Abstract
Objective
Rupture of abdominal aortic aneurysms (AAA) is a devastating event potentially preventable by therapies that inhibit growth of small aneurysms. Receptor of advanced glycation end products (RAGE) has been implicated in age related diseases including atherosclerosis and Alzheimer’s. Consequently, we explored whether RAGE may also contribute to the formation of AAAs.
Results
Implicating a role for RAGE in AAA, we found the expression of RAGE and its ligand AGE were highly elevated in human aneurysm specimens as compared to normal aortic tissue. In a mouse model of AAA, RAGE gene deletion (knockout) dramatically reduced the incidence of AAA to 1/3 of control (AAAs in 75.0% of controls versus 25.0% knockouts). Moreover, aortic diameter was markedly reduced in RAGE knockout animals versus controls. As to mechanism, we found that RAGE was co-expressed in AAA macrophages with MMP-9, a promoter of matrix degradation, which is known to induce AAA. In vitro, advanced glycation end products (AGE) induced the production of MMP-9 in macrophages in a dose-dependent manner while blocking RAGE signaling with a soluble AGE inhibitor prevented MMP-9 expression. In vivo, RAGE gene deficiency eliminated MMP-9 activity that was prevalent in aneurismal wall of the wild-type mice.
Conclusions
RAGE promotes the development of AAA by inducing MMP-9 expression. Blocking RAGE in a mouse aneurysm model has a dramatic inhibitory effect on the formation of aneurysms. These data suggest that larger animal and eventually human trials should be designed to test oral RAGE inhibitors and their potential to prevent progression of small aneurysms.
INTRODUCTION
Abdominal aortic aneurysm (AAA) is a significant medical problem with a mortality rate associated with rupture as high as 90%1. Rupture of AAAs is a devastating event that is potentially preventable by therapies that inhibit the growth of small aneurysms. Studies of the pathophysiology of aneurysms have shown that the aortic wall of aneurysms is characterized by chronic inflammation2, loss of the extracellular protein elastin3, increased collagen metabolism, elevated activities of matrix-degradating enzymes matrix metalloproteinases (MMPs) suchas MMP-9 and MMP-24, and changes in cellular content, including inflammatory cell infiltration and apoptosis of vascular smooth muscle cells (SMCs)5. However, the precise mechanism responsible for AAA formation and progression is still under debate, and developing a better of understanding of this mechanism could lead to the development of novel strategies for the prevention of this life-threatening vascular disease.
MMP-9 is considered to be an important mediator for the development of aneurysms. MMP-9 not only degrades the extracellular matrix, but it also plays an important role in many physiologic responses such as the control and regulation of the inflammation, wound healing, and remodeling relevant for atherosclerosis and restenosis6–8. MMPs destroy elastin and collagen, which is fundamental to the development of aneurysms2. However, knowledge about the stimuli for induction of MMP-9 during the process of aneurismal formation remains limited.
Advanced glycation end products (AGEs) are formed from proteins and peptides by non-enzymatic glycoxidation after contact with aldose sugars9, 10. AGE as well as its receptor (RAGE) has been implicated in disease of the elderly age including atherosclerosis and Alzheimer’s11, 12. AGEs are known to accumulate in the vessel wall, where they may act to alter the extracellular matrix (ECM), cell surface receptors, or impact the function of intracellular proteins13. AGE/RAGE interactions elicit oxidative stress and induceproinflammatory or procoagulant cellular responses including increases in the vascular cell adhesion molecule-1 (VCAM-1) and TNF-α expression14. These events are thought to be mediated by the activation of NF- κB by circulating AGEs. Moreover, previous studies have shown that RAGE−/− miceare protected from the lethal effects of septic shock, whichdepend largely on the innate immune response15,16. Thus, the emerging importance for AGE/RAGE in the pathophysiology of diseases of the elderly and in inflammatory processes led us to hypothesize that this ligand and receptor may contribute to the formation of AAAs.
In this study, we tested whether AGE/RAGE is involved in the formation of AAA by measuring the expression of AGE and RAGE in AAA tissues both in the mouse and in humans, and by testing whether gene deletion of RAGE affected the formation of aneurysms in a mouse AAA model. Moreover, we have identified a mechanism by which AGE/RAGE may promote aneurysms through the induction of MMP-9. Our results provide evidence that AGE/RAGE plays an important role in the development of aneurysms.
MATERIALS AND METHODS
Human Tissue Procurement
Human AAA specimens were obtained from patients undergoing surgical repair of AAA by the members of the Division of Vascular Surgery. As controls, normal human aorta samples were obtained from autopsy specimens (age ≥ 50) through Brain and Tissue Bank for Developmental Disorders (National Institute of Child Health and Human Development). No patients were known to have connective tissue disorders. The use of human samples in this study was approved by the Institutional Review Board at Weill Cornell Medical College and Columbia Medical Center.
