Abstract
BACKGROUND
Posttransplant cyclophosphamide (CY) has been shown to control graft-versus-host disease (GVHD) and facilitate engraftment in the major histocompatibility complex (MHC)-haploidentical transplant setting. Here we hypothesized that methotrexate (MTX) could be used in a similar fashion. In patients with genetic diseases, the use of MTX rather than an alkylating agent such as CY would be preferable due to its reduced risk of promoting secondary malignancies.
METHOD
Using our standard conditioning regimen consisting of a specific anti-CD44 MAb (S5) and 200 cGy total body irradiation (TBI) followed by postgrafting immunosuppression with cyclosporine (CSP) and mycophenolate mofetil (MMF) as a control group, we compared outcomes to experimental animals receiving the same regimen with the addition of a single, large dose of posttransplant MTX on day +3 (50–400 mg/m2).
RESULTS
Adding MTX at all dose levels did not abrogate initial engraftment and controlled GVHD in most cases. Dogs receiving MTX at the first dose level (50 mg/m2) improved time to rejection compared to controls (p=0.03) but did not decrease overall rates of rejection (p=0.56). However, increasing the dose of MTX beyond 50 mg/m2 appeared to have detrimental effects in both average (p=0.04) and peak (p=0.002) donor chimerism. Increasing the dose of MTX also promoted more profound lymphopenia. Finally, delaying CSP and MMF until after MTX administration did not appear to significantly improve engraftment kinetics.
CONCLUSION
Adding high-dose MTX appeared to benefit the duration of donor chimerism at the lowest dose studied, but there was no benefit when escalating MTX doses to toxicity.
Keywords: MHC-haploidentical, canine model, methotrexate
INTRODUCTION
The ability to engraft without causing severe GVHD has been the crux of developing successful transplant regimens in the dog leukocyte antigen (DLA)-haploidentical model. When pretreating recipient dogs with S5, a specific anti-CD44 MAb that sensitizes natural killer (NK) cells to radiation kill (1,2), we achieved virtually uniform sustained donor engraftment of DLA-haploidentical littermate cells after 4.5 Gy total body irradiation (TBI) (3) but the success rate declined to 50% after 2 Gy TBI (4). Additionally, GVHD control was not achieved uniformly. However, using a nonmyeloablative regimen that included 2 Gy TBI, Luznik and colleagues have effectively added a single dose of CY 50 mg/kg on day +3 to standard postgrafting immunosuppression in human leukocyte antigen (HLA)-haploidentical HCT resulting in high rates of engraftment and low rates of GVHD (5). By delaying immunosuppression until 3 days after HCT to generate highly proliferative, alloreactive lymphocytes, CY was then used to selectively delete those clones responsible for graft rejection and GVHD. Slower-growing cells, such as memory T cells or T cells derived from stem cells and ultimately important for immune reconstitution would then remain relatively preserved. However, as an alkylating agent, CY theoretically may not be the most optimal chemotherapeutic agent to use in the posttransplant setting, particularly for patients transplanted for non-malignant diseases. MTX, on the other hand, has been used with great experience in the posttransplant setting for both malignant and non-malignant diseases. As described in the canine model by Deeg and colleagues (6), its administration on days +1, +3, +6, and +11 alongside a calcineurin inhibitor has been the standard of care for postgrafting immunosuppression after myeloablative, MHC-matched hematopoietic cell transplantation (HCT). MTX is cell cycle (S-phase) specific, and thus its principal mechanism of action is to eradicate actively replicating cells by inhibiting DNA and RNA synthesis, making it an optimal drug to target alloreactive donor lymphocytes that proliferate after HCT. However, it is unclear whether efficacy can be improved or if additional toxicity would develop if these mini-doses were combined and given similarly as a single, large dose. Prior studies have incorporated MTX 200 mg/kg (equivalent to ~6000 mg/m2) with leucovorin rescue as GVHD prophylaxis after 9.2 Gy TBI with devastating results in terms of toxicity, GVHD, and marrow hypoplasia (7). To test whether single, smaller doses can be given in the nonmyeloablative setting, we added MTX 50 mg/m2 on day +3 to our established regimen of S5 and 2 Gy TBI followed by CSP and MMF. We further tested whether this anti-proliferative effect could be enhanced by increasing the dose of MTX (100–400 mg/m2) without affecting stem cell engraftment, delaying immune reconstitution, or promoting chemotoxicity. And finally, we tested whether delaying the start of CSP and MMF until after MTX administration could maximize the impact of MTX on these highly proliferating, non-immunosuppressed lymphocytes and enhance anti-GVHD effects and promote more sustainable engraftment.
MATERIALS AND METHODS
Laboratory Animals
Litters of hounds (beagles and beagle/mini-mongrel mixed breeds) were used. Thestudy was approved by the Institutional Animal Care and UseCommittee at the FHCRC, which is accredited by the Associationfor Assessment and Accreditation of Laboratory Animal Care International. Supportive care was administered. Selection of DLA-haploidentical littermate donor/recipient pairs was based on family studies showing haplo-disparity for highly polymorphic MHC class I and class II microsatellite markers (8,9). Results were confirmed by direct sequencing of DLA-DRB1 alleles (10). No pairs had coincidental sharing of DLA antigens on the mismatched haplotypes. Furthermore, mutual lymphocyte reactivity was verified by mixed lymphocyte reactions (MLR) (11).
Hematopoietic Cell Transplantation
All recipient dogs (n=29) were identically conditioned with 0.2 mg/kg of murine anti-CD44 MAb (S5) daily from days −7 to −2 before HCT and 2 Gy TBI on day 0 (1,4,12). TBI was delivered at 7 cGy/min using a linear accelerator (Varian CLINAC 4, Palo Alto, CA). The day of TBI and subsequent peripheral blood stem cell (PBSC) infusion was designated day 0. Recombinant canine (rc)-GCSF-mobilized PBSC were collected from DLA-haploidentical related donors as described (1). Cell subsets were characterized by either May-Giemsa stain or flow cytometry. Seven different postgrafting immunosuppressive regimens were tested. Details of these regimens can be found in Table 1.
