Abstract
A fluorous-linker-assisted solution-phase protocol has been developed and applied to parallel synthesis of a piperazinedione-fused tricyclic compound library. The one-pot [3 + 2] cycloaddition of fluorous amino esters, aldehydes, and maleimides afforded bicyclic proline derivatives. The intermediates were subjected to N-acylation with chloroacetyl chloride, followed by displacement reactions with amines. Linker cleavage with concomitant lactamization yielded the final products. Microwave heating was employed to facilitate several reaction steps and fluorous solid phase extraction (F-SPE) was employed to purify the intermediates. During the method development, a small library containing sixteen analogs was prepared. The optimized conditions were applied to the synthesis of a production library containing ninety analogs.
Introduction
Multicomponent reactions (MCRs) represent a convergent and atom economical way to construct molecules with complex structures.1 These reactions are increasingly popular in the synthesis of chemical libraries for lead generation programs at pharmaceutical companies.2 In MCRs, one or more components are usually used in excess to drive the reaction to completion. The excess of starting materials may complicate the product purification. Having one of the building blocks attached to solid support3 or attached to a fluorous linker4 can facilitate the separation of MCR products.
Fluorous separations are based on highly selective fluorophilic interactions between the fluorous molecules and the separation media.5 Fluorous solid phase extraction (F-SPE) separates a mixture based on the presence or absence of fluorinated components.6 In contrast to molecules bound to a solid support, fluorous molecules are soluble in many organic solvents, reactions can be monitored by techniques like TLC, NMR, LC-MS, fluorous reagents can be used in equimolar ratios, and conventional solution phase protocols can be applied to fluorous chemistry without significant optimization.
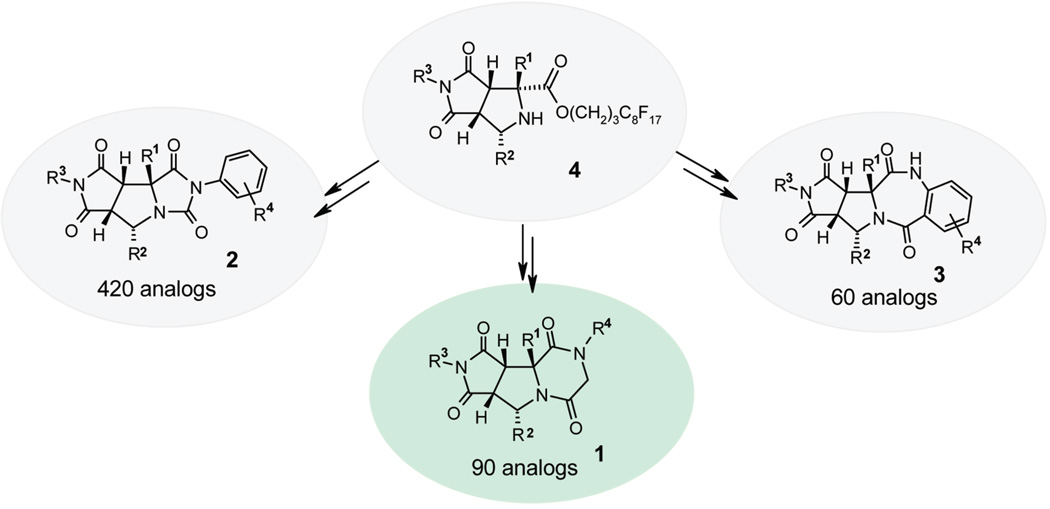
We recently reported a fluorous diversity-oriented synthesis of three novel heterocyclic scaffolds.7 The common bicyclic proline intermediates 4 were prepared by a onepot, three-component [3 + 2] cycloaddition involving fluorous amino esters, aldehydes, and maleimides.8 The intermediates then underwent different postcondensation modifications to form hydantoin-fused tricyclic compounds 2, piperazinedione-fused tricyclic compounds 1, and benzodiazapinedione-fused tricyclic compounds 3, respectively (Scheme 1). A 420-membered library of 2 and a 60-membered library of 3 have been prepared by fluorous mixture synthesis.9 Described in this paper is the development and production of a 90-membered library of 1 by fluorous parallel synthesis.
Scheme 1.
Diversity-Oriented Synthesis of Three Heterocyclic Library Scaffolds
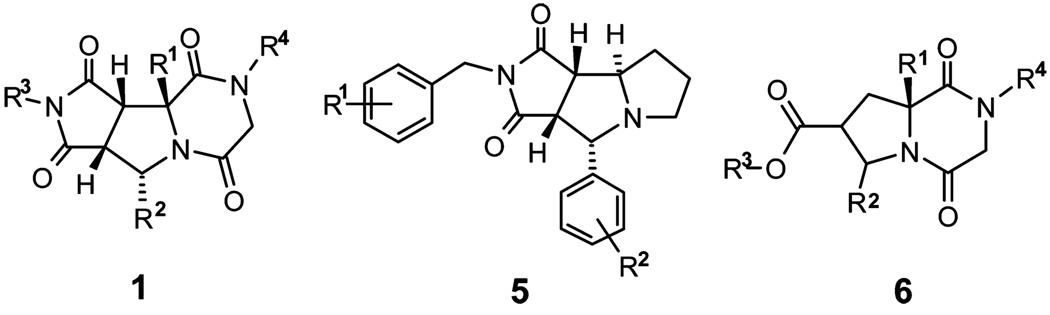
Tricyclic piperazinediones library 1 is structurally comparable to tricyclic thrombin inhibitors 510 and diketopiperazine-based inhibitors 6 of human hormone-sensitive lipase (Scheme 2).11 Synthesis and screening of tricyclic piperazinedione analogs may provide additional QSAR information.
Scheme 2.
