Abstract
The late-phase of long-term potentiation (L-LTP) in hippocampal area CA1 requires gene expression and de novo protein synthesis but it is expressed in an input-specific manner. The `synaptic tag' theory proposes that gene products can only be captured and utilized at synapses that have been `tagged' by previous activity. The mechanisms underlying synaptic tagging, and its activity dependence, are largely undefined. Previously, we reported that low-frequency stimulation (LFS) decreases the stability of L-LTP in a cell-wide manner by impairing synaptic tagging. We show here that a phosphatase inhibitor, okadaic acid, blocked homosynaptic and heterosynaptic inhibition of L-LTP by prior LFS. In addition, prior LFS homosynaptically and heterosynaptically impaired chemically induced synaptic facilitation elicited by forskolin / 3-isobutyl-1-methylxanthine, suggesting that there is a cell-wide dampening of cAMP / protein kinase A (PKA) signaling concurrent with phosphatase activation. We propose that prior LFS impairs expression of L-LTP by inhibiting synaptic tagging through its actions on the cAMP / PKA pathway. In support of this notion, we show that hippocampal slices from transgenic mice that have genetically reduced hippocampal PKA activity display impaired synaptic capture of L-LTP. An inhibitor of PKA, KT-5720, also blocked synaptic capture of L-LTP. Moreover, pharmacological activation of the cAMP / PKA pathway can produce a synaptic tag to capture L-LTP expression, resulting in persistent synaptic facilitation. Collectively, our results show that PKA is critical for synaptic tagging and for input-specific L-LTP. PKA-mediated signaling can be constrained by prior episodes of synaptic activity to regulate subsequent L-LTP expression and perhaps control the integration of multiple synaptic events over time.
Keywords: long-term potentiation, metaplasticity, mice, protein kinase A, synaptic tagging
Introduction
Activity-dependent changes in synaptic strength are believed to underlie information storage in the brain (Martin et al., 2000). Long-term potentiation (LTP) is a long-lasting increase in synaptic strength that occurs in response to brief, repetitive stimulation (Bliss & Lømo, 1973; Andersen et al., 1977). A neuron in the central nervous system typically receives inputs from thousands of synaptic contacts, yet changes in synaptic strength can be input-specific and are spatially restricted (Lynch et al., 1977; Andersen et al., 1977).
Long-lasting LTP [late-phase LTP (L-LTP)] requires gene expression and de novo protein synthesis, the products of which may be transported in a cell-wide manner (Krug et al., 1984; Stanton & Sarvey, 1984; Frey et al., 1988; Nguyen et al., 1994). To preserve the input specificity of L-LTP, a mechanism to mark, or `tag', active synapses has been proposed to allow newly synthesized gene products to be captured and utilized at appropriately activated synapses (Sossin, 1996; Schuman, 1997; Frey & Morris, 1998b). Frey & Morris (1997) first provided evidence for the synaptic tag theory in the rat hippocampus. They proposed that proteins synthesized in response to long-term synaptic changes at one set of synapses could be captured and utilized by other synapses to express L-LTP if a synaptic tag is generated by appropriate synaptic activity. In accordance with this idea, they found that transient potentiation resulting from weaker synaptic activation could be prolonged to resemble L-LTP if paired with established protein synthesis-dependent L-LTP at separate synaptic inputs (Frey & Morris, 1997, 1998a). Recently, PKMζ has been identified as a plasticity-related protein that may be captured by tagged synapses during the transformation of early into late LTP (Sajikumar et al., 2005). However, the nature and identities of synaptic tags, and how tagging is regulated by synaptic activity remain largely undefined.
Previously, we reported a novel form of homosynaptically and heterosynaptically expressed metaplasticity (Young & Nguyen, 2005). Low-frequency stimulation (LFS) impaired L-LTP subsequently induced at the previously activated synapses (homosynaptic inhibition), as well as at other synapses converging on the same post-synaptic cells (heterosynaptic inhibition). We extend these findings here by showing that homosynaptic and heterosynaptic inhibition of L-LTP by prior LFS require protein phosphatase activity, and that LFS also impairs signaling through the cAMP / protein kinase A (PKA) pathway in a non-input-specific manner. Our previous data suggested that prior LFS impairs L-LTP by inhibiting synaptic tagging and capture (Young & Nguyen, 2005). Indeed, in the present study, we found that pharmacological or genetic inhibition of PKA produced similar impairments in synaptic capture. Moreover, pharmacological activation of cAMP / PKA signaling is sufficient to generate a synaptic tag that can produce persistent synaptic facilitation. Our results implicate a role for PKA in synaptic tagging that may be a novel control point in the consolidation of L-LTP. PKA-mediated signaling can be constrained by prior episodes of synaptic activity to regulate subsequent L-LTP expression and the integration of multiple synaptic events over time.
Materials and methods
Animals
Experiments were performed on female C57BL / 6 mice (aged 10–14 weeks, Charles River, Montreal, Canada) housed at the University of Alberta under Canadian Council on Animal Care guidelines. Where indicated, female R(AB) transgenic mice and age-matched wildtype littermates of the transgenic mice were used (aged 12–14 months). Transgenic animals were derived from two independent lines that were previously characterized for neural expression of the R(AB) transgene (Clegg et al., 1987), hippocampal PKA activity, hippocampal synaptic physiology and hippocampus-dependent long-term memory (Abel et al., 1997). R(AB) transgenic mice are maintained in the hemizygous state by backcrossing to C57BL6 / J mice. The R(AB) colony is currently at backcross generation N10–N13 onto C57BL6 / J. For genotyping, tail DNA was analysed by southern blotting using a transgene-specific probe as described previously (Abel et al., 1997). R(AB) mice were housed at the University of Pennsylvania and University of Alberta under Institutional Animal Care and Use Committee and Canadian Council on Animal Care guidelines.
Protein kinase A assays
Mice were killed by cervical dislocation and decapitated. Hippocampi were dissected and homogenized in 300 μL of ice-cold extraction buffer (phosphate-buffered saline, 1 mM EGTA, 1 mM EDTA, 0.5% Triton X-100, 5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride and a cocktail of protease inhibitors). Hippocampal extracts were then diluted with extraction buffer to a protein concentration of 2 mg / mL (Bradford, 1976) and kept in ice until use. Reactions took place in 50-μL volumes containing 10 μL of the 2 mg / mL protein extract and 11.5 μL of assay buffer [37 mM MgOAc, 74 mM Tris-HCl, pH 7.4, 18.5 mM NaF, 2 mM 3-isobutyl-1-methylxanthine (IBMX), 5 mM dithiothreitol, 195.5 μm ATP, [γ-32P]ATP and 120 μm kemptide as the PKA substrate]. Some reactions included 10 μL cAMP (25 μm) and / or 4 μL of the PKA inhibitory peptide, PKI (0.5 mg / mL). Reactions were carried out in duplicate at 30 °C for 5 min and terminated by spotting 15 μL onto phosphocellulose discs that were washed five times with 0.5% phosphoric acid. Discs were air-dried and kemptide-incorporated 32P was determined by scintillation counting. To calculate PKA activities (pmol [γ-32P]ATP / min / mg protein), background activity from parallel reactions without added kemptide was subtracted. Four different PKA activities were determined: basal PKI (no PKI and no cAMP added), basal + PKI (with PKI and no cAMP), cAMP PKI (with cAMP and no PKI) and cAMP + PKI (with cAMP and PKI added). Basal PKA activity was calculated by subtracting values of the activity that was not inhibited by PKI (basal + PKI) from those obtained when no PKI was added (basal PKI). Likewise, cAMP-stimulated PKA activity was calculated by subtracting values of the activity that was not inhibited by PKI (cAMP + PKI) from those obtained when no PKI was added (cAMP PKI).
Student's t-test was used for statistical comparison of PKA activity levels.
