Abstract
The effects on thyroid function of an inhibitor of tyrosine dehalogenase, 3-nitro-L-tyrosine (MNT) have been investigated in rats. In preliminary studies, marked inhibition of iodotyrosine deiodination was demonstrated in rats drinking 8 mM MNT. A series of experiments was then performed in which rats received Remington low iodine diet and 8 mM MNT as drinking fluid. This regimen had the following effects, compared to the effects of a low iodine diet alone: (a) a decrease in serum protein-bound iodine, elevation of serum thyrotropin level, goiter, and growth inhibition all prevented or reversed by iodine supplements: (b) on initiation of MNT, a 2- to 3-fold increase in the rate of release of radioiodine from the thyroid and concomitant urinary excretion of large amounts of organic iodine: and (c) after 2 wk of MNT, a greatly increased rate of thyroidal uptake and release of 131I, an increase in the ratio of monoiodotyrosine-131I to diiodotyrosine-131I in thyroid proteolysates and the appearance of labeled iodotyrosines in serum.
Acute administration of MNT intraperitoneally to rats on either an iodine-deficient or iodine-sufficient diet did not inhibit thyroidal uptake of 131I or alter the distribution of 131I among thyroidal iodoamino acids.
It is concluded that MNT is an effective inhibitor of iodotyrosine deiodination in vivo, without other important actions on thyroid function. Thus, MNT treatment affords a model for the human dehalogenase defect. By provoking iodotyrosine secretion and consequent urinary loss of iodine, MNT can exaggerate the effects of a low iodine intake, producing goitrous hypothyroidism despite a rapid rate of iodine turnover in the thyroid.
Full text
PDF
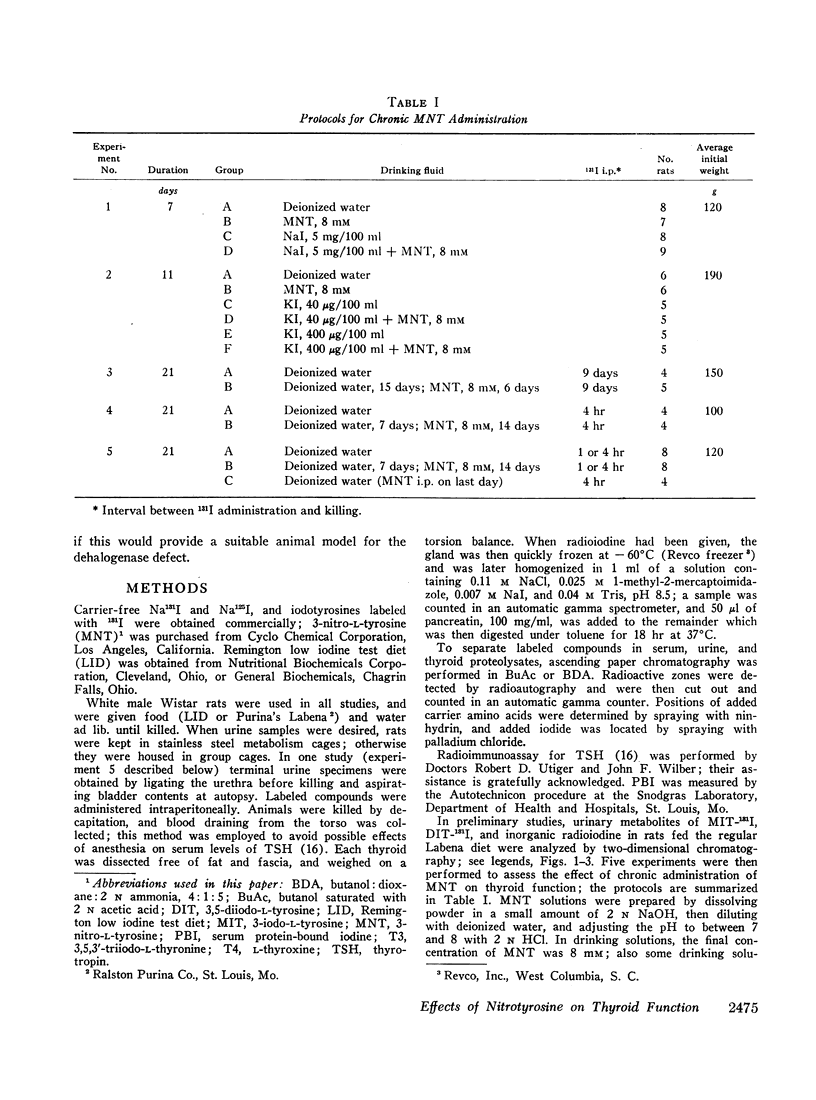
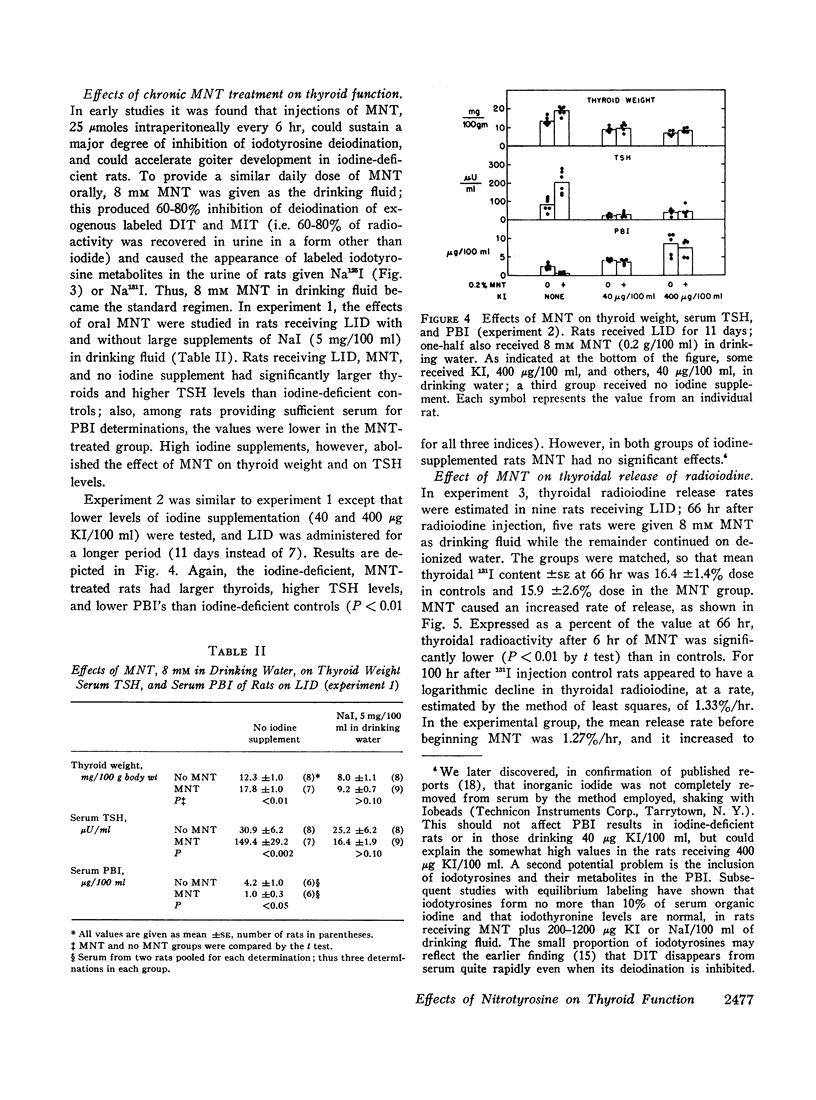
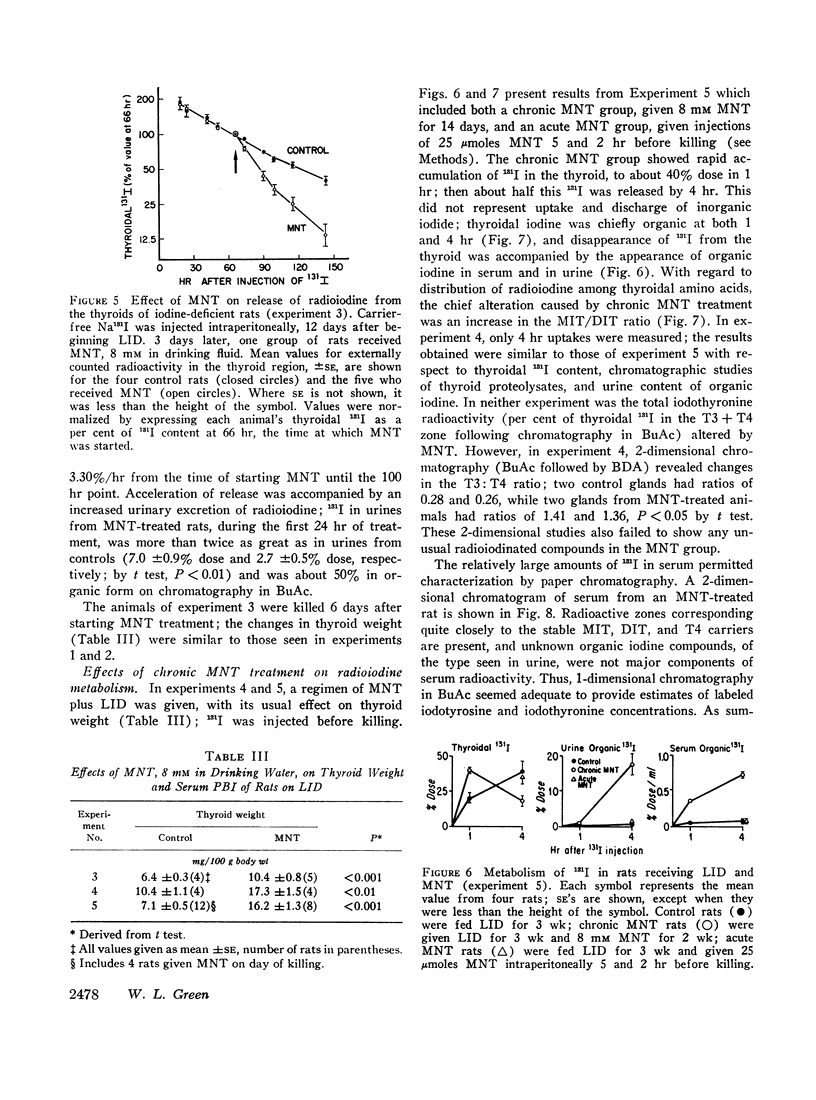



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander W. D., Wolff J. Thyroidal iodide tranpsort. 8. Endocrinology. 1966 Mar;78(3):581–590. doi: 10.1210/endo-78-3-581. [DOI] [PubMed] [Google Scholar]
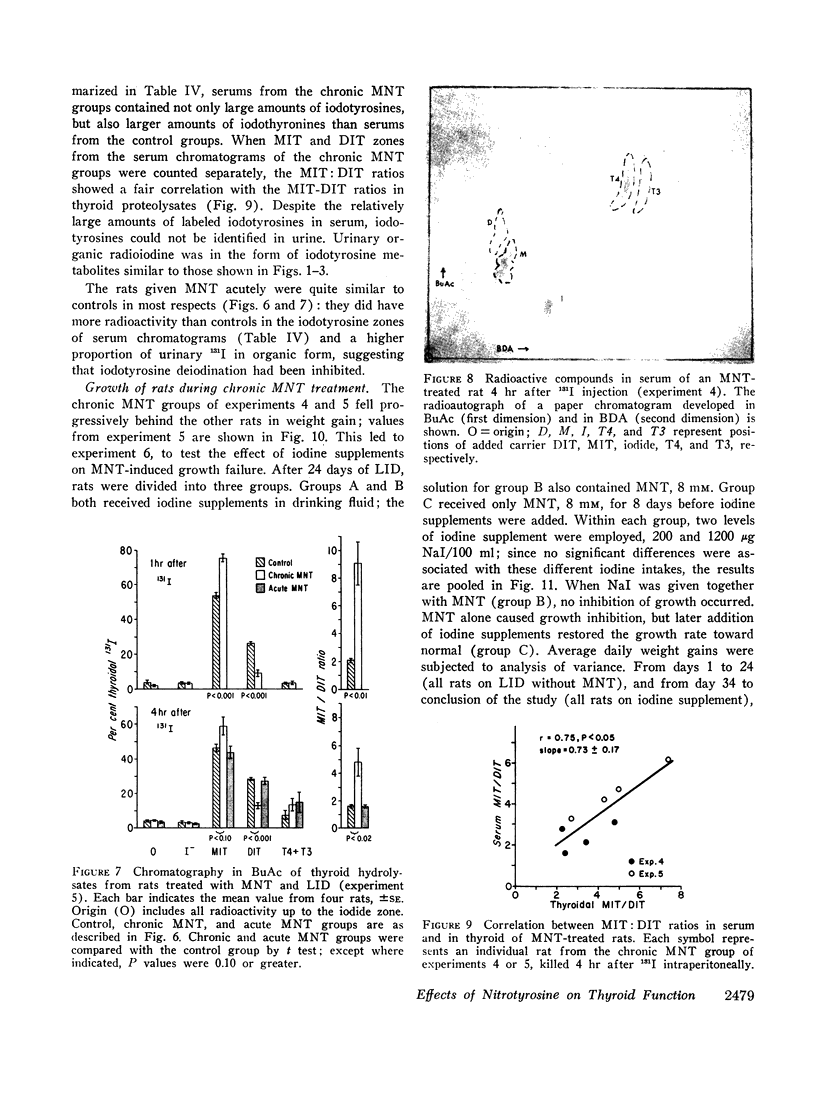
