Abstract
Papillon-Lefevre syndrome (PLS; OMIM 245000) is an autosomal recessive disease caused by cathepsin C (CTSC) gene and is clinically characterized by palmoplantar keratoderma, psoriasiform lesion over the extensor surfaces and ginigivitis followed by teeth loss. CTSC gene is known to be expressed in several tissues including the skin and immune system. In the skin CTSC is thought to play a role in differentiation and desquamation. In the immune system it activates serine proteases. Here we investigated a patient from Pakistan with features of PLS and determined a novel deletion mutation designated c.21delG (Leu7PhefsX57) in exon 1 of the CTSC gene, that most likely results in absent CTSC protein, further extending the spectrum of mutations in the CTSC gene.
Keywords: Papillon-Lefevre syndrome, Cathepsin C, Palmoplantar keratoderma, Gingivitis
Papillon Lefevre syndrome (PLS; OMIM 245000), is a rare autosomal recessive keratoderma affecting males and females equally and caused by mutations in the cathepsin C (CTSC) gene, GeneBank accession number(NM_001814.4), located on chromosome 11q14.1
CTSC is is a lysosomal enzyme expressed in several tissues including the skin, where it is thought to play a major role in epithelial differentiation and desquamation via activation of serine proteases.2 Thus, mutations in CTSC gene is expected to result in a hyperproliferative state, such as observed in the palms and soles of patients with PLS. Moreover, abnormal epithelial differentiation can affect the region adhering the ginigiva to the tooth surface, compromising the defense mechanical barrier against pathogens and leading to recurrent gingivitis and loss of teeth with time.2 CTSC is also highly expressed in the immune cells including both the myeloid and lymphoid lineages. These proteases have major roles in the phagocytic destruction of bacteria and activation of cytokines and chemokines. Surprisingly, patients with PLS do not present with any immune dysfunction, suggesting that other pathways, can compensate for the CTSC gene in the immune cells. This is in contrast to patients with Chediak-Highashi syndrome, which is also caused by a lysosomal enzyme defect and characterized by recurrent gingivitis and tooth loss, where patients usually die early secondary to severe immunodeficiency.3, 4
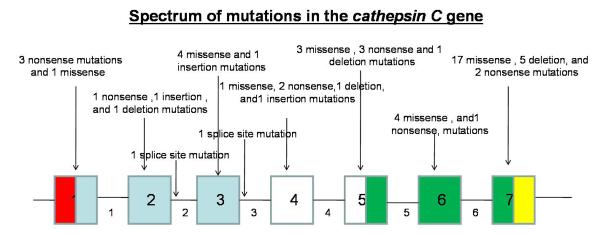
To date, more than 50 mutations (Fig.1) have been reported in the CTSC gene leading to PLS. Here, we report a novel mutation in the CTSC, leading to a mild skin phenotype in a Pakistani patient.
Figure1.
CTSC gene consists of 7 exons and 463 codons. To date, 55 different mutations have been detected in the Cathepsin C gene. (The numbers in the boxes indicate exons and numbers outside the boxes indicates introns. Red color indicates the region containing the signal peptide. The blue color indicates the dipeptidyl-peptidase 1 exclusion domain chain containing region. The white color indicates the propeptide chain region. The green color indicates the dipeptidyl-peptidase 1 heavy chain containing region. The yellow color indicates the dipeptidyl-peptidase 1 light chain containing region).