Table 1.
Immunosuppressive regimens.*
| Group | MTX (mg/m2) | CSP | MMF | ||
|---|---|---|---|---|---|
| Start (day) | Stop (day) | Start (day) | Stop (day) | ||
| 1 | 0 | −1 | +100 | 0 | +100 (tapered +41 to +100) |
| 2 | 50 | −1 | +100 | 0 | +100 (tapered +41 to +100) |
| 3 | 100 | −1 | +210 (tapered +181 to +210) | 0 | +180 (tapered +101 to +180) |
| 4 | 200 | −1 | +210 (tapered +181 to +210) | 0 | +180 (tapered +101 to +180) |
| 5 | 200 | +4 | +210 (tapered +181 to +210) | +4 | +180 (tapered +101 to +180) |
| 6 | 400 | −1 | +210 (tapered +181 to +210) | 0 | +180 (tapered +101 to +180) |
| 7 | 400 | +4 | +210 (tapered +181 to +210) | +4 | +180 (tapered +101 to +180) |
Each group received a different regimen. When MTX was given after transplant (Groups 2–7), leucovorin rescue was started 24 hours after MTX infusion at a dose of 3 mg/kg IV twice a day on days +4 and +5
Groups 1, 2:
CSP: 30 mg/kg [−1 to +100]
MMF: 20 mg/kg [0 to +40], 10 mg/kg [+41 to +100]
Groups 3, 4, 6:
CSP: 30 mg/kg [−1 to +180], 15 mg/kg [+181 to +190], 7.5 mg/kg [+191 to +200], 3 mg/kg [+201 to +210] MMF: 20 mg/kg [0 to +100],10 mg/kg [+101 to +130], 5 mg/kg [+131 to +160], 2.5 mg/kg [+161 to +180]
Groups 5, 7:
CSP: 30 mg/kg [+4 to +180], 15 mg/kg [+181 to +190], 7.5 mg/kg [+191 to +200], 3 mg/kg [+201 to +210]
MMF: 20 mg/kg [+4 to +100],10 mg/kg [+101 to +130], 5 mg/kg [+131 to +160], 2.5 mg/kg [+161 to +180]
Flow Cytometry
PBSC subsets were verified with flow cytometry using canine monoclonal antibodies directly conjugated to FITC, including CD34(1H6, IgG1) (13), CD3 (CA17.6F9, IgG2b) (14), TCRαβ (CA15.9D5, IgG1) (15), CD4 (CA13.1.E4, IgG1) (16), CD8 (CA17.6B3, IgG2a) (16), CD21 (CA2.1D6, IgG1) (16), granulocytes (DM5, IgG1) (17), and CD14 (TUK4, IgG2a; DAKO, Carpinteria, CA).
Methotrexate Levels
For all dogs receiving MTX dosed at 100, 200, or 400 mg/m2, fluorescence polarization immunoassay (FPIA) testing was performed at the University of Washington Medical Center Laboratories, Seattle, WA at 24 and 48 hours (drawn prior to the first and third doses of leucovorin, respectively) after infusion.
Chimerism Analysis
Contributions of recipient and donor cells to peripheralblood, marrow, and other tissues were quantified by variable number tandem repeat (VNTR) analysis using ABI Prism 310 Genetic Analyzer and Gene Scan 3.1 Software (Applied Biosystems, Foster City, CA). Engraftment was defined as ≥ 3% mononuclear cell (MNC) donor chimerism, and graft rejection was defined as the first day when donor MNC chimerism was <3% without subsequent increase in donor chimerism.
MTX effects, tolerance induction and immune reconstitution
Mixed lymphocyte reaction (MLR) was performed as described (11) to determine the degree of host lymphocyte proliferation in response to self, donor, 3rd party unrelated donor, and concanavalin A (ConA). A natural killer (NK) cell functional assay was performed as described (18). Absolute lymphocyte (ALC) and absolute neutrophil counts (ANC) were followed after transplant to determine immune reconstitution. In the experimental dogs, CD4+ and CD8+ lymphocyte numbers were also characterized using flow cytometry.
Statistical Methods
Proportions of failure (e.g., graft rejection, GVHD, lymphoproliferative disease) were compared between groups with Fisher’s exact test. Time to rejection amongst dogs that rejected was compared between groups using the two-sample t-test. The average chimerism values were computed for each dog, and the mean of these averages were compared between groups using the two-sample t-test. The two-sample t-test was also used to compare the mean in peak chimerism values and average number of cells for various PBSC subsets. To examine a dose effect of MTX on outcome, MTX was modeled as a continuous linear variable in the MTX groups. Cox regression was used to test for a dose effect on the risk of rejection, and linear regression was used to test for a dose effect on average and peak donor chimerism, weight loss, and MMF dose.
