Abstract
Self-assembled monolayer on mesoporous supports (SAMMS™) are hybrid materials created from attachment of organic moieties onto very high surface area mesoporous silica. SAMMS with surface chemistries including three isomers of hydroxypyridinone, diphosphonic acid, acetamide phosphonic acid, glycinyl urea, and diethylenetriamine pentaacetate (DTPA) analog were evaluated for chelation of actinides (239Pu, 241Am, uranium, thorium) from blood. Direct blood decorporation using sorbents does not have toxicity or renal challenges associated with traditional chelation therapy and may have potential applications for critical exposure cases, reduction of nonspecific dose during actinide radiotherapy, and for sorbent hemoperfusion in renal insufficient patients, whose kidney clear radionuclides at very slow rate. Sorption affinity (Kd), sorption rate, selectivity, and stability of SAMMS were measured in batch contact experiments. An isomer of hydroxypyridinone (3,4-HOPO) on SAMMS demonstrated the highest affinity for all four actinides from blood and plasma and greatly outperformed the DTPA analog on SAMMS and commercial resins. In batch contact, a fifty percent reduction of actinides in blood was achieved within minutes, and there was no evidence of protein fouling or material leaching in blood after 24 hr. The engineered form of SAMMS (bead format) was further evaluated in a 100-fold scaled-down hemoperfusion device and showed no blood clotting after 2 hr. A 0.2 g quantity of SAMMS could reduce 50 wt.% of 100 ppb uranium in 50 mL of plasma in 18 min and that of 500 dpm mL−1 in 24 min. 3,4-HOPO-SAMMS has a long shelf-life in air and at room temperature for at least 8 years, indicating its feasibility for stockpiling in preparedness for an emergency. The excellent efficacy and stability of SAMMS materials in complex biological matrices suggest that SAMMS can also be used as orally administered drugs and for wound decontamination. By changing the organic groups of SAMMS, they can be used not only for actinides but also for other radionuclides. By using the mixture of these SAMMS materials, broad spectrum decorporation of radionuclides is very feasible.
Keywords: actinide, plutonium, americium, uranium, thorium, chelation therapy, SAMMS
INTRODUCTION
Actinide isotopes are components in nuclear explosives, and could be used in dirty bombs. Uranium is readily absorbed by the body, and currently there is no chelating agents recommended for its removal from systemic circulation (Stradling and Taylor 2005). The diethylenetriamine pentaacetate (DTPA) recommended for thorium is still ineffective in animal studies at high doses (Stradling et al. 1991). Although DTPA has been reported to be effective for plutonium and americium, emerging ligands that are significantly more effective for plutonium and americium decorporation in animals have been reported (Stradling et al. 1993, Volf et al. 1996).
This work evaluates solid sorbents in search for better decorporating agents for actinides from blood than the currently used DTPA chelator. Actinides are hard Lewis acids and are larger than most transition metals, thus both ligands’ hardness/softness and synergy play a role in designing effective and selective ligands for actinides. Herein, our focus is evaluating novel sorbents for actinide removal from complex biological matrices, such as blood and plasma, which contain lipids, proteins, cells, electrolytes, and anions. The effective sorbents will potentially be used in hemoperfusion. In patients with renal insufficiency, free metals or metal-chelates (if chelating agent like DTPA is administrated) are cleared from the body at a much slower rate. For illustration, with normal renal function, the half-life of Gd is ~2 hours, with loss of renal function the half-life is prolonged reaching extremes of 34 hours in chronic kidney disease patients, during which Gd may dissociate from the chelate via transmetallation process (Penfield and Reilly 2007). Delayed clearance of actinides leads to their deposition in liver and bone, from which they are difficult to remove (Stradling and Taylor 2005).
Developed at the Pacific Northwest National Laboratory (PNNL) originally for environmental and nuclear waste cleanups, the self-assembled monolayers on mesoporous supports (SAMMS™) materials offer extremely large surface area (up to 500 m2/g) and functionalities that has been fine-tuned to selectively capture heavy metals (Feng et al. 1997, Yantasee et al. 2003), actinides (Fryxell et al. 2005, Lin et al. 2005), lanthanides (Fryxell et al. 2004, Yantasee et al. 2005), radioiodine (Mattigod et al. 2003), cesium (Lin et al. 2001), and oxometallate anions (Fryxell et al. 1999).
Herein, SAMMS having organic moieties known to be effective at capturing actinides were evaluated for the sorption of actinides from blood and plasma. SAMMS materials investigated include three isomers of hydroxypyridinone, diphosphonic acid, acetamide phosphonic acid, glycinyl urea, and a diethylenetriamine pentaacetate analog. Understanding how these SAMMS materials behave in complex biological matrices would lead to many potential applications such as blood decorporation using sorbent hemoperfusion, orally administered sorbents for safe and long term treatment of radionuclide poisoning, and wound decontamination of radionuclides, all of which will advance the field of Health Physics and protect health and safety of the public in events resulting in the release of radioactive materials.