Library Scaffold 1 and Related Biologically Active Molecules
Results and Discussion
Library Development
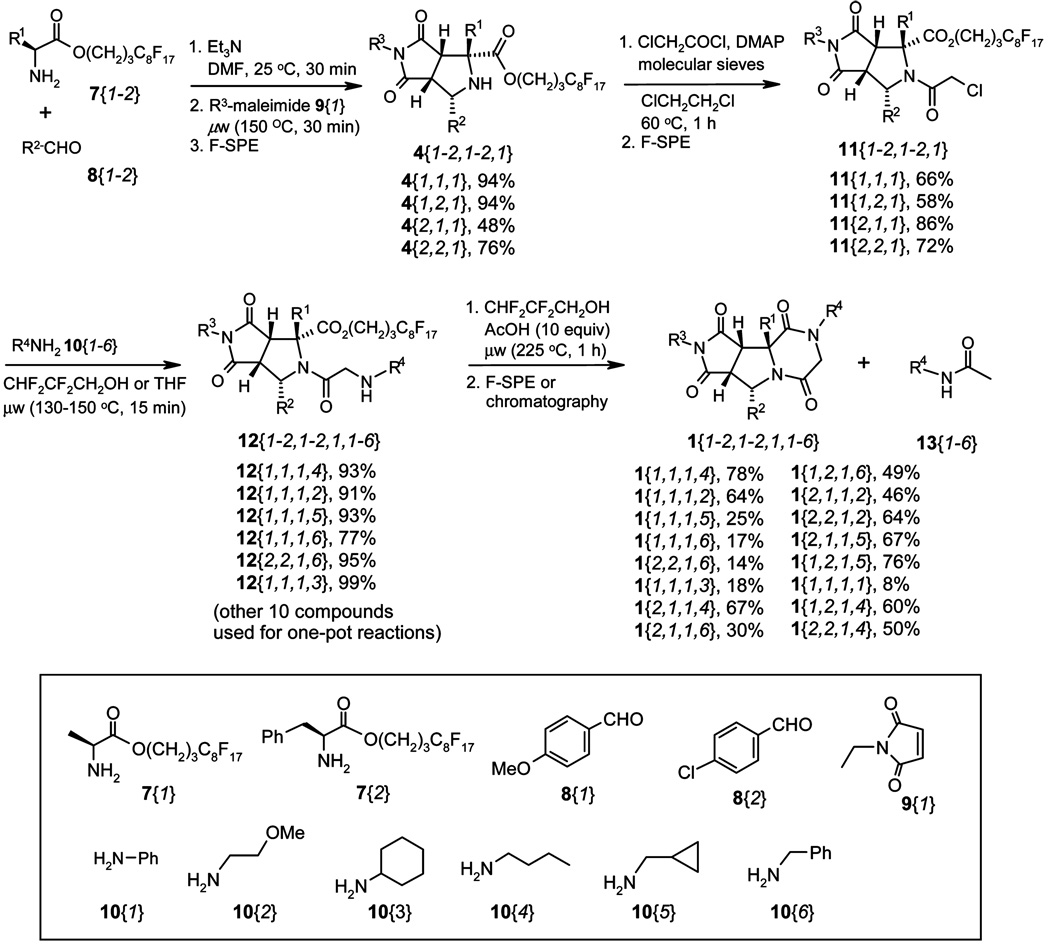
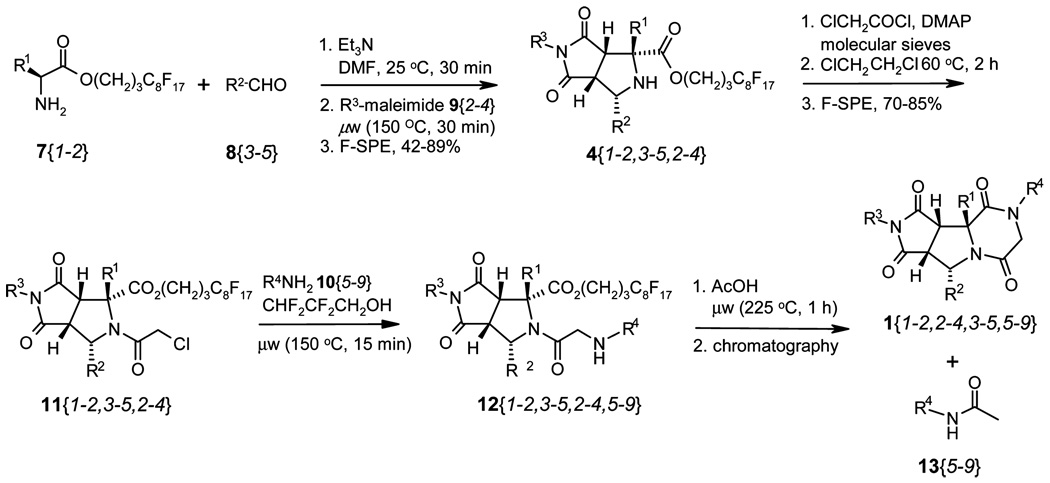
The synthesis of tricyclic piperazinediones has four steps: (1) one-pot [3 + 2] cycloaddition of fluorous amino ester 7, aldehyde 8, and maleimide 9 to afford bicyclic proline derivative 4; (2) N-acylation of 4 with chloroacetyl chloride to form 11; (3) displacement of chloride on 11 with amine 10 to form 12; and (4) linker cleavage with concomitant lactamization to yield final product 1. Each reaction step was optimized during the library development. Sixteen final products 1 were prepared during the method development by combining a selection of building blocks 7, 8, 9, and 10, as shown in Scheme 3.
Scheme 3.
Sixteen-Member Development Library of 1 and Building Blocks (7–10)
The one-pot, three component [3 + 2] cycloaddition reactions of 7, 8, and 9 were performed under microwave irradiation at 150 °C for 30 min to give four fluorous proline derivaties 4{1–2,1–2,1}.7 The use of oil bath heating was also tested but resulted in lower yields. For example, compound 4{1,1,1} was produced in 94% yield under microwave heating at 130 °C for 30 min, whereas the same compound was produced in 72% under oil bath heating at 130 °C for 1 h. We found that the yield of these [3 + 2] cycloaddition reactions could be improved by reacting the aldehyde and amine first in the presence of triethylamine, followed by the addition of the corresponding maleimide and continued heating in the microwave at 150 °C for 30 min. The fluorous amino esters 7{1–2} were used as the limiting reagents, and the other two building blocks were used in slight excess to ensure the consumption of the fluorous component so that the unreacted starting material, as well as base and solvent, could be easily removed by F-SPE. Nonfluorous components were eluted with 4:1 MeOH/H2O, and the fluorous product 4 was eluted with MeOH. In the case of R1 being a benzyl substituent, flash chromatography was used for additional purification. The purity of all four products was >90% as determined by 1H NMR.