RESULTS
Engraftment and GVHD
The addition of MTX to standard postgrafting immunosuppression prolongs the duration of mixed chimerism without GVHD
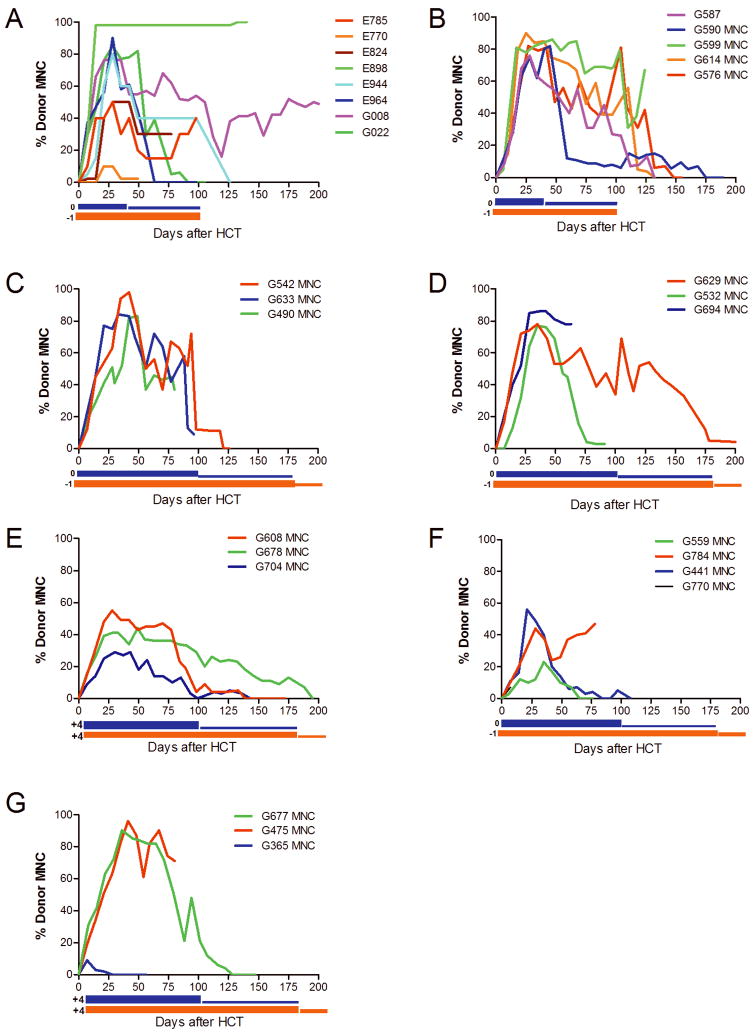
Dogs in Group 1 (n=8) underwent nonmyeloablative conditioning and received standard immunosuppression consisting of CSP and MMF. Dogs in Group 2 received, in addition, a single dose of MTX 50 mg/m2 on day +3, followed by leucovorin rescue. While 4 of 8 dogs (50%) eventually rejected their grafts in the control group (Group 1), 4 of 5 dogs (80%) rejected their grafts in the MTX group (Group 2) (p=0.56). However, when focusing on only the rejection dogs, the average time to rejection was longer with the addition of MTX. Specifically, in the control group, the average time to rejection of 4 dogs was 68 (range, 14–119) days, while in the MTX group, 4 dogs rejected on average of 139 (range, 122–168) days after HCT (p=0.03). The mean of the peak MNC chimerism was higher in Group 2, although this was not statistically significant (83.2% vs. 67.5%, p=0.26). Because at approximately day +40 after HCT donor chimerism appeared to decrease in temporal relation to the tapering of MMF, it was decided to increase the duration of both MMF and CSP in subsequent cohorts. One dog in the control group (E964) developed clinical and pathological changes consistent with GVHD, while no dogs in Group 2 developed GVHD. Figure 1 depicts donor engraftment kinetics for dogs on this study. Compared to donor MNC engraftment, VNTR performed on the granulocyte fractions at the same timepoints demonstrated more rapid engraftment with donor granulocyte persistence even after the MNC donor graft was lost (data not shown).
Figure 1. Donor mononuclear cell (MNC) chimerism kinetics vary based on treatment regimen.
Donor MNC chimerism was evaluated weekly for each dog during the course of the study. The blue bars below the x-axis represent duration of MMF therapy while the orange bars represent the duration of CSP therapy. Engraftment kinetics vary based on treatment regimen. A) Control group dogs that did not receive any post-transplant MTX. B) Dogs receiving MTX 50 mg/m2 on day +3. C) Dogs receiving MTX 100 mg/m2 on day +3. D) Dogs receiving MTX 200 mg/m2 on day +3. E) Dogs receiving MTX 200 mg/m2 on day +3, with delayed CSP and MMF beginning on day +4. F) Dogs receiving MTX 400 mg/m2 on day +3. G) Dogs receiving MTX 400 mg/m2 on day +3 with delayed CSP and MMF beginning on day +4.
Increasing the dose of posttransplant MTX does not add additional engraftment benefit
Dogs in Groups 2, 3, 4, and 6 received 50, 100, 200, and 400 mg/m2 of MTX on day +3, respectively. The observed proportions of rejection in these groups were 4/5, 1/3, 2/3, 2/4, respectively. A test of a dose effect yielded a positive association (increase in MTX, increase in rejection), although not statistically significant (p=0.11). Additionally, there was an inverse relationship between MTX dose and donor MNC chimerism, with both average (p=0.009) and peak (p<0.0001) donor chimerism decreasing with higher MTX doses.
Delaying CSP and MMF until after MTX administration has a detrimental effect on peak chimerism based on the dose of MTX used
Rather than starting CSP at day −1 and MMF on day 0 (“standard” dosing interval), we investigated the impact of starting CSP and MTX on day +4 (“delayed” dosing interval). Results showed that when MTX 200 mg/m2 was used, both the average and peak chimerism were worse with delayed compared to standard dosing. Specifically, the mean average MNC chimerism of Group 4 (standard) was 47.9% while for Group 5 (delayed) it was 20.0% (p=0.06). The mean peak chimerism was 80.3% in Group 4 and 42.7% in Group 5 (p=0.01). However, when using MTX 400 mg/m2, the delayed dosing resulted in higher chimerism compared to standard dosing, although the differences were not statistically significant. Specifically, the mean average chimerism in these groups were 36.9% and 16.7%, respectively (p=0.30), and the mean peak chimerism levels were 65.0% and 33.3%, respectively (p=0.29).