MATERIALS AND METHODS
Sorbent materials
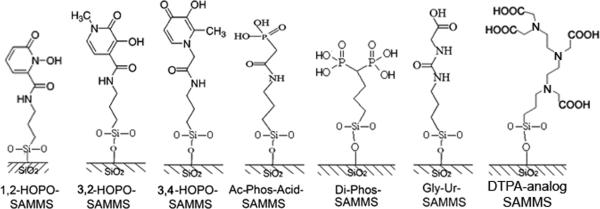
Synthesis and characterization of the SAMMS materials have been described elsewhere, including SAMMS immobilized with three analogs of hydroxypyridinones (1,2-HOPO, 3,2-HOPO, and 3,4-HOPO) (Lin et al. 2005, Yantasee et al. 2005), acetamide phosphonic acid (Ac-Phos), glycinyl-urea (Gly-Ur) (Fryxell et al. 2004, Fryxell et al. 2005), diphosphonic acid (Di-Phos), and DTPA-analog. The Na-DTPA derivatized silica gel (Silia Bond TAAcONa) was obtained from SiliCycle® Inc. (Quebec City, Canada).
Test matrices
For evaluation of SAMMS to be used in hemoperfusion of uranium, 239Pu, and 241Am, batch contact experiments were performed using human blood and plasma purchased from Golden West Biologicals, Inc. (Temecula, CA, USA). They were spiked with known amount of uranium, 239Pu, or 241Am prior to batch sorption experiments. Am and Pu stocks were kept in acid (e.g., 239Pu in 2 M HNO3 and 241Am in 0.5 M HCl) for long term stability. Before spiking to blood or plasma, the Pu and Am stocks were diluted in 0.1 M citrate buffer to avoid the precipitation of actinides while the pH was increased to ~ 7 using 1.0 M NaOH. Blood or plasma contained either 0.1 M sodium citrate or 15 unit mL−1 of sodium heparin as anticoagulant. Only small volume of uranium and thorium stock (1000 mg L−1 in 1-2% HNO3) was spiked directly into blood or plasma to achieve the desired concentration, with no impact on matrix pH. Seawater (used in stability study) was obtained from the Sequim Bay (WA) and was filtered with 0.45 μm cellulose acetate membranes prior to use. Its pH was adjusted to a desired pH value with HNO3 or NaOH.
Batch contact
Adsorption experiments were performed using the following conditions: 0.003 g of solid sorbent in 3 mL of fluid (liquid per solid ratio, L S−1, of 1000 mL g−1), shaken for 2 hrs at 250 rpm and 37 °C. Then the sorbent was removed using custom made filters (porous glass frit having pore size of ~ 8 micron), which allowed only fluid passing through but not sorbent. The metal content in the fluid before and after adding the sorbent was analyzed by an inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7500ce, Agilent Technologies, Santa Clara, CA, USA) for uranium and thorium, by gamma counts for 241Am (Wallac Wizard 1480 Gamma counter, Perkin-Elmer, Waltham, MA, USA), and by liquid scintillation counts for 239Pu (Wallac 1414 Liquid Scintillation Counter). All batch experiments were performed in triplicates and the averaged values were reported. The use of commercially available human blood fell under the Institutional Review Board (IRB) exempt classification.
Sorption rate
Kinetics experiments were carried out similarly to batch equilibrium experiments except that a 0.03 g of sorbent was dispersed in a 30 mL volume (L S−1 of 1000 mL g−1) of plasma containing 1000 dpm mL−1 of 241Am, 700 dpm mL−1 of 239Pu, or mixture of 100 ppb U/Th. Kinetics of U removal was also performed in blood using 0.06 g of sorbent and 30 mL of blood (L S−1 of 500 mL g−1). The aliquots were removed at 1, 2, 5, 10, 30, 60 min, 2, 8, and 24 hr, filtered through the custom made filters and subjected to ICP-MS analysis along with the initial solution (zero time point).
Material stability
Si dissolved from SAMMS was measured on various SAMMS as a function of pH (0-8) using pH-adjusted seawater at (L S−1 of 1000 mL g−1). The suspension was vigorously stirred for 2 hr at 37 °C. The solid was then removed and the filtrate was subject to ICP-MS analysis of Si. The percent dissolved Si (weight percent of dissolved Si per weight of SAMMS) was reported as an average value of three triplicates.
