Abstract
The contrast sensitivity function (CSF) is routinely assessed in clinical evaluation of vision and is the primary limiting factor in how well one sees. CSF improvements are typically brought about by correction of the optics of the eye with eyeglasses, contact lenses or surgery. We found that the very act of action video game playing also enhanced contrast sensitivity, providing a complementary route to eyesight improvement.
Contrast sensitivity, the ability to detect small increments in shades of gray on a uniform background, is one of the main limiting factors in a wide variety of visual tasks1. Unfortunately, it is one of the aspects of vision that is most easily compromised. This problem affects thousands of people worldwide, including those with professional activities requiring excellent eyesight, aging populations2 and individuals who are clinically evaluated for vision problems such as amblyopia3. Although deterioration of the optical quality of the eye can decrease contrast sensitivity3, optical changes alone cannot account for the diverse array of situations in which the CSF is compromised. Instead, neural factors also appear to be at work. It may therefore be possible to develop interventions that enhance the CSF through neural plasticity. Such an intervention would be of great clinical benefit as a complement to the standard clinical approaches, which are mainly directed at enhancing the optical quality of the eye.
Sizeable performance improvements, brought about through brain plasticity, have been documented in various aspects of vision after training4. Yet identification of a training regimen that can improve the CSF, or contrast detection, has remained elusive. Training-induced improvements have been reported for contrast discrimination5 but not for contrast detection6,7. In addition, the documented improvements are typically restricted to the trained stimulus, limiting their practical and theoretical value. The CSF per se has proven difficult to improve. There are some indications that radiologists may exhibit enhanced contrast sensitivity8, but the causal effect of experience remains to be established. Studies that directly address training-induced changes in contrast detection show improvements only when using experimental conditions that are known to produce poor performance, such as testing away from fixation or using diagonal orientations9,10. Yet, to be clinically relevant, CSF improvements need to be documented in foveal vision and with cardinal orientations, where human performance is at its best. To the best of our knowledge, CSF improvements under these optimal conditions have not previously been demonstrated. Here, we identified action video game playing as an efficient tool to enhance the CSF.
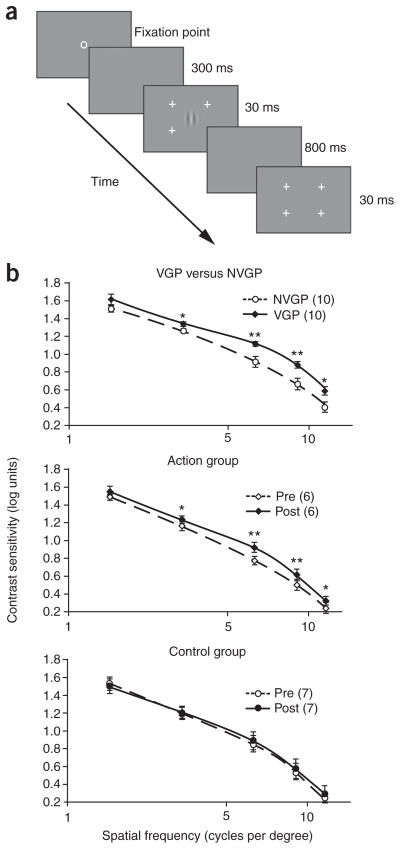
Expert action video game players (VGPs) were compared to gender- and age-matched non–action game players (NVGPs) in a CSF procedure11 (Fig. 1a and Supplementary Note 1 online). Because we were interested in the effect of gaming on everyday eyesight, participants were tested binocularly with their current eye prescription. We were interested in whether vision, which should not be far from optimal under such conditions in young adults, may be further heightened by action video game practice. The VGP group showed enhanced contrast sensitivity as compared with the NVGP group, and this population difference interacted with spatial frequency, indicating a greater group difference at intermediate and higher spatial frequencies than at the lowest spatial frequency (Fig. 1b). A similar spatial-frequency dependency has been reported in the aging and in the amblyopia literature and has been attributed to changes in cortical processing rather than to alterations of peripheral, eye-related factors. We propose that the changes that we observed after action game playing also reflect cortical plasticity, but for the better in this case.
Figure 1.
Improved CSF as a result of action video game experience. (a) The CSF was assessed at five different spatial frequencies (1.5, 3, 6, 9 and 12 cycles per degree) by using a two-interval forced-choice procedure in which subjects had to decide which of two intervals, each marked by the presence of four peripheral cross-hairs, contained a Gabor patch. Unlike in the typical clinical procedure, the size of the Gabor patch was scaled with frequency so that the space constant of the Gabor was equal to one period of the grating at all frequencies. A trial consisted of a 30-ms Gabor signal and a 30-ms blank screen, separated by an 800-ms interval. Participants were asked to indicate which 30-ms interval marked by the cross-hairs contained the Gabor signal11. Contrast of the Gabor was modulated in 0.1-log-unit steps following a 3-up–1-down staircase to find the 79% threshold. (b) Contrast sensitivity as a function of spatial frequency in VGPs (n = 10) versus NVGPs (n = 10) and in the action-trained (n = 6) and control-trained groups (n = 7) pre- and post-training. VGPs showed higher contrast sensitivity (plotted in log units) than NVGPs (group effect: F1,18 = 9.37, P = 0.007, partial eta squared (ηp2) = 0.34). This group difference was greater at intermediate and higher spatial frequencies (F4,72 = 2.48, P = 0.05, ηp2 = 0.12). In the training experiment, the action-trained group showed a significant improvement in contrast sensitivity as a result of training, whereas no such change was noted in the control-trained group (pre/post × group interaction: F1,11 = 5.65, P = 0.04, ηp2 = 0.34). Curves were obtained by smoothed interpolation between data points. Error brackets are s.e.m. * P < 0.05, ** P < 0.01.