Electrophysiology
Following cervical dislocation and decapitation, transverse hippocampal slices (400 μm thickness) were cut, transferred to an interface chamber and maintained at 28 °C. Artificial cerebrospinal fluid, used for dissection and superfusion, was consistent with previous studies (Young & Nguyen, 2005) and contained the following (in mM): NaCl, 124; KCl, 4.4; MgSO4.7H2O, 1.3; NaH2PO4.H2O, 1.0; NaHCO3, 26.2; CaCl2, 2.5 and D-glucose, 10. Slices were allowed to recover for at least 60 min before experiments commenced. For experiments involving pre-incubation in okadaic acid (OA), slices were allowed to recover for 30 min before transfer to drug solutions.
Recordings of extracellular field excitatory post-synaptic potentials (fEPSPs) were obtained with a glass microelectrode (electrical resistance 2–4 MΩ) filled with artificial cerebrospinal fluid and placed in stratum radiatum of area CA1. fEPSPs were elicited by using two bipolar nickel-chromium electrodes placed in stratum radiatum to stimulate two separate sets of inputs converging onto the same post-synaptic population of neurons. The independence of the two pathways was confirmed by the absence of interpathway paired-pulse facilitation elicited by successive stimulation through the two electrodes at 40-, 50-, 75-, 100-, 150- and 200-ms intervals. Interpathway paired-pulse facilitation was assessed during baseline acquisition and at the conclusion of experiments. Test stimuli were given once per minute at a stimulus intensity that evoked fEPSP amplitudes of ~40% of the maximum size (0.08 ms pulse width). The `weak' LTP protocol consisted of a single 100-Hz train (1 s duration) and it elicited transient early LTP (E-LTP; Huang & Kandel, 1994). `Strong' tetanus to induce L-LTP consisted of four stimulus trains (100 Hz each) with an intertrain interval of 3 s. This protocol was also used to induce L-LTP in R(AB) transgenic mice; L-LTP induced with four 100-Hz trains spaced 5 min apart is impaired in R(AB) transgenic mice but a compressed multitrain stimulation protocol (four trains, intertrain interval 3 s) can overcome the PKA signaling impairments in R(AB) mutants to elicit stable L-LTP (Woo et al., 2003). LFS at 5 Hz for 3 min was used to induce metaplasticity and also to elicit depotentiation (DPT) (reversal of LTP; Barrioneuvo et al., 1980; Staübli & Lynch, 1990; Woo & Nguyen, 2002). fEPSPs were monitored for 60 min (E-LTP and DPT experiments) or 120 min [L-LTP and forskolin (FSK) / IBMX experiments] post-LTP induction.
Drugs
Forskolin (50 μM; Sigma, St Louis, MO, USA), an adenylyl cyclase activator, and IBMX (50 μM; Sigma), a phosphodiesterase inhibitor, were applied together to elicit PKA-dependent chemical L-LTP (Chavez-Noriega & Stevens, 1992; Nguyen et al., 2000). Lower concentrations of FSK and IBMX (25 μM each) in combination with a transcriptional inhibitor, actinomycin D (Act D, 25 μM; Bioshop Canada, Burlington, Ontario, Canada), were used in experiments that required only transient cAMP-induced synaptic facilitation (Fig. 5). At the concentration used here (25 μM), Act D has been shown to inhibit transcription in hippocampal slices by approximately 70–80% (Nguyen et al., 1994). KT-5720 (KT, 1 μM; Biomol, Plymouth, PA, USA) inhibits PKA by blocking activity of catalytic subunits of PKA (Kase et al., 1987). FSK, IBMX, Act D and KT were prepared as concentrated stock solutions (50 mM FSK, 50 mM IBMX, 25 mM Act D and 1 mM KT) in dimethylsulfoxide (Sigma Aldrich). These drugs were diluted with artificial cerebrospinal fluid to their final concentrations and bath applied. Sodium OA (Sigma) was prepared as a 1 mM concentrated stock solution in distilled water and then diluted to a final concentration of 1 μM in artificial cerebrospinal fluid. At this concentration, OA inhibits protein phosphatases 1 and 2A (PP1 and PP2A, respectively; Ishihara et al., 1989; Cohen et al., 1990). All slices in the OA study were pre-incubated in OA for 90–180 min before transfer back to the interface chamber where they were given 10 min to recover before experiments commenced. Experiments involving light-sensitive drugs were carried out in dim-light conditions.
Fig. 5.
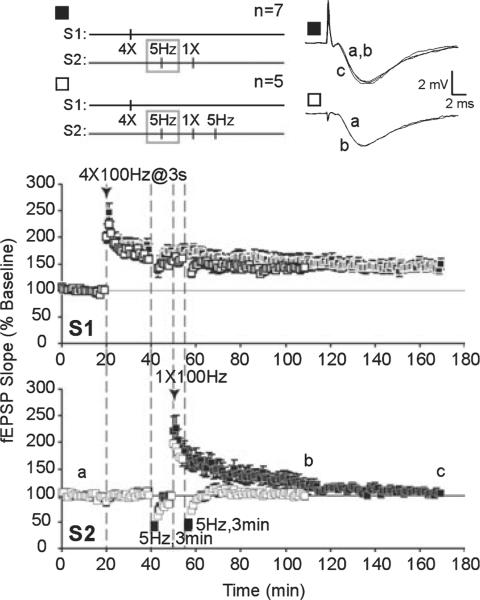
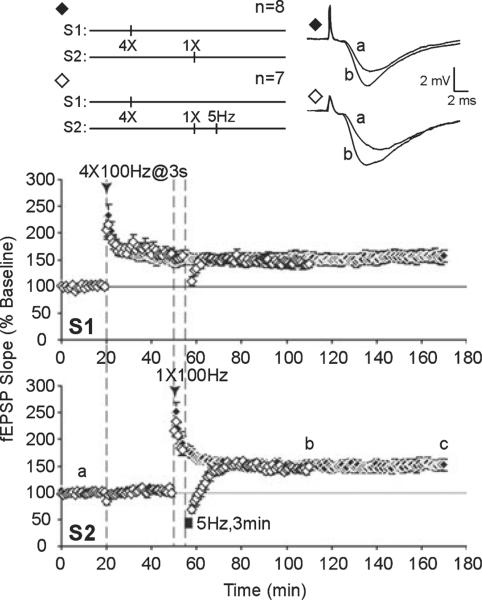
Low-frequency stimulation (LFS) impairs synaptic capture of late-phase long-term potentiation (L-LTP) expression and blocks transfer of immunity to depotentiation (DPT). Strong stimulation to S1 was paired with weak stimulation to S2 to examine synaptic tagging and capture of L-LTP expression in S2. LFS was applied to S2 10 min prior to weak tetanus in S2. Long-term potentiation of S2 (∎) decayed to baseline values (a) within 120 min post-tetanus (c). Transferred immunity to DPT (□) was also blocked by prior LFS; following depotentiating LFS, mean field excitatory post-synaptic potential (fEPSP) slopes were depressed and stabilized at baseline levels (b).
Data analysis
In general, our data analysis followed procedures described in Woo & Nguyen (2003). The initial slope of the fEPSP was measured as an index of synaptic strength. Average `baseline' slope values were acquired over 20 min and LTP graphs were plotted by expressing fEPSP slopes as percentages of the averaged baseline slopes. Student's t-test was used for statistical comparison of mean fEPSP slopes within paired data sets, with a significance level of P < 0.05 (denoted on graphs with an *). Data sets with more than two comparison groups were analysed with ANOVA. A Tukey-Kramer multiple comparisons test was completed if ANOVA analysis indicated a significant difference between groups (P < 0.05, denoted on graphs with an *). Kolmogorov-Smirnov and Bartlett's tests were performed to determine normality and to analyse SDs, respectively, of all test groups. All values shown are mean ±SEM with n, number of slices and, where indicated, N, number of animals.