RESULTS AND DISCUSSION
Adsorption affinity (Kd)
The chemical binding affinity of a sorbent can be expressed in term of the distribution coefficient (Kd), (in mL g−1), which is simply a mass-weighted partition coefficient between the solid sorbent and liquid supernatant phase (see eq. 1).
| (1) |
where Co and Cf are the initial and final concentrations in the solution of the target species, V is the solution volume in mL, and M is the mass in gram of the sorbent. Kd is the solid phase equivalent of the more widely known liquid phase partition coefficient (typically represented with D). For the test conditions the Kd value represents the affinity of a sorbent for an analyte and the higher the Kd value, the more effective the sorbent material is at capturing and holding the target species. Kd values are appropriate performance measures of sorbent materials since the relevant levels of radionuclide exposure (that is still treatable) are often at very dilute conditions (e.g., radionuclide content is well below the saturation binding capacity of the sorbent). In biological fluids Kd values are typically much lower than in simple aquatic systems (i.e. drinking water) due to their higher ionic strength and competition from proteins. Ineffective sorbent materials have poor Kd even in simple aquatic systems since they cannot overcome the mass transport limitations, especially when the analyte is very dilute (like in case of radionuclide exposure).
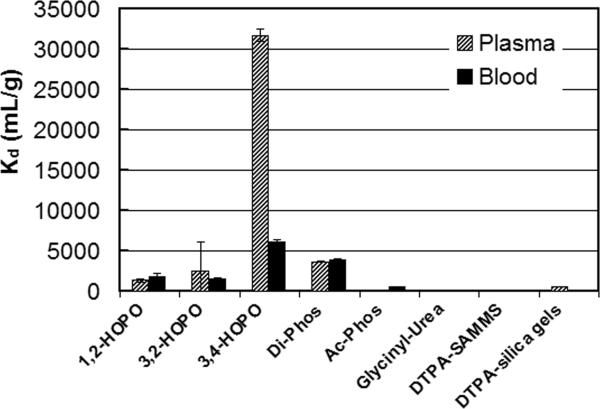
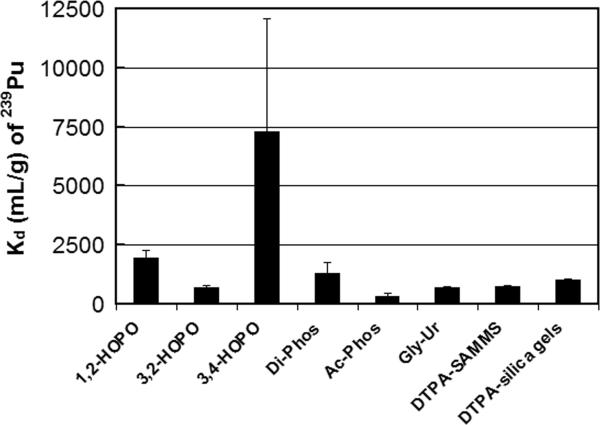
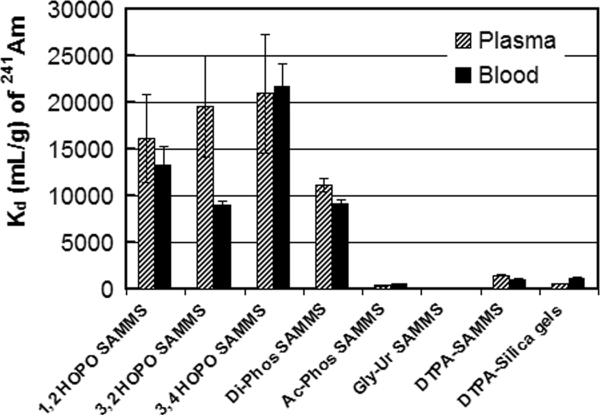
Preliminary screening (which will be published elsewhere) was carried out in pH-adjusted seawater since this would allow us to evaluate the affinity of the various ligands for the actinides in a high ionic strength matrix, without the complications of competitive binding, protein fouling, etc. The second tier screening was performed in human blood and plasma since, in these biological fluids, sorption affinity (Kd) of radionuclides may be significantly different from aqueous solutions. In this regard, complexation of radionuclides affects their solubility and chemical properties and hence their sorption affinity to a given ligand. For example, plutonium and uranium nitrate and chloride complexes are relatively more soluble than oxide complexes, thus they are able to adsorb better on sorbent materials. Other ligands and organic molecules in blood and plasma also interact with radionuclides and may affect their binding affinity to sorbent materials; for example, 47% of U is found as bicarbonate complexes, 32% binds with proteins, and 20% binds to red blood cells (Chevari and Likhner 1968). A series of experiments was performed using various SAMMS materials to capture uranium, thorium, 241Am, and 239Pu from blood and plasma. Performance was compared among the SAMMS materials and commercial resins (e.g., DTPA analog on silica gels) as well as the DTPA analog on mesoporous silica (e.g., DTPA-SAMMS). The Kd values are presented in Figs. 2-4 for uranium, 241Am, and 239Pu, respectively. It is obvious that 3,4-HOPO-SAMMS outperformed every other sorbent evaluated. The Kd values for uranium were 32,000 (plasma) and 6,100 (blood), those of 241Am were 21,000 (plasma) and 22,000 (blood), and that of 239Pu was 9,400 (blood). The large difference in blood and plasma Kd values of uranium suggests that uranium binds