:268–274. doi: 10.1002/pros.10193. [DOI] [PubMed] [Google Scholar]
- Mayahara H, Ito T, Nagai H, Miyajima H, Tsukuda R, Taketomi S, et al. In vivo stimulation of endosteal bone formation by basic fibroblast growth factor in rats. Growth Factors. 1993;9:73–80. doi: 10.3109/08977199308991583. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E. Effects of fibroblast growth factors on deoxyribonucleic acid and collagen synthesis in rat parietal bone cells. Endocrinology. 1989;125:2118–2126. doi: 10.1210/endo-125-4-2118. [DOI] [PubMed] [Google Scholar]
- Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- Nelson JB, Hedican SP, George AH, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature Med. 1995;1:944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- Nelson JB, Nguyen SH, Wu-Wong JR, Opgenorth TJ, Dixon DB, Chung LW, et al. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999;53:1063–1069. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129:1421–1432. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, et al. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007;67:2800–2808. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993;14:424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–972. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakar-Lopez F, Cheng C-J, Kim J, Shi GG, Troncoso P, Tu S-M, et al. Up-regulation of MDA-BF-1, a secreted isoform of ErbB3, in metastatic prostate cancer cells and activated osteoblasts in bone marrow. J Pathol. 2004;203:688–695. doi: 10.1002/path.1568. [DOI] [PubMed] [Google Scholar]
- Ye XC, Choueiri M, Tu SM, Lin SH. Biology and clinical management of prostate cancer bone metastasis. Front Biosci. 2007;12:3273–3286. doi: 10.2741/2311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PMOs (50,000 cells) were infected with copGFP lenti viral particles for 24 hrs. (A) Expression of copGFP after the PMOs were incubated for five days in growth medium. ×200. (B) Expression of copGFP after the PMOs were incubated in the growth medium for 3days followed in differentiation medium for 2 days. ×200. The PMOs in the differentiation medium assumed a flattened and extended morphology and appeared bigger in their sizes compared to those PMOs in growth medium.
Absence of osteoclasts in the PMOs. PMOs or RAW264.7 cells were treated without or with various concentrations of RANKL as indicated for 96 hrs. The presence of osteoclasts was detected by TRAP staining. (A) Quantitative TRAP assay. (B) TRAP staining.