Recipients of high-dose MTX have low rates of GVHD
One of 8 dogs (13%) in the control group and 1 of 21 dogs (5%) in the MTX groups developed clinical or pathological changes consistent with GVHD (p=0.48) (Table 2). In the control group, E964 developed skin erythema and was euthanized 8 weeks after HCT due to pneumonia. Histopathological findings confirmed GVHD in the skin and stomach. In the MTX group, G475 received MTX 400 mg/m2. Beginning at day +50 after HCT, transaminitis (alanine aminotransferase 221) and jaundice (total bilirubin 10.8, conjugated 7.5) developed. On necropsy, widespread abnormalities of small bile ducts were consistent with liver GVHD. VNTR performed on the infiltrating hematopoietic cells in the liver showed a donor chimerism of 66% (whole blood chimerism of 77%; whole marrow chimerism of 87%).
Table 2.
Dogs given PBSC grafts from DLA-haploidentical littermates after conditioning with anti-CD44 MAb S5, 2 Gy TBI, and post-grafting immunosuppression with MMF, CSP, and MTX
| Group | Post-transplant Immunosuppression (days) | Dog # | Rejection | Duration of Donor Chimerism (Wks) | Clinical/Pathological GVHD | % Donor MNC Chimerism (Max-Final) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | MMF (0 to +100) CSP (−1 to +100) |
E785 | No | >14 | No / No | 40-40 | ET1 due to PTLD |
| E770 | Yes | 5 | No/Unknown | 10-0 | ET2 | ||
| E824 | No | >11 | No / No | 50-30 | ET1due to pneumonia | ||
| E898 | No | >24 | No / No | 98-100 | Adopted | ||
| E944 | Yes | 16 | No / No | 80-0 | ET2 | ||
| E964 | Yes | 8 | Yes / Yes | 90-0 | ET1due to GVHD / pneumonia | ||
| G008 | No | >50 | No ? No | 76-48 | Transferred to other study | ||
| G022 | Yes | 11 | No / No | 85-0 | ET2 | ||
| 2 | MMF (0 to +100) CSP (−1 to +100) MTX 50 mg/m2 (+3) |
G587 | Yes | 18 | No / No | 76–0 | ET2 (PTLD found on necropsy) |
| G590 | Yes | 24 | No / No | 82–0 | ET2 | ||
| G599 | No | >18 | No / No | 86–67 | ET1 due to PTLD | ||
| G614 | Yes | 18 | No / Unknown | 90–0 | Adopted | ||
| G576 | Yes | 20 | No / No | 82–0 | ET2 | ||
| 3 | MMF (0 to +100) CSP (−1 to +100) MTX 100 mg/m2 (+3) |
G542 | Yes | 16 | No / No | 98-0 | ET1 due to exogenous leukemia* |
| G633 | No | >14 | No / No | 84-9 | ET1 due to PTLD | ||
| G490 | No | >11 | No / No | 83-37 | ET1 due to exogenous leukemia* | ||
| 4 | MMF (0 to +180) CSP (−1 to +210) MTX 200 mg/m2 (+3) |
G629 | Yes | 30 | No / No | 78-0 | ET2 |
| G532 | No | >13 | No / No | 77-3 | ET2 | ||
| G694 | No | >9 | No / No | 86-78 | ET1 due to PTLD | ||
| 5 | MMF (+4 to +180) CSP (+4 to +210) MTX 200 mg/m2 (+3) |
G608 | Yes | 19 | No / No | 55-0 | ET2 |
| G678 | Yes | 27 | No / No | 41-0 | ET2 | ||
| G704 | Yes | 19 | No / No | 29-0 | ET2 due to PTLD | ||
| 6 | MMF (0 to +180) CSP(−1 to +210) MTX 400 mg/m2 (+3) |
G559 | Yes | 8 | No / No | 23-0 | ET2 |
| G784 | No | >11 | No / No | 44-47 | ET1due to sepsis | ||
| G441 | Yes | 14 | No / No | 56-0 | ET2 | ||
| G770 | NE | NE | NE | NE | ET1 due to sepsis | ||
| 7 | MMF (+4 to +180) CSP (+4 to +210) MTX 400 mg/m2 (+3) |
G677 | Yes | 18 | No / No | 90-0 | ET2 |
| G475 | No | >11 | Yes / Yes | 96-71 | ET1due to liver GVHD | ||
| G365 | Yes | 3 | No / No | 9-2 | ET2 |
ET1: euthanized in poor condition; ET2: euthanized in good condition due to rejection; NE: not evaluable; PTLD: Post-transplant lymphoproliferative disease
Refer to Thakar et al. (28).
Drug Levels Demonstrate Appropriate Clearance of Methotrexate
Pharmacokinetic sampling of one dog was undertaken at 15, 30, and 60 minutes, and 24 and 48 hours after MTX 100 mg/m2 was infused. Levels were detected as early as 15 minutes after infusion (24 umol/L) and showed complete clearance 24 hours later, prior to receiving the first dose of leucovorin. These results confirmed the ability to use this assay in canine blood samples. All dogs receiving greater than or equal to MTX 100 mg/m2 had MTX levels sent at 24 (prior to receiving leucovorin) and 48 hours after MTX infusion. At the 24-hour time point, blood levels were between <0.02 to 0.08 umol/L. By the 48-hour time point, all dogs had undetectable MTX levels (<0.02 umol/L).
rcG-CSF-mobilized PBSC Subsets Show Some Differences Between Groups
Group 1 (control) received higher numbers of total nucleated cells (TNC, p=0.11), mononuclear cells (MNC, p=0.0005), CD34 cells (p=0.004), and CD4 cells (p=0.004). The average number of CD8 and CD14 cells, on the other hand, were lower in the control group (p=0.006 and 0.02, respectively), while the number of CD3 cells appeared to be similar (p=0.65). TNC, MNC, CD3, and CD34 graft compositions were relatively similar between Groups 4 and 5 (p=0.82; 0.99; 0.32; 0.68, respectively) and between Groups 6 and 7 (p=0.35; p=0.53; p=0.95; p=0.85, respectively). Additionally, there were no statistically significant differences between Groups 2, 3, 4, and 6 in terms of TNC (p=0.81), MNC (p=0.33), or CD3 (p=0.12) populations, although there was a difference in CD34 counts (p=0.01) (ANOVA test).