- BARAKAT R. M., INGBAR S. H. THE EFFECT OF ACUTE IODIDE DEPLETION ON THYROID FUNCTION IN MAN. J Clin Invest. 1965 Jul;44:1117–1124. doi: 10.1172/JCI105218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENT W. E., HUTCHISON J. H., MCGIRR E. M. Sporadic non-endemic goitrous cretinism; identification and significance of monoiodotyrosine and diiodotyrosine in serum and urine. Lancet. 1956 Nov 3;271(6949):906–908. [PubMed] [Google Scholar]
- ESCOBAR DEL REY F., MORREALE DE ESCOBAR G. The effect of propylthiouracil, methylthiouracil and thiouracil on the peripheral metabolism of 1-thyroxine in thyroidectomized, 1-thyroxine maintained rats. Endocrinology. 1961 Sep;69:456–465. doi: 10.1210/endo-69-3-456. [DOI] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- GARDNER J. U., HAYLES A. B., WOOLNER L. B., OWEN C. A., Jr Iodine metabolism in goitrous cretins. J Clin Endocrinol Metab. 1959 Jun;19(6):638–657. doi: 10.1210/jcem-19-6-638. [DOI] [PubMed] [Google Scholar]
- Green W. L. Inhibition of thyroidal iodotyrosine deiodination by tyrosine analogues. Endocrinology. 1968 Aug;83(2):336–347. doi: 10.1210/endo-83-2-336. [DOI] [PubMed] [Google Scholar]