strongly to red blood cells, while the similarity in blood and plasma Kd values of 241Am suggests that 241Am does not (which might be attributed to higher oxidation states of uranium (+6) than those of 241Am (+3)). In plasma, this corresponds to 97% removal of uranium, suggesting that SAMMS can outcompete proteins and other ligands in the plasma for uranium. Interestingly, the Kd values suggest that the 3,4-HOPO SAMMS was best at chelating all of the actinides tested (uranium, americium, and plutonium and thorium, not shown), which is a desirable trait since (1) exposure to all these radionuclides at once is possible (e.g., in nuclear explosion) and (2) when exposure to exact radionuclide (out of this class) cannot be identified in a timely manner prior to starting the treatment. The Kd values suggest that 3,4-HOPO has higher decorporation efficacy than 1,2-HOPO and 3,2-HOPO isomers. The reason for that is not well understood at this point. It is not likely due to steric effects since all three isomers have plenty of room to flex in the monolayer (which is very important for binding species with the equatorial plane like uranyl ions), but is perhaps due to the 3,4-HOPO being less prone to biofouling than the others. The Kd values also suggest that the 3,4-HOPO-SAMMS has higher decorporation efficacy than the DTPA analog on a commercial sorbent (e.g., Na-DTPA analog on silica gels; note that this chemically tethered DTPA analog contains only four carboxylic acid groups, and not the five carboxylic acids of the parent DTPA ligand).
Figure 2.
Kd of uranium (U) in human blood and plasma measured on various sorbents, initial U of 50 ppb, L S−1 of 1300 mL g−1.
Figure 4.
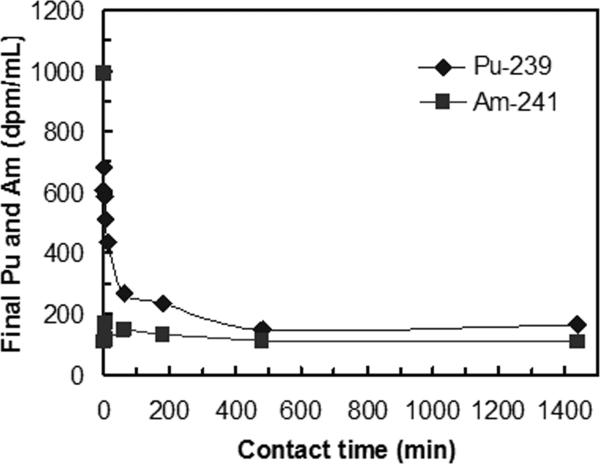
Kd of plutonium (239Pu) in human blood measured on various sorbents (initial 239Pu of 1000 dpm mL−1, L S−1 of 1000 mL g−1).
Rare earths and actinides like U, Th, Pu, and Am are hard Lewis acids and are considerably larger than the typical transition-metal cations, thus both hard anionic ligands and ligand synergy are important attributes in designing effective complexing agents for the rare earth metals. The HOPO are hard ligands and highly selective f-block chelators (inspired by iron binding siderophores secreted by bacteria (Gorden et al. 2003)). It is not surprising that HOPO ligands are most effective for these actinides than other ligands. The Raymond group has evaluated liquid formulations of HOPO ligands in rodent models and found them to be highly effective for facilitating the excretion of absorbed Th, Am, and Pu (Stradling et al. 1993, Stradling and Moody 1995, Volf et al. 1996).
Attaching the ligands on mesoporous silica substrate creates a new sorbent that could potentially be used in sorbent hemoperfusion. These sorbents can also potentially be used as orally administered drugs to limit absorption of ingested radionuclides or absorbed radionuclides from other routes of exposures (inhaled or dermal), which undergo enterohepatic recirculation. On the silica substrate of SAMMS, the HOPO ligands are in relatively close proximity, allowing multiple ligand-metal interactions, and hence strong binding affinity and stability; for example chelation ratio up to 4:1 has been observed in similar systems (Fryxell et al. 2004). Thus, 3,4-HOPO-SAMMS were evaluated further with ultimate goal of using it in sorbent hemoperfusion of actinides and lanthanides.
