Abstract
Monocyte derived tissue effector cells, macrophages, are present in large numbers in all forms of kidney disease with inflammation. Their roles in inflammation and the molecular effectors of macrophage function have been difficult to decipher. With the advent of modern genetic tools and mouse models of human disease, great insight into monocyte/macrophage biology has been forthcoming. In this review we will place macrophage study in its historical context, define immunological diseases of the kidney, and broaden its definition to encompass current thinking of the immune response to kidney injury, highlight key advances of the study of monocyte/macrophages in kidney diseases, and identify new therapeutic pathways and targets that hinge around macrophage function. Here we advance the case that targeting macrophage activation and phenotype is leading to new therapies in treatment of many acute and chronic kidney diseases.
Keywords: macrophage, activation, injury, fibrosis, kidney, subpopulations
Introduction
Monocyte/macrophages play roles in many aspects of experimental and human renal disease and are implicated in the induction of injury and fibrosis as well as renal repair. This review will examine the role of Mφ in kidneys affected by immunological inflammation. Although conditions such as autoimmune glomerulonephritis have previously been considered to represent classical immunological inflammation of the kidney, it is now apparent that disparate renal conditions such as ischemia-reperfusion injury and even fibrosis may involve key components of the innate or adaptive immune system such as lymphocytes together with humoral mediators such as complement and antibody. As a consequence, this review will adopt a relatively broad definition of immunological disease of the kidney and this will serve to underscore the key importance of monocyte/macrophage biology in these pathological processes.
Macrophages: inflammatory cells of acute and chronic disease as well as repair of the kidney
Tissue effector cells of the monocyte lineage (macrophages [Mϕs]) have been increasingly detected as a major, or the major subset of recruited ‘inflammatory’ cells in inflammatory diseases of the kidney. These discoveries were possible through development of monoclonal antibodies, in the 1970’s, against cell surface myeloid cells epitopes such as CD11b (Mac-1), a component of the integrin co-receptor that forms the complement receptor for C3, and the myeloid lysosomal membrane protein CD68. In addition, seminal studies from the Rockefeller Institute linking monocyte trafficking from bone marrow (BM) via the circulation, to the tissues where monocytes differentiate into tissue macrophages (Mϕs) also enabled our understanding of the origins and identification of tissue Mϕs 1,2 In many examples Mϕs are the major inflammatory cell in the kidney outnumbering T lymphocytes (T-cells), B lymphocvtes (B-cells), Natural Killer (NK) cells or neutrophils (PMN). Mϕs have been recorded in large numbers in both acute diseases such as post-streptococcal glomerulonephritis (GN) ANCA associated GN, or in chronic diseases such as IgA nephropathy or Systemic Lupus Erythematosis (SLE) (Figure 1A). Mϕs are found in both acute and chronic diseases of kidney transplantation. Moreover they have been detected not only in the glomerulus of all inflammatory glomerular diseases but also in the interstitium of kidney cortex and medulla 3–11. In many human biopsy studies, glomerular or interstitial Mϕs correlate numerically with poor outcomes, including disease progression, severity of presentation, likelihood of future fibrosis or tubular atrophy suggesting possible roles in these processes/outcomes12–16.
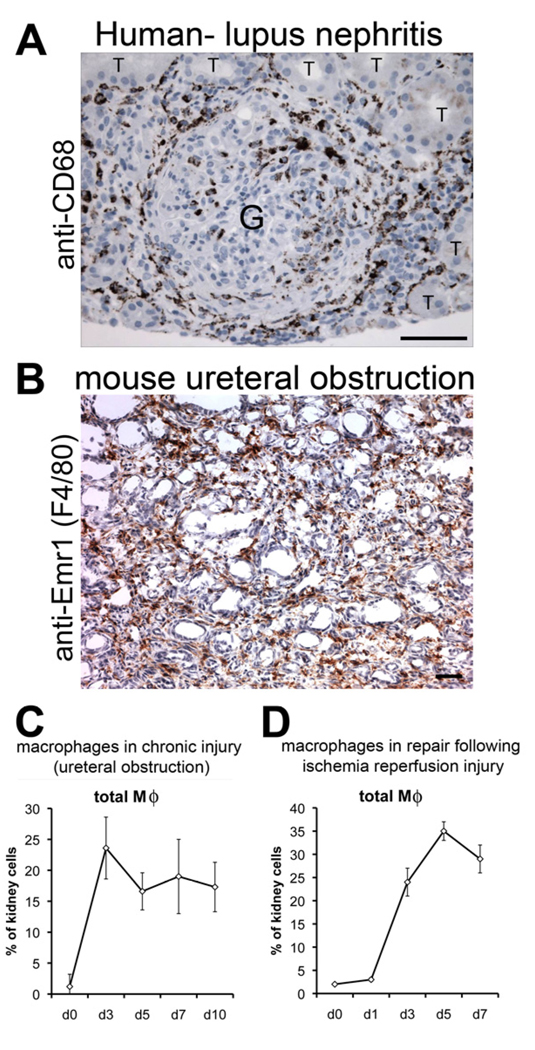
Figure 1. Macrophages are present in all forms of kidney disease with inflammation.