Animal AAA model
ApoE−/− mice were obtained from The Jackson Laboratories (Bar Harbor, Maine, USA). Homozygous RAGE−/− mice, backcrossed more than 12 generations into C57BL/6, were bred into the apoE−/− background to obtain apoE−/− RAGE−/− mice. ApoE −/− RAGE−/− mice exhibit normal reproductive capacity, physical fitness, and overall phenotype under nominal conditions17. ApoE−/− RAGE−/− and apoE−/− RAGE+/+ mice were anesthetized and AngII (1000 ng/kg/min;Sigma, St. Louis, MO) or phosphate buffered saline (PBS) was administered by Alzet osmoticminipumps (model 2004) implanted subcutaneously, as describedpreviously18. The maximum diameter of the infrarenal aorta and the suprarenal aorta were measured 4 weeks after the pump implantation. AAA tissues were harvested after animals were anesthetized. The suprarenal aorta was removed and tissue was frozen for protein extraction or fixed with 4% PFA for cross section preparation. Tissues for cross section were then paraffin embedded for histological analysis. All procedures were approvedby the Institutional Animal Care and Use Committee at the University of Columbia.
Cell Culture
The murine macrophage cell line RAW 264.7 and rat aortic vascular smooth muscle cells were obtained from American type Culture Collection (ATCC, Manassas, VA, USA). RAW 264.7 cells and rat vascular smooth muscle cells were maintained in DMEM supplemented with 10% fetal calf serum (FCS), 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2/water-saturated incubator at 37 °C. Treatments with vehicle(PBS), AGE (Sigma), and S100b (Calbiochem) were carried out in 1% FCS.
Gelatin Zymography
For the in vitro studies, conditioned media were collected at the indicated time points and centrifuged for 5 min at 500×g to remove cells and debris. For the in vivo studies, the supera renal segments of aortas were harvested from mice 28 day after pump implantation. The tissues were homogenized in phosphate-buffered saline buffer (PBS) (pH 7.4) on ice, and homogenates were centrifuged at 10,000 rpm for 5 min, and the supernatants were collected. The total protein concentration in supernatants was determined using the Bio-Rad protein assay. Supernatants or tissue extracts containing equal amounts of protein (10 μg) were mixed with SDS gel-loading buffer and then loaded without reduction or heating onto 10 % SDS-polyacrylamidegels containing 0.1% gelatin. After electrophoresis, SDS was removed by washing with buffer (50 mm Tris/HCl, pH 7.6, 150 mM NaCl, 5 mM CaCl2, 2 μM ZnCl2, 0.1%, Triton X-100) for 30 min at room temperature with gentle agitation to renature enzymes. The gels were subsequently incubated in zymogen development buffer (50 mM Tris-Cl, pH 7.5; 10 mM CaCl2; 1μM ZnCl2; 1% Triton X-100; 0.02% NaN3) at 37°C for 16 to 24 h . After briefly washing in water, gels were stained with Coomassie blue R-250 for 1 h. Gels were destained with 40% methanol and 5% acetic acid until clear white bands against a blue background were visible.
Western Blotting Analysis
Fifty micrograms of protein from each human aortic sample or an equal protein content of supernatants from control and AGE-treated samples were resolved on 10% SDS PAGE gels. Proteins were then transferred onto nitrocellulose membranes by electroblotting using an electroblotting apparatus. Nonspecific binding of the membranes was blocked with wash buffer containing 5% (w/v) nonfat dry milk for one hour. Membranes were washed with wash buffer three times for 10 min and incubated with appropriate dilution of RAGE, MMP-9 or MMP-2 antibodies in wash buffer overnight at 4 °C. Subsequently, the membranes were washed with wash buffer and incubated with an appropriate secondary antibody (horseradish peroxidase-conjugated goat antimouse or antirabbit IgG) for 1 h. After washing the membrane three times for 10 min, band detection was revealed by enhanced chemiluminescence using ECL Western blotting detection reagents and exposed to ECL hyperfilm.
RT-PCR
Total RNA from macrophages culture treated with AGE and S100b was isolated using the RNeasy® Mini kit following the instructions of the manufacturer (Qiagen). Messenger RNA of MMP-9 and GAPDH expression in each sample was determined by reverse transcription-polymerasechain reaction (RT-PCR) using GeneAmp RNA PCR Core Kit (PerkinElmer Life Sciences).
ELISA assay of AGE
Aortic tissues lysates were prepared using cell lysis buffer for determining protein concentrations and measure AGE product. The competitive AGE-ELISA procedure was performed as described previously19.
Immunohistochemistry
Immunostaining for AGE and RAGE using monoclonal anti-AGE and anti-RAGE antibody (a gift from A. M. Schmidt) were performed as described previously20. Slides were visualized with a Nikon Eclipse E800 upright microscope. Digital images were acquired using a RetigaEXi CCD digital camera and processed and analyzed using IPLab software.