Regimen-related Toxicity
Weight
All dogs lost weight after HCT, although some dogs regained or even surpassed their baseline weights by the end of the study. When evaluating weight loss as the difference between baseline and lowest study weights, dogs in the MTX 50, 100, 200, and 400 mg/m2 groups lost an average of 22.2 (range, 17.5–29.6)%, 14.4 (range, 9.4–22.1)%, 29.5 (range, 20.4–35.9)%, and 20.1 (range, 4.2–33.0)% of their baseline weights, respectively. With MTX modeled as a continuous linear variable, an increasing dose of MTX was correlated with a more profound weight loss from baseline to end of study (p=.02). Furthermore, dogs receiving 400 mg/m2 had, on average, a change of weight 12.3% higher than that among dogs treated at the lower doses of MTX (p=.01).
Diarrhea
All dogs receiving posttransplant MTX tolerated the infusion with no acute toxicity. However, despite effectively clearing the drug by 48 hours after infusion, dogs receiving MTX 400 mg/m2 had more diarrhea. Using the surrogate marker of decreasing MMF doses as an indicator of gut toxicity, dogs receiving MTX 400 mg/m2 received less MMF during the first 30 days after HCT. Specifically, dogs receiving MTX 50, 100, and 200 mg/m2 received a higher average percentage of MMF at 76.3 (range, 69.0–89.9)%, 78.9 (range, 47.6–100)%, and 82.6 (range, 58.8–100)% of doses respectively, compared to an average percentage of 63.2 (range, 51.4–73.8)% in the MTX 400 mg/m2 group. With MTX modeled as a continuous linear variable, an increasing dose of MTX was suggestively associated with a decrease in MMF dose (p=.07). Comparing the dose of 400 mg/m2 to all other doses, these dogs had, on average, an MMF dose 16.3% lower than those treated at the lower doses of MTX (p=.02).
Fever and Infections
Bacteremia or sepsis was documented in nine dogs and upper respiratory infections in 16 dogs during the posttransplant course. There did not appear to be any predilection for more severe infections in dogs receiving higher doses of MTX. There was an inverse relationship between days with fever and dose of MTX. Dog receiving MTX 50, 100, 200, and 400 mg/m2 had median percentages of days with fevers of 10.2 (range, 0.7–10.9), 3.8 (range, 3.2–6.9), 4.2 (range, 1.1–25.2), and 2.8 (range, 1.3–40)%, respectively.
Transfusion requirements
Transfusion requirements decreased as the MTX dose increased. The average percentages of time dogs received transfusions after transplant was 10.8 (range, 0–21.8)%, 4.5 (range, 1–11.1)%, 3.7 (range, 0–9.4)%, and 2.6 (range, 0–10)%.
Posttransplant lymphoproliferative disease (PTLD)
PTLD was seen in 1 of 8 dogs in the control group (12.5%) and 5 of 21 dogs in the MTX groups (24%) (p=0.65). There was no predilection for developing PTLD based on the dose of MTX (Table 2). Median time to development of PTLD was 124 (range, 70–139) days after HCT in the MTX groups. Lymphoma tissue was available for analysis in all five dogs from the MTX group, and in four cases found to be of a mixed donor-host chimerism, while in one case was 100% host.
Immune Reconstitution
Neutropenia and lymphopenia
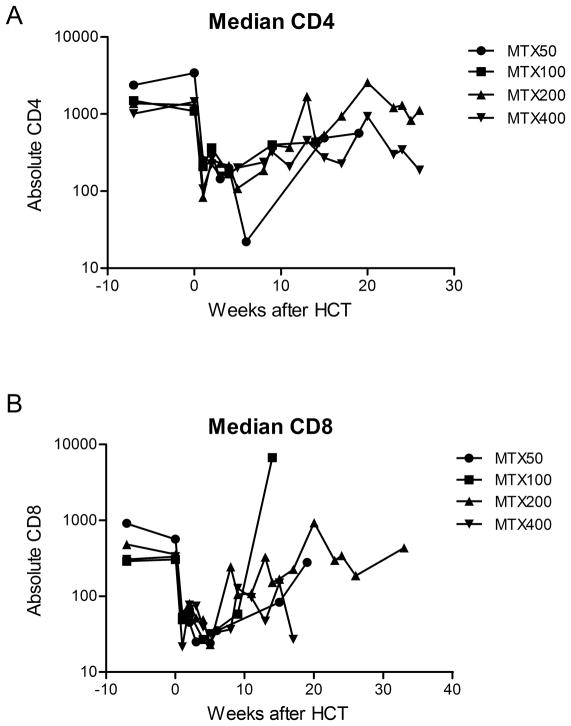
No dogs developed marrow aplasia or graft failure after transplant, and all dogs that rejected had autologous recovery. While there was no suggestion of a dose-dependent effect of MTX on neutropenia (p=0.92), there was a trend of higher MTX doses leading to increased lymphopenia after transplant (p=0.07). Additionally, flow cytometry performed at several timepoints after transplant identifying CD4 and CD8 T cell subsets showed profound depletion with recovery over time (Figure 2).
Figure 2.
A) Median CD4 and B) Median CD8 T cell subpopulations are selectively depleted after receiving non-myeloablative conditioning followed by increasing doses of post-transplant MTX. There was a trend of more profound lymphodepletion when dogs received higher doses of MTX.