The acylation of 4 with chloroacetyl chloride was performed under conventional heating at 60 °C for 1 h. High yields were obtained in dichloroethane in the presence of molecular sieves and a catalytic amount of N,N-dimethylaminopyridine (DMAP). The addition of 15 equiv of chloroacetyl chloride was completed in two portions and the starting material was usually consumed after 1 h. Purification by flash chromatography gave four products 11{1–2,1–2,1} in 58–86% yields and ELS purities >90%.
Two different approaches were developed for the last two steps. We only prepared sixteen of possible twenty-four compounds of 12{1–2,1–2,1,1–6}. Among them, six were isolated and then used for the next round of cyclization reactions to produce the final products. Ten were used in a one-pot procedure for the synthesis of the final products. For the approach involving the isolation of 12, α-chloroamide 11 and primary amine 10 in THF were heated under microwave irradiation at 150 °C for 15 min. The crude product was purified by F-SPE. The first elution solvent 4:1 MeOH/H2O contained 10 equiv of tetramethylguanidine to remove the HCl formed in the displacement reaction and to ensure that the product 12 was obtained as the free amine. The products 12 were produced in high yields with excellent purities. The lactamizition of 12 to form the final products 1 proved to be a difficult step, and a screening of a range of different reaction conditions was carried out with substrate 12{1,1,1,4}. This compound was converted to 1{1,1,1,4} by heating in isopropanol under microwave at 180 °C for 12 h. For the lactamizations of other substrates 12, they were heated in 2,2,3,3-tetrafluoropropanol with 10 equiv of acetic acid at 250 °C for 1 h. The acetic acid prevented the decomposition of the starting material. The products were purified by F-SPE to give corresponding tricyclic piperazinediones 12 in modest to good yield and excellent purity.
For the one-pot displacement and lactamization reactions, a solution of α-chloroamide 11 and primary amine 10 in 2,2,3,3-tetrafluoropropanol were heated under microwave conditions at 150 °C for 15 min, then 10 equiv of acetic acid were added, and the mixture was heated in the microwave at 250 °C for 1 h. The corresponding tricyclic piperazinediones 1 were isolated in modest to good yields after chromatography. However, in this one-pot procedure acetylamine 13 was observed as a side product and sometimes coeluted with the final compound.
Compounds 4, 11, 12, and 1 were prepared during the library development stage and were characterized by 1H and 13C NMR spectroscopy and mass spectrometry. The aromatic resonances in the 1H NMR spectra of compounds 11, 12, and 1 (see Supporting Information) displayed various stages of coalescence. Resonances for pairs of symmetry related protons on the aryl ring of R2 (H2/H5 and H3/H4) appeared as either broad two-proton signals or more or less broad pairs of one-proton signals. Clearly the rotation of the CH–Ar bond of the R2 substituent is occurring on the NMR time scale at 300 MHz.12 We will report in more detail on this unusual phenomenon in a later paper.
Library Production
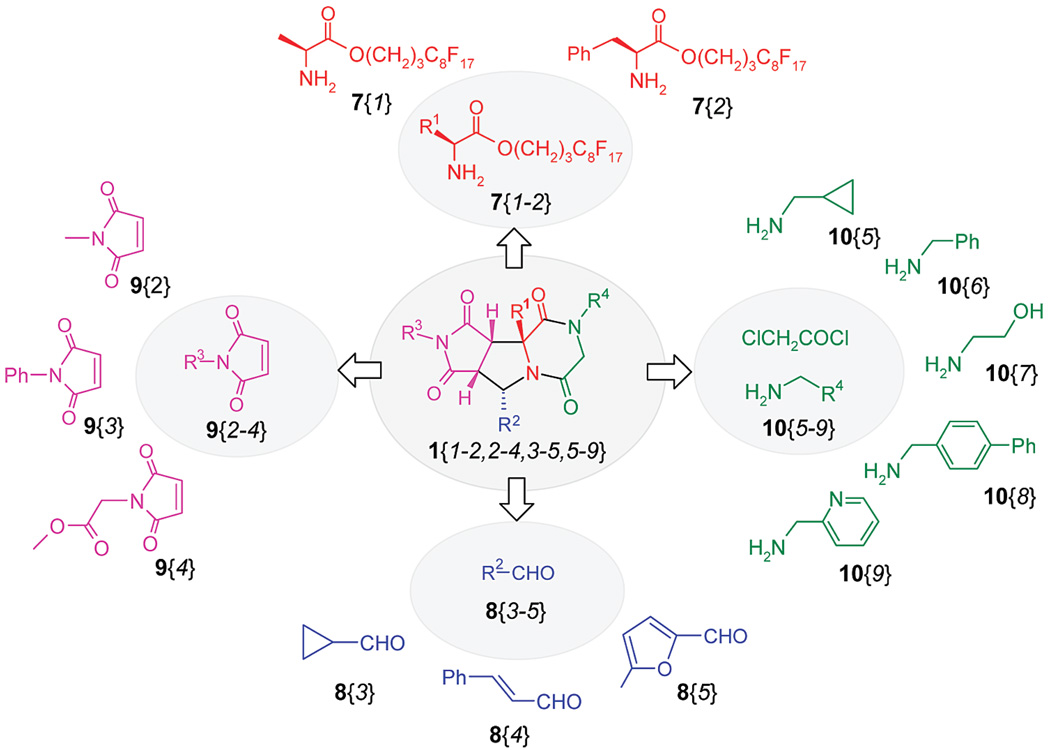
The synthesis of a 90-compound library of piperazinedione-fused tricyclic compounds 1 was undertaken. Four sets of building blocks were selected which included two fluorous amino esters 7{1–2}, three aldehydes 8{3–5}, three maleimides 9{2–4}, and five amines 10{5–9} (Scheme 4). With the exception of compounds 7{1–2} and 10{5–6}, all other building blocks are different from those used in the library development stage.
Scheme 4.