Photomicrographs of (A) human glomerulonephritis labeled with antibodies (brown) against the macrophage marker CD68 (T = tubule, G = glomerulus). (B) mouse model of chronic kidney disease showing macrophages (brown) labeled with the F4/80 antibody against EMR1. (C-D) Graph showing the proportion kidney cells that are macrophages in two mouse models of kidney disease. Note that macrophages are present in chronic disease (C) but also in repair after injury (D). Marker = 100µm
With the development of antibodies specific to murine monocytes and Mϕs such as the surface marker Emr1 detected by F4/80 antibody or CD68 detected by ED1, and the coincident development of models of kidney diseases in rodents in the 1980’s heralded an explosion of knowledge about the immune-response in the kidney (Figure 1B–D) 11,17–29.
The term ‘immunologically-mediated diseases’ of the kidney, refers principally to glomerulonephritides, where lymphocytes, monocytes/Mϕs, NK cells, PMN and immune-complexes are detected in the glomerulus and also kidney cortex. By implication the disease is caused by the immune system in an unspecified way. The term, coined in the 1970’s, implies something special about these diseases, but as this article hopefully uncovers, despite the unusual histological manifestations, there is little unique about the inflammation in the kidney. In addition to the immunologically-mediated diseases, Mϕs are present in large numbers in acute and chronic allo-immune diseases in kidney transplants and are recruited following all manner of kidney injuries including ischemic or toxic renal injuries that comprise acute tubular necrosis (Figure 1D). The fact that Mϕs are present not only in diseases where aberrant immune activation is noted but also in a broad range of injuries implicates Mϕs in diverse processes and outcomes in the kidney and blurs the definition of immunologically mediated disease.
Kidney macrophages show evidence of activation and correlate numerically with disease outcomes
When cultured in vitro Mϕs may be activated by a range of stimuli. Most notably bacterial cell wall proteins such as lipopolysaccharide (LPS), flagellin, and CpG microbial oligodeoxynucleotides, collectively known as pathogen associated molecular patterns (PAMPs), potently activate Mϕs by engagement of specific receptors including but not restricted to Toll-like receptors (TLRs), receptors that are collectively known as pattern recognition receptors (PRRs) 30–33. Through intracellular signaling pathways including NFκB and MAP kinase, Mϕs ‘spew out’ a broad range of pro-inflammatory cytokines including TNFα, IL1β, IL12, IL18, IL23, IL6, pro-inflammatory chemokines including MIP1, MIP2, MCP, KC, and they generate Reactive Oxygen Species (ROS) and reactive nitrogen species including nitric oxide (NO). In addition to foreign proteins, immune-complexes (ICs) (comprising immunoglobulins, antigens, complement components, pentraxins and other plasma proteins of the innate immune system), that frequently deposit in the glomerulus and bind to leukocyte receptors including activating immunoglobulin Fc receptors (FcRs) and complement receptors (CRs) also have the capacity, in certain circumstances, to activate Mϕs with broadly similar activation and pattern of cytokine release to that described for pathogens 34. Certain pathogens such as amoebe and schistosomes activate Mϕs, but the pattern of cytokine release is quite distinct with high levels of Tgfβ, IL13, and chemokines such as CCL17, CCl22 being released 35,36. The presence of cell surface ED3 antigen (CD163) in rats or Mac2 (galectin-3) in mice has been implicated as a marker of activated Mϕs in tissues, although expression of NOS2 or IL1β proteins is probably a more reliable marker of activation 37,38.