Statistical analysis
Data are expressed as mean ± SE. Unpaired Student's t test was used to evaluate the statistical differences between normal and AAA patient groups. Values of p < 0.05 were considered significant.
Results
AGE and RAGE are highly expressed in human AAA
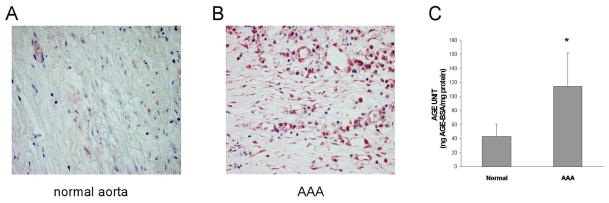
Previous reports have implicated AGE to be involved in the pathogenesis of vascular inflammation as well as a number of diseases of the elderly. Since AAA is a disease of the elderly, we began our studies by determining whether the expression of AGE is altered in AAA samples from humans. Immunostaining revealed a markedly increased accumulation of AGE in AAA tissues compared to normal aortic tissue (Fig. 1A and B). The expression of AGE was then quantified in human AAA tissues and normal human aortic tissues by ELISA. As shown in Fig. 1C, the accumulation of AGEs in AAA tissues was 2.7 fold higher than that found in normal aortic tissues. Since the expression of RAGE is often upregulated in parallel with its ligands, we next evaluated RAGE expression in human AAA tissues by immunostaining and Western blotting. As shown in Fig. 2A–C, human AAA tissue contained significantly higher levels of RAGE expression compared with tissue from normal aorta. Increased staining for RAGE was predominantly observed in CD68-positive macrophage cells and to a lesser extent in smooth muscle cell specific α-actin (SMA)-positive SMC (Fig. 2D–I).
Fig. 1. AGE expression in human AAAs.
(A) Normal aortic tissue from cadaveric specimens and (B) human AAA tissue collected during surgical repair. Samples were stained with an antibody specific for AGE (magnification: x200). (C) Tissue samples were lysed and subjected to an antibody specific for AGE and quantified by ELISA.
Fig. 2. RAGE expression in macrophages and SMCs of tissue from human AAA.
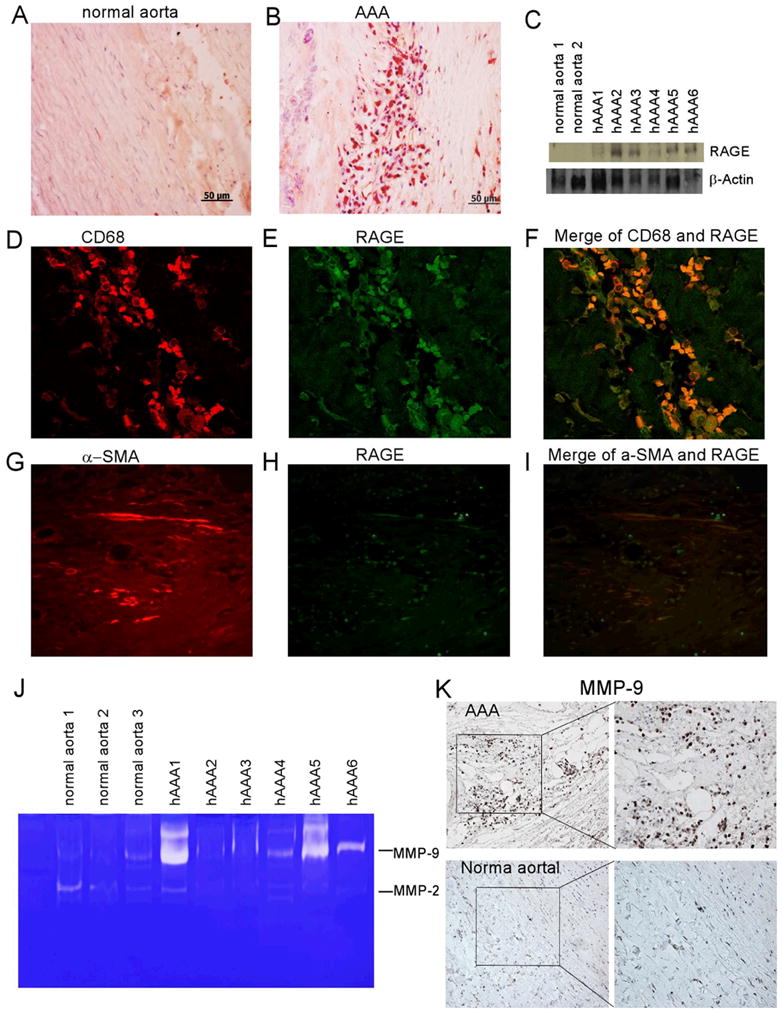
(A) Immunohistochemical staining of RAGE in normal aorta or (B) tissue from human aneurysms (magnification: x200). (C) Western blot showing RAGE expression in human AAA samples (n = 6) and no expression in normal human aorta tissue. Equal protein loading was confirmed by re-probing with β-actin. (D–F) Double-immunofluorescent staining for RAGE and CD68, a marker for macrophages. (G–I) Double- immunofluorescent staining for RAGE and α-SMA, a marker for SMC (magnification: x200). (J) Gelatin zymography for MMP-2 and MMP-9 in protein extracts from normal human aorta and AAA tissue. (K) Immunohistochemistry for MMP-9 in human normal and AAA tissues (magnification: left x100; right x200).
