Abstract
Objective
To investigate the biological significance of Smad3 in the progression of osteoarthritis (OA), the crosstalk between Smad3 and ATF-2 in the TGF-β signaling pathway, and the effects of ATF-2 overexpression and p38 activation in chondrocyte differentiation.
Methods
Joint disease in Smad3 knockout (Smad3−/−) mice was examined by micro-CT and histology. Numerous in vitro methods including immunostaining, real-time PCR, Western blotting, an ATF-2 DNA-binding assay and a p38 kinase activity assay were used to study the various signaling responses and protein interactions underlying the altered chondrocyte phenotype in Smad3−/− mice.
Results
Smad3−/− mice gradually developed an end-stage OA phenotype. TGF-β-induced TAK1-ATF-2 signaling was disrupted in Smad3−/− chondrocytes at the level of p38 MAP kinase activation resulting in reduced ATF-2 phosphorylation and transcriptional activity. Re-introduction of Smad3 into the Smad3−/− cells restored the normal p38 response to TGF-β. Phospho-p38 formed a complex with Smad3 by binding to the Smad3 MH1-linker domains. Additionally, Smad3 inhibited the dephosphorylation of p38 by MAP kinase phosphatase-1 (MKP-1). Both ATF-2 overexpression and p38 activation repressed type X collagen expression in wild type and Smad3−/− chondrocytes. p38 was detected in articular cartilage and perichondrium; articular and sternal chondrocytes expressed p38 isoforms α, β and γ, but not δ.
Conclusions
Smad3 is involved in both the onset and progression of OA. Loss of Smad3 abrogates TAK1-ATF-2 signaling, most likely by disrupting the Smad3-phospho-p38 complex and, thereby, promoting p38 dephosphorylation and inactivation by MKP-1. p38 and ATF-2 activation inhibit chondrocyte hypertrophy. Modulation of p38 isoform activity may provide a new therapeutic approach for OA.
Osteoarthritis (OA) is a chronic joint disease characterized by progressive degenerative changes in the composition, structure and function of articular tissues. OA primarily affects articular cartilage although pathologic changes are also found in the synovial membrane and subchondral bone [1, 2]. Chondrocytes in normal articular cartilage have a stable phenotype without apparent mitotic activity. A slow turnover of extracellular matrix helps maintain articular cartilage homeostasis. Certain stimuli, however, can induce articular chondrocytes to undergo an abnormal differentiation process similar to that in the epiphyseal growth plate, culminating in ossification of articular cartilage and reactive bone formation [3–5]. In spite of intense research, the detailed molecular mechanism underlying these processes has not been fully defined.
Transforming growth factor-β (TGF-β) superfamily members play a critical role in maintaining articular chondrocytes in a prehypertrophic stage. Bone morphogenetic protein (BMP) induces hypertrophic changes in articular chondrocytes while TGF-β counteracts this effect [6, 7]. Classical TGF-β signaling involves TGF-β binding to its cell surface type II receptor (TβRII). TβRII then recruits and activates the type I receptor (TβRI). The activated TβRI phosphorylates receptor-regulated Smads (R-Smads), Smad2 and Smad3, allowing them to form a complex with co-mediator Smad, Smad4. The Smad2/4 or Smad3/4 complexes then translocate to the nucleus where they regulate gene expression [8, 9]. The R-Smads and Smad4 contain conserved N-terminal and C-terminal MAD-homology domains designated MH1 and MH2, respectively. A variable linker region separates these two domains and is known to be the site of regulation by several protein kinases [10–13]. The linker region also houses the PY motif that is important for regulation of Smad ubiquitin-mediated proteolysis [14–16]. The MH1 domains of all R-Smads, except for that of Smad2, bind to DNA while the MH2 domains facilitate Smad oligomerization and contain the site of direct phosphorylation by the activated type I receptors. Both the MH1 and MH2 domains are involved in numerous interactions with other transcription factors. The MH2 domain interacts with several transcriptional co-activators and co-repressors as well [8, 9].
Mice expressing a dominant negative TβRII exhibit articular cartilage degeneration similar to that observed in human OA. Abnormal expression of type X collagen, an indicator of chondrocyte hypertrophy, is found in the articular cartilage of these mice [17]. Mutant mice with targeted disruption of Smad3 (Smad3−/−) show a similar pathology in articular chondrocytes including aberrant type X collagen expression in articular chondrocytes in vivo [18]. Primary chondrocytes isolated from Smad3−/− mice demonstrate an accelerated differentiation process with upregulated BMP signaling events [19]. These findings suggest that the TGF-β-Smad signaling pathway plays a pivotal role in the maintenance of normal articular cartilage and that its disruption leads to OA.
In addition to the canonical TβRII-TβRI-Smad2/3 pathway, numerous non-Smad pathways also transduce TGF-β signals [20]. Mitogen-activated protein kinase (MAPK) pathways are one example. MAPK pathways are arrayed in the prototypical triple kinase phospho-relay structure, namely, MAP kinase kinase kinase (MAP3K), MAP kinase kinase (MAP2K) and MAP kinase (MAPK). To date, three major MAP kinase pathways, ERK, JNK and p38, have been investigated in chondrocytes. While contradictory results have been reported about the effects of ERK and JNK on chondrocytes, the role of the TAK1 (MAP3K) - MKK3/6 (MAP2K) - p38 (MAPK) - ATF-2 (transcription factor) pathway appears to be more consistent with regard to chondrocyte differentiation.