Mixed lymphocyte reactions
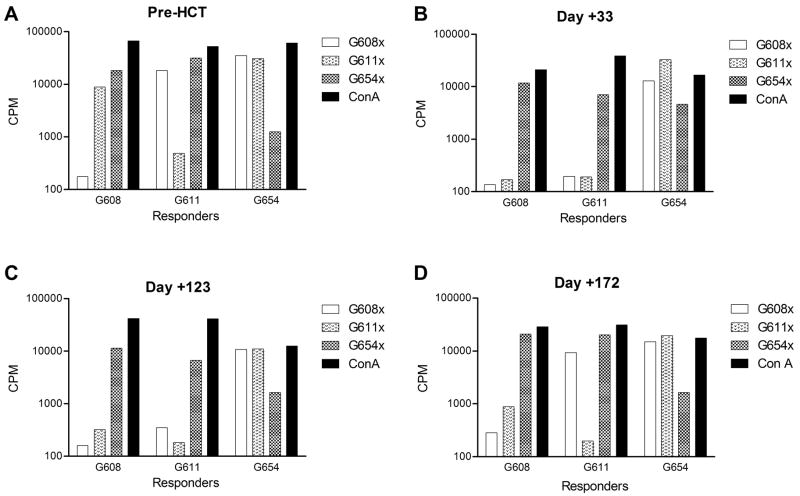
MLR demonstrated a strong proliferative response to donor MNC in all recipients as expected. The proliferation, as measured by 3H incorporation, became suppressed as early as 1 week after HCT, which correlated with the emergence of donor engraftment. This persisted throughout the posttransplant course and, in almost all cases, continued to be observed despite the loss of donor engraftment (Figure 3).
Figure 3.
A) Prior to HCT, MLR in recipient G608 was performed showing profuse 3H uptake in allo-response to the DLA-haploidentical donor (G611), 3rd party unrelated dog (G654), and the mitogen ConA. B) At day +33 when the MNC chimerism was 50%, G629 showed suppressed 3H uptake in response to irradiated donor stimulator cells similar to that seen in autologous controls, demonstrating donor tolerance induction. Concurrently, there was suppressed 3H uptake in donor responder cells in response to host stimulators due to the large number of donor mononuclear cells in host peripheral blood C) At day +123 when MNC chimerism was 4%, this same pattern of tolerance remains in the recipient. Although the overwhelming majority of MNC is host-derived, the donor does not mount an alloresponse to the recipient. D) However, 49 days later at day +172, at which point the recipient had fully rejected with complete autologous recovery, G609 still demonstrated host tolerance as indicated by surpressed 3H uptake while the donor mounts an alloresponse to the recipient similar to what was seen pre-HCT.
Natural killer cytolytic assays
In select dogs, NK functional assays were performed weekly during the first month after HCT showing robust NK cell reconstitution that surpassed the activity of controls as donor chimerism increased. In some dogs, the effect of MTX on early NK function was also performed demonstrating a relative resistance of NK cells to MTX. Specifically, despite lymphodepletion as suggested by low absolute peripheral blood lymphocyte counts after MTX was given, NK cell activity appeared similar to baseline levels (Figure 4).
Figure 4.
A) Prior to HCT, the recipient’s (G694) NK activity was lower than that of two normal controls, G697 (donor) and G654 (unrelated 3rd party). B) On day +3 (while receiving CSP and MMF immunosuppression), NK function was tested just prior to and 4 hours after MTX administration showing no change in NK activity, and again lower than control (G699; unrelated 3rd party). C) Twenty-four hours after receiving MTX, NK activity is again evaluated showing strong persistence of cytolytic activity against targets similar to controls. D) One month after HCT, NK activity in the recipient is much higher than controls, indicative of NK cell reconstitution. E) By 2 months after HCT, NK activity has normalized and is similar to controls. Percent specific lysis was expressed as: [(experimental CPM − spontaneous CPM)/maximum release CPM − spontaneous release CPM]] × 100, where CPM represents the mean of triplicate counts per minute.
DISCUSSION
The addition of short-course MTX as postgrafting immunosuppression is considered standard-of-care in the MHC-matched related and unrelated donor, myeloablative HCT setting. When using MHC-haploidentical donors, more potent immunosuppressive strategies must be explored to promote engraftment in the nonmyeloablative setting and prevent GVHD across MHC barriers. Here we describe results of improving postgrafting immune suppression by adding high-dose MTX to CSP and MMF. First, we showed that adding MTX at any of the doses studied did not interfere with initial engraftment. Furthermore, adding a single dose of MTX 50 mg/m2 is efficacious in prolonging duration of chimerism amongst those dogs that rejected, although it did not improve the overall rate of long-term engraftment in this group. However, intensifying the dose of MTX led to increasing toxicity with no apparent benefit on donor chimerism. Finally, delaying the start of CSP and MMF made no statistically significant difference in outcomes.
Other groups have attempted using a large bolus of CY to decrease GVHD rates with success (5). The benefit of using CY after HCT is its limited effect on stem cells, which are naturally protected from damage due to the presence of large amounts of aldehyde dehydrogenase, while mature cells, such as T and NK cells with lower enzyme levels, are deleted (19,20). However, there has been concern that incoming donor cells would be exposed to an alkylating agent which could then potentially result in an increased risk of secondary malignancy in donor cells. This would be of particular concern for patients with genetic diseases and thus our impetus to explore the use of MTX. MTX has been used extensively in patients with nonmalignant diseases and has not been associated with secondary malignancies (21).
In our study, we showed that incoming stem cells exposed to high-dose MTX maintain enough ability and integrity to engraft in all 21 dogs, thus confirming that this remains a nonmyeloablative HCT model. In further support, all dogs that rejected donor grafts had autologous recovery. Additionally, compared to controls, we showed an advantage for prolonging duration of mixed chimerism when MTX 50 mg/m2 was added to CSP/MMF. Although the rate of overall sustained engraftment did not improve, the ability to prolong the duration of mixed chimerism is beneficial in providing an opportunity to add other immunotherapy platforms, such as donor natural killer cell infusions, to promote long-term engraftment (22).
