Abstract
IL 4 receptor α (IL-4Rα) expression by non-bone marrow (BM)-derived cells is required to protect hosts against several parasitic helminth species. In contrast, we demonstrate that IL-4Rα expression by BM-derived cells is both necessary and sufficient to prevent Schistosoma mansoni-infected mice from developing severe inflammation directed against parasite ova, whereas IL-4Rα expression by non-BM-derived cells is neither necessary nor sufficient. Chimeras that express IL-4Rα only on non-BM-derived cells still produce Th2 cytokines, but overproduce IL-12p40, TNF, and IFN-γ, fail to generate alternatively activated macrophages, and develop endotoxemia and severe hepatic and intestinal pathology. In contrast, chimeras that express IL-4Rα only on BM-derived cells have extended survival, even though the granulomas that they develop around parasite eggs are small and devoid of collagen. These observations identify distinct roles for IL-4/IL-13 responsive cell lineages during schistosomiasis: IL-4Rα-mediated signaling in non-BM-derived cells regulates granuloma size and fibrosis, whereas signaling in BM-derived cells suppresses parasite egg-driven inflammation within the liver and intestine.
Interleukins 4 and 13 drive many immune and inflammatory responses that are associated with Th2 cytokines (1). IL-4 receptor α-chain (IL-4Rα)3 is essential for signaling by both cytokines and pairs with cytokine receptor common γ-chain to form the type 1 IL-4R, which is activated uniquely by IL-4, or with IL-13 Rα1 to form the type 2 IL-4 receptor, which is activated by both IL-4 and IL-13 (2). Although IL-4Rα is required for protective immunity against most intestinal and some nonintestinal parasitic helminths, it does not protect against all helminth parasites through a single mechanism (3). For example, expulsion of the intestinal nematodes Nippostrongylus brasiliensis and Strongyloides venenzuelensis only requires IL-4Rα expression by non-bone marrow (BM)-derived cells, while Trichinella spiralis expulsion requires IL-4Rα expression by both BM- and non-BM-derived cells (4, 5). No situation has been described to date in which IL-4Rα expression by non-BM-derived cells is not required for host protection against a nematode parasite.
Infection of mice with Schistosoma mansoni, a parasitic blood fluke that infects 250 million persons worldwide, is characterized by markedly increased IL-4 and IL-13 production that drives extensive fibrosis and granulomatous immunopathology directed against parasite eggs that lodge in the liver and intestine (6, 7). from Parasite ova cause the recruitment of lymphocytes, eosinophils, and macrophages to the local microenvironment and this inflammatory response may damage the entire organ if left unchecked. In this model, IL-4Rα expression on macrophages (Mφ) is required to limit the severity of egg-induced inflammation (8), whereas T cell responsiveness to IL-4 is nonessential for host survival or www.jimmunol.org collagen deposition (9). Mφ IL-4/IL-13 responsiveness is sufficient to induce myeloid precursors to differentiate into alternatively activated macrophages (AAMφ) (10). These cells secrete several proteins involved in the wound healing response that are not produced by classically activated macrophages (CAMφ), including arginase 1, relm α (FIZZ-1), and CCL17 (11). Conversion on July 28, 2010 of the common substrate L-arginine to polyamines and proline via arginase 1 instead of NO via inducible NO synthetase (NOS-2) is one mechanism that may allow AAMφ to block the effects of CAMφ. Although our previous work supports a hypothesis that BM-derived cells are required to protect against severe inflammation during S. mansoni infection, it was not addressed whether host protection against this parasite shares the requirement for IL-4Rα expression by non-BM-derived cells that has been identified in all other investigations of nematode parasite infection.
We have now used irradiation BM chimeras to distinguish between the contributions of IL-4Rα on BM-derived vs non-BM-derived cells to host protection against S. mansoni egg-induced immunopathology. Our results demonstrate two surprising observations: 1) IL-4Rα expression by non-BM-derived cells is not necessary to protect against lethality; and 2) granulomas that develop around parasite eggs are very small and devoid of fibrosis in mice in which non-BM-derived cells lack IL-4Rα. Thus, contrary to expectations, the development of large, fibrotic egg granulomas during the initial phase of infection does not reduce hepatotoxicity or promote host protection during acute schistosomiasis.
Materials and Methods
Mice
Mice were purchased from Taconic Farms. All mouse experiments were approved by the Institutional Animal Care and Use Committee at the Cincinnati Veterans Affairs Medical Center.