Ninety-Member Production Library of 1 and Building Blocks (7–10)
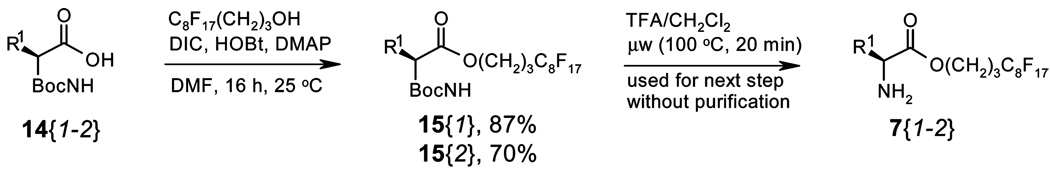
Fluorous amino esters 7{1–2} were prepared from the corresponding amino acids. 3-(Perfluorooctyl)propanol as the fluorous linker was attached to the Boc-protected amino acids 14{1–2} to form esters 15{1–2}under standard conditions using N,N′-diisopropylcarbodiimide (DIC) and 1-hydroxybenzotriazole (HOBt) as the coupling agents. The Boc groups were removed from 15{1–2} with 10% TFA/CH2Cl2 solution under microwave heating at 100 °C for 20 min to yield amino esters 7{1–2} (Scheme 5).
Scheme 5.
Preparation of Fluorous Amino Esters7{1–2}
The [3 + 2] cycloaddition reactions for the preparation of compound 4 were performed using the optimized conditions (Scheme 6 and Table 1). Aldehydes 8 and amines 7 were first reacted in the presence of triethylamine, followed by addition of maleimides 9. The mixtures were heated in the microwave reactor at 150 °C for 30 min and the products were purified by F-SPE. The fluorophilic fraction contained only the fluorous addition products 4{1–2,3–5,2–4}. With all alanine derivatives, bicycles 4{1,3–5,2–4} were obtained as single diastereomers in good yields (average 75%, including Boc-deprotection) and excellent purities (average 94%). The benzyl substituent at R1 led to a mixture of diastereomers and lower yields (average 49%, including Boc-deprotection). Traditional chromatography was used in several cases to obtain intermediates in sufficient purity (average 94%).
Scheme 6.
Preparation of Tricyclic Piperazinediones 1{1–2,2–4,3–5,5–9}
Table 1.
Yields [%] for the Synthesis of Fluorous Bicycles 4{1–2,3–5,2–4} from 7{1–2}, 8{3–5}, and 9{2–4} (Purity by ELSD [%])
| 4{1–2,3–5,2–4} | 7{1} | 7{1} | 7{1} | 7{2} | 7{2} | 7{2} |
|---|---|---|---|---|---|---|
| 9{2} | 9{3} | 9{4} | 9{2} | 9{3} | 9{4} | |
| 8{3} | 75 (93) | 79 (89) | 75 (98) | 78 | 64 (86)a | 35 (87)a |
| 8{4} | 85 (92) | 42 (86) | 84 (96) | 30 (94)b | 19 (99)a | 27a |
| 8{5} | 69 (97) | 81 (98) | 82 (96) | 50 (97)a | 50 (98)a | 80 (100) |
Yield after chromatography; a single diastereomer was obtained after chromatography.
Major diastereomer (second eluting).
The acylation reactions of 4 were performed in the presence of molecular sieves and catalytic amounts of DMAP under conventional heating at 60 °C for 1 h. The addition of 15 equiv of chloroacetyl chloride was completed in two portions. While the acylation of alanine derivatives led again to good yields and purities (average yield 70% and average purity 93% after F-SPE), it was observed that for R1 = benzyl only the major diastereomer of 4{2,3–5,2–4} could be acylated, probably because of steric reasons (Table 2). In the case of 4{2,4,2}, the two diastereomers had been separated. Akylation of the major diastereomer led to 11{2,4,2} in 88% yield (100% purity), while no conversion was observed for the minor diastereomer under otherwise identical reaction conditions. The minor diastereomer could be recovered from the reaction mixture.
Table 2.
Yields [%] for the Synthesis of Amides 11{1–2,3–5,2–4} from 4{1–2,3–5,2–4} (Purity by ELSD in [%])
| 11{1–2,3–5,2–4} | 7{1} | 7{1} | 7{1} | 7{2} | 7{2} | 7{2} |
|---|---|---|---|---|---|---|
| 9{2} | 9{3} | 9{4} | 9{2} | 9{3} | 9{4} | |
| 8{3} | 43 (89) | 76 (93) | 80 (83) | 72 (84) | 77 (93) | |
| 8{4} | 73 (88) | 80 (97) | 85 (99) | 88 (100)a | 44 (98) | 74 (100) |
| 8{5} | 62 (96) | 75 (99) | 61 (81) | 80 (100) | 10 (98) | 74 (100) |
From the second eluting diastereomer of 7{2,4,2}, acylation of minor diastereomer failed.
The nucleophilic substitution of the α-chloro amides 11{1–2,3–5,2–4} with primary amines 10{5–9}, followed by lactamization of 11{1–2,3–5,2–4,5–9} to form final products 1{1–2,3–5,2–4,5–9} was performed using the one-pot procedure. α-Chloroamides 11 and primary amine 10 in 2,2,3,3-tetrafluoropropanol were heated under microwave conditions at 150 °C for 15 min; then 10 equiv of acetic acid was added, and the mixture was heated in the microwave reactor at 250 °C for 1 h. Acylated amines 13{5–9} were observed as side products and coeluted with the target compounds in several cases. To solve this problem, the protocol was changed halfway through the library synthesis (see footnotes in Table 3). Insertion of a third F-SPE purification after the SN reaction removed the excess amine and prevented the formation of amides 15{5–9} after addition of acetic acid in the last step. Although the additional purification step led to lower isolated yields (average 30% instead 53% for the one-pot protocol, R1 = Me), the target compounds 1{1–2,3–5,2–4,5–9} were no longer contaminated with 13{5–9} (average purity 97% instead of 92% for the one-pot protocol, R1 = Me). These results emphasize the power of F-SPE as an orthogonal purification strategy to traditional chromatography on silica. Although thirty-eight out of fourty-five benzyl derivatives 1{2,3–5,2–4,5–9} could be isolated in excellent purities (average 96%), the yields were low for this branch of the library (average 28%).
Table 3.