Many studies in kidney diseases have indicated that a proportion of Mϕs in injured tissues are in fact not merely passive bystander cells, but are activated in similar ways to that which is achieved in vitro. These observations hold true not only in GN with autoimmunity or GN with immune-complexes but also in other diseases where renal injury of ‘non-immunological’ causes is implicated in the pathogenesis, such as diabetic nephropathy, ischemic/vascular kidney disease, and all forms of chronic kidney disease 39–48. In all of these diseases, Mϕ number, and Mϕ activation status have been reported to correlate negatively with outcome. In the subsequent sections we will explore more broadly how in the absence of PAMPs or immunecomplexes inflammatory Mϕs may become activated and how the pattern of activation affects Mϕ function.
Despite the correlation of Mϕ number and Mϕ activation with poor outcomes in many studies of kidney diseases, and despite the capacity for Mϕs to release cytokines that can impact the kidney deleteriously (IL1β, TNFα, IL12, NOS2, ROS, IL6, CCL17, TGFβ, PDGF), Mϕs also have the capacity to generate a broad range of cytokines that might impact the kidney beneficially (VEGF, TGFβ, IL10, ANG1, HGF, FGF2, WNT7B) Furthermore, a major function of Mϕs is their capacity for phagocytosis 49. Phagocytosis in many circumstances occurs without cellular activation or without release of proinflammatory cytokines 50,51. Phagocytosis is not limited to pathogens, but Mϕs scavenge many things from aged erythrocytes (spleen and liver) dying leukocytes, cellular debris, pathological matrix, and ICs 52. In all these circumstances, phagocytic clearance can occur without cellular activation and in the context of kidney disease would be beneficial to tissue remodeling and regeneration following injury53,54. Since in health most monocytes do not become significantly activated yet clear things away (erythrocytes, PMNs, ICs, pathogens invading gut and lung) it is likely that the major normal function of monocytes/ Mϕs one of repair and homeostasis. Only in overwhelming circumstances does cellular activation occur with release of pro-inflammatory cytokines/chemokines. In this context it is very striking that in models of single kidney injury and repair such as the ischemia reperfusion model (a model of human ATN), inflammatory Mϕs are recruited during the repair phase and these Mϕs correlate numerically with repair 55 (see later). One collective interpretation of these observations is that, analogous with wound healing, the Mϕ response to severe injury is initial sterilization and debridement of the tissue, followed by repair and rebuilding of the tissue56,57. In repetitive or chronic injury states however, the Mϕ is driven to become excessively or aberrantly activated with deleterious outcomes.
Innate response to injury vs autoimmunity in macrophage activation