MMP-9 expression is associated with AGE/RAGE signaling in AAA tissues
MMP-9 has been reported as an important factor responsible for the development of AAA2, 21, 22. Consistent with previous reports, we found significantly higher MMP-9 activity in AAA tissues compared to normal aortic tissues, measured by gelatin zymography (Fig. 2J). Immunostaining of AAA tissue showed that MMP-9 was mostly expressed in inflammatory cells, but not in SMCs nor in fibroblasts (Fig. 2K).
RAGE is up-regulated in mouse AAA model
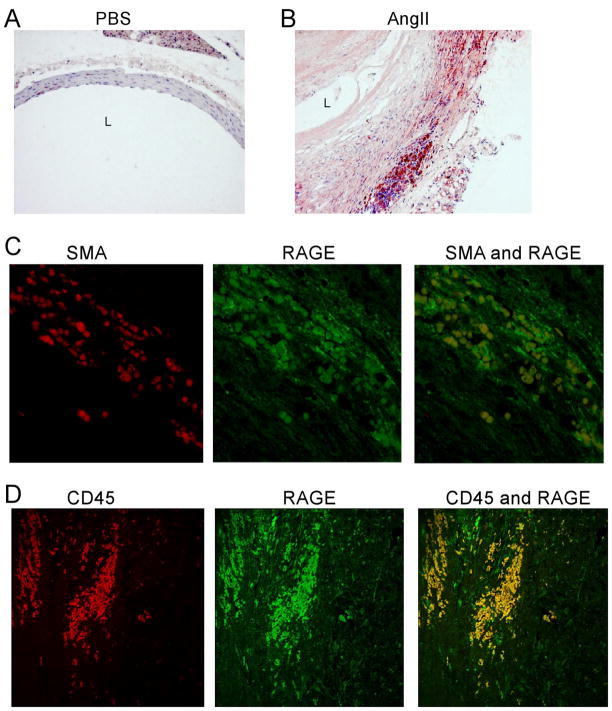
To further explore the potential involvement of AGE/RAGE signaling pathway in AAA development, we examined RAGE expression in a mouse model of AAA. The mouse model was created as described in methods by continuous infusion of Ang II into apoE null mice. Consistent with previous reports, Ang II produced an AAA-like dilation involving the suprarenal aorta in approximately 75 % of the treated mice. Immunostaining revealed a marked upregulation of RAGE expression in the suprarenal aorta of AngII-infused mice as compared to the aortic tissue of mice infused with saline (As shown in Fig. 3A–B). Similar to what was we observed in human AAA specimens, the highest expression of RAGE was found in CD45 labeled cells, a marker for leukecytes (Fig. 3D). RAGE was infrequently colocalized with cells positive for the SMC marker α-actin suggesting that RAGE may also emanate from vascular SMCs (Fig. 3C).
Fig. 3. RAGE expression in SMC and inflammatory cells in an Angiotensin mouse model of AAA.
(A–B) Immunohistochemistry for RAGE expression in ApoE−/− mice infused with PBS (A) or Angiotensin II (B) (black L indicates lumen). (C–D) Double immunofluorescence staining for RAGE with α-SMA (C) or CD45 (D).
RAGE is necessary for AAA development
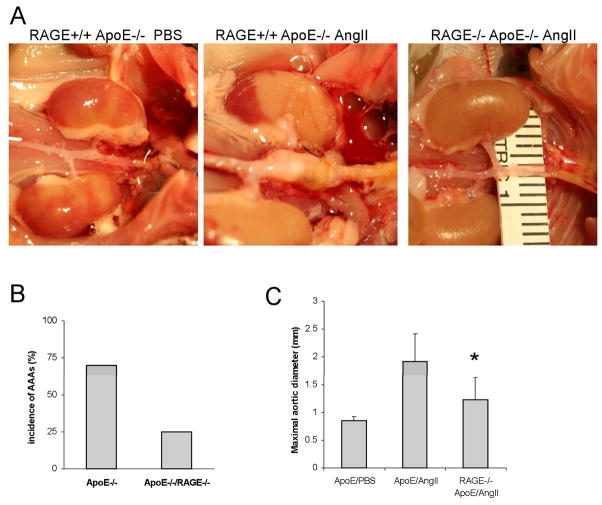
To further test the role of RAGE in the development of AAA, we generated a strain of mice that are deficient in both RAGE and apoE (RAGE−/− apoE−/−). Ang II was administered to double “knock-out” mice (RAGE knockout) or single “knock-out” (control or RAGE wild-type) mice for 28 days. As shown in Fig. 4, six out of the eight or 75% of the control mice developed aneurysms, defined as a 50% or larger increase in suprarenal aorta compared to infra renal aorta (the aneurysm model). In contrast, only two out of the eight, or 25%, of the RAGE knock-out mice developed AAA (Fig. 4A&B). Consistently, the maximum diameter of the suprarenal abdominal aorta in the RAGE knock-out mice was significantly diminished compared to the control mice (Fig. 4C). Furthermore, two of the control mice died prior prematurely due to ruptured aorta. No premature death occurred in RAGE knock-out mice.