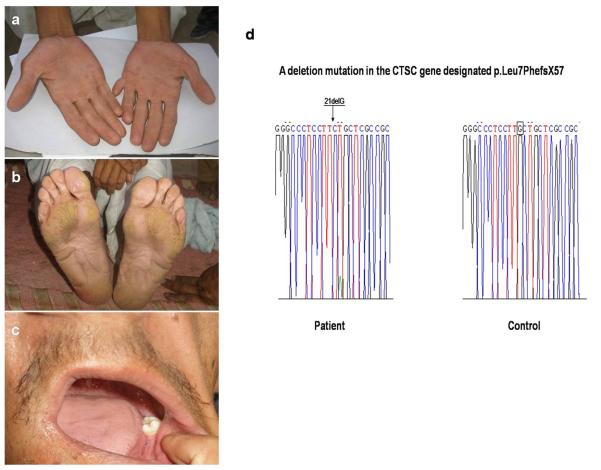
A 35 year old Pakistani individual, son of consanguineous parents, presented to us with several years history of skin thickening involving the palms and soles. He also reported having had two episodes of severe gingivitis, the first at 4 years of age and the second at 14 years with subsequent loss of teeth. Patient reported having a brother and a sister with the same features. On physical examination, we observed mild palmoplantar keratoderma affecting mainly the pressure areas (Fig.2a,b). No psoriasiform lesions were seen over the elbows or the knees. Postaxial polydactyly in the left hand was noted. Oral examination revealed loss of most of his teeth (Fig.2c). The patient was otherwise healthy. We collected peripheral blood sample from patient in EDTA-containing tubes (under institutional approval and in adherence to the Declaration of Helsinki Principles). Genomic DNA was isolated from the sample according to standard techniques. All exons of the CTSC gene with adjacent sequences of exon-intron borders were amplified by PCR with primers and conditions described previously.2 The amplified PCR products were directly sequenced in an ABI Prism 310 Automated Sequencer, using the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems). We detected a homozygous deletion mutation in exon 1, designated c.21delG, leading to a frameshift and premature termination codon (PTC) (p.Leu7PhefsX57) (Fig.2d). Screening assays with the restriction enzyme MwoI in 100 control individuals from the same population validated the mutation (data not shown).
Figure2.
a,b) Palmoplantar keratoderma affecting mainly the pressure regions in a man with PLS. c) Prominent tooth loss secondary to gingivitis occurring at 14 years of age. d) A deletion mutation designated c.21delG (p.Leu7PhefsX57) in the CTSC gene in the affected individual. The deleted nucleotide is boxed.
PLS usually manifests in the first two to three years of life. Clinically, it presents with palmoplantar hyperkeratosis and transgrediens spread with the soles being more extensively involved.1 Psoriasiform lesions may also develop over the elbows, knees, and knuckles.2 This is followed later on by periodontitis and gingivitis with subsequent loss of primary and permanent teeth.1 Patients typically report two episodes of gingivitis, the first one being at 3 years of age leading to primary teeth loss at the age of 5 years.5 This is followed by a period of remission until the age of 15 years, when a second episode of ginigivitis occur, leading to the loss of the permanent teeth.6 Although patients with PLS are immunocompetent, there is one report of a patient developing an internal fatal abscess,7 but this could be unrelated to his primary condition. It is worth noting that Japanese patients might be at an increased risk of developing melanomas at the sites of keratoderma.8
Here we detected a novel deletion mutation, c.21delG (p.Leu7PhefsX57), in exon 1 of the CTSC gene. To date, no deletion mutations have been reported in exon1, and no deletion mutations have been found in the Pakistani population. Mutations in exon1 including nonsense mutations9, 10 did not show more severe phenotypes than other mutations occurring in different exons. This suggests that there is no genotype-phenotype correlation for mutations in CTSC gene.11 The mutation c.21delG occured in the N-terminal signaling peptide sequences and is predicted to cause a frameshift and a PTC (p.Leu7PhefsX57). This mutation is expected to lead to the degradation of the mRNA through nonsense mediated mRNA decay or less likely to generate a truncated protein that is nonfunctional. Clinically, our patient had mild palmoplantar keratoderma with teeth loss. The mild palmoplantar involvement and the lack of the psoriasiform lesions over the extensor areas, suggest that the disease is heterogenous in the skin. Several factors may contribute to the disease modification including environmental factors and also genetic factors. There is a variant of late onset PLS that lacks the CTSC mutation12, suggesting that other genes might have similar functions to the CTSC gene in the skin and therefore compensating for its absence. This might explain for instance, why our patient despite having a null mutation in the CTSC gene still shows a mild cutaneous phenotype. The common denominators among the patients with PLS are gingivitis and tooth loss, therefore we suggest investigating patients presenting with only severe gingivitis especially in the setting of a positive family history of gingivitis for CTSC gene mutations as the possibility of having only gingival symptoms without cutaneous manifestations may be likely.