BM chimeras
Six- to 8-wk-old BALB/c male wild-type (WT) and IL-4Rα-deficient mice were used. Mice received two doses of 475 Rads 3 h apart with a 154Cs irradiator, and 4 h later were administered 3–5 × 106 BM cells i.v. Mice were subsequently fed doxycycline-containing food for 3–4 wk. Mice were inoculated with S. mansoni 4–5 wk post-BM reconstitution.
mAbs for flow cytometry
Spleen and PBMC were stained with fluorochrome-labeled rat mAbs to mouse CD3 (2C11) (12), B220 (6B2) (13), CD11b (M1/70) (14), Gr-1 (RB6 – 8C5) (15), IL-4Rα (M1) (16), and/or CD45 (30-F11) (17).
Infection of mice with S. mansoni
Chimeric mice were anesthetized and percutaneously infected with 50 – 60 S. mansoni cercariae as previously described (8). Parasites were provided by the National Institute of Allergy and Infectious Diseases Schistosomiasis Resource Center at the Biomedical Research Institute, through National Institute of Allergy and Infectious Diseases Contract N0-AI-30026.
Flow cytometry
PBMC, isolated by Ficoll gradient, and splenocytes were washed in FACS buffer (HBSS, 1% FBS, and 0.2% sodium azide) and incubated with anti-FcγRII/RIII mAb (2.4G2). Cells were then stained with the mAbs described and analyzed with a BD FACSCalibur and CellQuest software. The percent of donor cells in chimeric mice was determined as percentage of cells that appeared IL-4Rα+ or IL-4Rα− in the chimeric mouse, divided by the percentage of cells that appeared IL-4Rα+ in WT mice or the percentage of cells that appeared IL-4Rα− in IL-4Rα-deficient mice.
Evaluation of cytokine production and morbidity
IL-12 p40 levels were measured by ELISA (eBioscience). IL-4, TNF, and IFN-γ were measured by in vivo cytokine capture assay (18). Serum AST concentration was measured by the Cincinnati Veterans Affairs Medical Center clinical pathology laboratory. Serum LPS concentration was measured as described (8, 19).
Determination of S. mansoni tissue egg burden and granuloma measurement
Biopsies of liver or intestine (ileum) were collected from each mouse, weighed, and digested in 5% KOH at 37°C for 16 h, and eggs were counted at 40× magnification. Data were expressed as eggs per gram. Histologically processed sections of liver tissue (5 μm) were stained with H&E or Masson’s trichrome. Individual granuloma areas were measured on coded slides; only granulomas that possessed a central egg were evaluated. Quantitation of area was performed using a SPT Diagnostics imaging system and Simple PCI C-Imaging systems software. Data shown are the mean ± SE of 150 granulomas per group from two independent experiments.
Real-time PCR
RNA was obtained from hepatic tissue, DNase I-treated, and cDNA was generated using SuperScript II Reverse Transcriptase (Invitrogen Life Technologies). Real-time PCR was conducted on a Gene Amp 7500 instrument (PE Biosystems) with the Syber Green detection reagent. Cycle threshold values for NOS-2 and FIZZ-1 were determined and fold induction was compared with vimentin using the 1/ΔCT method (8) as follows: Vimentin Forward: 5′-TGA CCG GCT TGT ATG CTA TC-3′; Vimentin Reverse: 5′-CAG TGT GAG CCA GGA TAT AG-3′; NOS-2 Forward: 5′-CAG AAG AAT GGA AGA GTC AG-3′; NOS-2 Reverse: 5′-CAG ATA TGC AGG GAG TCA CC-3′; Relm-α/FIZZ-1 Forward: 5′-AGA TGG GCC TCC TGC CCT GCT GGG-3′; Relm-α/FIZZ-1 Reverse: 5′-ACC TGG TGA CGG GCG ACG ACG GTT-3′.
Determination of hydroxyproline content
Hepatic collagen content was measured as hydroxyproline concentration (8, 9).
Statistical analysis
Statistical significance was assessed by either one-tailed Student’s t test (two groups) or ANOVA for multiple groups and a post hoc Bonferroni’s test to determine significance, all performed using Prism Graph Pad software.