TGF-β activated kinase 1 (TAK1) was initially identified as a member of the MAP3K family involved in transduction of TGF-β and BMP signals [21]. Mice with a chondrocyte-specific deletion of TAK1 are runted with severe chondrodysplasia and display defects in both chondrocyte proliferation and maturation revealing the importance of TAK1 in normal cartilage development [22, 23]. Downstream of TGF-β, activated TAK1 directly phosphorylates and activates MAP2K family members MKK3 and MKK6 [24, 25]. MKK3/6, in turn, phosphorylate and activate the p38 family of MAP kinases [26]. MAP kinase p38 was originally reported as a stress and inflammation related kinase and has four isoforms, α, β, γ, and δ. p38 is a positive regulator of mesenchymal differentiation toward chondrogenesis and yet appears to be a negative regulator of chondrocyte maturation in vivo [27]. For example, mice over-expressing a constitutively active form of MKK6 in chondrocytes are dwarfed and exhibit a delayed onset of chondrocyte hypertrophy [28]. Furthermore, transgenic mice harboring a dominant negative p38 in cartilage develop an OA-like lesion [29].
ATF-2 is a transcription factor and direct target of p38 that is rapidly activated upon treatment of cells with TGF-β [30, 31]. ATF-2 is expressed in resting and proliferating chondrocytes, but not in hypertrophic chondrocytes [32]. ATF-2 mutant mice have a defect in endochondral ossification similar to human chondrodysplasia with disorganized growth plate structure [32]. ATF-2 regulates chondrocyte proliferation through the modulation of cyclins [33] and in cooperation with Smad3 to inhibit chondrocyte hypertrophy [30]. The duration and intensity of p38 MAP kinase signaling is regulated by MAPK phosphatases (MKPs) as well as negative feedback by ATF-2 [34, 35]. Collectively, the TAK1-ATF-2 pathway may be involved in OA pathogenesis by regulating the maturation of articular chondrocytes. This pathway consists a series of kinases, making chemical modulation a feasible approach for OA treatment.
The objectives of this study were to define the role of TGF-β-Smad signaling in the progression of OA lesions; to explore the possible crosstalk between the Smad3 and ATF-2 pathways using Smad3−/− chondrocytes; and to examine the effects of TAK1-ATF-2 signaling on chondrocyte maturation.
MATERIALS AND METHODS
Mouse model of injury-induced osteoarthritis
All studies were performed according to the guidelines of the University of Rochester Committee on Animal Resources. C57BL/6 mice (10-week-old) were anesthetized with ketamine (60 mg/kg) and xylazine (4 mg/kg) via intraperitoneal injection. The medial collateral ligament (MCL) was exposed through a midline parapatellar approach and incised proximally and distally at the joint margins. The anterior half of the medial meniscus was visualized by placing an external rotation and valgus stress on the knee and removed with careful dissection. The wounds were irrigated with sterile saline and closed with 3/0 fast absorbing suture. The sham group underwent a skin incision only. Twelve weeks after surgery, the knee joints were harvested and prepared for immunostaining.
Micro-CT analysis
Following sacrifice, knee joints of 13-month-old mice, either wild type (WT, Smad3+/+; n=5) or Smad3−/− (n=5), were examined by micro-computed tomography (μCT) with a VivaCT40 scanner (ScanCo Medical, Basserdorf, Switzerland).
Isolation of primary chondrocytes
The mouse primary sternal chondrocytes were isolated as previously described [19]. Chondrocytes were treated with the following reagents: TGF-β (5 ng/ml, R&D Systems, Minneapolis, MN), BMP-2 (50 ng/ml, R&D), or anisomycin (10−5 M, Sigma, St. Louis, MO). Mouse primary articular chondrocytes were prepared from femoral heads of 10-week-old mice using serial digestion in pronase (1 hour) and collagenase A (5 hours). After filtering and centrifugation, the cell pellet was resuspended in complete DMEM. Chick articular chondrocytes were isolated from the femora and tibiae of 5-week-old Gallus domesticus chickens as previously described [36].
Cell lines, transfection and retroviral infection
C5.18 and 293 cells were cultured in α-MEM (Invitrogen, Carlsbad, CA) containing 10% FBS. Transfections were performed with Lipofectamine™ 2000 (Invitrogen). The MKP-1 expression plasmid was a gift from Dr. Chi (Yale University) and the wild type Smad3 expression plasmid from Dr. Sun (University of Rochester). Expression plasmids encoding the GST-tagged full-length Smad3 and various Smad3 domains were created via the Gateway® Technology system (Invitrogen) with pDEST™27 as the destination vector. Retroviral plasmid LPCX-F-Smad3 was a gift from Dr. Derynck (UCLA). Retroviral plasmids pLHCX-ATF-2 and pLHCX-dn-ATF-2 were gifts from Dr. Mercola (Sidney Kimmel Cancer Center). Retroviral infection was performed as previously described [19].
Quantitative real-time PCR (qPCR)
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). SuperScript First-Strand Synthesis System (Invitrogen) was used to synthesize cDNA. qPCR was performed using the SYBR green PCR master mix (Abgene, Rockford, IL) with specific primers for Col2a1 and Col10a1 as previously described[19].
Western blotting, Immunoprecipitation (IP) and in vitro assays
Western blotting was performed as previously described [19]. All primary antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA) except for total Smad3 (Zymed, San Francisco, CA) and mouse β-actin (Sigma). IP was performed using the Catch and Release® Reversible Immunoprecipitation System from Millipore (Billerica, MA). The TransAM™ATF-2 DNA-binding assay was performed according the manufacturer’s protocol (Active Motif, Inc., Carlsbad, CA). The p38 MAP kinase assay was performed with a kit from Cell Signaling Technology, Inc. following the manufacturer’s protocol. Glutathione-coated magnetic particles from the MagneGST™ Protein Purification System (Promega, Madison, WI) were used to isolate the GST-tagged Smad3 domains directly from cell lysates. Briefly, lysates and particles were incubated together at room temperature for 30 minutes with gentle rocking. Particles were washed extensively before GST-fusion proteins were eluted and subjected to Western blotting.