Yields [%] for the Synthesis of Piperazindiones 1{1–2,3–5,2–4,5–9} from 11{1–2,3–5,2–4} and Amines 10{5–9} (Purity by ELSD [%], if the Purity Was Less than 50% the Reaction Was Considered As Failed)
| (a) reaction of 11{1–2,1–3,1–3} with cyclopropylmethlamine 10{5} | ||||||
| 1{1–2,3–5,2–4,5} | 7{1} | 7{1} | 7{1} | 7{2} | 7{2} | 7{2} |
| 9{2} | 9{3} | 9{4} | 9{2} | 9{3} | 9{4} | |
| 8{3} | 59 (97)a | 69 (97)a | failedb | 23 (100)b | failedb | |
| 8{4} | 56 (99)a | 24 (93)a | 40 (100)a | 54 (100)b | 34 (100)b | 16 (100)b |
| 8{5} | 59 (100)b | 59 (94)a | 18 (100)b | 21 (100)b | 20 (90)b | 21 (100)b |
| (b) reaction of 11{1–2,1–3,1–3} with ethanolamine 10{6} | ||||||
| 1{1–2,3–5,2–4,5} | 7{1} | 7{1} | 7{1} | 7{2} | 7{2} | 7{2} |
| 9{2} | 9{3} | 9{4} | 9{2} | 9{3} | 9{4} | |
| 8{3} | 64 (97)a | 60 (83)a | failedb | failedb | failedb | |
| 8{4} | 55 (100)a | 45 (100)a | 54 (89)a | 25 (100)b | failedb | 20 (100)b |
| 8{5} | 46 (100)b | 42 (95)a | 29 (99)b | 22 (100)b | 89 (89)b | 30 (98)b |
| (c) reaction of 13{1–2,1–3,1–3} with benzylamine 10{7} | ||||||
| 1{1–2,3–5,2–4,5} | 7{1} | 7{1} | 7{1} | 7{2} | 7{2} | 7{2} |
| 9{2} | 9{3} | 9{4} | 9{2} | 9{3} | 9{4} | |
| 8{3} | faileda | 62 (78)a | 19 (100)b | 24 (89)b | 20 (100)b | |
| 8{4} | 35 (100)a | 39 (100)a | 23 (78)a | 33 (100)b | 26 (100)b | 16 (100)b |
| 8{5} | 12 (96)b | 40 (62)a | 18 (98)b | 11 (100)b | 9 (98)b | 12 (100)b |
| (d) reaction of 11{1–2,1–3,1–3} with 4–phenylbenzylamine 10{8} | ||||||
| 1{1–2,3–5,2–4,5} | 7{1} | 7{1} | 7{1} | 7{2} | 7{2} | 7{2} |
| 9{2} | 9{3} | 9{4} | 9{2} | 9{3} | 9{4} | |
| 8{3} | 68 (95)a | failedb | 57 (68)b | 6 (100)b | 62 (98)b | |
| 8{4} | 58 (100)a | 33 (100)b | 26 (100)b | 30 (90)b | 88 (61)b | 32 (100)b |
| 8{5} | 51 (100)b | 42 (94)b | 25 (94)b | 39 (95)b | 78 (73)b | failedb |
| (e) reaction of 11{1–2,1–3,1–3} with 2-pyridylmethylamine 10{9} | ||||||
| 1{1–2,3–5,2–4,5} | 7{1} | 7{1} | 7{1} | 7{2} | 7{2} | 7{2} |
| 9{2} | 9{3} | 9{4} | 9{2} | 9{3} | 9{4} | |
| 8{3} | faileda | 96 (74)a | 24 (100)b | 20 (94)b | 24 (100)b | |
| 8{4} | 57 (95)a | 19 (79)b | faileda | 35 (99)b | 20 (100)b | 25 (100)b |
| 8{5} | 30 (100)b | 21 (100)b | 23 (97)b | 17 (100)b | 23 (73)b | 17 (98)b |
Protocol D: no FSPE on intermediate.
Protocol E: FSPE on intermediate prior to ring closure.
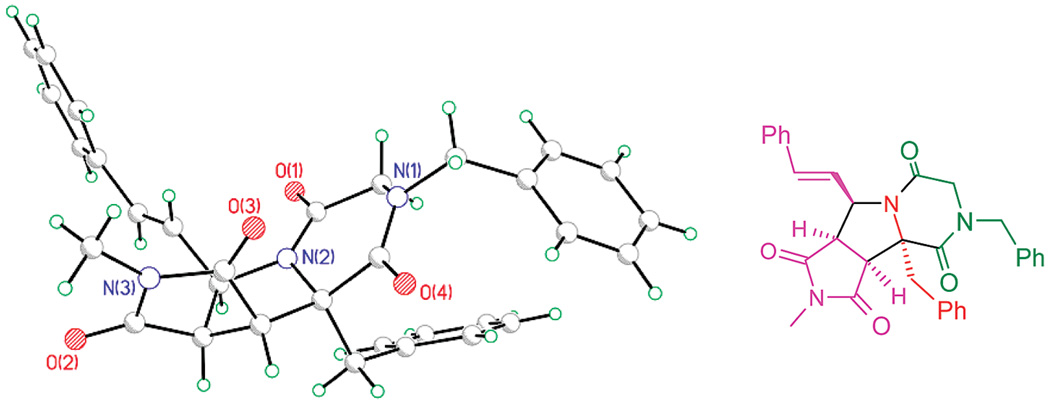
The configuration of the library members was determined with the help of a crystal structure analysis. The three-component [3 + 2] cycloaddition reaction was diastereoselective in the case of the alanine derivatives (R1 = Me), and the stereochemistry has been confirmed by an X-ray structure analysis described in our previous paper.7 A mixture of diastereomers was obtained in the case of the phenylalanine derivatives (R1 = Bn). Only the major diastereomer underwent the subsequent acylation and ring closure. This diastereomer shows the same configuration as the one obtained from the alanine series (Figure 1), so we conclude that all final library members have the same core configuration.
Figure 1.
Crystal structure analysis of 1{2,4,2,6}.
Purity Analysis
Altogether seventy-two tricyclic piperazinediones 1{1–2,3–5,2–4,5–9} were synthesized. All compounds were analyzed by LC/MS-ELSD (Tables 1–3). Sixty-three compounds passed the purity criteria of 85% by ELSD for the UPCMLD compound collection. The average purity by ELSD of the accepted library members was 98% (Table 3).