To unambiguously establish the causal effect of action gaming, we conducted an intensive training study (50 h over 9 weeks) on a small sample of NVGPs. Training consisted of one of two conditions: each trainee played either experimental, action video games (action group played Unreal Tournament 2004 by Atari and Call of Duty 2 by Infinity Ward) or a control, non–action video game (control group played The Sims 2 by Electronic Arts). Like the experimental games, the control game was chosen to be visually complex and engaging, but it differed by having a slower pace and by not requiring precise, visually guided aiming actions (Supplementary Note 2 online). A few days before and after the training period, participants’ CSFs were assessed as described above. Action-trained participants improved significantly more than the control-trained participants (P = 0.04), establishing the causal effect of game playing and ruling out any interpretation of these results in terms of a simple test-retest improvement. In addition, the use of another video game–trained group as a control group ensured that all participants received equal contact with experimenters, limiting any possible social attention effect, such as the so-called Hawthorne effect. Finally, the use of a video game as control training also ensured that the control group, like the experimental group, was engaged in a stimulating video game–related activity. The size of the effect, albeit small in log units (0.16–0.2), represented a large percentage improvement (43–58%) and was extremely robust across the population (Supplementary Note 3 online).
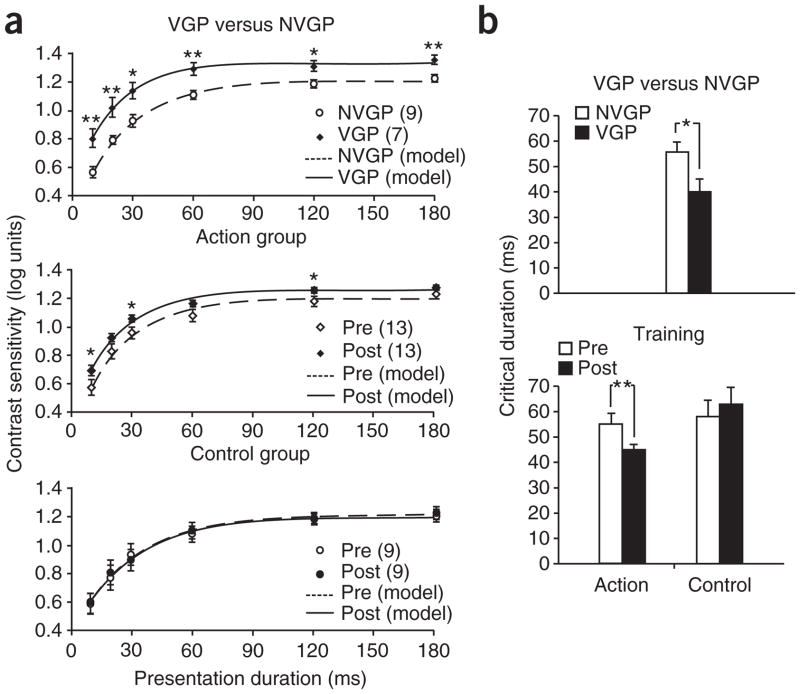
To further confirm a change in contrast sensitivity, we evaluated the integration time for contrast detection at an intermediate spatial frequency (6 cycles per degree) by measuring contrast sensitivity as stimulus duration varied. The contrast sensitivity–duration reciprocity function is well fit by an exponential saturation function representing the response of a linear low-pass filter12. Critical duration is defined as the exposure duration at which the contrast sensitivity reaches 90% of its asymptotic value and reflects the time constant for contrast sensitivity (Fig. 2a). A shorter critical duration indicates greater sensitivity, with less overall energy being necessary for detection to occur. As predicted, VGPs showed a significantly shorter critical duration than NVGPs (Fig. 2b, P = 0.02). A training study confirmed the causal effect of action game playing in the reduction in integration time (Fig. 2b and Supplementary Note 4 online).
Figure 2.