When increasing the MTX dose, detrimental effects were seen in terms of both duration and peak donor chimerism, suggesting that the improved duration of chimerism that MTX 50 mg/m2 provided to dogs that eventually rejected was counterproductive at higher doses. Additionally, transplant-related toxicity including diarrhea and weight loss was seen at higher dose levels. Not surprisingly, due to this toxicity at the highest MTX dose, the total MMF dose administered decreased to compensate for diarrhea, which may have contributed to poor long-term chimerism. Dogs receiving MTX 400 mg/m2 had the highest weight loss from baseline to end-of-study. As all MTX levels cleared based on FPIA testing by 24–48 hours after infusion, it is likely that peak MTX levels appeared to have promoted toxic effects in the dog.
Other factors, such as graft composition, can affect durable engraftment in HCT models. Dogs in the control group had higher CD34 and MNC doses compared to dogs receiving MTX 50 mg/m2. Although one dog in the control group developed durable engraftment, the duration of mixed chimerism was longer in the MTX 50 mg/m2 group. In groups receiving either 200 or 400 mg/m2 MTX, there did not appear to be any obvious differences in graft composition. Dogs receiving MTX 200 mg/m2 and delayed CSP/MMF experienced a detrimental effect of peak chimerism compared to the standard schedule CSP/MMF, while delayed vs. standard CSP/MMF made no difference in the MTX 400 mg/m2 group. Thus, this specific model shows that as a non-myeloablative regimen containing 2 Gy TBI, additional early immunosuppression (whether in the form of a higher-dose MTX or initiating CSP/MMF without delay) is required for improved engraftment kinetics.
Immune reconstitution was also studied. Dogs receiving higher doses of MTX did not appear to have worse neutropenia. However, lymphopenia was more profound as the MTX dose increased. Infections, both bacteremias and upper respiratory infections, were seen throughout all groups with no predilection based on MTX dose. More fevers and blood transfusion support was observed in the MTX 50 mg/m2 group for unclear reasons. As may be expected with increased immune suppression leading to immune dysregulation, PTLD was seen throughout the groups, including the control group (4). PTLD rates were no different between control and MTX groups. Likewise, there did not appear to be a dose-dependent increase of PTLD as the MTX dose increased. Thus, it appears that the immune dysregulation that presented after DLA-haploidentical HCT played a more significant role in PTLD development rather than the use of MTX. Furthermore, certain breeds of dogs (such as hounds) appear to have a natural predisposition to developing lymphomas, even in the non-transplant setting (23) and, thus, may have an additional inclination to developing PTLD. VNTR from lymphoma tissue showed mixed donor-host chimerism in four of five dogs from the MTX group.
T and NK cell functions were also studied. MLR showed that tolerance developed as early as 1 week after transplant and persisted despite graft rejection. This shows that these dogs, if given additional interventions such as immunotherapy with preceding chemotherapy, may have the ability to more easily accept a second graft from the same donor. Studies evaluating the use of NK cell based-DLI as a means to augment donor chimerism are currently in progress in our lab to test this hypothesis (unpublished).
Short-lived donor specific tolerance has also been explored in extending survival of transplanted solid organs. Our group has shown that, when performing skin grafts in dogs with previously-rejected hematopoietic stem cell grafts from the same DLA-identical littermate, graft survival was significantly prolonged compared to recipients without preceding marrow grafts (p=0.002) (24). In clinical medicine, the Boston group has used this immunological phenomenon by simultaneously transplanting marrow and kidney grafts from HLA-haploidentical related donors, which resulted in stable renal function and graft survival in 4 of 5 recipients despite very early hematopoietic rejection and discontinuation of all immunosuppression (25).
When performing NK cytolytic assays in response to MTX, it did not appear that NK cell activity was reduced at 4 and 24 hours after MTX infusion suggesting that posttransplant MTX did not appear to have imposed a significant effect on residual host NK cells, which may have additionally contributed to eventual graft rejection.
In addition to serving as an immunosuppressant, we are currently studying the use of MTX in selecting for exogenously-transduced dihydrofolate reductase-resistant CD34+ stem cells in the canine model. This strategy would allow escalation of therapy without promoting severe marrow or gastrointestinal tissue toxicity (26,27).
One major limitation of this study is the small number of animals evaluated in each individual cohort. This is a frequent limitation of large-animal studies due to the cost and efforts in maintaining a suitable colony. However, given the limited sample size, the failure to identify a statistically significant difference does not allow us to conclude that important differences did not exist.
In conclusion, intensifying immune suppression to promote engraftment after DLA-haploidentical transplantation is possible with the addition of MTX. Given the vast experience with posttransplant MTX in the canine model and strong precedence for translation of this animal model to clinical trials, these approaches should be investigated in human studies.
Acknowledgments
We would like to thank Brian Beard, PhD, for his thoughtful discussions during this project and George McDonald, MD, for his evaluation of canine liver GVHD. We thank Stacy Zellmer and Patrice Stroup for the DLA typing of dogs and selecting appropriate DLA-haploidentical littermates. We also thank Michele Spector, DVM, the canine facilities staff, and weekend investigators for their care of dogs on this study. CA17.6F9 was kindly provided by Dr. Peter Moore (School of Veterinary Medicine, University of California, Davis) and recombinant canine (rc)G-CSF was kindly provided by Amgen, Thousand Oaks, CA, USA. Finally, we thank Helen Crawford, Bonnie Larson, and Sue Carbonneau for help with manuscript preparation.
Grant support: This study was supported by NIH HL36444, CA15704, and AI067770.
Footnotes
Author Contributions:
- Monica S Thakardesigned and conducted the study, analyzed and interpreted data, drafted and revised the manuscript.
- Erlinda B Santos designed and conducted the study, analyzed and interpreted data, and revised the manuscript.
- Ted A Gooley did the statistical analysis of the data and revised the manuscript.
- George Sale did the pathology analysis for the study.
- Rainer Storb assisted in the design of the study, interpreted data, and revised the manuscript.