Fig. 4. Effect of RAGE deletion in the angiogtensin mouse AAA model.
RAGE+/+ApoE−/− (control) or RAGE−/− ApoE−/− (RAGE knockout) mice were treated with PBS vehicle or Ang II for 4 weeks, at which time aortas were dissected and removed. (A) Representative examples of abdominal aortas from control mice treated with PBS (left), Ang II (middle) and RAGE knockout mice treated with AngII (right). (B) Aneurysm incidence (mice with aneurysms/total mice examined) in control and RAGE knockout mice; suprerenal aortas with > 1.5-fold the diameter of the infrarenal aorta were considered aneurysms. (C) Maximum suprarenal/infrarenal diameter for each group; *p< 0.05.
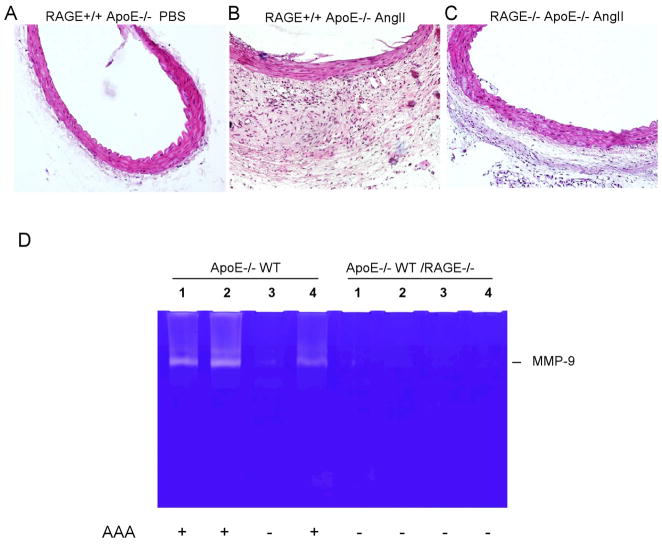
Examination of the histology of aortic sections revealed substantial expansion and cellularity of the tunic adventitia in the control mice that were treated with angiotensin II, a characteristic of AAA associated inflammation (Fig. 5A&B). This adventitial response was markedly diminished in RAGE knockout mice (Fig. 5C). To determine whether MMP-9 is involved in the development of aneurysms in the mouse AAA model, we examined levels of MMP-9 activity inaortic tissue from control and RAGE knock-out mice. In this particular experiment, we compared four samples from each. Three out of the four control mice developed AAA-like dilation in comparison to zero of four RAGE knockout mice. Elevated MMP-9 activity was identified in the three control mice with aortic dilatation, whereas MMP-9 was barely detectable in the control mouse without aortic dilatation as well as all of the aortic specimens from RAGE knock-out mice (Fig. 5D).
Fig. 5. Analysis of MMP-2 and 9 in mouse aortic tissues.
(A–C) Histological sections of the mouse aorta were stained with hematoxylin/eosin. Apo E−/− mice treated with PBS (A), ApoE−/− mice treated with AngII (B), and RAGE−/− ApoE−/− mice treated with AngII (C). (D) Gelatin zymography for MMP-9 of protein extracts from suprarenal aorta tissue in angiotensin treated ApoE−/− and ApoE−/− RAGE−/− mice.