Results
BM-derived cell IL-4Rα is required for host protection during natural infection with S. mansoni
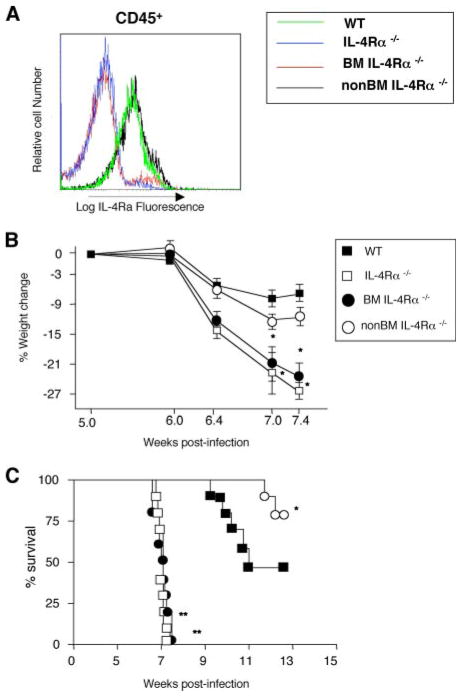
IL-4Rα-deficient mice, but not WT mice, usually die 6 – 8 wk after natural infection with S. mansoni (8). To determine whether IL-4Rα expression on BM-derived and/or non-BM-derived cells is required to protect against S. mansoni-induced pathology, irradiation BM chimeras were generated by reconstituting WT mice with IL-4Rα−/− BM (BMIL-4Rα−/− mice) and IL-4Rα−/− mice with WT BM (non-BMIL-4Rα−/− mice). These mice and control mice produced by reconstituting WT mice with WT BM, and IL-4Rα−/− mice with IL-4Rα−/− BM were infected with 50 – 60 S. mansoni cercariae 5 wk posttransplant and evaluated 1 wk later by flow cytometry for BM engraftment by determining IL-4Rα expression. Lymphocytes and monocyte/macrophages showed highly efficient donor BM reconstitution, while neutrophil engraftment was less complete (Fig. 1). Of note, neutrophils (CD11b+/Ly6Ghigh cells) express relatively low levels of IL-4Rα, which makes it difficult to distinguish between IL-4Rα+ and IL-4Rα− cells (Fig. 1). Evaluation of IL-4Rα levels on CD45+ (BM-derived) splenocytes 7 wk postinfection gave consistent results, with only a small residual population of host cells in chimeric mice (Fig. 2A). S. mansoni infection induced striking differences in the responses of the two chimeric strains: body weights of BMIL-4Rα −/− and IL-4Rα−/− mice rapidly declined starting 6 wk postinfection (Fig. 2B) that preceded death of these animals (Fig. 2C). In contrast, non-BMIL-4Rα−/− mice lost only slightly more weight than WT mice, and had a significantly extended rate of survival compared with WT controls at 13 wk postinfection (Fig. 2C). Thus, protection against cachexia and lethality during murine schistosomiasis requires an IL-4 and/or IL-13 effect on a BM-derived cell, but not on a non-BM-derived cell.
FIGURE 1.
IL-4Rα mean fluorescence intensity (MFI) (left panels) and percents of IL-4Rα+CD3+ (T) cells; B cells (B220+); monocytes/macrophages (CD11b+/Ly6G−low); and neutrophils (CD11b+/Ly6Ghigh) in peripheral blood 6 wk postinoculation with S. mansoni. (n = 10); experiment performed three times with similar results.
FIGURE 2.
BM cell IL-4Rα deficiency causes cachexia during S. mansoni infection. A, Splenocytes isolated from chimeric mice 7.4 wk post S. mansoni inoculation were stained with mAbs to CD45 (30-F11) and IL-4Rα (M1) and analyzed by flow cytometry. Representative of two experiments (n = 5). B, Weight loss by S. mansoni-infected WT, IL-4Rα, BMIL-4Rα, and non-BMIL-4Rα−/− mice. Experiment was performed three times with similar results. Means ± SE of 8–10 mice/group. (*, p < 0.05 compared with infected WT group). C, Survival kinetics of S. mansoni-infected WT, IL-4Rα, BMIL-4Rα, and non-BMIL-4Rα−/− mice. Representative of three independent experiments with 8–10 mice/group. (*, p < 0.05; **, p < 0.01, and ***, p < 0.001 compared with WT infected group).
BM derived IL-4Rα expression limits severe organ damage and Type 1 inflammation
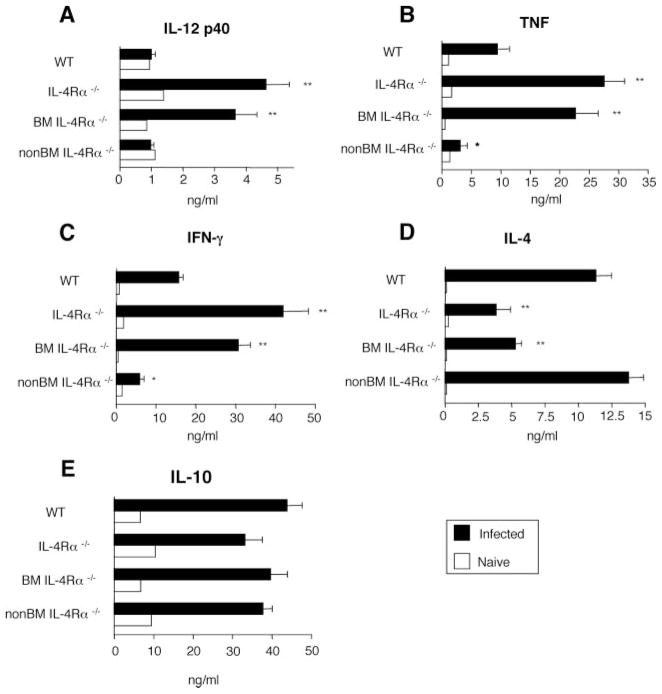
Because cytokine abnormalities are associated with increased inflammation during schistosomiasis (20), we determined whether BM IL-4Rα-deficiency causes proinflammatory cytokine overproduction during S. mansoni infection. S. mansoni-infected IL-4Rα−/− and BMIL-4Rα−/− mice produced significantly more IL-12p40, IFN-γ, and TNF and less but still considerable IL-4 as compared with WT mice (Fig. 3, A–D), as has been described (21). In contrast, even less TNF and IFN-γ were produced in infected non-BMIL-4Rα−/− mice than in WT mice, while IL-10 production was similarly elevated in all strains (Fig. 3, B, C, and E).