Since monocyte lineage tissue effector cells are present in diverse kidney diseases, there has been debate as to whether these Mϕs are tissue effectors activated and regulated by the adaptive immune response or whether they become activated as an innate response to local tissue injury 58–62. Several studies in mouse models of GN (nephrotoxic nephritis and anti-GBM disease) indicated that Mϕs are secondary effectors regulated or controlled by CD4 T cells reactive against foreign antibodies planted in the glomerulus 58. This type of monocyte activation (Figure 2) has been likened to delayed type hypersensitivity responses as seen in infections such as tuberculosis where the monocyte ‘plays foot soldier’ to the effector autoreactive T lymphocyte 63. Prevention of T lymphocyte-directed activation of monocytes is an attractive therapeutic target and it is likely that this type of T cell directed activation is by paracrine signaling via IFNγ, IL17, IL12 and other TH1 skewed lymphokines. While it is true that this mechanism of Mϕ activation likely is important in certain contexts in human glomerular diseases, it is unlikely that this is a major mechanism of Mϕ activation in the kidney, because there is relatively little evidence of cell-mediated autoimmunity (effector T cells) against the kidney itself in diseases such as ANCA associated GN, or in autoimmune diseases such as SLE. The exception to that is anti-GBM Disease and Goodpasture’s Syndrome where autoimmunity against the kidney is the root cause of the disease 64–66. Many ‘immunological diseases’ of the kidney feature IC deposition in the glomerulus. In these diseases, the glomerulus is in large part is a bystander, rather than target, of autoimmunity. Immune-complexes become trapped in the glomerulus by virtue of its highly specialized vasculature and sieving function. It is likely that the major physiological function of PMNs and mononocytes/ Mϕs in the glomerulus is safe, ‘non-phlogistic’, phagocytic clearance of formed immune-complexes from the glomerulus 67,68. This innate immune function is multifaceted in that it involves many innate immune proteins, receptors and regulated cell signaling pathways, with many systems in place to prevent myeloid leukocyte activation triggered by this ‘cleaning-up process’ including complement proteins and pentraxins 52,69. It is also clear, however, that these ‘non-phlogistic’ mechanisms of IC clearance are readily overwhelmed, as may be detected by complement consumption from plasma, with resultant inappropriate myeloid leukocyte activation, and liberation of local cytotoxic products and pro-inflammatory chemokines which contribute to local tissue injury, activation of the coagulation cascade, recruitment of further leukocytes and consequent loss of glomerular function (Figure 2) 26,58,70–72. The consequence for this is that in several models of GN the net effect of monocyte/Mϕ function is deleterious26,73,74. The activating Fcγ receptors (and FcαR in IgA nephropathy) and late complement components (including C5a) have been strongly implicated in this innate activation process 67,75–79. In addition to GN with immune-complexes, recent insights into the mechanisms of ANCA associated vasculitis (AAV) presenting as GN also suggest that local activation of neutrophils and monocytes in glomerular capillaries by ANCA ICs on the endothelial surface is an important part of the pathogenesis of glomerular injury in these diseases 80. Therefore in ANCA associated diseases, innate immune responses to aberrant IC formation also is central to the pathogenesis26,67,74 73.
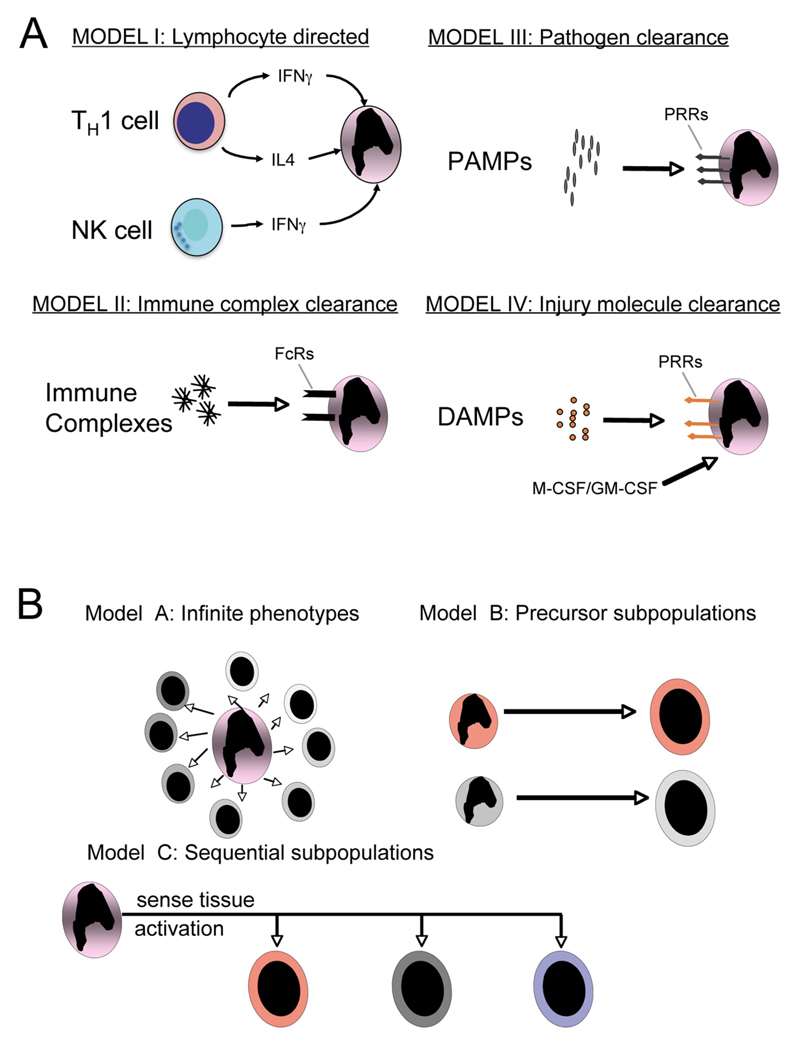
Figure 2. Schematics demonstrating likely mechanisms of.