Sorption rate
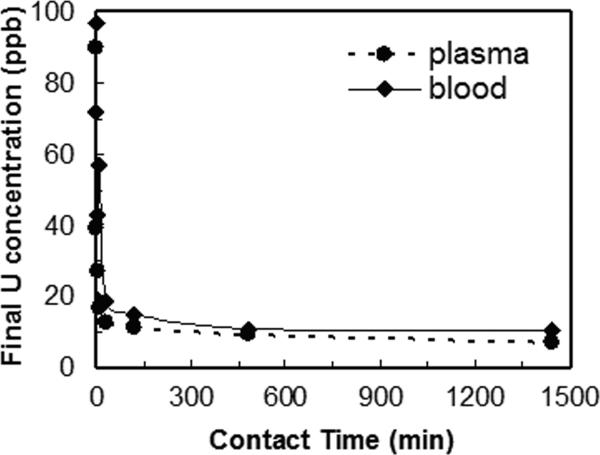
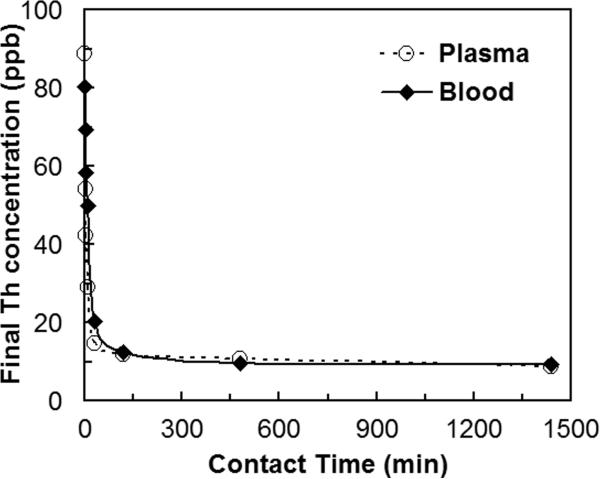
It is important that a sorbent material offers rapid sorption in order to allow the hemoperfusion treatment to be completed quickly and minimize the radionuclides leaving the intravascular space for other parts of the body. To determine the rates of radionuclide removal, the concentration profile of radionuclides were obtained in bodily fluids as a function of time that they were in contact with the 3,4-HOPO-SAMMS. The kinetics of adsorption of uranium in blood and plasma are shown in Fig. 5, those of thorium are shown in Fig. 6, and those of 241Am and 239Pu in plasma are shown in Fig. 7. In both blood and plasma, removal of uranium, thorium, and Am reached equilibrium within minutes, owing to the rigid, open pore structure of SAMMS that enhances ligand availability and facilitates mass transfer of ions to the binding sites. Such fast capture of toxic metals would be highly beneficial for chelation therapies. For Pu, the slower sorption kinetics may be a result of competitive binding by citrate (used to stabilize Pu and prevent it from precipitating in plasma) or by transferrin. After 24 hr contact, SAMMS retains the captured actinides in blood and plasma, suggesting good binding stability that is attributed to minimal protein fouling and no leaching of binding sites from SAMMS in blood over time. It is worth noting that the same removal rate of uranium was achieved in both blood and plasma simply by doubling the amount of 3,4-HOPO-SAMMS. Thorium behaved similarly.
Figure 5.
Removal rate of uranium (U) on 3,4-HOPO SAMMS, measured in human plasma and blood, initial U of 100 ppb, L S−1 of 1000 mL g−1 for plasma and 500 mL g−1 for blood.
Figure 6.
Removal rate of thorium (Th) on 3,4-HOPO SAMMS, measured in human plasma and blood, initial Th of 100 ppb, L S−1 of 1000 mL g−1 for plasma and 500 mL g−1 for blood.
Figure 7.
Adsorption kinetics of 239Pu and 241Am on 3,4-HOPO SAMMS™, measured in human plasma, initial Pu of 700 dpm mL−1 and Am of 1000 dpm mL−1, L S−1 of 1000 mL g-1.
Stability of SAMMS
Long shelf-life and stability of chelating agents are highly desirable for maintaining their stockpile in preparedness for emergency. Most SAMMS materials remain effective for many years (e.g., the HOPO-SAMMS were synthesized in 2001 and still show no measurable reduction in performance even today). Table 1 summarizes the chemical stability of 3,4-HOPO SAMMS (e.g., during use), which was measured in term of dissolved Si as a function of solution pH in pH-adjusted filtered seawater. From pH of −0.1 to physiological pH (7.4), the dissolution of Si was less than 0.4% (warranting that the efficacy will not be lost during use due to material degradation). This is attributed to SAMMS materials being composed of covalent bonding with high cross-linking of the ligand and the silica substrate.
Table 1.