Immunohistochemistry
Mouse knee joint samples were fixed, decalcified, embedded and cut. Sections from 5-month-old TNFα-Tg mouse knee joints were used as a positive control for some immunostaining. Sections were deparaffinized and rehydrated. Antigen retrieval was performed in 0.01 M sodium citrate buffer in a pressure chamber followed by quenching of endogenous peroxidase activity in 3% H2O2 for 20 minutes, and blocking of nonspecific antibody binding sites in a 1:20 dilution of normal goat serum for 20 minutes. Sections were incubated overnight with a polyclonal rabbit anti-phospho-ATF-2 antibody (1:100 dilution) or an anti-phospho-p38 antibody (1:50 dilution), both from Cell Signaling Technology, Inc., then rinsed with PBS and incubated for 30 minutes at room temperature with a biotinylated goat anti-rabbit secondary antibody (1:200 dilution). After a final rinse with PBS, antigen was detected following application of HRP-conjugated streptavidin and a 5-minute detection with Romulin AEC chromogen. Slides were counterstained with hematoxylin.
Immunofluorescence labeling
Mouse primary articular chondrocytes were cultured in chamber slides for 24 hours. After washing with PBS, the cells were fixed with 4% paraformaldehyde at 4°C for 1 hour. The cells were incubated with antibodies against different p38 isoforms (diluted in PBS containing 5% BSA, 0.5% Tween-20) at 4°C for 12 hours. This was followed by incubation with a FITC-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at room temperature for 1 hour. The chamber slides were rinsed with water, air-dried, and mounted with Vectashield (Vector Laboratories, Burlingame, CA).
Statistical Analysis
Student’s t-test (two-tailed) was used to analyze the differences between groups, p values of <0.05 were considered significant.
RESULTS
Smad3−/− mice develop end-stage osteoarthritis
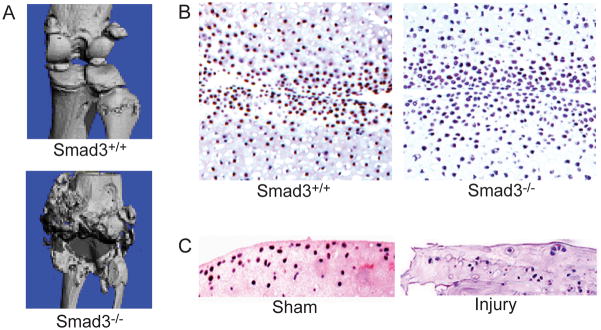
Micro-CT imaging showed that even at the age of 13 months, the knee joints of WT mice were largely intact with smooth articular surfaces and clear joint space. In contrast, Smad3−/− mice of the same age showed complete joint destruction with severe deformity similar to end-stage OA in humans (Figure 1A).
Figure 1.
Smad3−/− mice gradually develop an end-stage OA phenotype and phospho-ATF-2 immunostaining is not detected in OA-like lesions. (A) Smad3−/− mice (13-month-old) showed complete joint destruction closely mimicking the progression of human OA, while wild type (WT, Smad3+/+) mice of the same age showed intact knee joint structure as evidenced by micro-CT. (B) Immunostaining with an antibody against phospho-ATF-2 revealed some positive cells in the articular cartilage of 4-week-old WT mice, while this signal was not detectible in Smad3−/− mice. Representative images are shown at 200x original magnification. (C) Phospho-ATF-2 immunoreactivity was found in articular cartilage of the uninjured (sham) mice, while no phospho-ATF-2 was found in the articular cartilage of mice with a meniscus injury. Representative images are shown at 100x original magnification.
Loss of Smad3 and meniscal injury result in decreased expression of phospho-ATF-2 in articular cartilage
To investigate the function of the TAK1-ATF-2 pathway in Smad3−/− chondrocytes, we performed immunostaining in 4-week-old joint samples using an antibody against phospho-ATF-2. While numerous positive cells were found in articular cartilage of WT mice, phospho-ATF-2 immunoreactivity was rarely seen in the samples of Smad3−/− mice (Figure 1B). To further explore these changes in another experimental OA-like condition, we performed phospho-ATF-2 immunostaining in the joint samples from meniscal- or sham-injured mice. Phospho-ATF-2 signal was detected in the sham surgery group. In contrast, there was no phospho-ATF-2 immunoreactivity in injury-induced OA samples (Figure 1C).
The TAK1-ATF-2 signaling pathway is disrupted in Smad3−/− chondrocytes
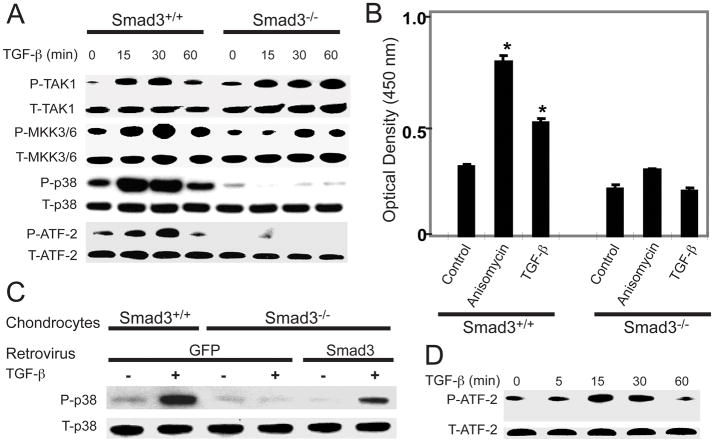
To confirm that the changes in phospho-ATF-2 expression were caused by loss of Smad3, we isolated sternal chondrocytes from WT and Smad3−/− mice and treated them with TGF-β. In WT chondrocytes, TGF-β induced a sequential phosphorylation of TAK1-MKK3/6-p38-ATF-2. The effect was seen at 15 minutes and peaked at 30 minutes. In Smad3−/− chondrocytes, TGF-β treatment resulted in the phosphorylation of TAK1 and MKK3/6, but not of p38 or ATF-2 (Figure 2A).
Figure 2.