Results
Homosynaptic and heterosynaptic inhibition of late-phase long-term potentiation by prior low-frequency stimulation requires protein phosphatases
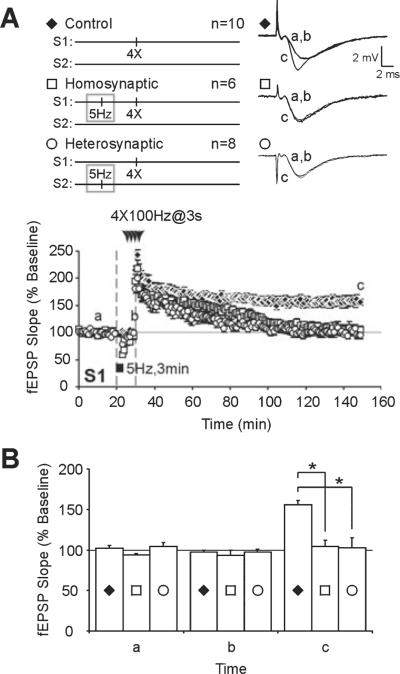
We replicated previous experiments and showed that LFS at 5 Hz for 3 min impairs L-LTP induced subsequently at those same synapses (i.e. homosynaptic inhibition; Woo & Nguyen, 2002) and at other synapses converging on the same post-synaptic cells (i.e. heterosynaptic inhibition; Young & Nguyen, 2005). We stimulated two independent pathways and recorded fEPSPs in stratum radiatum of area CA1. We applied 5-Hz LFS to one pathway. At 10 min after the beginning of LFS, a time point at which fEPSPs had recovered to control baseline values (S1, 30 min; controls, 97 ± 2%, n = 10; homosynaptic, 93 ± 6%, n = 6; heterosynaptic, 97 ± 3%, n = 8; F2,22 = 0.3259, P = 0.7255; Fig. 1A, time point b), four trains of tetanus were given either to the pathway that had received the LFS (i.e. homosynaptic) or to a separate pathway (i.e. heterosynaptic). Consistent with previous studies, prior LFS significantly decreased the amount of potentiation observed 120 min after L-LTP induction (S1, 150 min; controls, 156 ± 5%, n = 10; homosynaptic, 105 ± 8%, n = 6; heterosynaptic, 103 ± 12%, n = 8; F2,22 = 13.319,P < 0.0002; Fig. 1A, time point c). Post-hoc tests revealed significant impairment of homosynaptic (P < 0.01) and heterosynaptic (P < 0.001) L-LTP compared with control slices that received L-LTP stimulus without prior LFS (Fig. 1B, time point c).
Fig. 1.
Prior low-frequency stimulation (LFS) impairs subsequent induction of late-phase long-term potentiation (L-LTP) in homosynaptic and heterosynaptic inputs. (A) Four 100-Hz trains of stimuli were used to induce stable L-LTP (control, ◆). When L-LTP induction was preceded by LFS at 5 Hz for 3 min, L-LTP expression was significantly impaired in both homosynaptic (□) and heterosynaptic (◯) inputs. (B) Summary histogram showing homosynaptic (□) and heterosynaptic (◯) inhibition of L-LTP by prior LFS (control, ◆). LFS induced a transient synaptic depression that recovered to baseline values (a) within 10 min of initial LFS (b). L-LTP expression was significantly impaired at 120 min post-induction (c). Asterisks indicate statistical significance (*P < 0.05). fEPSP, field excitatory post-synaptic potential.
Protein phosphatase activity is enhanced following LFS and induction of long-term depression (Mulkey et al. 1993; Thiels et al., 1998), and PP1 / 2A have been implicated in homosynaptic inhibition of subsequent L-LTP (Woo & Nguyen, 2002). It is unclear whether PP1 / 2A are also critical for heterosynaptic metaplasticity. We used a PP1 / 2A inhibitor, sodium OA (1 μM; Clegg et al. 1987), to determine whether these phosphatases are needed for the inhibitory effects of LFS on subsequent L-LTP.
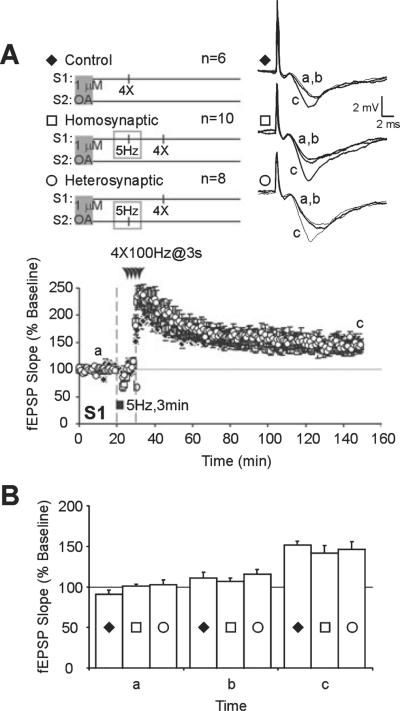
Slices were incubated in a separate holding chamber in artificial cerebrospinal fluid with OA for 90–180 min and then transferred to an interface chamber where they were allowed to recover for 10 min before experiments commenced. LFS at 5 Hz was applied to one pathway followed by L-LTP-inducing tetani to either homosynaptic or heterosynaptic inputs. To control for possible effects of OA, the incubation period, or transfer protocol on L-LTP, comparisons were made to control slices which underwent similar incubation in OA, transfer protocol and recovery period, and which received L-LTP-inducing stimuli but not prior LFS.
Pre-incubation in OA did not affect the stability of L-LTP or general health of slices but blocked the inhibitory effects of prior LFS on subsequent L-LTP (Fig. 2A). Mean fEPSP slopes in slices that received LFS pre-conditioning (S2, 150 min; homosynaptic, 142 ± 9%, n = 10; heterosynaptic, 147 ± 9%, n = 8; Fig. 2A, time point c) did not differ significantly from slices that received L-LTP tetanus without prior LFS (S2, 150 min; control, 151 ± 5%, n 6; F2,21 = 0.2943, P = 0.7481; Fig. 2A, time point c). Figure 2B shows a summary histogram of mean fEPSP slopes from the three treatment groups taken during baseline (time point a), 10 min after LFS (time point b) and 120 min after L-LTP induction (time point c). These data show that PP1 / 2A are required for homosynaptic and heterosynaptic inhibition of L-LTP by prior LFS.
Fig. 2.
Homosynaptic and heterosynaptic inhibition of late-phase long-term potentiation (L-LTP) by prior low-frequency stimulation (LFS) requires protein phosphatase 1/2A activation. (A) Pre-incubation of slices in okadaic acid (OA; 1 μM) blocked the homosynaptic (□) and heterosynaptic (◯) inhibitory effects of LFS on subsequently induced L-LTP. Comparisons were made to control slices which underwent similar incubation in OA, transfer protocol and recovery period, and received L-LTP-inducing stimuli but not prior LFS (◆). (B) Summary histogram of data from OA-treated slices. There was no significant difference in mean field excitatory post-synaptic potential (fEPSP) slopes at 120 min post-tetanus (c) between slices that received LFS prior to L-LTP induction (◯ and □) and those that did not (P > 0.01, ◆). Mean fEPSP slopes were similar during baseline (a) and 10 min after LFS onset (b).
Prior low-frequency stimulation homosynaptically and heterosynaptically impairs cAMP / protein kinase A signaling
Expression of synaptic plasticity involves a balance between the opposing actions of protein kinase and protein phosphatase activities (Lisman, 1989; Coussens & Teyler, 1996; Wang & Kelly, 1996; Blitzer et al., 1998). Stimulation at low frequencies (< 10 Hz) is usually associated with phosphatase activation and decreased synaptic strength (Mulkey et al., 1993, 1994). In contrast, stimulation at higher frequencies activates protein kinases and results in increased synaptic strength (Malinow et al., 1989; Matthies & Reymann, 1993). In particular, signaling through the PKA pathway is required for the consolidation of early to late LTP (Frey et al., 1993; Matthies & Reymann, 1993; Huang & Kandel, 1994; Abel et al., 1997; for review, see Nguyen & Woo, 2003). As LFS selectively impairs L-LTP while leaving E-LTP intact (Woo & Nguyen, 2002; Young & Nguyen, 2005), we hypothesized that LFS may impair expression of L-LTP by inhibiting cAMP / PKA signaling.