FIGURE 3.
S. mansoni infection causes increased Type 1 inflammation despite elevated IL-10 production. Analyses were performed on sera from naive (white bars) or S. mansoni-infected mice (black bars) mice 7 wk post S. mansoni inoculation. A, IL-12p40. B, TNF. C, IFN-γ. D, IL-4. E, IL-10 Means ± SE for 8 –10 mice per group. Representative of three independent experiments. (*, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with WT infected group).
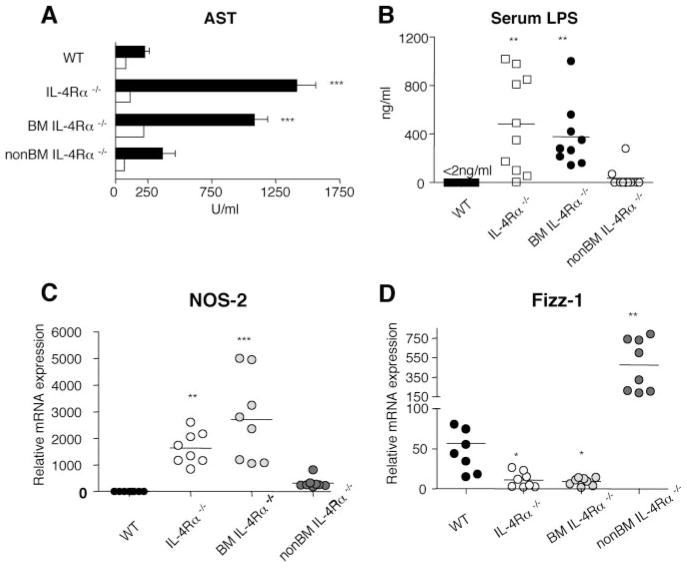
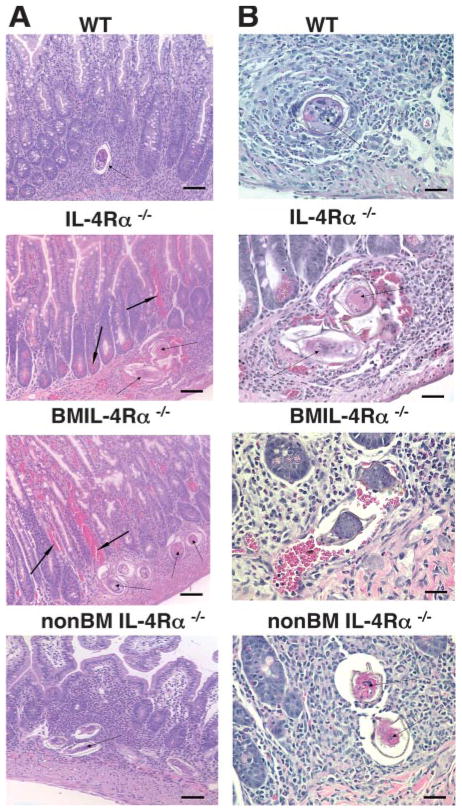
Studies also evaluated whether weight loss and mortality in S. mansoni-infected IL-4Rα−/− and BMIL-4Rα−/− mice was associated with liver damage (measured as increased serum aspartate transaminase (AST), an enzyme released by damaged hepatocytes (7) and/or a leak of intestinal bacteria or LPS into the systemic circulation (as reflected by increased serum LPS). AST levels were ~4 – 6-fold higher and serum LPS levels >100-fold higher in S. mansoni-infected IL-4Rα−/− and BMIL-4Rα −/− mice than in WT mice and non-BMIL-4Rα−/− mice at 7 wk postinfection (Fig. 4, A and B). Quantitation of liver mRNA transcript levels for NOS-2 and FIZZ-1 was used to investigate whether hepatic injury was associated with CAMφ (NOS-2) or AAMφ (FIZZ-1). S. mansoni-infected IL-4Rα−/− and BMIL-4Rα−/− mice produced 1,000 to >2,000-fold higher levels of NOS-2 compared with WT mice, while transcripts in non-BMIL-4Rα−/− were much less elevated (Fig. 4C). In contrast, FIZZ-1 gene expression was 50-fold higher in infected non-BMIL-4Rα−/− chimeras than in IL-4Rα−/− or BMIL-4Rα−/− mice and 8-fold higher than in WT mice (Fig. 4D). These observations are consistent with the observation that S. mansoni-infected mice that selectively lack IL-4Rα on Mφ (MφIL-4Rα−/− mice) develop a dramatic increase in serum LPS (8). Both MφIL-4Rα−/− mice and BMIL-4Rα−/− mice, like IL-4Rα−/− and IL-4/IL-13−/− mice, develop obvious gut hemorrhage during S. mansoni infection (Fig. 5) (8, 23).