AGE induces MMP-9 in Macrophages
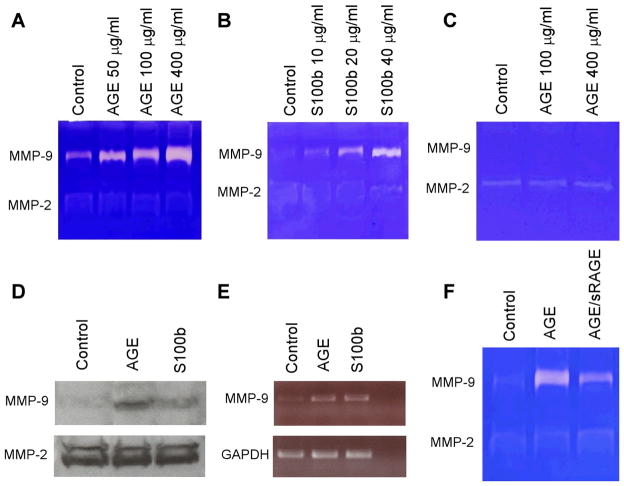
To further evaluate the relationship between AGE/RAGE and MMP-9 production, we stimulated rat vascular SMCs or a macrophage cell line (RAW 264.7) with increasing amounts of the RAGE ligand, AGE or S100b. S100b, a member of the S100 family of proteins containing two EF-hand-type calcium-binding domains, is another ligand of RAGE23. As shown in Fig. 6A and B, both AGE and S100b increased MMP-9 activity (gelatinolytic activity at 92-kDa) in macrophages in a concentration dependent manner without significantly affecting the activity of MMP-2, another MMP that has been associated with the development of aneurysms. In contrast, the SMCs treated with AGE did not evidence increased enzymatic activity of either MMP-9 or MMP-2 (Fig. 6C). The stimulatory effects of AGE and S100b on MMP-9 were confirmed by Western blotting (Fig. 6D). Totest whether AGE stimulates MMP-9 at a transcriptional level, we analyzed MMP-9 mRNA levels by RT-PCR. Compared to control cells, macrophages treated with AGE or S100b demonstrated significantly enhanced levels of MMP-9 mRNA (Fig. 6E). To determine whether the observed effect on MMP-9 is indeed mediated by AGE/RAGE signaling, we pre-treated macrophages with a RAGE inhibitor: soluble RAGE (sRAGE), a truncated form of RAGE that is composed of only the extracellular ligand-binding domain and lacking the cytosolic and transmembrane domains24. As shown in Fig. 6F, inhibition of RAGE signaling with sRAGE blocked MMP-9 induction in response to AGE.
Fig. 6. In vitro measurement of MMP-2 and 9 in macrophages and SMCs following stimulation with AGE.
(A) RAW 264.7 cells (macrophage cell line) were treated with 50, 100 and 400μg/mL of AGE for 24 h, then assessed for MMP-9 and MMP-2 activity by gelatin zymography. (B) RAW 264.7 cells were treated with 10 to 40μg/mL of S100b for 24 h. Supernatant was collected and assessed for MMP-9 and MMP-2 activity by gelatin zymography. (C) Rat SMCs cells were treated with 100 or 400μg/mL of AGE for 24 h, then assessed for MMP-9 and MMP-2 activity by gelatin zymography. (D–E) In RAW 264.7 cells, protein levels of MMP-9 and MMP-2 in response to AGE and S100b were confirmed by Western blotting (D) or mRNA expression of MMP-9 was measured by RT-PCR (E). (F) RAW 264.7 cells were treated with AGE (200 μg/ml) and 100 μM sRAGE or solvent for 24 h then assessed for MMP-9 and MMP-2 activity by gelatin zymography.
Discussion
In this study, we clearly demonstrate a significant association between AGE/RAGE and aneurysm formation in humans as demonstrated by the presence of these two proteins in human aneurysmal tissues. To determine cause and effect, we employed a mouse model of AAA produced by the infusion of angiotensin II in ApoE deficient mice. We were able to demonstrate a markedly reduced incidence of AAA in mice that were lacking the gene for RAGE. To our knowledge, this is the first study to report an association between AGE/RAGE and aneurysms. Our findings suggest a novel association between AGE and RAGE and the pathogenesis of this important and lethal condition.
It is well known that the degradation of AGE is diminished with aging which in turn results in its tissue accumulation25. Indeed, several studies have reported that AGE and RAGE are involved in age related diseases such as Alzheimer’s, diabetes and atherosclerosis26, 27. AGE has a number of actions that contribute to its provocative effects on disease including effects on extracellular matrix as well as its ability to induce inflammation and the generation of reactive oxygen species.
Likely the most prevalent risk factor for aneurysms is age. Aneurysms become increasingly prevalent with age with the incidence in the 9th decade being almost 10 times that in the 6th decade. Thus, it is a plausible hypothesis that AGE and RAGE might play a role in the development of AAA. Previous investigators have reported that RAGE and AGE are involved in other vascular diseases specifically restenosis and atherosclerosis. In fact our laboratory has demonstrated an important role for RAGE in restenosis17, 28, 29. However, over the past several years it has become clear that aneurysms once thought to be a form of atherosclerosis, have a pathogenesis that is distinct from that of occlusive vascular disease.
We chose in our initials studies of RAGE to employ a model that uses angiotensin-II to induce the development of aneurysms. This is a unique model that is characterized by the initial formation of dissection followed by dilatation of the supera renal aorta. Interestingly, this model may more closely parallel in humans, aortic dissection which produces aneurysms secondary to a weakened dissected wall; a process that may differ from the one that predisposes to the traditional infrarenal aneurysm in humans. There are two additional animal models of AAA, one resulting from the external application of calcium chloride and a second that is produced by the intraluminal infusion of elastase. These models are similar in that in all three, inflammation is essential. However, the differences are sufficiently great that the role of RAGE in the formation of AAA should confirmed using conditions that more precisely model those that produce the traditional infrarenal aneurysm.