(A) macrophage activation in vivo. In addition to pathogens (Model III), lymphocytes, immune complexes and molecules released from damaged tissue all have the capacity to activate macrophages. (B) Three models proposed to explain macrophage heterogeneity in tissue inflammation. In model A an infinite number of Mϕ phenotypes can occur, in model B there are subsets of monocytes that are preprogrammed with a stereotyped response and in model C monocytes differentiate into a restricted number of phenotypes depending on both the tissue environment and an activating stimulus.
In addition to the ‘immune mediated’ kidney diseases, many other kidney diseases feature glomerular and interstitial Mϕs. Diabetic nephropathy, chronic kidney disease of any initial etiology, acute interstitial nephritis, and acute tubular necrosis all feature marked recruitment of interstitial Mϕs (Figure 1). For some time it has been unclear what role these inflammatory cells play in renal pathology. However increasing evidence particularly from animal models indicates that these leukocytes are also activated and play active roles in renal pathology 52. While it has been easier to understand how glomerular Mϕs clearing ICs or those adjacent to activated T cells might become activated it has been less clear how interstitial or glomerular Mϕs in ‘non-immune’ kidney disease become activated. One possibility is that NK cells, recruited to sites of injury generate IFNγ which activates monocytes. Another is that chemokines released from injured parenchymal cells not only recruit Mϕs but activate them 81. However in many diseases of kidney there are very few NK cells and the data that chemokine ligation of monocyte chemokine receptors triggers activation has not been forthcoming 58. More likely is that factors released from injured cells or extracellular factors oxidized or modified during injury function as ligands for activating receptors on monocytes82. These factors, known as danger associated molecular patterns (DAMPs) have been increasingly described and bind to receptors of the innate immune response known as pattern recognition receptors such as Toll-like receptors triggering leukocyte activation with a similar pattern of activation to PAMPS and ICs (Figure 2). This group of danger molecules includes advanced glycation end products (AGE), HMGB1, S100A8, S100A9, adenosine and others 83–85.
Macrophage heterogeneity and polarization
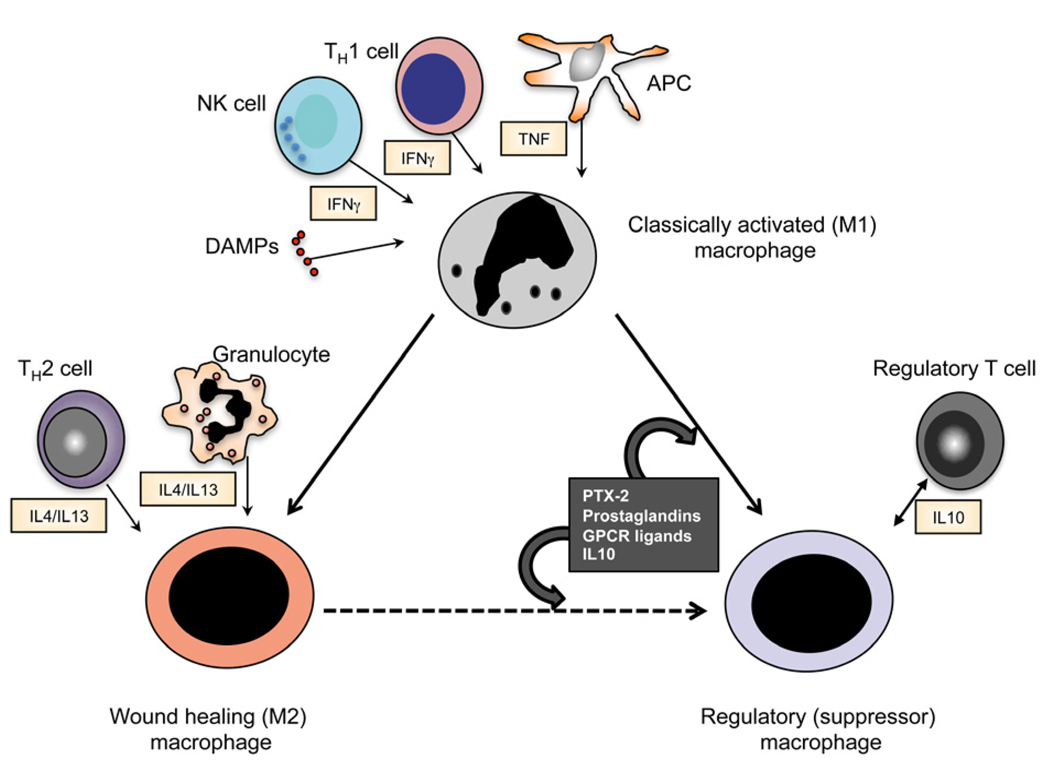
As an increasing number of cell-surface Mϕ markers have been identified with commercially available antibodies, labeling studies in tissues have identified different populations of Mϕs in kidney and elsewhere. Some of these subpopulations have been reported to be differentially activated, some more activated and some activated to produce different cytokines. One thing is clear: not all Mϕs are the same. In vitro studies indicate that Mϕs can be polarized by activation with different cytokines. Polarized activated Mϕs have been ascribed different functions largely based on in vitro studies (Figure 2B, Figure 3). Polarization was initially described as classical vs alternate activation, but more recently the former has been ascribed M1 and the latter M2, reminiscent of classifications of T lymphocytes 49,86,87. Several problems with the in vitro models exist. Firstly, the in vitro activation is highly artificial and secondly, the in vitro cultured Mϕ may bear limited resemblance to their in vivo cousins: The M1 macrophage can be differentiated with IFNγ or LPS neither of which may be present significantly in tissue injury, the M2 macrophage generated by exposure to IL4 or Il13, neither