Transient application of an adenylyl cyclase activator, FSK, and a phosphodiesterase inhibitor, IBMX, can induce long-lasting facilitation at CA3–CA1 synapses (Chavez-Noriega & Stevens, 1992). This form of synaptic facilitation is mediated by the cAMP / PKA signaling pathway as it is blocked by a PKA inhibitor, Rp-cAMPS (Nguyen et al., 2000; Woo et al. 2002). In addition, R(AB) transgenic mice that have reduced hippocampal PKA activity also show impaired FSK / IBMX facilitation (Abel et al., 1997; Woo et al., 2002).
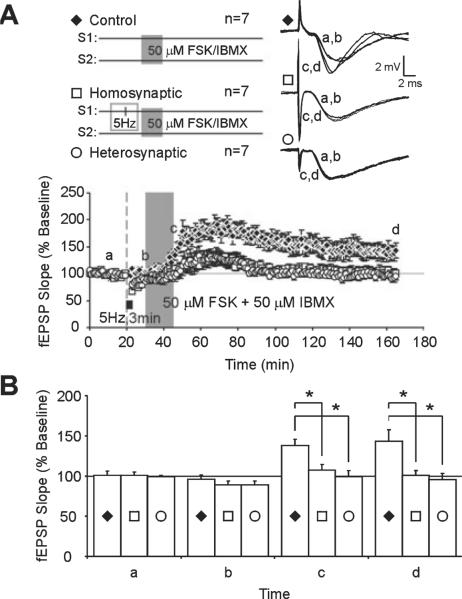
We found that chemical LTP induced by activation of the cAMP / PKA pathway was significantly impaired homosynaptically and heterosynaptically following LFS. Brief application of FSK / IBMX (50 μM each) produced stable, persistent facilitation lasting 2 h after drug application (165 min; control, 143 ± 15%, n = 7; Fig. 3A, time point d). LFS at 5 Hz for 3 min induced a transient homosynaptic and heterosynaptic synaptic depression, in accordance with previous results (Woo & Nguyen, 2002; Young & Nguyen, 2005). Mean fEPSP slopes recovered within 10 min of LFS onset and did not differ significantly from pre-LFS baseline slopes (30 min; homosynaptic, 89 ± 5%, n = 7; heterosynaptic, 89 ± 4%, n = 7; control, 96 ± 5%, n = 7; F2,18 = 0.7057, P = 0.5069; Fig. 3A, time point b). Application of FSK / IBMX following LFS and recovery induced a smaller facilitation of fEPSPs than in control slices that did not receive LFS prior to FSK / IBMX application (45 min; homosynaptic, 107 ± 7%, n = 7; heterosynaptic, 99 ± 8%, n = 7; control, 138 ± 8%, n = 7; Fig. 3A, time point c). Mean fEPSP slopes in slices that received prior LFS decayed to baseline within 2 h of drug application (165 min; homosynaptic, 100 ± 6%, n = 7; heterosynaptic, 96 ± 7%, n = 7; Fig. 3A, time point d). ANOVA analysis indicated that mean fEPSP slopes of LFS-treated slices were significantly less than controls that did not receive LFS (45 min, F2,18 = 7.069, P = 0.0054; 165 min, F2,18 = 5.982, P = 0.0102). Figure 3B summarizes LTP data with post-hoc analysis of mean fEPSP slopes taken at baseline (time point a), after LFS (just prior to FSK / IBMX application; time point b), following FSK / IBMX washout (time point c) and 120 min after application of FSK / IBMX (time point d). Prior LFS significantly impaired cAMP / PKA-mediated facilitation in both homosynaptic and heterosynaptic pathways immediately following FSK / IBMX application (45 min; homosynaptic, P < 0.05; heterosynaptic, P < 0.01; Fig. 3B, time point c) and 2 h after (165 min; homosynaptic, P < 0.05; heterosynaptic, P < 0.05; Fig. 3B, time point d).
Fig. 3.
Prior low-frequency stimulation (LFS) impairs homosynaptic and heterosynaptic cAMP/protein kinase A signaling. (A) Chemical long-term potentiation induced by application of forskolin with 3-isobutyl-1-methylxanthine (FSK/IBMX; 50 μM each) is stable over 120 min (control, ◆). Low-frequency stimulation prior to FSK/IBMX application significantly impaired facilitation in homosynaptic (S1, □) and heterosynaptic (S2, ◯) inputs. (B) Summary histogram of mean field excitatory post-synaptic potential (fEPSP) slopes from FSK/IBMX-treated slices (◆) and FSK/IBMX with prior LFS (◯ and □). Drug application began 10 min after initial LFS, when fEPSP values recovered to baseline values (b). Impairments in FSK/IBMX facilitation were evident immediately following FSK/IBMX washout (c) and 120 min after FSK/IBMX application. Asterisks indicate statistical significance (*P < 0.05).
We show that pharmacological activation of the cAMP / PKA pathway is sufficient to elicit long-lasting facilitation at CA3–CA1 synapses, in accordance with previous reports (Chavez-Noriega & Stevens, 1992; Frey et al., 1993; Nguyen et al., 1994; Duffy & Nguyen, 2003). With prior LFS, long-lasting facilitation induced by chemical activation of cAMP / PKA signaling decayed to baseline values within 2 h (Fig. 3A, time point d). These results mirror the decay of electrically induced L-LTP after prior LFS (Woo & Nguyen, 2002; Young & Nguyen, 2005). Significantly, prior LFS also impaired FSK / IBMX facilitation during the initial development of facilitation (Fig. 3A, time point c), indicating that cAMP / PKA signaling is important during the initial stages of L-LTP expression.
Pharmacological inhibition of protein kinase A blocks synaptic capture of late-phase long-term potentiation expression and acquired immunity to depotentiation
Previous work showed that LFS impairs subsequently induced L-LTP by inhibiting synaptic tagging and capture of L-LTP-stabilizing gene products (Young & Nguyen, 2005). As LFS may inhibit cAMP / PKA signaling to suppress subsequent L-LTP, we next asked whether PKA is critical for synaptic tagging and capture of L-LTP. In area CA1 of hippocampal slices, E-LTP can be induced with one high-frequency train of stimulation (typically 100 Hz, 1 s duration) that decays to baseline within 1–2 h (Reymann et al., 1985; Huang & Kandel, 1994). With multiple trains of 100-Hz stimulation, L-LTP is recruited and the duration of potentiation is considerably extended through transcription- and translation-dependent processes (Stanton & Sarvey, 1984; Krug et al., 1984; Frey et al., 1988; Nguyen et al., 1994). However, if transcription-dependent L-LTP is first established at one set of inputs (S1), weak tetanization at a separate set of inputs (S2) can generate a synaptic tag to capture plasticity-related proteins that have been mobilized in response to L-LTP in S1 (Frey & Morris, 1997). We paired multiple tetanic trains (`strong' stimulation) in S1 with a single tetanus (`weak' stimulation) to S2, given 30 min later. Potentiation of the `weak' S2 pathway was persistent at 120 min post-tetanus (S2, 170 min; control, 152 ± 11%, n = 8; Fig. 4, time point b) and resistant to reversal by LFS given 5 min post-tetanus (DPT) after an initial depression below baseline (S2, 58 min; DPT, 75 ± 8%, n = 7), mean fEPSP levels recovered within 60 min and stabilized at pre-depotentiated levels (S2, 110 min; DPT, 149 ± 6%, n = 7; Fig. 4, time point b).
Fig. 4.
Synaptic capture of late-phase long-term potentiation (L-LTP) and acquired immunity to depotentiation. Four trains of 100-Hz tetani (`strong' stimulation) were applied to S1 followed by one train of 100-Hz tetanus (`weak' stimulation) to S2 to examine synaptic tagging and capture of L-LTP expression in S2. Long-term potentiation in S2 resembled L-LTP; field excitatory post-synaptic potential (fEPSP) values remained stable (◆) in S2 for 120 min post-tetanus (c) and long-term potentiation in S2 was resistant to depotentiation (DPT) by subsequent low-frequency stimulation (LFS) (◇); following depotentiating LFS, mean fEPSP slopes were depressed and recovered to potentiated levels (b).