- Greer M. A., Grimm Y. Changes in thyroid secretion produced by inhibition of iodotyrosine deiodinase. Endocrinology. 1968 Sep;83(3):405–410. doi: 10.1210/endo-83-3-405. [DOI] [PubMed] [Google Scholar]
- Harden R. M., Alexander W. D., Papadopoulos S., Harrison M. T., Macfarlane S. The influence of the plasma inorganic iodine concentration on thyroid function in dehalogenase deficiency. Acta Endocrinol (Copenh) 1967 Jun;55(2):361–368. doi: 10.1530/acta.0.0550361. [DOI] [PubMed] [Google Scholar]
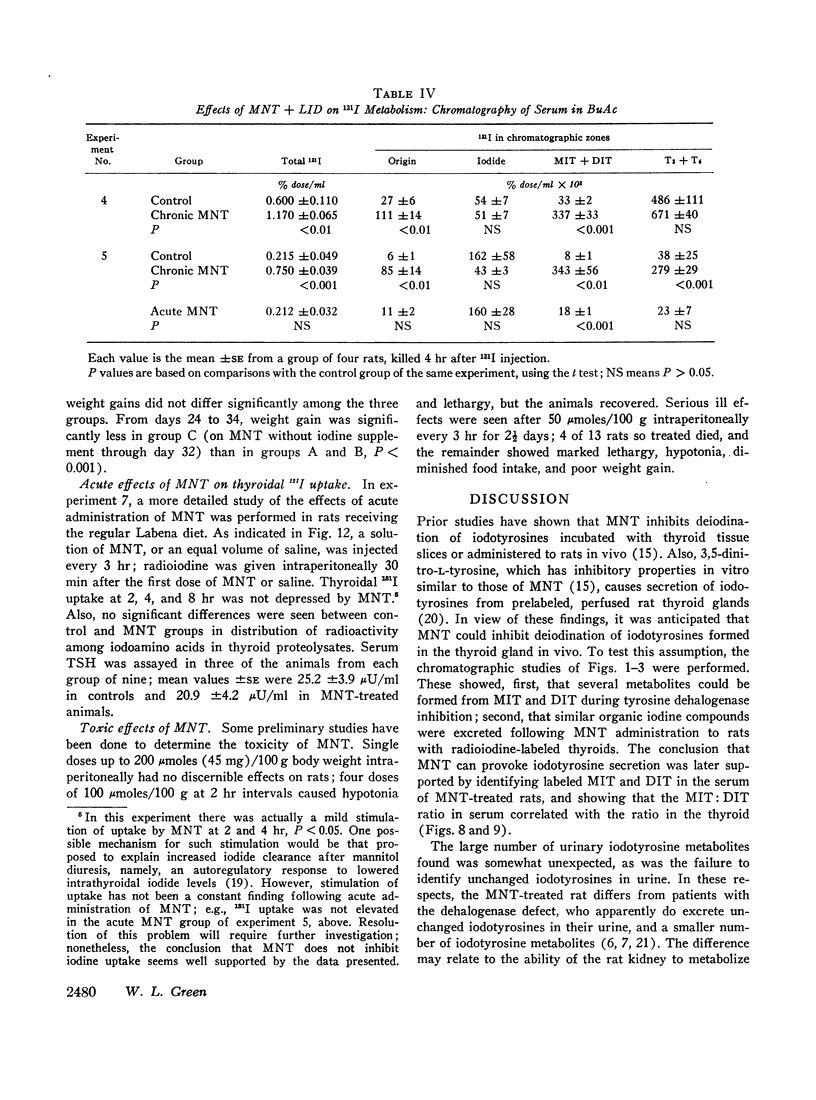
- Inoue K., Taurog A. Acute and chronic effects of iodide on thyroid radioiodine metabolism in iodine-deficient rats. Endocrinology. 1968 Aug;83(2):279–290. doi: 10.1210/endo-83-2-279. [DOI] [PubMed] [Google Scholar]
- JOSEPH R., TUBIANA M., JOB C. J. L'hy-L'hypothyroïdie par anomalie congénitale de la thyroxinogenèse. Rev Fr Etud Clin Biol. 1958 Feb;3(2):167–175. [PubMed] [Google Scholar]
- Lissitzky S., Comar D., Rivière R., Codaccioni J. L. Etude quantitative du métabolisme de l'iode dans un cas d'hypothyroïdie avec goitre due à un défaut d'iodotyrosine-déshalogénase. Rev Fr Etud Clin Biol. 1965 Jun-Jul;10(6):631–639. [PubMed] [Google Scholar]