of which are abundant in tissue injury in the kidney. Thirdly the correlation between in vitro markers (e.g. nitric oxide vs arginase) and in vivo function is poor. Nevertheless increasing evidence that this type of functional heterogeneity exists in vivo has accumulated. More recently this classification has been modified to reflect the increasing controversy in this area and the increased awareness that other discrete macrophage phenotypes may exist. The M2 population of Mϕs may be better described as wound healing since depending on the injury and organ context the M2 Mϕ may promote wound healing, angiogenesis or fibrosis (Figure 3). In addition, exposure to, the antiinflammatory cytokine IL10, pentraxin-2 (also known as serum amyloid P), adenosine or in certain circumstances apoptotic cells, and ICs can result in Mϕs that generate high levels of IL10 themselves and are actively involved in the suppression of immune responses. This macrophage subpopulation might be better identified as a regulatory Mϕ 52,88,89.
Figure 3. Subpopulations of inflammatory macrophages in vivo.
Schematic showing three different types of inflammatory macrophage, and factors that regulate their activation/differentiation in sterile inflammation in vivo. Although cell-derived and tissue-derived factors can regulate recruited monocytes to differentiate into different macrophage subtypes, regulatory macrophages also differentiate from M1 and M2/wound healing activated macrophages, triggered by mechanisms that are poorly understood.
To explain heterogeneity further, three hypotheses have evolved: (1) monocytes differentiate into an infinite number of phenotypes depending on the environment (Hume Hypothesis) 90 (Figure 2B); (2) there are pre-existing subpopulations of monocytes that are functionally prescribed (Geissman Hypothesis) 91–93; (3) there are discrete functional populations that can change (switch) from one form to the other 56. Evidence supports all three of these hypotheses. It is clear that depending on the cytokine mixture applied in vitro cultured Mϕs acquire different phenotypes transcriptionally that are not polarized, rather show many patterns of activation suggesting infinite possibilities. Furthermore, increasing evidence from in vivo studies points to monocytes not only sensing danger or injury but also sensing and responding to the tissue specific environment, providing multiple ‘phenotypes’ 60,94–96. In contrast however, studies from the 1990’s revealed two or more discrete populations of human monocytes and studies of Geissman and colleagues provided evidence of clear functional differences between subpopulations of circulating monocytes in mice, in keeping with the second hypothesis 91,97,98 (Figure 2B). Our own recent studies using the marker Ly6C to define Mϕ subpopulations confirm the second hypothesis but show that the third hypothesis holds true in vivo, that is that a single monocyte subset differentiates sequentially into functionally discrete populations rather than infinite phenotypes (Figure 2B, 4) 60. In order for this to occur, either cellular activation engages a transcriptional program which regulates a sequential or phenoypic switch, or environmental triggers (i.e. within the injured tissue) activate a transcriptional and functional switch. Several studies support the idea that a phenotypic switch is triggered by environmental factors. Local release of Adenosine in injured tissues binds adenosine receptors on Mϕs and can trigger Mϕ polarization 81. Further studies to define a role for Adenosine receptors and other injury-released compounds (DAMPs) and their cognate receptors in this switch are required.