Stability of various SAMMS™, presented in term of Wt.% Si dissolved per gram of material, measured in pH adjusted seawater (L/S of 1000 mL g−1).
| pH | Wt.% of Dissolved Si |
|---|---|
| −0.1 | 0.11 |
| 1.8 | 0.11 |
| 2.8 | 0.13 |
| 3.5 | 0.14 |
| 6.5 | 0.23 |
| 7.4 | 0.39 |
Application of SAMMS materials
The effectiveness of SAMMS in capturing of actinides in blood will render them viable for a range of applications in the field of Health Physics. Treatment using direct sorption from blood or plasma does not have toxicity or renal challenges associated with traditional chelation therapy. Presently envisioned potential applications include direct filtration for critical exposure cases, reduction of nonspecific dose during actinide radiotherapy and sorbent hemoperfusion.
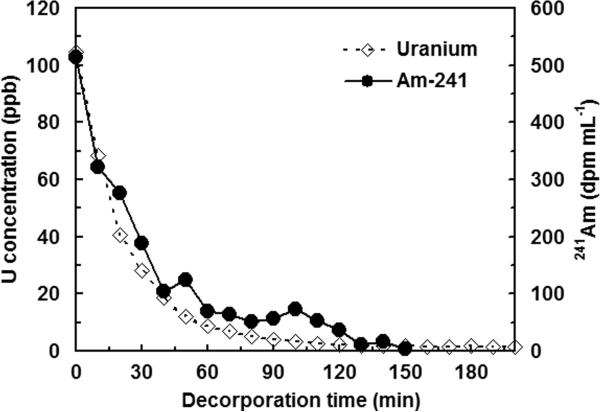
An issue with using SAMMS as sorbents for hemoperfusion devices, and other direct blood sorption applications is the potential damage the sorbent may cause to blood in a flow device due to their mineral and crystalline nature. To make SAMMS blood-compatible and minimize blood damage, an engineered form of SAMMS was created by attachment of selected functional group (e.g., 3,4-HOPO) on porous silica beads (particle size > 75 micron and porosity of 60 angstrom). A 0.2 gram quantity of the beads was then packed between two porous silica discs having pore size of 40-60 angstrom. A 50 mL volume of heparinized blood or plasma containing actinides (uranium and 141Am) stored in a stirred reservoir was continuously pumped through the bed in a close-loop manner at the flow rate of 2 ml min−1. This system corresponds to a 100-fold scaled-down of a hemoperfusion device, which normally filters 5 L of blood (adults) at the blood flow rate of 200 mL/min (Kokot et al. 1979). A 50% reductions of 100 ppb uranium and 500 dpm mL−1 of 241Am were achieved in 18 min and 24 min, respectively as shown in Fig. 8. In a separate experiment, after 3 hours contacting with blood, the flow remained constant, indicating that there was no blood clotting, which is a major issue in sorbent hemoperfusion (Stamatialis et al. 2008). This is likely due to suitable particle size and shapes (round), pore size (excluding large molecules), and chemistry of SAMMS (i.e., HOPO groups are not prone to binding proteins). Our recent work (Yantasee et al. In Press) shows that HOPO-SAMMS can capture both free and chelated-Gd (e.g., gadodiamide Gd) from blood, which could enable the fast removal of this Gd-based contrast agent after a contrast MRI procedure. If not removed, Gd is believed to dissociate from the chelate via transmetallation and trigger a fatal skin disease namely Nephrogenic Systemic Fibrosis (NSF) (High et al. 2007).
Figure 8.
Removal of uranium and 241Am from plasma using 3,4-HOPO-SAMMS in a 100-fold scaled-down hemoperfusion device.
SAMMS materials maintain their efficacy and stability in biological matrices. In our separate study, SAMMS attached with copper ferrocyanide has shown to be successful at preventing gut absorption of cesium (Timchalk et al., published in this special issue). In the event of a nuclear or dirty bomb explosion, it is very likely that the public will be exposed to a number of these radionuclides concurrently, yet it will be difficult to discern to which radioisotopes there was exposure in a timely manner. In this regard, we are developing the decorporation material that will provide broad spectrum decorporation of radionuclides that are most often encountered in nuclear reactor incidents, nuclear explosions, and/or dirty bombs. The broad spectrum performance will be achieved by mixing the best SAMMS material for each class of radionuclides (e.g., heavy metals like Po, transition metals like Co, actinides, Cs, I, and Sr) together in a form suitable for simple oral delivery and could easily be taken in response to suspected exposure, or as preventative mitigation treatment for first responders. Our separate study has shown that SAMMS can be mixed together without interaction or loss of activity. Results of this study will be reported in due course.