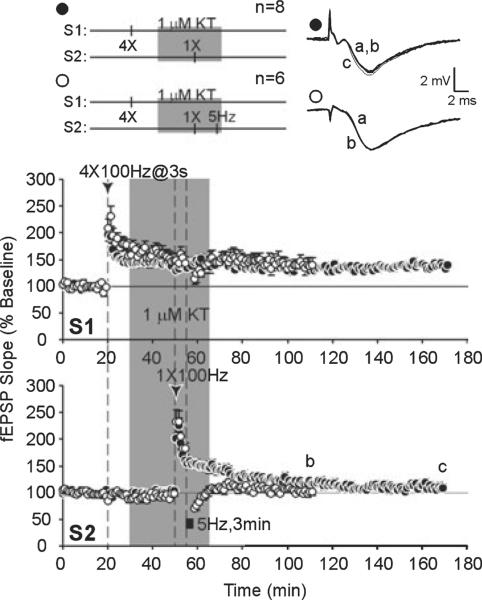
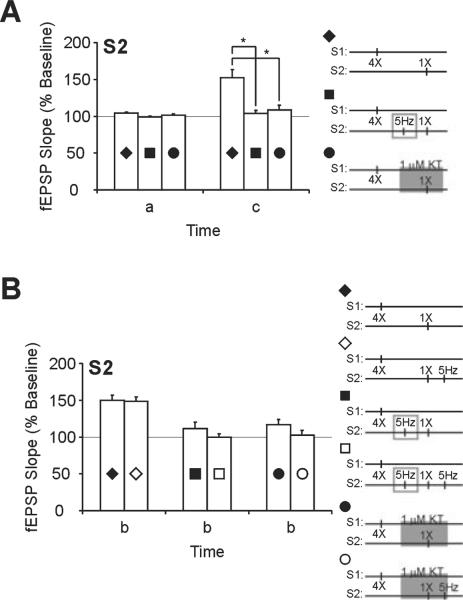
With the same strong plus weak pairing, application of 5-Hz LFS prior to, or a PKA inhibitor (KT, 1 μM) during, weak tetanus in S2 resulted in a transient potentiation of S2 inputs (S2, 170 min; LFS, 104 ± 4%, n = 7; KT, 109 ± 7%, n = 8; Figs 5 and 6, time point c) that was significantly less than controls that did not receive LFS or a PKA inhibitor (F2,20 = 11.109, P < 0.0006). Post-hoc tests showed that strong plus weak stimulation with LFS prior to weak stimulation (P < 0.01), or with KT application during weak stimulation (P < 0.01), elicited significantly less potentiation in the weak S2 pathway than in control slices that had strong plus weak paired without treatment (Fig. 7A).
Fig. 6.
A protein kinase A (PKA) inhibitor impairs synaptic capture of late-phase long-term potentiation (L-LTP) expression and blocks transfer of immunity to depotentiation (DPT). Strong stimulation to S1 was paired with weak stimulation to S2 to examine synaptic tagging and capture of L-LTP expression in S2. A PKA inhibitor, KT-5720 (1 μM), was applied during weak tetanus in S2. Long-term potentiation of S2 (●) decayed to baseline values (a) within 120 min post-tetanus (c). KT-5720 application prevented transfer of immunity to DPT (◯); following depotentiating low-frequency stimulation, mean field excitatory post-synaptic potential (fEPSP) slopes were depressed and stabilized at baseline levels (b).
Fig. 7.
Electrical or pharmacological inhibition of protein kinase A impairs synaptic tagging and capture of late-phase long-term potentiation (L-LTP) expression. (A) Summary of strong stimulation plus weak stimulation long-term potentiation data from the S2 pathway. Mean field excitatory post-synaptic potential (fEPSP) slopes from slices that received one-train tetanus paired with four-train tetanus (◆) are significantly higher at 120 min after one-train tetanus (c) when compared with mean fEPSP slopes from slices that received low-frequency stimulation (LFS) prior to one-train tetanus (∎) or KT-5720 (KT) application during one-train tetanus (●). Mean fEPSP slopes during baseline were similar between all three groups (a). (B) Summary of data from pairing strong plus weak stimulation followed with depotentiating LFS. Mean fEPSP slopes are compared within treatment groups (control, diamonds; LFS, squares; KT, circles) with (open symbols) and without (filled symbols) depotentiating LFS. Mean fEPSP slopes of the S2 pathway recovered to potentiated levels following depotentiation if one-train was paired with four-train L-LTP (diamonds) but remained at baseline values if LFS was given prior to one-train tetanus (squares) or if KT was applied during one-train tetanus (circles). Asterisks denote statistical significance (*P < 0.05).
When strong plus weak tetanus was paired with LFS or KT application, the resulting LTP induced by weak tetanus was also sensitive to DPT. Following DPT, initial levels of depression were similar between control, LFS and KT treatment groups (S2, 58 min; control, 75 ± 8%. n = 7; LFS, 62 ± 5%, n = 5; KT, 71 ± 5%, n 6; F2,15 = 1.163, P 0.3393). However, slices in the LFS and KT treatment groups did not acquire immunity to DPT and mean fEPSP slopes remained close to baseline values (S2, 110 min; LFS, 100 ± 5%, n = 5; KT, 102 ± 7%, n = 6; Figs 5 and 6, time point b). A comparison of depotentiated slices and non-depotentiated controls within treatment groups is summarized in Fig. 7B.
Our data show that PKA is required for synaptic capture of long-lasting potentiation following weak stimulation, consistent with previous studies (Barco et al., 2002). Heterosynaptic transfer of somatic gene products can stabilize LTP expression by conferring immunity of LTP to DPT (Woo & Nguyen, 2003). We demonstrate that capture of immunity to DPT also requires PKA activity. Significantly, L-LTP in S1 was unaffected by LFS (20 min post-tetanus) or KT application (which started at 10 min post-tetanus). Mean fEPSP slopes in S1 of control, LFS and KT-treated slices did not differ significantly at 2.5 h post-induction (S1, 170 min; control, 153 ± 12%, n = 8; LFS, 150 ± 11%, n = 7; KT, 142 ± 6%, n = 8; F2,20 = 0.3235, P = 0.7273). LFS or a PKA inhibitor applied after L-LTP induction did not affect previously established LTP, suggesting that there is a limited time period during which the synaptic tag is set following synaptic activity.
Impairment of synaptic capture of late-phase long-term potentiation expression in R(AB) transgenic mice
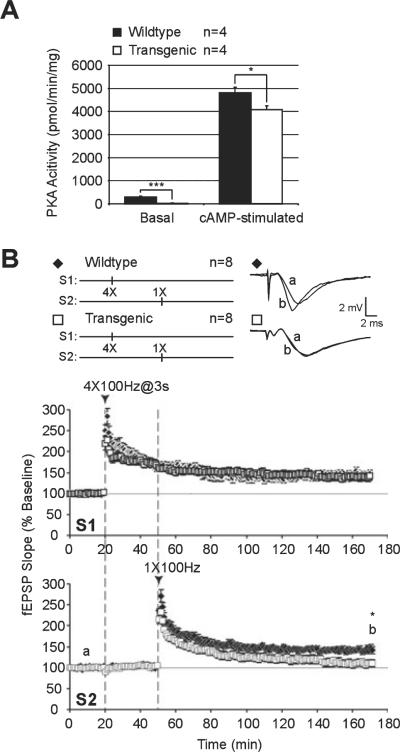
To further examine the role of PKA in synaptic tagging, we measured L-LTP in slices from mutant PKA mice [R(AB) transgenic mice (Abel et al., 1997)] that have genetically reduced hippocampal PKA activity due to expression of an inhibitory form of a regulatory subunit of PKA (RIα; Clegg et al., 1987). The R(AB) transgene is now on a C57BL6 / J genetic background. To verify the decrease in hippocampal PKA activity originally described in the R(AB) transgenic mice (Abel et al., 1997), we recently measured PKA activity in hippocampal extracts from these mice and their wildtype littermates (Fig. 8A). Transgenic animals exhibited more than a 10-fold reduction in basal PKA activity [wildtype, 289 ± 45 pmol / min / mg, n = 4, N = 4; R(AB), 22 ± 11 pmol / min / mg, n = 4, N = 4; P < 0.001]. When the kinase was activated by 5 μM cAMP, PKA activity was significantly reduced by 16% in the mutant mice [wildtype, 4803 ± 240 pmol / min / mg, n = 4, N = 4; R(AB), 4072 ± 176 pmol / min / mg, n = 4, N = 4; P < 0.04]. These results show that the PKA deficits previously described in Abel et al. (1997) are still present in the R(AB) transgenic mice studied here.