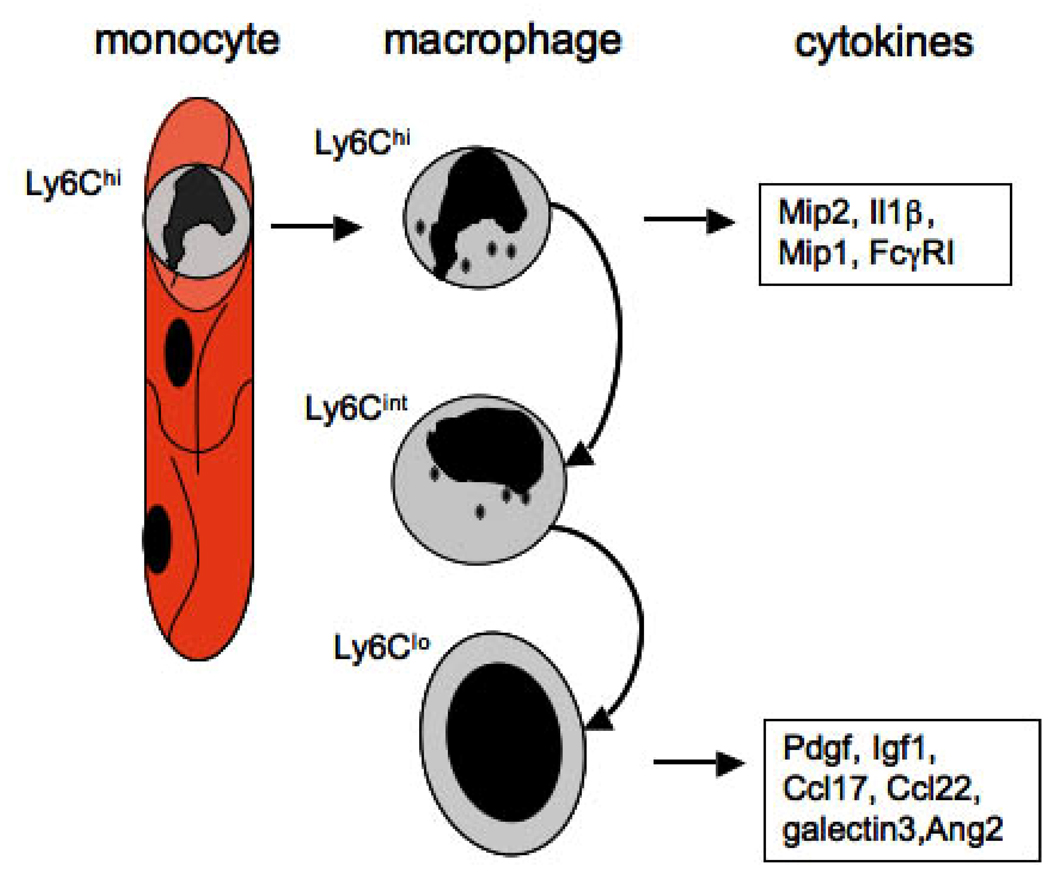
Figure 4. Ly6C, a marker of monocyte and macrophage heterogeneity.
Schematic showing recruitment of Ly6Chi monocytes selectively into the kidney from capillaries, which differentiate into three populations of kidney macrophages, Ly6Chi, Ly6Cint and Ly6Clo. These kidney macrophage subpopulations generate discrete M1 biased (Ly6Chi) and M2 (Ly6Clo) biased cytokines in vivo. The Ly6Cint subpopulation comprises both macrophages derived from activation of resident macrophages and also macrophages in transition between with Ly6Chi and Ly6Clo subpopulations.
Lessons from genetic models in rodents
Until recently, the function of Mϕs in tissue injury has largely been inferred by their presence in injured tissues and the cytokines that they can generate in vitro when activated. A limited number of studies using polyclonal anti-Mϕ sera suggested deleterious functions for Mϕs in glomerular diseases, but these have to be interpreted with caution due to lack of specificity of such preparations 27,72. In the 1990’s liposomal encapsulated clodronate was developed as a strategy to ablate Mϕs in vivo. This strategy relies on the selective uptake of liposomes by monocytes and Mϕs, delivering toxic levels of the bisphosphonate clodronate. However liposomes are endocytosed by many cells including neutrophils and endothelial cells, and clodronate has anti-inflammatory effects of its own. Nevertheless, lipsomal clodronate is effective and has been widely used to study Mϕ function in vivo despite potential lack of specificity.