Loss of Smad3 results in disruption of the TAK1-ATF-2 signaling pathway at the level of p38 activation and does involve Smad4. (A) WT and Smad3−/− chondrocytes were treated with TGF-β (5 ng/ml) for the indicated amounts of time. Cell lysates were examined by Western blotting for total and phosphorylated TAK1, MKK3/6, p38, and ATF-2. (B) An ELISA-based ATF-2 DNA-binding assay was performed on cell lysates from WT and Smad3−/− chondrocytes following a 60-minute treatment with media alone (control), anisomycin, or TGF-β. Results are shown as the mean ± SEM. *P<0.05 compared to Smad3+/+ control. (C) WT or Smad3−/− chondrocytes were infected with either retro-GFP or retro-Smad3 and then treated with TGF-β for 30 minutes. Immunoblots of the cell lysates were performed with antibodies to total and phospho-p38. (D) SW480 Smad4-deficient cells were treated with TGF-β for the indicated amounts of time. Cell lysates were examined by Western blotting with antibodies to total or phospho-ATF-2.
After activation, phosphorylated ATF-2 translocates into the nucleus and regulates gene expression by binding to the promoters of its target genes. To examine the possible changes in ATF-2 DNA-binding ability in Smad3−/− chondrocytes, we performed the TransAM™ ATF-2 binding assay. In WT cells, treatment with anisomycin, a potent activator of p38 MAP kinases, or TGF-β for 60 minutes significantly increased ATF-2 DNA-binding activity. In Smad3−/− chondrocytes, the basal ATF-2 DNA-binding activity was slightly lower than in WT chondrocytes and the effects of TGF-β and anisomycin were abrogated. (Figure 2B). Together, these data show that Smad3 is necessary for the activation of the p38 MAPK pathway by TGF-β in chondrocytes.
A gain-of-function study was performed by retroviral delivery of Smad3 to Smad3−/− chondrocytes with retro-GFP as a control. TGF-β induced p38 phosphorylation in WT chondrocytes infected with retro-GFP. Smad3−/− chondrocytes infected with retro-GFP did not respond to TGF-β. Re-introduction of Smad3 into Smad3−/− chondrocytes partially restored their response to TGF-β regarding p38 phosphorylation (Figure 2C).
Following TGF-β stimulation, Smad3 is phosphorylated and complexes with Smad4 before translocating to the nucleus to regulate gene expression. To determine if Smad4 also plays a role in regulation of the TAK1-ATF-2 pathway, we treated Smad4-deficient (SW480) cells with TGF-β and observed ATF-2 activation. The results showed that TGF-β induced ATF-2 phosphorylation in Smad4-deficient cells (Figure 2D), excluding the involvement of Smad4 in regulation of the TAK1-ATF-2 pathway at the level of p38 activation.
Smad3 binds to phospho-p38 and inhibits its dephosphorylation
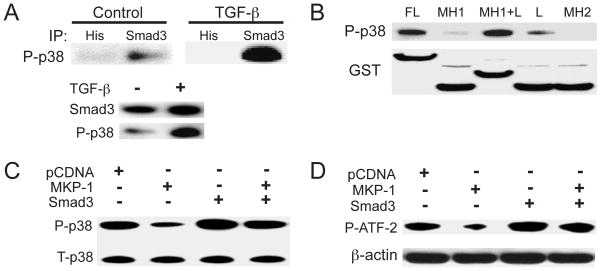
To explore the mechanism of the disruption of the TAK1-ATF-2 signaling pathway in Smad3−/− chondrocytes, we performed an immunoprecipitation assay. Lysates from C5.18 cells treated with TGF-β were precipitated with an anti-Smad3 antibody. A larger quantity of phospho-p38 was detected in the resulting immunocomplex from TGF-β treated cells compared to untreated controls, demonstrating the interaction between Smad3 and phospho-p38 in these chondrogenic cells (Figure 3A).
Figure 3.
Complex formation between Smad3 and phospho-p38 prevents p38 dephosphorylation. (A) Cell lysates from untreated or TGF-β-treated C5.18 cells were precipitated with either anti-Smad3 or anti-His antibodies (control). Immunoprecipitated complexes were immunoblotted for phospho-p38. Western blotting with antibodies against Smad3 and phospho-p38 verified the existence of both proteins in the original cell lysates. (B) GST-tagged full-length Smad3 or different Smad3 domains were transfected into 293 cells. The Smad3 proteins were purified from cell lysates with glutathione-coated magnetic particles. The eluates were subjected to immunoblotting analysis with an anti-phospho-p38 antibody. (C) C5.18 cells were transfected with MKP-1, Smad3 or the combination, in the presence of TGF-β. Cell lysates were examined by immunoblotting with antibodies to total or phospho-p38. (D) C5.18 cells were transfected as in C. Phospho- p38 was immunoprecipitated from the cell lysates and used in a kinase assay with ATF-2 as substrate. Phospho-ATF-2 was detected by immunoblotting. β–actin levels from cell lysates before IP are shown.
To identify the binding site for phospho-p38 on Smad3, we constructed GST-tagged plasmids expressing either full-length Smad3 or different Smad3 domains including MH1, MH1+linker, linker, and MH2. Strong phospho-p38 bands were found in the lysates from the cells transfected with either full-length or MH1+linker domains, while a weaker band was found in the cells transfected with the linker domain only. No phospho-p38 was immunoprecipitated in the cells transfected with MH1 and MH2 domains (Figure 3B).
We next examined the consequences of complex formation between Smad3 and phospho-p38 on MKP-1-mediated p38 dephosphorylation. C5.18 cells were transfected with MKP-1, Smad3, or both, for 24 hours. Cells were treated with TGF-β for 30 minutes before lysis for either Western blot analysis or a p38 kinase assay. MKP-1 transfection significantly decreased the level of phospho-p38, while co-transfection with Smad3 blocked the MKP-1-mediated dephosphorylation of phospho-p38 (Figure 3C). Furthermore, a p38 kinase assay showed that MKP-1 greatly reduced p38 kinase activity toward ATF-2. Over-expression of Smad3 prevented this effect (Figure 3D).