- MATSUDA K., GREER M. A. NATURE OF THYROID SECRETION IN THE RAT AND THE MANNER IN WHICH IT IS ALTERED BY THYROTROPIN. Endocrinology. 1965 Jun;76:1012–1021. doi: 10.1210/endo-76-6-1012. [DOI] [PubMed] [Google Scholar]
- MAYBERRY W. E., ASTWOOD E. B. The effect of propylthiouracil on the intrathyroid metabolism of iodine in rats. J Biol Chem. 1960 Oct;235:2977–2980. [PubMed] [Google Scholar]
- Nakano M. Purification and properties of halogenated tyrosine and thyroid hormone transaminase from rat kidney mitochondria. J Biol Chem. 1967 Jan 10;242(1):73–81. [PubMed] [Google Scholar]
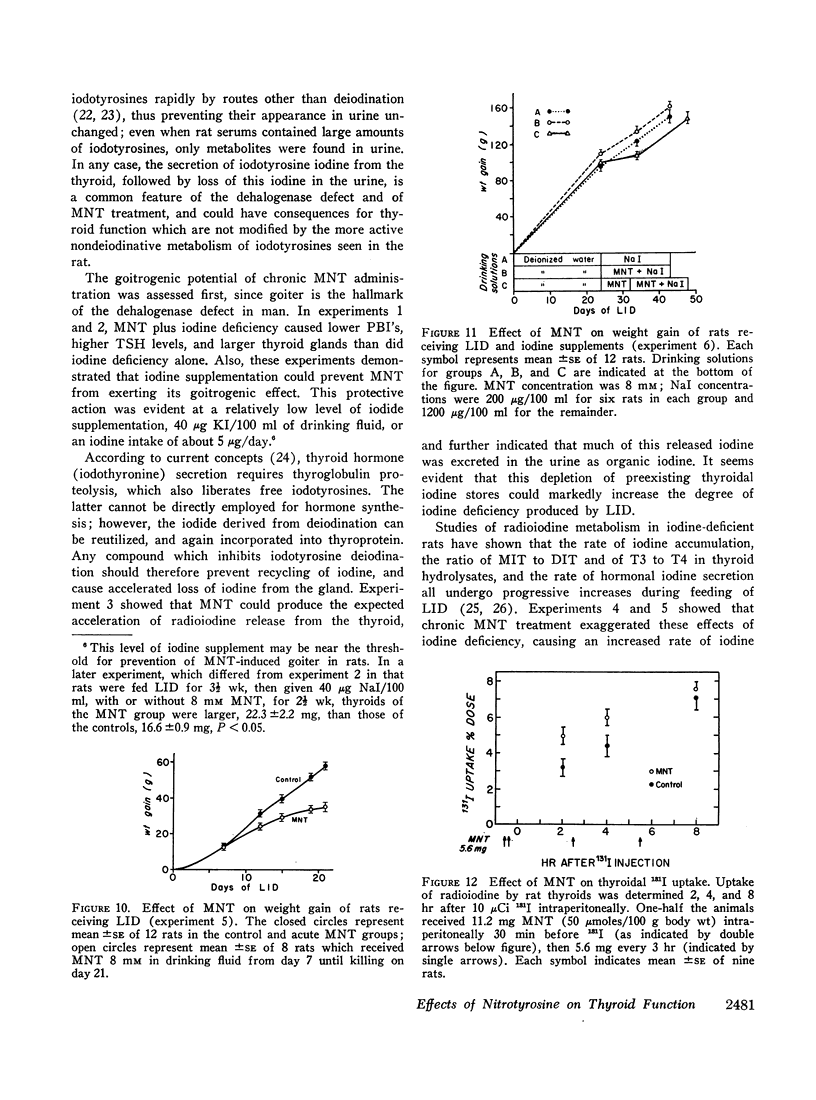
- Orsini A., Pierron H., Delire M. Hypothyroïdie par carence en iodo-tyrosine désiodase. Résultats après 7 ans de traitement par l'iode. Mars Med. 1969;106(1):47–49. [PubMed] [Google Scholar]
- RICHARDS J. B., INGBAR S. H. The effects of propylthiouracil and perchlorate on the biogenesis of thyroid hormone. Endocrinology. 1959 Aug;65(2):198–207. doi: 10.1210/endo-65-2-198. [DOI] [PubMed] [Google Scholar]