In conclusion, we have detected a novel deletion mutation the CTSC gene, extending the spectrum of mutations in the CTSC gene.
Acknowledgments
We gratefully acknowledge the families for having participated in this study. We also thank Helen Lam for expert technical assistance. This study was supported in part by a research career development award from the Dermatology Foundation (YS) and by USPHS NIH grant from NIH/NIAMS RO1 AR44924 (to A.M.C.)
Footnotes
Conflict of interest:None.
References
- 1.Canger EM, Celenk P, Devrim I, Yenisey M, Gunhan O. Intraoral findings of Papillon-LeFevre syndrome. J Dent Child. 2008;75(1):99–103. [PubMed] [Google Scholar]
- 2.Toomes C, James J, Wood AJ, et al. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet. 1999;23(4):421–424. doi: 10.1038/70525. [DOI] [PubMed] [Google Scholar]
- 3.Fukai K, Oh J, Karim MA, et al. Homozygosity mapping of the gene for Chediak-Higashi syndrome to chromosome 1q42-q44 in a segment of conserved synteny that includes the mouse beige locus (bg) Am J Hum Genet. 1996;59(3):620–624. [PMC free article] [PubMed] [Google Scholar]
- 4.Karim MA, Nagle DL, Kandil HH, et al. Mutations in the Chediak-Higashi syndrome gene (CHS1) indicate requirement for the complete 3801 amino acid CHS protein. Hum Mol Genet. 1997;6(7):1087–1089. doi: 10.1093/hmg/6.7.1087. [DOI] [PubMed] [Google Scholar]
- 5.Lundgren T, Renvert S. Periodontal treatment of patients with Papillon-Lefévre syndrome: a 3-year follow-up. J Clin Periodontol. 2004;31(11):933–938. doi: 10.1111/j.1600-051X.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 6.Fardal O, Drangsholt E, Olsen I. Palmar plantar keratosis and unusual periodontal findings: Observations from a family of 4 members. J Clin Periodontol. 1998;25(2):181–184. doi: 10.1111/j.1600-051x.1998.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 7.Pham CT, Ivanovich JL, Raptis SZ, Zehnbauer B, Ley TJ. Papillon-Lefévre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immunol. 2004;173(12):7277–7281. doi: 10.4049/jimmunol.173.12.7277. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima K, Nakano H, Takiyoshi N, et al. Papillon-Lefévre syndrome and malignant melanoma: A high incidence of melanoma development in Japanese palmoplantar keratoderma patients. Dermatology. 2008;217(1):58–62. doi: 10.1159/000124340. [DOI] [PubMed] [Google Scholar]
- 9.Lefévre C, Blanchet-Bardon C, Jobard F, et al. Novel point mutations, deletions, and polymorphisms in the cathepsin C gene in nine families from Europe and North Africa with Papillon-Lefévre syndrome. J Invest Dermatol. 2001;117(6):1657–1661. doi: 10.1046/j.0022-202x.2001.01595.x. [DOI] [PubMed] [Google Scholar]
- 10.Selvaraju V, Markandaya M, Prasad PV, et al. Mutation analysis of the cathepsin C gene in Indian families with Papillon-Lefévre syndrome. BMC Med Genet. 2003;4:5. doi: 10.1186/1471-2350-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noack B, Görgens H, Hempel U, et al. Cathepsin C gene variants in aggressive periodontitis. J Dent Res. 2008;87(10):958–63. doi: 10.1177/154405910808701017. [DOI] [PubMed] [Google Scholar]
- 12.Pilger U, Hennies HC, Truschnegg A, Aberer E. Late-onset Papillon-Lefévre syndrome without alteration of the cathepsin C gene. J Am Acad Dermatol. 2003;49(5 Suppl):S240–243. doi: 10.1016/s0190-9622(03)01558-5. [DOI] [PubMed] [Google Scholar]