The accumulation of natural RAGE ligands, such as AGE as well as the HMGB-1 and S100A12 polypeptides, has been associated with the activation of inflammatory cells and the production of proinflammatory mediators30. Amongst these ligand-RAGE effects are the cellular activation of signal transduction cascades that generate reactive oxygen species which in turn trigger inflamation31. Multiple intracellular pathways including the MAP kinases and PI3 kinase have been reported to be activated by RAGE32. Activation of these signal transduction pathways in turn turns on the redox-sensitive transcription nuclear factor NK-κB31, which in turn leads to gene expression of pro-inflammatory cytokines and leukocyte adherence molecules33.
Extracellular matrix (ECM) proteins, such as collagens, elastin and proteoglycans, are important structural components in arteries34. Degradation of these structural components by matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9, has been reported as important contributor to the pathogenesis of AAA in various animal models of AAA35, 36 and in humans2, 37. Moreover, systemic administration of MMP antagonists can decrease aneurysmal dilatation in the elastase-induced rodent model of AAA38. There is preliminary data in humans that that treatment of patients with small aneurysms with the MMP inhibitor, doxycycline, may decrease the growth rate of aneurysms39. In the current study, we demonstrate that AGE is a potent inducer of MMP-9 production in macrophages in vitro. Moreover, the formation of aneurysms in the angiotensin model is associated with enhanced expression of MMP-9 and this enhanced expression is eliminated in the RAGE knockout model. It is likely that AGE/RAGE at least in part affects the formation of aneurysms via the induction of MMP-9. As a possible mechanism for AGEs’ effect on MMP-9, it has been demonstrated that AGE/RAGE induces NF-κB activation through a variety of signal transduction pathways40–42. The NF-κB in turn, through activation of a protein 1 (AP-1) binding site in the MMP-9 promoter, is essential for activation of MMP-9 gene43. Nevertheless, the precise mechanism of AGE/RAGE induced MMP-9 needs to be further studied.
The soluble form of RAGE (sRAGE) can potentially bind to an AGE ligand thereby acting as a decoy, preventing the AGE–RAGE interaction and RAGE activation44. Administration of a recombinant soluble form of RAGE (sRAGE) consisting of the extracellular ligand-binding domain has been shown not only to suppress the development of atherosclerosis, but also to stabilize established atherosclerosis in diabetic apolipoprotein E null mice45, 46. Inhibition of the AGE/RAGE interaction opens new possibilities for the treatment of small aneurysms. An unanswered question is the timing of therapy. Animal models allow institution of preventative therapies at the inception of the processes that lead to aortic dilatation. However, most aneurysms are discovered after aortic dilatation has already occurred and presumably many years after the inciting events. Whether RAGE inhibitors will be useful at the later stages of AAA development remains to be determined. Encouraging is the fact that increased levels of RAGE remain present in the tissue of the relatively large aneurysms sampled in this study and inflammation also appears to play an important role in aneurysms of all sizes.
In summary, our results suggest that AGE and RAGE are involved in the development of AAAs. These findings contribute to a better understanding of the mechanisms underlying the pathogenesis of AAAs. Manipulation of the interaction between AGE and RAGE may ultimately lead to novel therapies to treat and prevent AAA progression.
Acknowledgments
This work was supported by a Public Health Service Grants R01 HL-68673 and R01-HL 081424 (K. Kent and B. Liu) from National Heart, Lung, and Blood Institute, and Startup grant from Columbia University Department of Surgery (F. Zhang) and National Institute of Health Training grants F32 HL088818-01 (Shirling). We are grateful to Yu Shan Zou and Wu Qu for technical assistance, to Dr. Shi-Fang Yan for advice on the use of RAGE antibody.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- 1.Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep. 2002;50(16):1–85. [PubMed] [Google Scholar]
- 2.Freestone T, Turner RJ, Coady A, et al. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15(8):1145–51. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 3.Huffman MD, Curci JA, Moore G, et al. Functional importance of connective tissue repair during the development of experimental abdominal aortic aneurysms. Surgery. 2000;128(3):429–38. doi: 10.1067/msy.2000.107379. [DOI] [PubMed] [Google Scholar]
- 4.Sinha S, Frishman WH. Matrix metalloproteinases and abdominal aortic aneurysms: a potential therapeutic target. J Clin Pharmacol. 1998;38(12):1077–88. [PubMed] [Google Scholar]
- 5.Ocana E, Bohorquez JC, Perez-Requena J, et al. Characterisation of T and B lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis. 2003;170(1):39–48. doi: 10.1016/s0021-9150(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 6.Van den Steen PE, Dubois B, Nelissen I, et al. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37(6):375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 7.Kuzuya M, Iguchi A. Role of matrix metalloproteinases in vascular remodeling. J Atheroscler Thromb. 2003;10(5):275–82. doi: 10.5551/jat.10.275. [DOI] [PubMed] [Google Scholar]