- ROCHE J., MICHEL R., MICHEL O., LISSITZKY S. Sur la déshalogénation enzymatique des iodotyrosine par le corps thyroïde et sur son rôle physiologique. Biochim Biophys Acta. 1952;9(2):161–169. doi: 10.1016/0006-3002(52)90143-1. [DOI] [PubMed] [Google Scholar]
- STANBURY J. B. Deiodination of the iodinated amino acids. Ann N Y Acad Sci. 1960 Apr 23;86:417–439. doi: 10.1111/j.1749-6632.1960.tb42820.x. [DOI] [PubMed] [Google Scholar]
- Saari W. S., Williams J., Britcher S. F., Wolf D. E., Kuehl F. A., Jr Tyrosine hydroxylase inhibitors. Synthesis and activity of substituted aromatic amino acids. J Med Chem. 1967 Nov;10(6):1008–1014. doi: 10.1021/jm00318a005. [DOI] [PubMed] [Google Scholar]
- Simpson D. An automated method for the determination of serum organic iodine. Clin Chem. 1967 Oct;13(10):890–899. [PubMed] [Google Scholar]
- Spector S., Mata R. O., Sjoerdsma A., Udenfriend S. Biochemical and pharmacological effects of iodo-tyrosines; relation to tyrosine hydroxylase inhibition in vivo. Life Sci. 1965 Jul;4(13):1307–1311. doi: 10.1016/0024-3205(65)90081-0. [DOI] [PubMed] [Google Scholar]