FIGURE 4.
Severe liver and intestinal injury in BMIL-4Rα−/− mice correlates with increased CAMφ and decreased AAMφ. Analyses were performed on sera from naive (white bars) or S. mansoni-infected mice (black bars) mice 7 wk post S. mansoni inoculation showing levels of AST (A) and endotoxin (LPS) (B). Hepatic mRNA transcripts for NOS-2 (C) and FIZZ-1 (D) were quantitated by real time PCR 7.4 wk postinoculation and expressed as fold increase compared with naive WT tissue. Experiment performed twice with similar results. (n = 6 – 8 mice/group). In all experiments (*, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with WT infected group).
FIGURE 5.
H&E staining of ileal tissue sections from chimeras at 7.4 wk postinfection. A, Original magnification, ×200. Thin arrows point to parasite ova. Arrows in bold indicate areas of hemorrhage in intestines of IL-4Rα and BMIL-4Rα−/− groups. B, Original magnification, ×400. Representative photomicrographs shown.
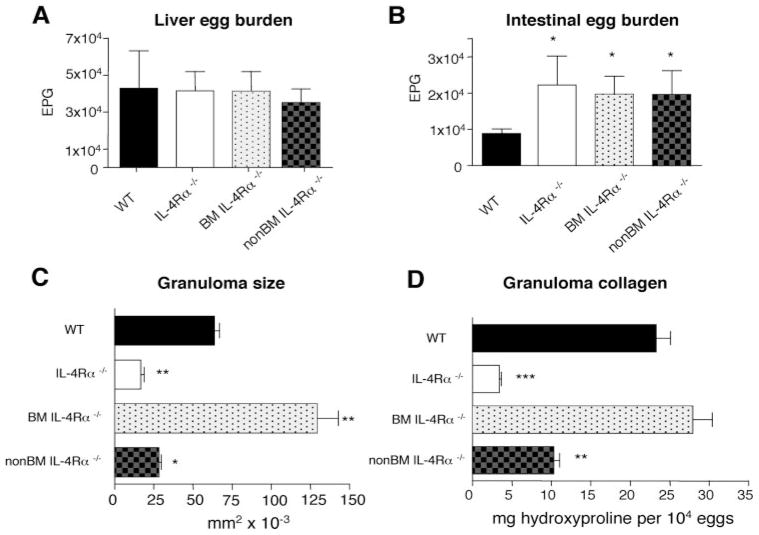
The increased parasite ova-induced intestinal and liver damage in S. mansoni-infected BMIL-4Rα−/− mice was not due to increased egg production, inasmuch as liver egg burden was similar in WT, IL-4Rα−/−, non-BM-IL-4Rα−/−, and BM-IL-4Rα−/− mice (Fig. 6A). Furthermore, although intestinal egg burden was similarly increased in both chimeric mouse strains (Fig. 6B), the failure of S. mansoni-infected non-BMIL-4Rα−/− to develop increased immunopathology indicates that increased intestinal egg burden is not sufficient to induce severe immunopathology and that IL-4Rα-expressing BM-derived cells exclusively suppress egg-induced intestinal inflammation and breakdown of mucosal barrier function.
FIGURE 6.
Non-BM-derived cell IL-4Rα deficiency reduces granuloma size and collagen content without affecting parasite fecundity. S. mansoni eggs were counted in liver (A) and intestine (B) 7.4 wk postinoculation with 50 – 60 cercariae. Data are means ± SE of eight mice per group. Representative of three experiments. C, Liver granuloma cross-sectional area was determined 7.4 wk postinoculation. Data represent 150 granulomas per group. D, Liver hydroxyproline levels 7.4 wk postinfection. Experiment performed twice with similar results (n = 8). In all experiments (*, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with WT infected group).