Conclusions
With the help of the fluorous linker and F-SPE for intermediate purification, a library containing 90 tricyclic piperazinediones was synthesized, and 63 final products were obtained with in greater than 85% purity. The key steps of three-component [3 + 2] cycloaddition reaction and detag lactamization cyclization have been optimized for library production. All compounds have been submitted to the NIH repository and are currently being evaluated in several high-throughput screening programs. Results on their biological activities can be found in PubChem (http://pubchem.ncbi.nlm.nih.gov).
Experimental Section
General
All solvents or reagents were used without further purification. Reactions were monitored by TLC analysis (EM Science precoated silica gel 60 F254 plates, 250 mm layer thickness), and visualization was accomplished with a 254 nm UV light and by staining with Vaughn’s reagent (4.8 g (NH4)6Mo7O24 • 4H2O, 0.2 g Ce(SO4)2 • 4H2O in 10 mL conc. H2SO4 and 90 mL H2O) and KMnO4 (1.0 g KMnO4, 1.0 g K2CO3, 2 mL 5% aqueous NaOH, 100 mL H2O). NMR spectra were recorded in CDCl3 or DMSO-d6 (298 K) at 300.1 MHz (1H) or 75.5 MHz (13C) using a Bruker Avance 300 with XWIN-NMR software. Chemical Shifts (δ) are reported in parts per million (ppm). Tetramethylsilane (1H), chloroform-d (13C), or DMSO-d6 (13C) were used as internal standards. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = dublet, t = triplet, q = quartet, m = multiplet, bs = broad singlet, app = apparent), integration and coupling constants. IR spectra were obtained on a Nicolet AVATAR 360 FTIR ESP Spectrometer. Mass spectra were obtained on a Micromass Autospec double focusing instrument (EI) or a Waters Q-Tof mass spectrometer (ESI). Compounds were analyzed by reverse-phase HPLC (Alltech Prevail C-18, 100 × 4.6 mm, 1 mL/min, 20–80% MeCN, 80–20% H2O) with UV (210 and 254 nm), ELS (Nebulizer 45 °C, Evaporator 45 °C, N2 flow 1.25 SLM), and MS detection using a Thermo Finnigan Surveyor LC and LCQ Advantage MS system (ESI positive mode) or a Waters LC-MS system.
General Procedure for the Preparation of Fluorous Esters 15{1–2}
A solution of Boc-l-alanine 14{1} (11.0 g, 58.3 mmol, 1.5 equiv) in DMF (85 mL) was treated with 3-(perfluorooctyl)propanol (18.9 g, 38.9 mmon, 1.0 equiv), N,N′-diisopropylcarbodiimide (7.36 g, 58.3 mmol, 1.5 equiv), HOBt (7.88 g, 58.3 mmol, 1.5 equiv), and DMAP (0.713 g, 5.83 mmol, 0.15 equiv) and was stirred overnight at room temperature. The reaction mixture was diluted with ethyl acetate (100 mL) and washed three times with water (100 mL). The organic fraction was washed with brine (100 mL), dried over magnesium sulfate, and concentrated. Purification by chromatography over SiO2 provided 21.9 g (34.0 mmol, 87%) of fluorous ester 15{1}, while 1.67 g of 3-(perfluorooctyl) propanol (3.50 mmol, 9%) was recovered.
General Procedure for the Preparation of Bicycles 4{1–2,3–5,2–4}
Fluorous ester 15{1} (2.49 g, 4.54 mmol, 1.0 equiv) was dissolved in a solution of TFA in DCM (10 wt%, 15 mL) and irradiated at 100 °C for 20 min in a Biotage Initiator microwave reactor. All volatile components were removed in a Genevac HT-4 parallel evaporation system, and the residue was dissolved in DMF (15 mL). Cinnamaldehyde 8{4} (0.720 g, 5.45 mmol, 1.2 equiv) and triethylamine (1.38 g, 13.6 mmol, 3.0 equiv) were added, and the reaction mixture was stirred for 30 min at room temperature. Then, N-methylmaleimide 9{2} (0.757 g, 6.81 mmol, 1.5 equiv) was added, and microwave irradiation was continued at 150 °C for 30 min.
F-SPE Purification
A plastic SPE cartridge (150 mL volume) was filled with FluoroFlash silica gel (50 g)13 and preconditioned with 80:20 MeOH/H2O. The crude reaction mixture was loaded in DMF onto the cartridge. Elution with 80:20 MeOH/H2O (200 mL) provided all nonfluorous materials, including the DMF, these fractions were discarded. Next, the cartridge was eluted with diethylether (200 mL). The resulting ether fraction was concentrated to provide the bicycle 4{1,2,1} (2.97 g, 3.84 mmol) in 85% yield and 92% purity by ELS detection. The cartridge was washed with THF (200 mL) and diethylether (100 mL) prior to reuse; it was reused up to 15 times.
(1R,3R,3aR,6aS)-4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-Heptadecafluoroundecyl 1,5-Dimethyl-4,6-dioxo-3-styryloctahydropyrrolo[3,4-c]pyrrole-1-carboxylate 4{1,4,2}
IR (film) 3336, 2982, 2953, 1703, 1437, 1208 cm−1; 1H NMR (CDCl3) δ 7.43–7.20 (m, 5 H), 6.69 (d, 1 H, J = 15.9 Hz), 6.29 (dd, 1 H, J = 15.9, 6.9 Hz), 4.37–4.25 (m, 3 H), 3.47 (app. t, 1 H, J = 8.0 Hz), 3.25 (d, 1 H, J = 7.5 Hz), 2.93 (s, 3 H), 2.40–2.15 (m, 2 H), 2.15–2.00 (m, 2 H), 1.58 (s, 3 H); 13C NMR (CDCl3) δ 175.7, 174.9, 172.1, 136.4, 132.4, 128.5, 127.9, 126.7, 125.0, 68.2, 64.5, 60.5, 56.4, 50.2, 27.9 (t, J = 20.3 Hz), 25.0, 24.0, 19.8 (t, J = 3.8 Hz); MS (ES) m/z (rel. intensity) 775 ([M + 1]+, 100), 671 (60); HRMS (ES) m/z calculated for C28H24N2O4F17 775.1465, found 775.1431.