- Hans-Peter Kiem assisted in the design of the study, interpreted data, and revised the manuscript.
- Brenda M Sandmaier designed and conducted the study, supervised the analysis and interpretation of data, and revised the manuscript.
References
- 1.Schuening F, Storb R, Goehle S, et al. Facilitation of engraftment of DLA-nonidentical marrow by treatment of recipients with monoclonal antibody directed against marrow cells surviving radiation. Transplantation. 1987;44:607–613. doi: 10.1097/00007890-198711000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Tan PHS, Santos EB, Rossbach HC, Sandmaier BM. Enhancement of natural killer activity by an antibody to CD44. J Immunol. 1993;150:812–820. [PubMed] [Google Scholar]
- 3.Sandmaier BM, Fukuda T, Gooley T, Yu C, Santos EB, Storb R. Dog leukocyte antigen-haploidentical stem cell allografts after anti-CD44 therapy and reduced-intensity conditioning in a preclinical canine model. Exp Hematol. 2003;31:168–175. doi: 10.1016/s0301-472x(02)01022-6. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda T, Kerbauy FR, Gooley T, Santos EB, Storb R, Sandmaier BM. Dog leukocyte antigen-haploidentical stem cell allografts after anti-CD44 therapy and nonmyeloablative conditioning in a preclinical canine model. Transplantation. 2006;82:332–339. doi: 10.1097/01.tp.0000228908.10775.b0. [DOI] [PubMed] [Google Scholar]
- 5.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeg HJ, Storb R, Appelbaum FR, Kennedy MS, Graham TC, Thomas ED. Combined immunosuppression with cyclosporine and methotrexate in dogs given bone marrow grafts from DLA-haploidentical littermates. Transplantation. 1984;37:62–65. doi: 10.1097/00007890-198401000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Storb R, Kolb HJ, Deeg HJ, et al. Prevention of graft-versus-host disease by immunosuppressive agents after transplantation of DLA-nonidentical canine marrow. Bone Marrow Transplant. 1986;1:167–177. [PubMed] [Google Scholar]
- 8.Wagner JL, DeRose SA, Burnett RC, Storb R. Brief Communication: Nucleotide sequence and polymorphism analysis of canine DRA cDNA clones. Tissue Antigens. 1995;45:284–287. doi: 10.1111/j.1399-0039.1995.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 10.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 11.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–373. [PubMed] [Google Scholar]
- 12.Sandmaier BM, Storb R, Appelbaum FR, Gallatin WM. An antibody that facilitates hematopoietic engraftment recognizes CD44. Blood. 1990;76:630–635. [PubMed] [Google Scholar]
- 13.McSweeney PA, Rouleau KA, Wallace PM, et al. Characterization of monoclonal antibodies that recognize canine CD34. Blood. 1998;91:1977–1986. [PubMed] [Google Scholar]
- 14.Zaucha JM, Zellmer E, Georges G, et al. G-CSF-mobilized peripheral blood mononuclear cells added to marrow facilitates engraftment in nonmyeloablated canine recipients: CD3 cells are required. Biol Blood Marrow Transplant. 2001;7:613–619. doi: 10.1053/bbmt.2001.v7.pm11760149. [DOI] [PubMed] [Google Scholar]
- 15.Barsoukov AA, Moore PF, Storb R, Santos EB, Sandmaier BM. The use of an anti-TCRab monoclonal antibody to control host-versus-graft reactions in canine marrow allograft recipients conditioned with low dose total body irradiation. Transplantation. 1999;67:1329–1335. doi: 10.1097/00007890-199905270-00007. [DOI] [PubMed] [Google Scholar]
- 16.Moore PF, Rossitto PV, Danilenko DM, Wielenga JJ, Raff RF, Severns E. Monoclonal antibodies specific for canine CD4 and CD8 define functional T-lymphocyte subsets and high density expression of CD4 by canine neutrophils. Tissue Antigens. 1992;40:75–85. doi: 10.1111/j.1399-0039.1992.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 17.Sandmaier BM, Schuening FG, Bianco JA, et al. Biochemical characterization of a unique canine myeloid antigen. Leukemia. 1991;5:125–130. [PubMed] [Google Scholar]
- 18.Sandmaier BM, Storb R, Santos EB, et al. Allogeneic transplants of canine peripheral blood stem cells mobilized by recombinant canine hematopoietic growth factors. Blood. 1996;87:3508–3513. [PubMed] [Google Scholar]
- 19.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 20.Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75:1947–1950. [PubMed] [Google Scholar]
- 21.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research (Review) Ann Rheum Dis. 2009;68:1100–1104. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 23.Modiano JF, Breen M, Burnett RC, et al. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005;65:5654–5661. doi: 10.1158/0008-5472.CAN-04-4613. [DOI] [PubMed] [Google Scholar]
- 24.Yunusov MY, Kuhr CS, Georges GE, et al. Partial donor-specific tolerance to delayed skin grafts after rejection of hematopoietic cell graft. Transplantation. 2006;82:629–637. doi: 10.1097/01.tp.0000229449.09622.28. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corey CA, DeSilva AD, Holland CA, Williams DA. Serial transplantation of methotrexate-resistant bone marrow: protection of murine recipients from drug toxicity by progeny of transduced stem cells. Blood. 1990;75:337–343. [PubMed] [Google Scholar]
- 27.Belur LR, Boelk-Galvan D, Diers MD, McIvor RS, Zimmerman CL. Methotrexate accumulates to similar levels in animals transplanted with normal versus drug-resistant transgenic marrow. Cancer Res. 2001;61:1522–1526. [PubMed] [Google Scholar]
- 28.Thakar MS, Zhang X-B, Beard BC, et al. Transmission and expansion of HOXB4-induced leukemia in two immunosuppressed dogs: implications for a new canine leukemia model. Exp Hematol. 2009;37:1157–1166. doi: 10.1016/j.exphem.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]