Non-BM derived IL-4Rα expression is required for liver granuloma formation and egg-induced fibrosis
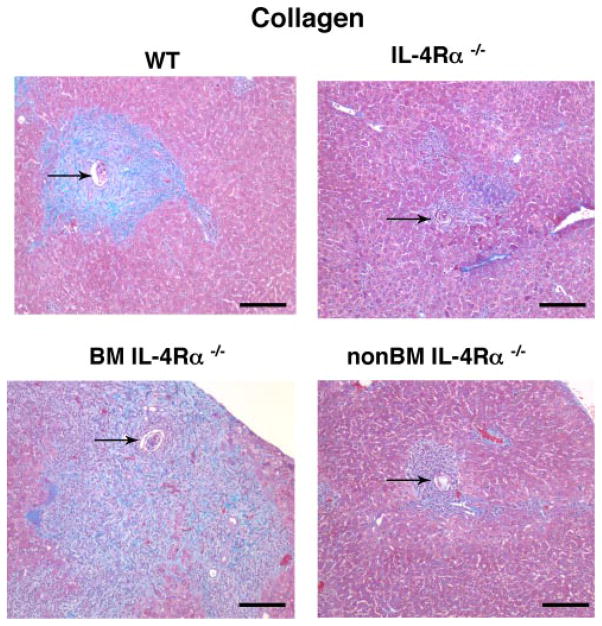
Both Mφ (BM-derived) and fibroblasts (non-BM-derived) have been shown to respond to IL-13 and participate in collagen biosynthesis (24). Studies were performed to distinguish whether BM-derived or non-BM-derived cells were essential for granuloma size and hepatic collagen (measured by hydroxyproline concentration) (22). Results demonstrate that liver granuloma size and fibrosis were suppressed in non-BMIL-4Rα−/− mice and IL-4Rα−/− mice to nearly the same extent and were increased in BMIL-4Rα−/− mice compared with WT mice (Figs. 6, C and D and 7). These results dissociate IL-4Rα-dependent hepatic granuloma formation and collagen deposition from IL-4Rα-dependent control of inflammation and mortality during S. mansoni infection.
FIGURE 7.
S. mansoni-induced fibrosis requires non-BM-derived cell IL-4Rα expression. Staining for collagen (blue) and hepatocytes (reddish/purple) in liver granulomas from S. mansoni infected mice 7.4 wk postinoculation. Arrows point to S. mansoni ova. Original magnification, ×100.
Discussion
Our observations establish that IL-4Rα expression by non-BM-derived cells is neither necessary nor sufficient for survival of mice during acute infection with S. mansoni, while IL-4Rα expression by BM-derived cells is both necessary and sufficient. Although a requirement for IL-4Rα-expressing BM-derived cells for host protection against this parasite was obvious from our previously demonstrated requirement for IL-4Rα-expressing macrophages (8), the lack of a requirement for IL-4Rα-expressing non-BM-derived cells was surprising. IL-4 and IL-13, signaling through IL-4Rα and STAT6, have previously been shown to protect mice from infection against several different nematode parasites, including Nippostrongylus brasiliensis, Trichuris muris, and Trichinella spiralis (5, 25–27). IL-4Rα expression by non-BM-derived cells was required for host protection against each of these parasites, while the requirement for IL-4Rα expression by BM-derived cells was variable. Thus, our studies with S. mansoni provide the first example where IL-4Rα-expression by BM-derived cells is sufficient to protect mice against a parasitic helminth.
One reason for this difference between S. mansoni and all other parasitic worms studied to date may be that studies of host protection against other worms used worm expulsion as a readout, while the immune system protects hosts against S. mansoni by limiting inflammation against worm ova rather than by hastening parasite death or expulsion. Adult S. mansoni worm number is no greater in IL-4Rα-deficient mice than in WT mice (28), but IL-4Rα-deficient mice develop lethal inflammation that primarily affects the intestines and liver, while inflammation is controlled in wild-type mice. IL-4Rα-mediated STAT-6 activation is not required for Th2 polarization in S. mansoni-infected mice (29); neither does it promote the development of T cells that secrete the immunosuppressive cytokine IL-10. Thus, although mice doubly deficient in IL-4 and IL-10 develop more severe inflammation than mice lacking either cytokine alone (20), the IL-4/IL-13 limitation of inflammation by activation of AAMφ during acute S. monsoni infection does not appear to involve increased secretion of IL-10.