Fig. 8.
Synaptic tagging and capture of late-phase long-term potentiation (L-LTP) are impaired in transgenic mice that have reduced protein kinase A (PKA) activity. (A) PKA activity is reduced in hippocampal extracts from R(AB) transgenic mice. PKA activity was measured in hippocampi of R(AB) transgenic mice (n = 4) and wildtype littermate controls (n = 4) as described in Materials and methods. Both basal and cAMP-stimulated PKA activities were significantly reduced in the R(AB)-2 transgenic mice compared with the controls. Asterisks denote statistical significance (***P < 0.001, *P < 0.05). (B) Pairing four-train (S1) and one-train (S2) tetanic stimulation elicited persistent L-LTP at both sets of inputs in slices from wildtype mice (◆). In contrast, slices from R(AB) transgenic mice have significantly lower mean field excitatory post-synaptic potential (fEPSP) slopes in S2 after identical pairing of one and four trains of 100-Hz stimulation (□). Asterisk denotes statistical significance (*P < 0.05).
We examined synaptic capture of L-LTP using the previous protocol of pairing strong tetanization to one pathway (S1) followed by weak tetanization to another pathway (S2). Wildtype littermates showed persistent L-LTP in both pathways (170 min; S1, 147 ± 10%, n = 6, N = 6; S2, 142 ± 8%, n = 6, N = 6; Fig. 8B, time point b). Transgenic mice showed similar levels of potentiation in response to strong tetanization (S1, 170 min, 142 ± 9%, n = 10, N = 8; P > 0.05; Fig. 8B, time point b). However, subsequent weak tetanization to S2 given 30 min later yielded LTP that decayed to near-baseline levels within 120 min (S2, 170 min; 109 ± 9%, n = 10, N = 8; Fig. 8B, time point b); this LTP was significantly lower than wildtype controls (P < 0.05).
Our results show that synaptic capture is impaired in hippocampal slices from mice with genetically reduced PKA activity. These data are consistent with our pharmacological data obtained with KT and with previously published results (Barco et al., 2002). The time course of LTP decay in the weak S2 pathway was similar between slices that received LFS prior to weak tetanus (S2, 110 min, 111 ± 9%, n = 7; Fig. 5), the KT treatment group (S2, 110 min, 117 ± 7%, n = 8; Fig. 6) and R(AB) transgenic mice (S2, 110 min, 122 ± 8%, n = 10, N = 8; F2,22 = 0.4335, P = 0.6536; Fig. 8B). Taken together, our results, obtained from pharmacological and genetic approaches, show that PKA is required for synaptic capture of L-LTP expression.
Transient activation of cAMP / protein kinase A signaling can generate a synaptic tag to capture long-lasting facilitation
Synaptic capture of L-LTP probably encompasses many different processes, including cell-wide distribution of plasticity-related gene products, generation of synaptic tags and capture of plasticity-related proteins at tagged synapses to enable transfer of L-LTP expression between distinct inputs (reviewed by Martin & Kosik, 2002; Kelleher et al., 2004a). Although prior LFS impairs synaptic tagging during subsequently induced L-LTP (Young & Nguyen, 2005), it is unclear whether pharmacological or genetic inhibition of PKA signaling impairs synaptic tagging or synaptic capture. To differentiate between the requirement for PKA in the capture process vs. its role in synaptic tagging, we examined whether activation of the cAMP / PKA pathway is sufficient for synaptic tagging (Fig. 9).
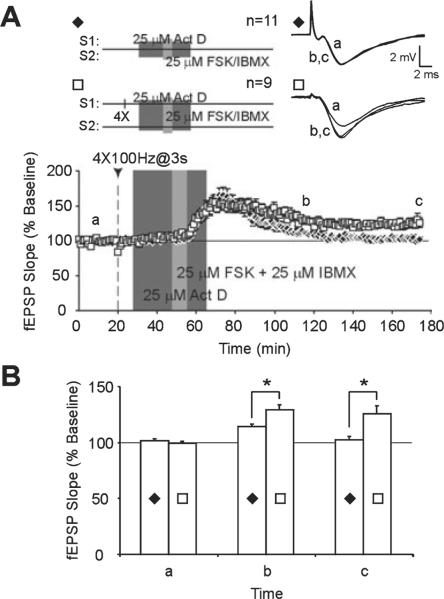
Fig. 9.
Pharmacological activation of the cAMP/protein kinase A pathway can produce a synaptic tag that captures persistent synaptic facilitation. (A) Application of forskolin/3-isobutyl-1-methylxanthine (FSK/IBMX) (25 μM each) in the presence of actinomycin D (Act D; 25 μM) produces a cell-wide transient synaptic facilitation (S1 and S2; ◆). When late-phase long-term potentiation (L-LTP) is first induced by applying four-train tetanus to one pathway (S1), FSK/IBMX + Act D elicits persistent synaptic facilitation in the non-tetanized pathway (S2, □). (B) Comparison of mean field excitatory post-synaptic potential (fEPSP) slopes in slices that received FSK/IBMX + Act D alone (◆) with slices that received four-train tetanus prior to drug application (□). Mean fEPSP slopes are significantly higher at 60 min (b) and 120 min (c) after FSK/IBMX application when FSK/IBMX + Act D is paired with pre-established L-LTP. Asterisks denote statistical significance (*P < 0.05).
As activation of PKA can generate transcription-dependent L-LTP (Frey et al., 1993; Nguyen et al., 1994; Fig. 3A), we used a transcription inhibitor, Act D (25 μM), to reduce transcriptional effects of PKA activation. At this concentration, Act D has been shown to inhibit transcription by > 70% in hippocampal slices (Nguyen et al., 1994). Brief application of FSK / IBMX in the presence of Act D produces a transient facilitation of fEPSPs that decays back to baseline within 120 min of FSK / IBMX application (175 min, S2, 103 ± 3%, n = 11; Fig. 9A, time point c). However, if L-LTP was first established at a separate set of inputs, Act D + FSK / IBMX produced facilitation that persisted for 2 h post-FSK / IBMX application (175 min, S2, 126 ± 7%, n = 9; Fig. 9A, time point c). Post-hoc tests showed that facilitation of fEPSPs by ActD + FSK / IBMX (control) was significantly greater when drug application was paired with strong tetanus at a separate set of converging inputs (P < 0.01; Fig. 9B, time point c). A significant difference in mean fEPSP slopes of the two treatment groups was also evident at 60 min post-FSK/IBMX application (115 min, S2: control, 114 ± 2%, n = 11; L-LTP, 129 ± 4%, n = 9; P < 0.01; Fig. 9B, time point b). These results demonstrate that cAMP/PKA activation is sufficient to generate a synaptic tag that can capture long-lasting facilitation from previously activated L-LTP.
Discussion
Late-LTP in hippocampal CA1 requires gene expression and de novo protein synthesis but is input specific. The `synaptic tag' theory proposes that L-LTP-associated gene products can only be captured and utilized at synapses that have been `tagged' by previous activity (Frey & Morris, 1997). Our principal finding is that PKA is critical for synaptic tagging. Furthermore, PKA-dependent synaptic tagging can be inhibited by prior synaptic activity and may serve as a critical control point to regulate subsequent L-LTP expression.