Since SAMMS are effective in highly complex matrices like blood, plasma, and urine, they may potentially be used for selective preconcentration of actinides and toxic metals from these fluids in order to improve the detection limits of the analytical instruments. Preconcentration of U from water (Yantasee et al. 2004) and Cd/Pb from urine (Yantasee et al. 2008) using SAMMS followed by their direct detection with analytical devices was recently demonstrated in our lab. Portable system based on built-in metal preconcentration with SAMMS developed in our lab (Crow 22 February 2008) will enable early detection of metal exposures by analyzing patient's blood and urine. Lastly, being able to effectively capture actinides from blood and plasma, SAMMS might be useful for wound decontamination of actinides (e.g., SAMMS powders would be applied to the contaminated wound in a fluid matrix, allowed to sit for a period of time to allow the radionuclides to diffuse into the SAMMS, and then the radionuclide-laden SAMMS and colloidal contaminants would be removed by irrigation). Since SAMMS are not absorbed through skin tissues, they could be removed after they have captured the actinides.
CONCLUSION
SAMMS effectively capture actinides in blood and plasma in terms of affinity, selectivity, capture kinetics, and stability. Among the various SAMMS materials with surface chemistries known to have high affinity for actinides, the 3,4-HOPO-SAMMS stands out as being best for capturing plutonium, americium, uranium, and thorium. 3,4-HOPO SAMMS also greatly outperformed a commercial DTPA-analog on silica gels. This makes the 3,4-HOPO-SAMMS viable for applications in Health Physics ranging from direct blood decorporation in patients with renal insufficiency, and also potentially for sensing, diagnostics, and wound decontamination of actinides. We have also extended the applications of SAMMS to other radionuclides beyond actinides by changing the organic groups on SAMMS materials. These endeavors are being pursued in our lab and the results will be reported in due course.
Figure 1.
Chemical structures of SAMMS having various organic groups.
Figure 3.
Kd of americium (241Am) in human blood and plasma measured on various sorbents (initial 241Am of 1000 dpm mL−1, L S−1 of 1000 mL g−1).
ACKNOWLEDGEMENTS
The work was supported by the National Institute of Allergy and Infectious Disease (NIAID), grant# R01 AI074064 and R01 AI080502, the National Institute of Environmental Health Sciences (NIEHS), grant# R21 ES015620, and PNNL's LDRD program. The authors thank Drs. Ken Raymond and Jide Xu of UC Berkeley for the HOPO ligands. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. A portion of research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Chevari S, Likhner D. Complex Formation of Natural Uranium in Blood. Moscow: 1968. [PubMed] [Google Scholar]
- Crow JM. [22 February 2008];Toxin test in a lunchbox. http://www.rsc.org/chemistryworld/News/2008/February/22020801.asp.
- Feng X, Fryxell GE, Wang LQ, Kim AY, Liu J, Kemner KM. Functionalized monolayers on ordered mesoporous supports. Science. 1997;276:923–926. [Google Scholar]
- Fryxell GE, Lin Y, Fiskum S, Birnbaum JC, Wu H, Kemner K, Kelly S. Actinide sequestration using self-assembled monolayers on mesoporous supports. Envi. Sci. Technol. 2005;39:1324–1331. doi: 10.1021/es049201j. [DOI] [PubMed] [Google Scholar]
- Fryxell GE, Liu J, Hauser TA, Nie Z, Ferris KF, Mattigod SV, Gong M, Hallen RT. Design and synthesis of selective mesoporous anion traps. Chem. Mater. 1999;11:2148–2154. [Google Scholar]
- Fryxell GE, Wu H, Lin Y, Shaw WJ, Birnbaum JC, Linehan JC, Nie Z, Kemner K, Kelly S. Lanthanide selective sorbents: self-assembled monolayers on mesoporous supports (SAMMS). J. Mater. Chem. 2004;14:3356–3363. [Google Scholar]
- Gorden AEV, Xu J, Raymond KN, Durbin P. Rational design of sequestering agents for plutonium and other actinides. Chem. Rev. 2003;103:4207. doi: 10.1021/cr990114x. [DOI] [PubMed] [Google Scholar]
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J. Am. Acad. Dermatol. 2007;56:21–26. doi: 10.1016/j.jaad.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Kokot F, Pietrek J, Seredyski M. Influence of hemoperfusion on the concentration of methyldigoxin and hormones in plasma. Kidney Inter. 1979;15:404. doi: 10.1038/ki.1979.52. [DOI] [PubMed] [Google Scholar]