During the course of infection, worm ova are first produced 5– 6 wk after mice are inoculated with cercariae (30). Ova must pass from the host veins, where the adult worms reside, through vein walls and intestinal mucosa to be expelled in host feces and complete the worm life cycle (30). In this process, eggs have the potential to induce a severe intestinal inflammatory response. In normal mice, this inflammatory response is kept in check by macrophages that are responsive to IL-4 and IL-13, as shown by the development of intense, hemorrhagic intestinal inflammation in S. mansoni-infected mice that lack IL-4Rα on all cells, all BM-derived cells, or selectively on macrophages (8). This intense intestinal inflammation is accompanied by an increase in intestinal permeability that allows bacteria and/or bacterial products to enter the systemic circulation, as shown by a large increase in serum levels of LPS. The increased inflammatory response is also accompanied by increased production of inflammatory cytokines, including TNF, IFN-γ, and IL-12 p40 (a component of both IL-12, which stimulates IFN-γ production, and IL-23, which promotes the production of IL-17) (31). Increased production of these inflammatory cytokines probably contributes to the development of lethal inflammation in mice that lack IL-4Rα on BM-derived cells because anti-TNF mAb can prolong survival in these mice (32) and excessive IL-12 p40 production exacerbates hepatic immunopathology in S. mansoni-infected mice in association with expansion of IL-17 producing cells (33).
Although an increased number of worm eggs are trapped in the intestines of S. mansoni-infected IL-4Rα-deficient mice, our observations demonstrate that this abnormality is not sufficient to cause lethal inflammation; increased trapping of worm eggs also is observed in non-BMIL-4Rα−/− chimeras, which are protected against lethal inflammation. Thus, although an IL-4/IL-13-responsive non-BM-derived cell must contribute to the passage of eggs from veins into the gut lumen, an anti-inflammatory mechanism mediated by IL-4/IL-13-responsive BM-derived cells can compensate for the increased intestinal egg burden. Interestingly, chimeras that expressed IL-4Rα only on BM derived cells (non-BMIL-4Rα−/−) generated FIZZ-1 mRNA transcripts that were 8-fold higher than WT mice. This suggests that Mφ activated by production of IL-4/IL-13 by cells of the adaptive immune system are involved in the compensatory mechanism that protects non-BMIL-4Rα−/− chimeras. Consistent with this, Loke et al. recently demonstrated that MHC class II restricted CD40T cell help is required to maintain AAMφ following helminth infection (34) and S. mansoni-infected RAG2-deficient mice develop severe tissue injury and significant mortality by 9 wk postinfection, despite the presence of IL-4Rα+ macrophages in these mice (35).
In addition to their increased intestinal pathology, BMIL-4Rα−/− chimeras, like fully IL-4Rα-deficient mice, develop severe liver disease, with substantial increases in serum levels of AST, which is released by damaged and dying hepatocytes (7). Previous studies with T cell-deficient and globally IL-4Rα-deficient mice were consistent with the possibility that liver damage resulted from the failure to develop perioval granulomas that might contain toxic products of the worm egg (36). In contrast, other studies demonstrated that mouse strains that produce liver egg granulomas of excessive size also suffer increased morbidity (37). Our new observations are consistent with the latter observations and indicate that large, fibrotic liver granulomas do not necessarily protect against host morbidity. Fully formed hepatic granulomas and increased serum levels of AST coexist in S. mansoni-infected BMIL-4Rα−/− chimeras, while infected non-BMIL-4Rα−/− chimeras have only slightly elevated serum AST levels despite their development of only rudimentary liver granulomas and their failure to develop egg-induced hepatic fibrosis. Thus, although hepatic granuloma formation in S. mansoni-infected mice depends on an IL-4/IL-13 effect on a non-BM-derived liver cell, hepatic protection against worm egg-induced toxicity during acute infection does not result from granuloma- or fibrosis-mediated containment and may be mediated by IL-4/IL-13-induced macrophage production of anti-inflammatory products. Indeed, the locus of protection may not even be the liver; we cannot currently rule out the possibility that hepatocyte toxicity results from the elevated levels of LPS or enteric bacteria that are caused by the increase in intestinal permeability.
Demonstration that macrophage-specific IL-4Rα-deficient mice (8) and BMIL-4Rα−/− chimeras produce as much liver collagen as WT mice argues against a hypothesis that AAMφ are required to promote egg-induced liver fibrosis during acute schistosomiasis (24). Instead, our studies are consistent with a hypothesis that IL-4/IL-13 act directly upon liver fibroblasts (hepatic stellate cells) to drive collagen synthesis (19, 38). Fibroblasts express the IL-13Rα1 chain and both IL-4 and IL-13 can induce type 1 collagen synthesis in murine fibroblast cell lines (39). This issue has considerable practical importance, because hepatic fibrosis (cirrhosis) is an important cause of morbidity and mortality during chronic murine schistosomiasis and in humans infected with S. mansoni (7). Our dissociation of fibrosis from host protection against lethal worm-induced inflammation suggests that it may be possible to inhibit hepatic cirrhosis without inducing the inflammatory effects that are normally prevented by IL-4Rα signaling.