General Procedure for the Preparation of Acylated Bicycles 11{1–2,3–5,2–4}
To a solution of bicycle 4{1,5,3} (1.17 g, 1.44 mmol, 1.0 equiv) in dichloroethane (35 mL) was added DMAP (0.018 g, 0.144 mmol, 0.1 equiv), molecular sieves (2.93 g), and chloroacetyl chloride (1.63 g, 14.4 mmol, 10.0 equiv). The reaction mixture was heated for at 70 °C for 1 h; 5 more equiv of chloroacetyl chloride was added, and heating was continued for 1 h. It was proven to be advantageous to add the chloroacetyl chloride in two portions. The molecular sieves were filtered off, and the reaction mixture was concentrated and redissolved in DMF (15 mL). The DMF reaction mixture was purified by F-SPE as described in Protocol B. Concentration of the ether fraction provided the acylated bicycle 13{1,5,3} (962 mg, 1.08 mmol) in 75% yield and 99% purity by ELS detection.
(1R,3S,3aR,6aS)-4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-Heptadecafluoroundecyl 2-(2-Chloroacetyl)-1-methyl-3-(5-methylfuran-2-yl)-4,6-dioxo-5-phenyloctahydropyrrolo[3,4-c]pyrrole-1-carboxylate 13{1,5,3}
1H NMR (CDCl3) δ 7.40–7.33 (m, 3 H), 6.97–6.92 (m, 2 H), 6.64 (d, 1 H, J = 3.0 Hz), 5.97 (dd, 1 H, J = 3.0, 0.9 Hz), 5.71 (d, 1 H, J = 10.5 Hz), 4.40–4.20 (m, 2 H), 4.12 (dd, 1 H, J = 10.2, 9.0 Hz), 3.83 (d, 1 H, J = 13.2 Hz), 3.65 (d, 1 H, J = 12.9 Hz), 3.52 (d, 1 H, J = 9.0 Hz), 2.21 (s, 3 H), 2.22–2.08 (m, 2 H), 2.08–1.95 (m, 2 H), 1.82 (s, 3 H); 13C NMR (CDCl3) d 172.4, 171.3, 168.9, 165.4, 153.7, 147.5, 131.1, 129.0, 128.8, 126.0, 111.4, 107.1, 69.2, 64.6, 57.8, 54.2, 48.7, 41.8, 27.7 (t, J = 22.5 Hz), 23.6, 19.7 (t, J = 3.4 Hz), 13.3.
General Procedure for One-Pot Diaplacement/Lactamization Reaction to Form 1{1–2,3–5,2–4,5–9}
A solution of the acylated bicycle 11{1,3,2} (0.201 g, 0.261 mmol, 1.0 equiv) in 2,2,3,3-tetrafluoropropanol (TEFPO) was treated with 4-phenylbenzylamine 10{8} (0.191 g, 1.04 mmol, 4.0 equiv), and microwave irradiation was continued for 15 min at 150 °C. After the mixture was cooled to room temperature, acetic acid (0.105 g, 1.82 mmol, 7.0 equiv) was added, and the reaction mixture was irradiated at 22 5°C for 1 h under microwave. All volatile components were removed in vacuo, and the residue was purified by chromatography over SiO2 to provide 0.0809 g (0.177 mmol, 68%) of the target compound 1{1,3,2,8} in 95% purity by ELS detection.
In several cases, the acylated amine 13{5–9} coeluted with the target compound 1{1–2,3–5,2–4,5–9}. This problem was solved by introducing a F-SPE purification prior to the addition of acetic acid. After the first microwave irradiation, all volatile components were removed in vacuo, and the residue was loaded in THF (0.5 mL) on a preconditioned F-SPE cartridge (5 g FluoroFlash silica gel). Elution with 80:20 MeOH/H2O provided 12{1–2,3–5,2–4,5–9} containing fraction. Methanol was removed in vacuo and the aqueous fraction was extracted with ethyl acetate (2 × 20 mL). Both organic fractions were combined, washed with brine, dried over magnesium sulfate, and concentrated. The residue was dissolved in 2,2,3,3-tetrafluoropropanol and treated with acetic acid in the microwave as described above.
(3aR,4R,9aR,9bS)-8-Aza-8-(biphenyl-4-ylmethyl)-4-cyclopropyl-2,9a-dimethyltetrahydro-1H-pyrrolo[3,4-a]indolizine-1,3,6,9(2H,9aH,9bH)-tetraone 1{1,3,2,8}
IR (film) 3007, 1712, 1673 cm−1; 1H NMR (CDCl3) δ 7.62–7.55 (m, 4 H), 7.47–7.39 (m, 4 H), 7.38–7.33 (m, 1 H), 4.82 (d, 1 H, J = 14.4 Hz), 4.56 (d, 1 H, J = 14.4 Hz), 3.88 (d, 1 H, J = 18.0 Hz), 3.79 (d, 1 H, J = 18.0 Hz), 3.58–3.50 (m, 2 H), 3.35 (app. t, 1 H, J = 9.3 Hz), 2.98 (s, 3 H), 1.51 (s, 3 H), 0.88–0.78 (m, 1 H), 0.78–0.70 (m, 2 H), 0.50–0.40 (m, 2 H); 13C NMR (CDCl3) δ 173.8, 173.5, 166.2, 163.6, 140.9, 140.4, 134.1, 129.0, 128.7, 127.5, 127.3, 126.9, 65.9, 65.4, 53.2, 50.3, 48.4, 46.2, 25.1, 24.8, 11.3, 9.4, 5.9; MS (ES) m/z (rel. intensity) 458 ([M + 1]+, 100); HRMS (ES) m/z calculated for C27H28N3O4 458.2080, found 458.2079.
Supplementary Material
Acknowledgment
the Authors gratefully acknowledge financial support provided by NIGMS (P50-GM067082).We also thank Ms. Stephanie Nicolay for performing LC-MS/UV/ELSD analyses.