Improved critical duration as a result of action video game experience. (a) Contrast sensitivity (plotted in log units) at 6 cycles per degree as a function of display duration (10, 20, 30, 60, 120 and 180 ms) for VGPs (n = 7) versus NVGPs (n = 9), and the action-trained (n = 13) and control-trained groups (n = 9) pre- and post-training. Curves are maximum-likelihood fits of the exponential saturation function log(CS) = log(CSmax) − ae−t/τ, where t is the time in milliseconds, CSmax is the asymptote, a is the amplitude and τ is the time constant. Exponential fits to the individual data were good (VGP, r2 = 0.99; NVGP, r2 = 0.98). (b) Critical duration was shorter in VGPs than NVGPs (group effect: F1,14 = 6.60, P = 0.02, ηp2 = 0.30); in the training study, the action-trained group showed reduced critical duration post-training, whereas the control-trained group did not (pre/post × group interaction: F1,20 = 6.35, P = 0.02, ηp2 = 0.24). Error brackets are s.e.m. * P < 0.05, ** P < 0.01.
Playing action video games resulted in an enhanced CSF and a shorter integration time for contrast sensitivity. To the best of our knowledge, this is the first report to identify a training regimen that improves performance over nearly the entire CSF in adults. These improvements were induced in central vision in young, healthy adults, supposedly at the prime of their visual abilities. This is of practical importance when driving at night or under degraded conditions, as well as during activities such as reading, which are known to correlate with CSF at 6 cycles per degree13. More generally, our results establish that time spent in front of a computer screen is not necessarily detrimental to vision. The positive effect remained months and even years after training, indicating long-lasting gains (Supplementary Note 5 online). Although the mechanism of this generalized enhancement remains to be elucidated, potential candidates for such perceptual learning include sharpening, gain enhancement or template retuning through feedback and/or lateral connections14.
Our results reveal previously unsuspected plastic potentiality in the adult visual system. We found that the benefits of action video game playing are not restricted to attentionally demanding situations (Supplementary Note 6 online), in which the target has to be selected from among distractors15. Instead, improvements generalized to a visual skill as basic as detecting a single low-contrast Gabor patch. Video game training, therefore, may become a useful complement to eye-correction techniques that are routinely used in the clinic to improve eyesight. Although the underlying cortical plasticity that is induced is likely to be most beneficial for central deficits such as amblyopia, video game playing may also compensate to some extent for optical and retinal defects by retraining the visual cortex to make a better use of the information that it receives, however degraded. Notably, our data establish that not all video games induce such a benefit, calling for special care in the choice of a clinically relevant training regimen.
Supplementary Material
Acknowledgments
We thank C.S. Green for his invaluable help and advice throughout this project, and A. Anderson, S. Bailey, A. Katz, M. Maciejewski, A. States, P. Santos and B. Hubert-Wallander for their help in running the training studies. This work was supported in part by grants from the US National Institutes of Health (EY016880), the James S. McDonnell Foundation and the Office of Naval Research to D.B. and the Israel Science Foundation to U.P.
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Campbell FW. Behav Brain Res. 1983;10:87–97. doi: 10.1016/0166-4328(83)90154-7. [DOI] [PubMed] [Google Scholar]
- 2.Sekuler R, Sekuler AB. Visual perception and cognition. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, editors. Oxford Textbook of Geriatric Medicine. Oxford University Press; New York: 2000. pp. 874–880. [Google Scholar]
- 3.Weale RA. The Senescence of Human Vision. Oxford University Press; Oxford: 1992. [Google Scholar]
- 4.Fahle M, Poggio T. Perceptual Learning. MIT Press; Cambridge, Massachusetts, USA: 2002. [Google Scholar]
- 5.Yu C, Klein SA, Levi DM. J Vis. 2004;4:169–182. doi: 10.1167/4.3.4. [DOI] [PubMed] [Google Scholar]
- 6.Adini Y, Sagi D, Tsodyks M. Nature. 2002;415:790–793. doi: 10.1038/415790a. [DOI] [PubMed] [Google Scholar]
- 7.Maehara G, Goryo K. Percept Psychophys. 2007;69:1009–1021. doi: 10.3758/bf03193939. [DOI] [PubMed] [Google Scholar]
- 8.Sowden PT, Davies IR, Roling P. J Exp Psychol Hum Percept Perform. 2000;26:379–390. doi: 10.1037//0096-1523.26.1.379. [DOI] [PubMed] [Google Scholar]
- 9.Sowden PT, Rose D, Davies IR. Vision Res. 2002;42:1249–1258. doi: 10.1016/s0042-6989(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 10.Mayer MJ. Vision Res. 1983;23:547–550. doi: 10.1016/0042-6989(83)90130-x. [DOI] [PubMed] [Google Scholar]
- 11.Polat U, Sagi D. Vision Res. 1993;33:993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- 12.Fiser J, Bex PJ, Makous W. Vision Res. 2003;43:2637–2648. doi: 10.1016/s0042-6989(03)00441-3. [DOI] [PubMed] [Google Scholar]
- 13.Patching GR, Jordan TR. Invest Ophthalmol Vis Sci. 2005;46:2219–2224. doi: 10.1167/iovs.03-1247. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert CD, Sigman M, Crist RE. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 15.Achtman RL, Green CS, Bavelier D. Restor Neurol Neurosci. 2008;26:435–446. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.