Activity-dependent regulation of protein phosphatase and kinase signaling
Using two-pathway extracellular field recordings, we showed that protein phosphatases can be recruited by synaptic activity to regulate L-LTP expression in a cell-wide manner. LFS-mediated homosynaptic and heterosynaptic inhibition of subsequent L-LTP was blocked by pre-incubation with OA, an inhibitor of PP1/2A (Fig. 2). Although we did not directly measure hippocampal levels of PP1/2A activity following LFS, our results are consistent with previous studies which have shown increased phosphatase activity during long-term depression (Mulkey et al., 1993; Thiels et al., 1998; Morishita et al., 2001) and activity-dependent translocation of PP1 to synapses using an LFS paradigm identical to that used in our study (5 Hz for 3 min; Morishita et al., 2001). PP1/2A activity has also been linked to decreased stability of L-LTP induced in the same set of inputs after LFS (homosynaptic inhibition; Woo & Nguyen, 2002). We extend these findings by showing that PP1/2A also regulate L-LTP expression at other synapses converging on the same post-synaptic cells (heterosynaptic inhibition).
Our results also show that LFS impairs signaling through the cAMP/PKA pathway (Fig. 3), which has been implicated in the cellular consolidation and stabilization of L-LTP (Frey et al., 1993; Matthies & Reymann, 1993; Abel et al., 1997). Although we did not directly measure PKA activity, we examined the effect of LFS on chemical LTP induced with FSK in combination with IBMX. FSK/IBMX facilitation is mediated by activation of the cAMP/PKA cascade; it is completely blocked by PKA inhibitors and is impaired in transgenic mice that have reduced hippocampal PKA activity (Abel et al., 1997; Nguyen et al., 2000; Woo et al., 2002). We found that giving LFS prior to induction of chemical LTP significantly attenuated its expression. Our results support previous findings which showed that glutamate treatments that result in synaptic depression similar to LFS-induced long-term depression also disrupt PKA anchoring to post-synaptic targets (Gomez et al., 2002). Impairments in FSK/IBMX facilitation were also evident immediately following drug application. As prior LFS selectively impairs L-LTP while leaving E-LTP intact (Woo & Nguyen, 2002; Young & Nguyen, 2005), this suggests that early inhibition of cAMP/PKA signaling by prior LFS can substantially reduce the consolidation of L-LTP.
The rapid impairment of PKA-dependent synaptic facilitation mirrors previously reported activation profiles of phosphatases following LFS. Pharmacological and biochemical studies show enhanced PP1 activity lasting 35–40 min following long-term depression induction in vivo and in vitro (Mulkey et al., 1993; Thiels et al., 1998). This temporal window of PKA inhibition and PP1/2A activation following LFS is consistent with previous studies of metaplasticity which revealed that LFS maintains its inhibitory effect on L-LTP for 20–40 min after LFS (Woo & Nguyen, 2002; Young & Nguyen, 2005). Taken together, our data suggest that LFS-mediated homosynaptic and heterosynaptic inhibition of L-LTP requires PP1/2A. Furthermore, our results indicate that such inhibition occurs through reduction of cAMP/PKA signaling to impair L-LTP expression in a cell-wide manner.
Our results are consistent with the notion that the direction of changes in synaptic efficacy is determined by the relative balance of signaling through protein phosphatases and kinases (Lisman, 1989; Coussens & Teyler, 1996; Wang & Kelly, 1996; Blitzer et al., 1998). Signaling complexes often contain both kinases and counterbalancing phosphatases that converge on specific synaptic plasticity substrates. For example, PKA and PP2A work directly in opposition to regulate class C L-type calcium channel activity by determining the phosphorylation level of serine 1928 (Davare et al., 2000). However, synaptic activity can also regulate anchoring proteins that target kinases and phosphatases to specific subcellular locations (Morishita et al., 2001; Gomez et al., 2002). The mechanisms through which LFS regulates PKA and PP1/2A activity, and the specific nature of the interaction between these two signaling pathways remain to be determined.
Critical role for protein kinase A in synaptic capture and tagging
We found that signaling through the cAMP/PKA cascade is critical for synaptic tagging and capture of L-LTP expression. If transcription-dependent L-LTP is first induced at one set of inputs (S1) with strong tetanic stimulation, weak LTP induced at a second set of inputs on the same post-synaptic neurons (S2) can be transformed to resemble L-LTP by generating a synaptic tag that enables capture of L-LTP-stabilizing proteins (Frey & Morris, 1997; Barco et al., 2002; Young & Nguyen, 2005; Fig. 4). With successful synaptic capture, transient LTP in S2 becomes persistent, lasting for 2 h post-induction. In addition, whereas LTP induced by a single high-frequency train is susceptible to DPT, pairing weak plus strong stimulation confers an immunity to DPT to the weak S2 pathway (Barco et al., 2002; Woo & Nguyen, 2003; Young & Nguyen, 2005; Fig. 4).
We show that a PKA inhibitor, KT, prevented synaptic capture of L-LTP expression and blocked acquired immunity to DPT (Fig. 6). Furthermore, R(AB) transgenic mice that have impaired cAMP/PKA signaling show deficient synaptic capture when tested with the strong plus weak LTP pairing protocol (Fig. 8). LFS has previously been shown to impair synaptic tagging and capture of L-LTP expression (Young & Nguyen, 2005; Fig. 5), and we show in the current study that LFS also impairs cAMP/PKA signaling (Fig. 3). Thus, synaptic capture of L-LTP expression is impaired by electrical (i.e. LFS), pharmacological or genetic inhibition of PKA signaling. Collectively, our data indicate that PKA is required for synaptic tagging and capture of L-LTP expression. Consistent with this interpretation, transient activation of the cAMP/PKA cascade is sufficient to generate a synaptic tag and capture prolonged facilitation when paired with electrically induced L-LTP (Fig. 9).
Protein kinase A activation leading to cAMP response element-mediated gene expression is a critical step in the consolidation of E-LTP to L-LTP (Bourtchouladze et al., 1994; Impey et al., 1996; Matsushita et al., 2001). In the present study, we show that PKA is also critical for synaptic tagging. This dual role for PKA in L-LTP is supported by a type of prolonged synaptic facilitation that results from pharmacological activation of the cAMP/PKA cascade. Previous studies have shown that this type of chemical LTP requires transcription and translation, and can occlude electrically induced L-LTP (Frey et al., 1993; Huang & Kandel, 1994). Significantly, several studies have shown that activation of transcription and translation alone is not sufficient to induce L-LTP (Barco et al., 2002; Dudek & Fields, 2002). For example, antidromic stimulation is sufficient for activating extracellular signal-regulated kinase and cAMP response element-binding protein (CREB; Dudek and Fields, 2002), two pathways associated with translational and transcriptional activation (Impey et al., 1998; Kelleher et al., 2004a, b). However, antidromic stimulation does not elicit LTP (Dudek & Fields, 2002). Similarly, temporally restricted expression of constitutively active CREB (VP16-CREB) does not alter basal synaptic transmission in transgenic mice (Barco et al., 2002). On the other hand, both antidromic stimulation and VP16-CREB expression reduce the threshold for inducing long-lasting plasticity. Weak LTP stimulation, when paired with antidromic stimulation or VP16-CREB expression (in transgenic mice), elicits stable L-LTP (Barco et al., 2002; Dudek & Fields, 2002). These results show that, in addition to transcription and translation, a synaptic tag (e.g. generated by weak LTP stimulation) is required to permit capture of plasticity-related proteins for L-LTP expression. This suggests that, during FSK/IBMX chemical LTP, PKA signaling may engage other processes besides transcription and translation. Indeed, post-synaptic infusion of PKA catalytic subunits is sufficient for initiating persistent synaptic facilitation (Duffy & Nguyen, 2003), consistent with a dual role for PKA in synaptic tagging and transcriptional control. Activity-dependent regulation of transcription and translation by PKA has been previously reported (for reviews, see West et al., 2002; Kelleher et al., 2004a). Our data identify a novel role for PKA in synaptic plasticity, i.e. regulation of synaptic tagging and expression of L-LTP.