ATF-2 overexpression inhibits chondrocyte maturation and abolishes BMP-2 effects
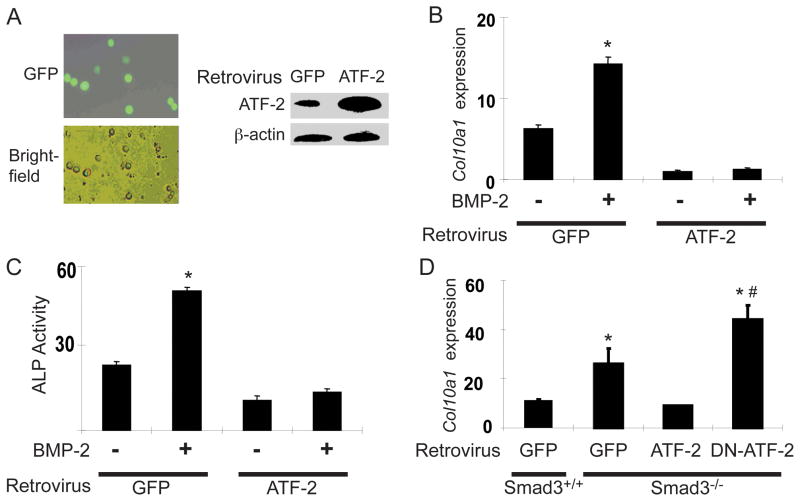
The direct effect of ATF-2 on chondrocyte maturation was tested by retroviral delivery of ATF-2 into mouse sternal chondrocytes. Retro-GFP was used as the infection control. The infection efficiency in primary chondrocytes was over 60% and ATF-2 expression was significantly increased (Figure 4A). WT chondrocytes infected with retro-GFP exhibited a normal BMP-2 response as shown by increased type X collagen mRNA (Col10a1) expression. ATF-2 infection greatly reduced the basal Col10a1 level and abolished the BMP-2 effect (Figure 4B). ATF-2 overexpression similarly inhibited alkaline phosphatase activity (Figure 4C). Smad3−/− chondrocytes showed a higher level of basal Col10a1 expression compared to WT chondrocytes. ATF-2 infection reduced Col10a1 expression in Smad3−/− chondrocytes to a level similar to that in WT chondrocytes, while infection of dn-ATF-2 further increased Col10a1 expression in Smad3−/− chondrocytes (Figure 4D). These findings show that aberrant chondrocyte maturation caused by loss of Smad3 can be rescued by ATF-2.
Figure 4.
Retroviral delivery of ATF-2 inhibits chondrocyte maturation. (A) Retroviral delivery of GFP (retro-GFP) revealed an infection efficiency of over 60%. Retro-ATF-2 infection of mouse primary sternal chondrocytes was confirmed by Western blotting, which showed a significant increase in ATF-2 protein levels. (B) Expression of Col10a1 in WT or Smad3−/− chondrocytes infected with either retro-GFP or retro-ATF-2 with or without BMP-2 (50 ng/ml) stimulation was measured by real-time qPCR. (C). An alkaline phosphatase (ALP) activity assay was carried out with extracts from the same treatment groups as in (B). (D). Primary mouse sternal chondrocytes from WT or Smad3−/− mice were infected with retro-GFP, retro-ATF-2, or retro-dn-ATF-2, and then assayed for Col10a1 expression by qPCR.
Anisomycin represses chondrocyte maturation
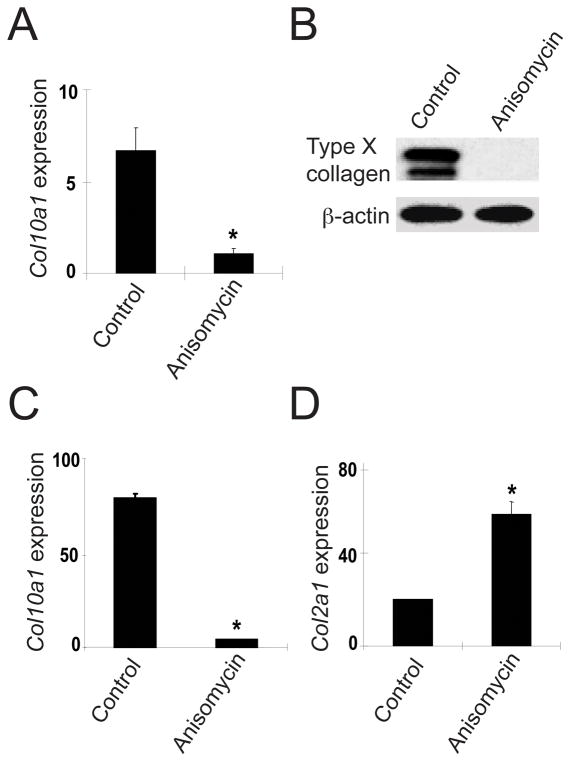
The effect of anisomycin on chondrocyte differentiation was tested in primary murine sternal chondrocytes and chicken articular chondrocytes. Anisomycin treatment significantly decreased Col10a1 mRNA expression in mouse sternal chondrocytes and significantly repressed type X collagen protein expression in chicken articular chondrocytes (Figure 5A, 5B). In Smad3−/− mouse sternal chondrocytes, the effect of anisomycin was even more dramatic with regard to Col10a1 expression (Figure 5C). In contrast, anisomycin treatment increased the expression of Col2a1 mRNA in WT mouse sternal chondrocytes (Figure 5D). These findings suggest that anisomycin, similar to ATF-2, prevents abnormal maturation of articular chondrocytes.