- Studer H., Kohler H., Bürgi H., Dorner E., Forster R., Rohner R. Goiters with high radioiodine uptake and other characteristics of iodine deficiency in rats chronically treated with aminoglutethimide. Endocrinology. 1970 Nov;87(5):905–914. doi: 10.1210/endo-87-5-905. [DOI] [PubMed] [Google Scholar]
- TAUROG A., WHEAT J. D., CHAIKOFF I. L. Nature of the I131 compounds appearing in the thyroid vein after injection of iodide-I131. Endocrinology. 1956 Jan;58(1):121–131. doi: 10.1210/endo-58-1-121. [DOI] [PubMed] [Google Scholar]
- TONG W., TAUROG A., CHAIKOFF I. L. The metabolism of I131-labeled diiodotyrosine. J Biol Chem. 1954 Mar;207(1):59–76. [PubMed] [Google Scholar]
- Udenfriend S., Zaltzman-Nirenberg P., Nagatsu T. Inhibitors of purified beef adrenal tyrosine hydroxylase. Biochem Pharmacol. 1965 May;14(5):837–845. doi: 10.1016/0006-2952(65)90103-6. [DOI] [PubMed] [Google Scholar]
- Wilber J. F., Utiger R. D. Immunoassay studies of thyrotropin in rat pituitary glands and serum. Endocrinology. 1967 Jul;81(1):145–151. doi: 10.1210/endo-81-1-145. [DOI] [PubMed] [Google Scholar]