Footnotes
Supporting Information Available. NMR spectra of representative compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.(a) Dömling A, Ugi I. Angew. Chem., Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (b) Ugi I, Heck S. Comb. Chem. High Throughput Screening. 2001;4:1–34. doi: 10.2174/1386207013331291. [DOI] [PubMed] [Google Scholar]; (c) Dömling A. Curr. Opin. Chem. Biol. 2002;6:306–313. doi: 10.1016/s1367-5931(02)00328-9. [DOI] [PubMed] [Google Scholar]; (d) Zhu J. Eur. J. Org. Chem. 2003;7:1133–1144. [Google Scholar]; (e) Zhu J, Bienayme H, editors. Multicomponent Reactions. Weinheim, Germany: Wiley; 2005. [Google Scholar]; (f) Dömling A. Chem. ReV. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 2.(a) Armstrong RW, Combs AP, Tempest PA, Brown SD, Keating TA. Acc. Chem. Res. 1996;29:123–131. [Google Scholar]; (b) Bienaymé H, Hulme C, Oddon G, Schmitt P. Chem.—Eur. J. 2000;6:3321–3329. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]; (c) Tietze LF, Modi A. Med. Res. Rev. 2000;20:304–322. doi: 10.1002/1098-1128(200007)20:4<304::aid-med3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]; (d) Weber L. Drug Discovery Today. 2002;7:143–147. doi: 10.1016/s1359-6446(01)02090-6. [DOI] [PubMed] [Google Scholar]; (e) Hulme C, Gore V. Curr. Med. Chem. 2003;10:51–80. doi: 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]; (f) Wipf P. Pharm. News. 2002;9:157–169. [Google Scholar]; (g) Werner S, Turner DM, Lyon MA, Huryn DM, Wipf P. Synlett. 2006;14:2334–2338. [Google Scholar]
- 3.(a) Sutherlin DP, Stark TM, Hughes R, Armstrong RW. J. Org. Chem. 1996;61:8350–8354. doi: 10.1021/jo960119j. [DOI] [PubMed] [Google Scholar]; (b) Lee D, Sello JK, Schreiber SL. Org. Lett. 2000;2:709–712. doi: 10.1021/ol005574n. [DOI] [PubMed] [Google Scholar]; (c) Samanta SK, Kylänlahti I, Yli-Kauhaluoma J. Bioorg. Med. Chem. Lett. 2005;15:3717–3719. doi: 10.1016/j.bmcl.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 4.(a) Studer A, Jeger P, Wipf P, Curran DP. J. Org. Chem. 1997;62:2917–2924. doi: 10.1021/jo970095w. [DOI] [PubMed] [Google Scholar]; (b) Zhang W, Tempest P. Tetrahedron Lett. 2004;45:6757–6760. [Google Scholar]; (c) Lu Y, Zhang W. QSAR Comb. Sci. 2004;23:827–835. doi: 10.1901/jaba.2004.23-827. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhang W, Lu Y, Geib S. Org. Lett. 2005;7:2269–2272. doi: 10.1021/ol0507773. [DOI] [PubMed] [Google Scholar]; (e) Zhang W. Comb. Chem. High Throughput Screening. 2007;10:219–229. doi: 10.2174/138620707780126697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For reviews and protocols on fluorous separation techniques, see: Curran DP. Angew. Chem., Int. Ed. Engl. 1998;37:1175–1196. Gladysz JA, Curran DP, Horvath IT, editors. Handbook of Fluorous Chemistry. Weinheim, Germany: Wiley-VCH; 2004. Zhang W. Tetrahedron. 2003;59:4475–4489. Zhang W. Chem. Rev. 2004;104:2531–2556. doi: 10.1021/cr030600r. Curran DP. Aldrichm. Acta. 2006;39:3–9. Zhang W. Chem. Rev. 2009;109:749–795. doi: 10.1021/cr800412s..
- 6.(a) Curran DP. Synlett. 2001:1488–1496. [Google Scholar]; (b) Curran DP. In: Handbook of Fluorous Chemistry. Gladysz JA, Curran DP, Horvath IT, editors. Weinheim, Germany: Wiley-VCH; 2004. pp. 101–127. [Google Scholar]; (c) Zhang W, Curran DP. Tetrahedron. 2006;62:11837–11865. doi: 10.1016/j.tet.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Lu Y, Chen CH-T, Curran DP, Geib S. Eur. J. Org. Chem. 2006:2055–2059. doi: 10.1002/ejoc.200600077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Zhang W, Chen CHT.Tetrahedron Lett 20051807–1810.18079977 [Google Scholar]; (b) Zhang W, Lu Y, Geib S. Org. Lett. 2005;7:2269–2272. doi: 10.1021/ol0507773. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Lu Y, Chen CH-T, Zeng L, Kassel DB. J. Comb. Chem. 2006;8:687–695. doi: 10.1021/cc060061e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen J, Seiler P, Wagner B, Fisher H, Tschopp T, Obst-Sander U, Banner DW, Kansy M, Muller K, Diederrich F. Org. Biomol. Chem. 2004;2:1339–1352. doi: 10.1039/b402515f. [DOI] [PubMed] [Google Scholar]
- 11.(a) Slee DH, Bhat AS, Nguyen TN, Kish M, Lundeen K, Newman MJ, McConnell SJ. J. Med. Chem. 2003;46:1120–1122. doi: 10.1021/jm020460y. [DOI] [PubMed] [Google Scholar]; (b) Furutsuka K, Hayashi H, Shiono Y. J. Nat. Prod. 1999;62:315–317. doi: 10.1021/np9802623. [DOI] [PubMed] [Google Scholar]; (c) Roe JM, Webster RAB, Ganesan A. Org. Lett. 2003;5:2825–2827. doi: 10.1021/ol034822n. [DOI] [PubMed] [Google Scholar]; (d) Ley SV, Cleator E, Hewitt PR. Org. Biomol. Chem. 2003;1:3492–3494. doi: 10.1039/b308288a. [DOI] [PubMed] [Google Scholar]
- 12.(a) Gust D, Mislow K. J. Am. Chem. Soc. 1973;95:1535–1547. [Google Scholar]; (b) Modarresi-Alam AR, Khamoosh F, Rostamizadeh M, Keykha H, Nasrollahzadeh M, Bijanzadeh HR, Kleinpeter E. J. Mol. Struct. 2007;841:61–66. [Google Scholar]
- 13. Available from Fluorous Technology Inc. ( www.fluorous.com).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.