Figure 5.
p38 activation prevents chondrocyte maturation. (A) WT mouse sternal chondrocytes were treated with anisomycin and analyzed for expression of Col10a1 mRNA by qPCR. (B) Cell lysates from chicken articular chondrocytes treated with anisomycin were examined by Western blotting for Type X collagen protein levels. (C) Smad3−/− mouse sternal chondrocytes were treated with anisomycin and analyzed for expression of Col10a1 mRNA by qPCR. (D) WT mouse sternal chondrocytes were treated with anisomycin and analyzed for expression of Col2a1 mRNA by qPCR. The results are shown as mean ± SEM. *P<0.05 compared to untreated control.
p38 is expressed in the articular surface/perichondrium and multiple p38 isoforms are expressed in articular chondrocytes
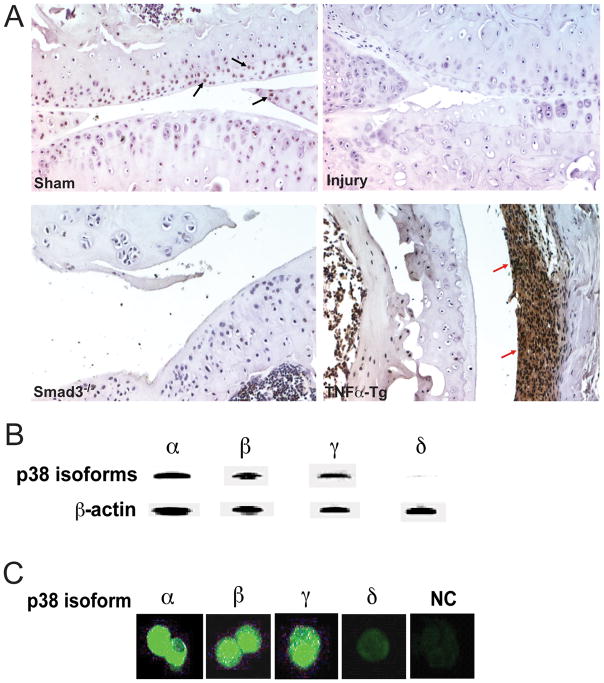
For p38 activation to be a reasonable therapeutic goal for OA, we first needed to demonstrate that p38 is expressed in the joint and that this kinase is dysregulated in arthritic phenotypes. To determine the expression of p38 in the joint, we performed immunohistochemical staining of joint tissue obtained from sham or meniscal-injured WT mice, Smad3−/− mice, or TNFα-Tg mice (as a positive control for a rheumatoid arthritis (RA) phenotype). An antibody against phospho-p38 revealed that activated p38 was located in articular cartilage of healthy wild type mice but not in arthritic articular surfaces (Figure 6A). Interestingly, the TNFα-Tg mice showed intense p38 staining in the inflamed synovium, which was not observed in our genetic or injury-induced models of OA. Correlation of these differences in expression pattern with specific p38 isoform immunostaining was not possible due to lack of specific antibodies. However, Western blotting with cell lysates from sternal chondrocytes of neonatal mice or immunofluorescence staining of articular chondrocytes with p38 isoform-specific antibodies showed that p38 isoforms α, β, and γ were expressed, while the expression of isoform δ was hardly detectible (Figure 6B and 6C).
Figure 6.
p38 isoforms are differentially expressed in arthritic cartilage and other knee joint tissues. (A). Immunohistochemical staining for phospho-p38 in articular cartilage and synovium sections from WT sham or meniscal-injured mice, Smad3−/− mice, or TNFα-Tg mice. Representative images are shown at 200x original magnification. (B) Western blotting analysis of the cell lysates from mouse primary sternal chondrocytes with different p38 isoform-specific antibodies. (C) Immunofluorescence labeling of articular chondrocytes isolated from 10-week-old WT mice using antibodies against different p38 isoforms, NC: negative control.
DISCUSSION
Abnormal hypertrophy with subsequent ossification of articular chondrocytes plays a pivotal role in OA pathogenesis. Therefore, prevention of such changes is a rational strategy for the development of novel OA therapeutics. Studies using different genetic mouse models demonstrate that intact TGF-β signaling is essential for the maintenance of healthy articular cartilage [17–19].
Treatment of OA by intra-articular injection of TGF-β has produced contradictory results, suggesting the involvement of several different downstream effectors [3, 37, 38]. In addition to the classical TGF-β-Smad pathway, the TAK1-MKK3/6-p38-ATF-2 signaling pathway also mediates the effect of TGF-β on chondrocytes. Notably, constitutive activation of MKK6 in cartilage inhibits chondrocyte hypertrophy [28]. Consistent with this finding, the genetic inhibition of p38 activity in cartilage leads to the development of severe OA [29]. In addition, ATF-2 deficient mice show a dysregulated differentiation process in the epiphyseal growth plate leading to a phenotype similar to human chondrodysplasia [32]. Previous studies suggest that the target genes of ATF-2 include chondrocyte/osteoblast-related genes such as fibronectin, osteopontin, osteocalcin, and alkaline phosphatase; as well as cell cycle related genes such as cyclin D1, cyclinA, cFos and pRb [32, 33, 39, 40]. Our present study shows that retroviral delivery of ATF-2 to mouse primary chondrocytes inhibits their terminal differentiation and blocks BMP-2-mediated effects. In addition, ATF-2 over-expression represses abnormal hypertrophic changes in Smad3−/− chondrocytes, while dn-ATF-2 has the opposite effect. Collectively, TAK1-ATF-2 signaling inhibits chondrocyte maturation and disturbance of this pathway may, therefore, play a role in the pathogenesis of OA.
We have previously reported that ATF-2 works synergistically with Smad3 to inhibit hypertrophic changes in chicken chondrocytes [30]. The present study provides further insight into the molecular basis for the crosstalk between the Smad3 and ATF-2 pathways. Taking advantage of the Smad3 mutant mouse model, we demonstrate here that TAK1-ATF-2 signaling is disrupted in Smad3−/− chondrocytes at the level of p38 activation. Consequently, the DNA-binding ability of ATF-2 is decreased in Smad3−/− cells. Reintroducing Smad3 into Smad3−/− chondrocytes restores their response to TGF-β with regard to p38 phosphorylation. The results indicate that Smad3 is indispensable for TGF-β signal transduction along the TAK1-ATF-2 pathway.