- Lin Y, Fiskum SK, Yantasee W, Wu H, Mattigod SV, Vorpagel E, Fryxell GE, Raymond KN, Xu J. Incorporation of hydroxypyridinone ligands into self-assembled monolayers on mesoporous supports for selective actinide sequestration. Envi. Sci. Technol. 2005;39:1332–1337. doi: 10.1021/es049169t. [DOI] [PubMed] [Google Scholar]
- Lin Y, Fryxell GE, Wu H, Engelhard M. Selective sorption of cesium using self-assembled monolayers on mesoporous supports (SAMMS). Envi. Sci. Technol. 2001;35:3962–3966. doi: 10.1021/es010710k. [DOI] [PubMed] [Google Scholar]
- Mattigod SV, Fryxell GE, Serne RJ, Parker KE, Mann FM. Evaluation of novel getters for adsorption of radioiodine from groundwater and waste glass leachates. Radiochim. Acta. 2003;91:539–545. [Google Scholar]
- Penfield JG, Reilly RF. What nephrologists need to know about gadolinium. Nat. Clin. Pract. Nephrol. 2007;3:654–668. doi: 10.1038/ncpneph0660. [DOI] [PubMed] [Google Scholar]
- Stamatialis DF, Papenburg BJ, Gironés M, Saiful S, Bettahalli SNM, Schmitmeier S, Wessling M. Medical applications of membranes: drug delivery, artificial organs and tissue engineering. J Membrane Sci. 2008;308:1. [Google Scholar]
- Stradling GN, Gray SA, Moody JC, Pearce MJ, Wilson I, Burgada R, Bailly T, Leroux Y, Raymond KN, Durbin PW. Comparative efficacies of 3,4,3-LIHOPO and DTPA for enhancing the excretion of plutonium and americium from the rat after simulated wound contamination as nitrates. Inter. J. Rad. Bio. 1993;64:133. doi: 10.1080/09553009314551191. [DOI] [PubMed] [Google Scholar]
- Stradling GN, Moody JC. Use of animal studies for assessing intakes of inhaled actinide bearing dusts. Radioanal. Nucl. Chem. 1995;197:309. [Google Scholar]
- Stradling GN, Moody JC, Gray SA, Ellender M, Hodgson A. The efficacy of DTPA treatment after deposition of thorium nitrate in the rat lung. Human Toxicolo. 1991;10:15. doi: 10.1177/096032719101000103. [DOI] [PubMed] [Google Scholar]
- Stradling GN, Taylor DM. Decorporation of radionuclides. In: Kaul A, Becker D, editors. Radiological Protection. Springer; New York: 2005. pp. 295–328. [Google Scholar]
- Volf V, Burgada R, Raymond KN, Durbin PW. Chelation therapy by DFO-HOPO and 3,4,3-LIHOPO for injected Pu-238 and Am-241 in the rat: effect of dosage, time and mode of chelate administration. Inter. J. Rad. Bio. 1996;70:765–772. doi: 10.1080/095530096144653. [DOI] [PubMed] [Google Scholar]
- Volf V, Burgada R, Raymond KN, Durbin PW. Treatment with 3,4,3-LIHOPO of simulated wounds contaminated with plutonium and americium in rat. Inter. J. Rad. Bio. 1996;70:109. doi: 10.1080/095530096145382. [DOI] [PubMed] [Google Scholar]
- Yantasee W, Charnhattakorn B, Fryxell GE, Lin Y, Timchalk C, Addleman RS. Detection of Cd, Pb, and Cu in non-pretreated natural waters and urine with thiol functionalized mesoporous silica and Nafion composite electrodes. Anal. Chim. Acta. 2008;620:55–63. doi: 10.1016/j.aca.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantasee W, Fryxell GE, Lin Y, Wu H, Raymond KN, Xu J. Hydroxypyridinone functionalized self-assembled monolayers on nanoporous silica for sequestering lanthanide cations. J. Nanosci. Nanotech. 2005;5:527–529. doi: 10.1166/jnn.2005.096. [DOI] [PubMed] [Google Scholar]
- Yantasee W, Fryxell GE, Porter GA, Pattamakomsan K, Sukwarotwat V, Chouyyok W, Koonsiripaiboon V, Xu J, Raymond KN. Novel sorbents for removal of gadolinium-based contrast agents in sorbent dialysis and hemoperfusion: preventive approaches to nephrogenic systemic fibrosis (NSF) NBM; Nanomed: In Press. doi:10.1016/j.nano.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantasee W, Lin Y, Fryxell GE, Busche BJ, Birnbaum JC. Removal of heavy metals from aqueous solution using novel nanoengineered sorbents: self-assembled carbamoylphosphonic acids on mesoporous silica. Sep. Sci. Technol. 2003;38:3809–3825. [Google Scholar]
- Yantasee W, Lin Y, Fryxell GE, Wang Z. Carbon paste electrode modified with carbamoylphosphonic acid functionalized mesoporous silica: a new mercury-free sensor for uranium detection. Electroanal. 2004;16:870–873. [Google Scholar]