Functional significance
Acute activation of cAMP/PKA is critical for many types of long-lasting synaptic plasticity and long-term memory. However, several studies suggest that up-regulation of the cAMP/PKA pathway beyond a certain optimal range can also have deleterious effects on long-term memory. Genetic manipulations that enhance cAMP/PKA signaling by removing inhibitory constraints of Gia1 on adenylyl cyclase impaired hippocampus-dependent memory formation (Pineda et al., 2004). Significantly, ablation of Gia1 in these mutants also resulted in a twofold increase in basal adenylyl cyclase activity (Pineda et al., 2004). Pharmacological enhancement of cAMP/PKA signaling can improve spatial memory in young mice and reverse age-related deficits in senescent mice (Barad et al., 1998; Bach et al., 1999; Hsu et al., 2002). However, pharmacological up-regulation of PKA activity is most effective at doses that amplify cAMP/PKA signaling without affecting basal cAMP levels and therefore preserve the signal-to-noise ratio of cAMP/PKA signaling induced by synaptic activity (Barad et al., 1998; Bach et al., 1999). Thus, memory formation depends on a fine balance between opposing mechanisms that increase and decrease cAMP/PKA signaling. Indeed, in vivo saturation of LTP with electrical stimulation or FSK application interferes with hippocampus-dependent memory (Moser et al., 1998; Pineda et al., 2004). Our data show that PKA plays a critical role in synaptic tagging and in input-specific long-lasting potentiation. Therefore, we speculate that manipulations that produce a general increase in cAMP/PKA activation may result in a non-specific setting of synaptic tags that may interfere with establishing input-specific plasticity required for making new memories.
Acknowledgements
This work was supported by grants (to P.V.N.) from the Canadian Institutes of Health Research (CIHR) and the Alberta Paraplegic Foundation. P.V.N. is a Faculty Senior Scholar of the Alberta Heritage Foundation for Medical Research. J.Z.Y. was supported by CIHR operating grants. T.A. and C.I. are supported by grants from the National Institutes of Health. T.A. is a David and Lucile Packard Foundation Fellow. We thank L. Schimanski and J. Gelinas for helpful discussions and critical reading of the manuscript, and Ted Huang for comments on the manuscript.
Abbreviations
- Act D
actinomycin D
- CREB
cAMP response element-binding protein
- DPT
depotentiation
- E-LTP
early phase long-term potentiation
- fEPSP
field excitatory post-synaptic potential
- FSK
forskolin
- IBMX
3-isobutyl-1-methylxanthine
- KT
KT-5720
- LFS
low-frequency stimulation
- L-LTP
late-phase long-term potentiation
- LTP
long-term potentiation
- OA
okadaic acid
- PKA
protein kinase A
- PKI
PKA inhibitory peptide
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Andersen P, Sundberg SH, Sveen O. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977;266:736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related deficits in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenutated by drugs that enhance the cAMP signaling pathway. Proc. Natl Acad. Sci. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel ER. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc. Natl Acad. Sci. 1998;95:15 020–15 025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and `potentiated' synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Stevens CF. Modulation of synaptic efficacy in field CA1 of the rat hippocampus by forskolin. Brain Res. 1992;574:85–92. doi: 10.1016/0006-8993(92)90803-h. [DOI] [PubMed] [Google Scholar]
- Clegg CH, Correll LA, Cadd GG, McKnight GS. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J. Biol. Chem. 1987;262:13 111–13 119. [PubMed] [Google Scholar]
- Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem. Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Coussens CM, Teyler TJ. Protein kinase and phosphatase activity regulate the form of synaptic plasticity expressed. Synapse. 1996;24:97–103. doi: 10.1002/(SICI)1098-2396(199610)24:2<97::AID-SYN1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Davare MA, Horne MC, Hell JW. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J. Biol. Chem. 2000;275:39 710–39 717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Fields RD. Somatic action potentials are sufficient for late-phase LTP-related cell signaling. Proc. Natl Acad. Sci. 2002;99:3962–3967. doi: 10.1073/pnas.062510599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SN, Nguyen PV. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J. Neurosci. 2003;23:1142–1150. doi: 10.1523/JNEUROSCI.23-04-01142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 1998a;37:545–552. doi: 10.1016/s0028-3908(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998b;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. Regulation of A-kinase anchoring protein 79 / 150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J. Neurosci. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Liang YC, Wu HM, Chen YL, Lo SW, Ho WC. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus. 2002;12:787–802. doi: 10.1002/hipo.10032. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn. Mem. 1994;1:74–81. [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser C, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser C, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemera D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Comm. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Comm. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms and of synaptic plasticity. Neuron. 2004a;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004b;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res. Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc. Natl Acad. Sci. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of LTP. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien JZ. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Martin KC, Kosik KS. Synaptic tagging – who's it? Nat. Rev. Neurosci. 2002;3:813–820. doi: 10.1038/nrn942. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:648–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Tomizawa K, Moriwaki A, Li ST, Terada H, Matsui H. A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation. J. Neurosci. 2001;21:6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H, Reymann KG. Protein kinase A inhibitors prevent the maintenance of hippocampal long-term potentiation. Neuroreport. 1993;4:712–714. doi: 10.1097/00001756-199306000-00028. [DOI] [PubMed] [Google Scholar]
- Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RGM. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcinuerin / inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog. Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Duffy SN, Young JZ. Differential maintenance and frequency-dependent tuning of LTP at hippocampal synapses of specific strains of inbred mice. J. Neurophysiol. 2000;84:2484–2493. doi: 10.1152/jn.2000.84.5.2484. [DOI] [PubMed] [Google Scholar]
- Pineda VV, Athos JI, Wang H, Celver J, Ippolito D, Boulay G, Birnbaumer L, Storm DR. Removal of G(ialpha1) constraints on adenylyl cyclase in the hippocampus enhances LTP and impairs memory formation. Neuron. 2004;41:153–163. doi: 10.1016/s0896-6273(03)00813-4. [DOI] [PubMed] [Google Scholar]
- Reymann KG, Malisch R, Schulzeck K, Broedemann T, Ott T, Matthies H. The duration of long-term potentiation in the CA1 region of the hippocampal slice preparation. Brain Res. Bull. 1985;15:249–255. doi: 10.1016/0361-9230(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: the role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. J. Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM. Synapse specificity and long-term information storage. Neuron. 1997;18:339–342. doi: 10.1016/s0896-6273(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Sossin WS. Mechanisms for the generation of synapse specificity in long-term memory: the implications of a requirement for transcription. Trends Neurosci. 1996;19:215–218. doi: 10.1016/0166-2236(96)20016-5. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J. Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli U, Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 1990;513:113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- Thiels E, Norman ED, Barrionuevo G, Klann E. Transient and persistent increases in protein phosphatase activity during long-term depression in the adult hippocampus in vivo. Neuroscience. 1998;86:1023–1029. doi: 10.1016/s0306-4522(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. The balance between postsynaptic Ca(2+)-dependent protein kinase and phosphatase activities controlling synaptic strength. Learn. Mem. 1996;3:170–181. doi: 10.1101/lm.3.2-3.170. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Woo NH, Nguyen PV. `Silent' metaplasticity of the late phase of long-term potentiation requires protein phosphatases. Learn. Mem. 2002;9:202–213. doi: 10.1101/lm.498402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Nguyen PV. Protein synthesis is required for synaptic immunity to depotentiation. J. Neurosci. 2003;23:1125–1132. doi: 10.1523/JNEUROSCI.23-04-01125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Abel T, Nguyen PV. Genetic and pharmacological demonstration of a role for cyclic AMP-dependent protein kinase-mediated suppression of protein phosphatases in gating the expression of late LTP. Eur. J. Neurosci. 2002;16:1871–1876. doi: 10.1046/j.1460-9568.2002.02260.x. [DOI] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, Nguyen PV. Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus. 2003;13:293–300. doi: 10.1002/hipo.10086. [DOI] [PubMed] [Google Scholar]
- Young JZ, Nguyen PV. Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of LTP by prior synaptic activity. J. Neurosci. 2005;25:7221–7231. doi: 10.1523/JNEUROSCI.0909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]