In the classical TGF-β signaling pathway phosphorylated Smad3 forms a complex with Smad4 and this complex then translocates to the nucleus to regulate gene expression. The present study shows that Smad4-deficient cells can still activate p38 in response to TGF-β. These experiments imply that the disruption of p38 activation in Smad3-deficient cells involves a cytoplasmic mechanism. These findings are somewhat surprising because the classic role of Smad3 is that of transcriptional regulation in the nucleus. However, based on our observations, we hyposthesize that Smad3 maintains p38 in its phosphorylated state. Indeed, our immunoprecipitation experiments reveal that Smad3 forms a complex with phospho-p38. Whether TGF-β-inducible phosphorylation of Smad3 is also involved in this interaction remains to be determined. Transfection with either full-length Smad3 or different Smad3 domains indicates that the phospho-p38 binding site in the Smad3 molecule is located between the MH1 and linker domains. Thus, Smad3 has genomic and non-genomic functions that are necessary for the maintenance of healthy articular cartilage.
The magnitude and duration of MAP kinase activity is regulated by MKPs. MKPs dephosphorylate and inactivate MAP kinases through a complex negative regulatory network [41]. TGF-β-induced p38 activation is associated with a concomitant induction of MKP-1 [42]. Our results show that in the presence of TGF-β, MKP-1 transfection significantly reduces the level of phosphorylated p38. Co-transfection of Smad3 with MKP-1 partially blocks MKP-1 mediated dephosphorylation of phospho-p38. The p38 kinase assay further confirms that upon over-expression, Smad3 prevents MKP-1-induced p38 dephosphorylation and thus sustains the activation of p38. We, therefore, conclude that the physical interaction of Smad3 with phospho-p38 prevents its dephosphorylation by MKP-1 and that this mechanism accounts for the disruption of the TAK1-ATF-2 signaling pathway in Smad3-deficient chondrocytes.
While Smad3 and ATF-2 are likely important in the pathogenesis of OA, transcription factors are poor drug targets. Modulation of the upstream regulatory components, however, may be a more practical approach for the development of novel OA therapies. Downregulation of Col10a1 expression and upregulation of Col2a1 expression in chondrocytes was achieved by treating cells with anisomycin, an activator of p38 kinase activity. Additionally, we showed that anisomycin abrogates the enhanced Col10a1 expression observed in Smad3−/− chondrocytes. These effects closely mimic the effect of ATF-2 in chondrocyte differentiation, suggesting a possible application in OA. Our immunohistochemical analysis also revealed that p38 is expressed in articular cartilage and perichondrium (data not shown), strengthening the rationale for use of p38 activators to treat OA.
Matrix metalloproteinases (MMPs) may be involved in the pathogenesis of OA and their regulation by p38 kinase in chondrocytes should be considered. MMP-13, for example, metabolizes type II collagen and is upregulated in osteoarthritic articular chondrocytes [43]. Mice deficient in MMP-13 exhibit decreased cartilage erosion in a surgically induced model of OA while mice over-expressing MMP-13 in chondrocytes develop an osteoarthritic phenotype [44, 45]. Several signaling pathways, including TGF-β, are shown to induce MMP13 expression dependent upon p38 activation. In human gingival fibroblasts, however, activation of p38 alone was not sufficient to induce MMP-13 expression [46]. Our previous studies show no change in MMP13 mRNA levels in chondrocytes from Smad3−/− mice when compared to WT mice even though Smad3-deficiency leads to an osteoarthritic phenotype and, as shown here, a decrease in p38-ATF-2 signaling [19]. Additionally, treatment of mouse limb bud chondrocytes with a p38 inhibitor produced only a modest decrease in levels of MMP13 mRNA [47]. Collectively, these data suggest that p38 signaling alone may not largely affect MMP13 expression in chondrocytes. Nevertheless, this will need to be confirmed in future experiments should an activator of p38 be considered for use in treatment of OA.
It is known that p38 activation can incite an inflammatory response such that p38 inhibitors are currently studied for their potential use in the treatment of RA [48, 49]. Compared to RA, OA shows more severe articular cartilage destruction but much milder synovial inflammation. With regard to the clinical application of a p38 activator for OA treatment, the potential complication of local inflammation must be overcome. In this respect, the avascular and aneural hyaline cartilage structure may respond differently than the cells residing in the well-vascularized and well-innervated synovial membrane. However, to completely eliminate the potential adverse effects of synovial inflammation, therapeutics must target a specific p38 isoform that is able to prevent abnormal maturation/ossification of articular chondrocytes without inciting joint inflammation if delivered intra-articularly. Therefore, four currently known p38 isoforms, namely, α(MAPK14), β(MAPK11), γ(MAPK12), and δ(MAPK13), were studied in chondrocytes. We examined the expression of p38 isoforms in mouse sternal chondrocytes by immunoblotting and detected isoforms α, β, and γ, but not δ, in these cells. Immunofluorescence labeling revealed a similar expression pattern in mouse articular chondrocytes. Future work will focus on the viral delivery of different p38 isoforms intra-articularly and observation of changes in articular cartilage and the synovial membrane to test their chondroprotective vs. inflammatory effects on cartilage and synovium, respectively. Once the most suitable isoform is identified, isoform-specific activators can be developed as potential new OA therapeutics.
In summary, the present findings establish that Smad3 is a critical molecule in the TGF-β pathway that is involved in both the initiation and progression of OA. Loss of Smad3 not only results in reduced TGF-β-Smad3 signaling but also leads to the disruption of TAK1-ATF-2 signaling. Our results demonstrate that Smad3 forms a complex with phosho-p38 preventing its dephosphorylation by MKP-1. Both loss of Smad3 and meniscal injury result in a significant repression of phospho-ATF-2 expression in articular cartilage. ATF-2 and its upstream activating kinase, p38 maintain cartilage homeostasis by inhibiting abnormal maturation of articular chondrocytes. Cartilage-specific activators of p38 isoforms may in the future provide a new avenue for the development of chondroprotective drugs for OA.