Although our current and previous observations promote our understanding of how hosts respond to worm infection by identifying responses to worm eggs that are mediated by both BM-derived cells (inhibition of lethal inflammation) and non-BM-derived cells (granuloma formation, fibrosis, intestinal egg expulsion), they also raise several important issues: 1) what cell types are directly responsible for IL-4/IL-13-induced granuloma formation and fibrosis; 2) are macrophages the only IL-4/IL-13-responsive BM-derived cell that is required to inhibit lethal inflammation (our previous studies demonstrate that IL-4/IL-13-responsive T cells are not required); and 3) how do IL-4/IL-13-stimulated, “alternatively activated” macrophages protect against lethal inflammation. Each of these issues is currently under study.
Acknowledgments
We thank J. Bailey for assistance with bone marrow chimera generation, M. Chiaramonte for help with hydroxyproline measurements, and M. Khodoun for critical reading of this manuscript.
Footnotes
This work was supported by the U.S. Department of Veterans Affairs and National Institutes of Health Grants R01 GM49758 and R01 AI052099, which included a diversity supplement.
Abbreviations used in this paper: IL-4Rα, IL-4 receptor α-chain; BM, bone marrow; Mφ, macrophage; AAMφ, alternatively activated macrophage; CAMφ, classically activated macrophage; NOS-2, inducible NO synthase; WT, wild type; FIZZ-1, relm-α; AST, aspartate transaminase.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 4.Negrao-Correa D, Pinho V, Souza DG, Pereira AT, Fernandes A, Scheuermann K, Souza AL, Teixeira MM. Expression of IL-4 receptor on non-bone marrow-derived cells is necessary for the timely elimination of Strongyloides venezuelensis in mice, but not for intestinal IL-4 production. Int J Parasitol. 2006;36:1185–1195. doi: 10.1016/j.ijpara.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Urban JF, Jr, Noben-Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol. 2001;167:6078–6081. doi: 10.4049/jimmunol.167.11.6078. [DOI] [PubMed] [Google Scholar]
- 6.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273–282. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 7.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 8.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 9.Leeto M, Herbert DBR, Marillier R, Schwegmann A, Fick L, Brombacher F. TH1-dominant granulomatous pathology does not inhibit fibrosis or cause lethality during murine schistosomiasis. Am J Pathol. 2006;169:1701–1712. doi: 10.2353/ajpath.2006.060346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon S. Alternative activation of macrophages. Nat Rev. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 11.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miescher GC, Schreyer M, MacDonald HR. Production and characterization of a rat monoclonal antibody against the murine CD3 molecular complex. Immunol Lett. 1989;23:113–118. doi: 10.1016/0165-2478(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 13.Morse HC, III, Davidson WF, Yetter RA, Coffman RL. A cell-surface antigen shared by B cells and Ly2+ peripheral T cells. Cell Immunol. 1982;70:311–320. doi: 10.1016/0008-8749(82)90332-x. [DOI] [PubMed] [Google Scholar]
- 14.Springer T, Galfre G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 15.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6 – 8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 16.Beckmann MP, Schooley KA, Gallis B, Vanden Bos T, Friend D, Alpert AR, Raunio R, Prickett KS, Baker PE, Park LS. Monoclonal antibodies block murine IL-4 receptor function. J Immunol. 1990;144:4212–4217. [PubMed] [Google Scholar]
- 17.Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 18.Finkelman FD, Wynn TA, Donaldson DD, Urban JF. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr Opin Immunol. 1999;11:420–426. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 19.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 21.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 22.Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of Schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465–466. doi: 10.1016/s0167-5699(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 23.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 24.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 25.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, et al. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urban JF, Jr, Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, Gamble HR, Byrd C, Donaldson D, Else K, Finkelman FD. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 27.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis . Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 28.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 29.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, Reilly NL, Schopf L, Urban JF., Jr Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 30.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 31.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 32.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-α-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 33.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–3926. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 34.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 35.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 36.Hanna S, Gharib B, Lepidi H, Montet JC, Dumon H, de Reggi M. Experimental schistosomiasis, protective aspects of granulomatous reaction in the mouse liver. Parasitol Res. 2005;96:6–11. doi: 10.1007/s00436-005-1319-5. [DOI] [PubMed] [Google Scholar]
- 37.Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci USA. 2001;98:13243–13248. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartley PB, Ramm GA, Jones MK, Ruddell RG, Li Y, McManus DP. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol. 2006;36:993–1001. doi: 10.1016/j.ijpara.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Jakubzick C, Choi ES, Kunkel SL, Joshi BH, Puri RK, Hogaboam CM. Impact of interleukin-13 responsiveness on the synthetic and proliferative properties of Th1- and Th2-type pulmonary granuloma fibroblasts. Am J Pathol. 2003;162:1475–1486. doi: 10.1016/S0002-9440(10)64280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]