- 8.Pearce WH, Shively VP. Abdominal aortic aneurysm as a complex multifactorial disease: interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann N Y Acad Sci. 2006;1085:117–32. doi: 10.1196/annals.1383.025. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt AM, Hori O, Brett J, et al. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14(10):1521–8. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Walker DG, Schmidt AM, et al. RAGE: a potential target for Abeta-mediated cellular perturbation in Alzheimer's disease. Curr Mol Med. 2007;7(8):735–42. doi: 10.2174/156652407783220741. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Shimogaito N, Wu X, et al. Toxic advanced glycation end products (TAGE) theory in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2006;21(3):197–208. doi: 10.1177/1533317506289277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 14.Valencia JV, Mone M, Zhang J, et al. Divergent pathways of gene expression are activated by the RAGE ligands S100b and AGE-BSA. Diabetes. 2004;53(3):743–51. doi: 10.2337/diabetes.53.3.743. [DOI] [PubMed] [Google Scholar]
- 15.Liliensiek B, Weigand MA, Bierhaus A, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113(11):1641–50. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutterloh EC, Opal SM, Pittman DD, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007;11(6):R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi T, Yan SF, Yan SD, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111(7):959–72. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105(11):1605–12. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang JS, Wendt T, Qu W, et al. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102(8):905–13. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 20.Hollenbeck ST, Sakakibara K, Faries PL, et al. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120(2):288–94. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118(9):3012–24. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson WR, Anderton M, Schwalbe EC, et al. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation. 2006;113(3):438–45. doi: 10.1161/CIRCULATIONAHA.105.551572. [DOI] [PubMed] [Google Scholar]
- 23.Kim W, Hudson BI, Moser B, et al. Receptor for advanced glycation end products and its ligands: a journey from the complications of diabetes to its pathogenesis. Ann N Y Acad Sci. 2005;1043:553–61. doi: 10.1196/annals.1338.063. [DOI] [PubMed] [Google Scholar]
- 24.Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res Ther. 2005;7(4):R817–24. doi: 10.1186/ar1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99(3):457–68. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luth HJ, Ogunlade V, Kuhla B, et al. Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer's disease brains. Cereb Cortex. 2005;15(2):211–20. doi: 10.1093/cercor/bhh123. [DOI] [PubMed] [Google Scholar]
- 27.Suliman ME, Heimburger O, Barany P, et al. Plasma pentosidine is associated with inflammation and malnutrition in end-stage renal disease patients starting on dialysis therapy. J Am Soc Nephrol. 2003;14(6):1614–22. doi: 10.1097/01.asn.0000067413.32377.cf. [DOI] [PubMed] [Google Scholar]
- 28.Wendt T, Bucciarelli L, Qu W, et al. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4(3):228–37. doi: 10.1007/s11883-002-0024-4. [DOI] [PubMed] [Google Scholar]
- 29.Naka Y, Bucciarelli LG, Wendt T, et al. RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24(8):1342–9. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 31.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582–92. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280(5):E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498(2–3):99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 34.Aziz F, Kuivaniemi H. Role of matrix metalloproteinase inhibitors in preventing abdominal aortic aneurysm. Ann Vasc Surg. 2007;21(3):392–401. doi: 10.1016/j.avsg.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pyo R, Lee JK, Shipley JM, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–9. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longo GM, Xiong W, Greiner TC, et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110(5):625–32. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMillan WD, Pearce WH. Increased plasma levels of metalloproteinase-9 are associated with abdominal aortic aneurysms. J Vasc Surg. 1999;29(1):122–7. doi: 10.1016/s0741-5214(99)70363-0. discussion 127–9. [DOI] [PubMed] [Google Scholar]
- 38.Moore G, Liao S, Curci JA, et al. Suppression of experimental abdominal aortic aneurysms by systemic treatment with a hydroxamate-based matrix metalloproteinase inhibitor (RS 132908) J Vasc Surg. 1999;29(3):522–32. doi: 10.1016/s0741-5214(99)70281-8. [DOI] [PubMed] [Google Scholar]
- 39.Abdul-Hussien H, Hanemaaijer R, Verheijen JH, et al. Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. J Vasc Surg. 2009;49(3):741–9. doi: 10.1016/j.jvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 40.Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269(13):9889–97. [PubMed] [Google Scholar]
- 41.Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274(28):19919–24. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 43.Takeshita H, Yoshizaki T, Miller WE, et al. Matrix metalloproteinase 9 expression is induced by Epstein-Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J Virol. 1999;73(7):5548–55. doi: 10.1128/jvi.73.7.5548-5555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370(Pt 3):1097–109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4(9):1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 46.Bucciarelli LG, Wendt T, Qu W, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106(22):2827–35. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]