Acknowledgments
Supported by the grants from the National Institute of Health (NIH), Sigrid Juselius Foundation, and Evo-grants and MATERA BioNanoCoRe
The authors thank Erica Dussman and Ryan Tierney for their technical assistance.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. O’Keefe had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. O’Keefe, Schwarz, Li, Chen
Acquisition of Data. Li, Gao, Sampson, Sheu, Zuscik
Analysis and interpretation of data. O’Keefe, Li, Gao, Sheu, Schwarz
Manuscript preparation. Li, O’Keefe, Konttinen, Sampson, Flick, Jonason
References
- 1.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 2.Hardingham T. Extracellular matrix and pathogenic mechanisms in osteoarthritis. Curr Rheumatol Rep. 2008;10(1):30–6. doi: 10.1007/s11926-008-0006-9. [DOI] [PubMed] [Google Scholar]
- 3.Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15(6):597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Drissi H, et al. Transcriptional regulation of chondrocyte maturation: potential involvement of transcription factors in OA pathogenesis. Mol Aspects Med. 2005;26(3):169–79. doi: 10.1016/j.mam.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto M, et al. Molecular network of cartilage homeostasis and osteoarthritis. Med Res Rev. 2008;28(3):464–81. doi: 10.1002/med.20113. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, et al. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci U S A. 2005;102(50):18023–7. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li TF, O’Keefe RJ, Chen D. TGF-beta signaling in chondrocytes. Front Biosci. 2005;10:681–8. doi: 10.2741/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 9.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 10.Grimm OH, Gurdon JB. Nuclear exclusion of Smad2 is a mechanism leading to loss of competence. Nat Cell Biol. 2002;4(7):519–22. doi: 10.1038/ncb812. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura I, et al. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430(6996):226–31. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 12.Alarcon C, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139(4):757–69. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, et al. Transforming growth factor-{beta}-inducible phosphorylation of Smad3. J Biol Chem. 2009;284(15):9663–73. doi: 10.1074/jbc.M809281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, et al. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400(6745):687–93. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 15.Lin X, Liang M, Feng XH. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. J Biol Chem. 2000;275(47):36818–22. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell. 2009;36(3):457–68. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra R, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139(2):541–52. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, et al. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li TF, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21(1):4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118(Pt 16):3573–84. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi K, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270(5244):2008–11. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 22.Shim JH, et al. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J. 2009;28(14):2028–41. doi: 10.1038/emboj.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunnell LM, et al. TAK1 regulates cartilage and joint Development via the MAPK and BMP signaling pathways. J Bone Miner Res. 2010 doi: 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanafusa H, et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274(38):27161–7. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi T, et al. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271(23):13675–9. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 26.Raingeaud J, et al. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16(3):1247–55. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanton LA, Underhill TM, Beier F. MAP kinases in chondrocyte differentiation. Dev Biol. 2003;263(2):165–75. doi: 10.1016/s0012-1606(03)00321-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, et al. Constitutive activation of MKK6 in chondrocytes of transgenic mice inhibits proliferation and delays endochondral bone formation. Proc Natl Acad Sci U S A. 2006;103(2):365–70. doi: 10.1073/pnas.0507979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namdari S, et al. Reduced limb length and worsened osteoarthritis in adult mice after genetic inhibition of p38 MAP kinase activity in cartilage. Arthritis Rheum. 2008;58(11):3520–9. doi: 10.1002/art.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ionescu AM, et al. ATF-2 cooperates with Smad3 to mediate TGF-beta effects on chondrocyte maturation. Exp Cell Res. 2003;288(1):198–207. doi: 10.1016/s0014-4827(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 31.Sano Y, et al. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem. 1999;274(13):8949–57. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- 32.Reimold AM, et al. Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature. 1996;379(6562):262–5. doi: 10.1038/379262a0. [DOI] [PubMed] [Google Scholar]
- 33.Beier F, et al. Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes. Proc Natl Acad Sci U S A. 1999;96(4):1433–8. doi: 10.1073/pnas.96.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breitwieser W, et al. Feedback regulation of p38 activity via ATF2 is essential for survival of embryonic liver cells. Genes Dev. 2007;21(16):2069–82. doi: 10.1101/gad.430207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119(Pt 22):4607–15. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 36.Zhu M, et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58(7):2053–64. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimaud E, Heymann D, Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002;13(3):241–57. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 38.van Beuningen HM, et al. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 2000;8(1):25–33. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- 39.Beier F, Taylor AC, LuValle P. Activating transcription factor 2 is necessary for maximal activity and serum induction of the cyclin A promoter in chondrocytes. J Biol Chem. 2000;275(17):12948–53. doi: 10.1074/jbc.275.17.12948. [DOI] [PubMed] [Google Scholar]
- 40.Vale-Cruz DS, et al. Activating transcription factor-2 affects skeletal growth by modulating pRb gene expression. Mech Dev. 2008;125(9–10):843–56. doi: 10.1016/j.mod.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–13. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 42.Tong XK, Hamel E. Transforming growth factor-beta 1 impairs endothelin-1-mediated contraction of brain vessels by inducing mitogen-activated protein (MAP) kinase phosphatase-1 and inhibiting p38 MAP kinase. Mol Pharmacol. 2007;72(6):1476–83. doi: 10.1124/mol.107.039602. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell PG, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–8. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Little CB, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–33. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuhold LA, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leivonen SK, et al. Smad3 mediates transforming growth factor-beta-induced collagenase-3 (matrix metalloproteinase-13) expression in human gingival fibroblasts. Evidence for cross-talk between Smad3 and p38 signaling pathways. J Biol Chem. 2002;277(48):46338–46. doi: 10.1074/jbc.M206535200. [DOI] [PubMed] [Google Scholar]
- 47.Stanton LA, et al. p38 MAP kinase signalling is required for hypertrophic chondrocyte differentiation. Biochem J. 2004;378(Pt 1):53–62. doi: 10.1042/BJ20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korb A, et al. Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2745–56. doi: 10.1002/art.22080. [DOI] [PubMed] [Google Scholar]
- 49.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67(7):909–16. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]