Macrophage ablation in vivo
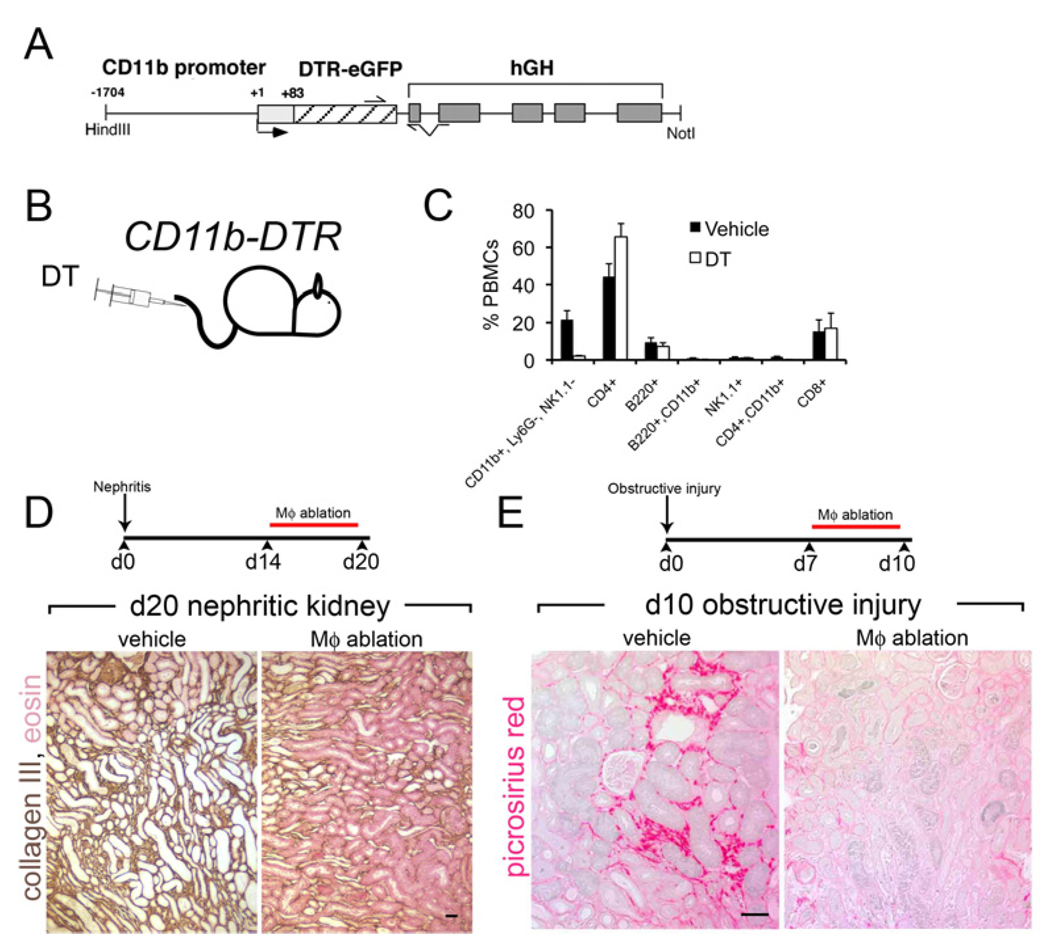
To circumvent these problems we developed a genetic approach to Mϕs ablation in vivo, relying on the selective susceptibility of human cells but not mouse cells to the toxic effects diphtheria toxin (DT) 26,99. Humans are greater than 1000×\ more susceptible to DT than rodents due to the cell surface expression of the human heparin binding epithelial growth factor receptor which is a receptor for DT (DTR) and transports DT to the cytosol where it is rapidly lethal. Transgenic expression of this human receptor in mouse cells renders those cells uniquely susceptible to DT. We generated a mouse model where the DTR was expressed under a Mϕ-specific promoter for the integrin CD11b, CD11b-DTR. Although CD11b is expressed by other cells including neutrophils only monocytes, Mϕs, dendritic cells and a small population of NKT cells are susceptible to the effects of DT (Figure 5A–C)
Figure 5. Studying macrophages in vivo by ablation using the CD11b-DTR mouse model.
(A) The CD11b-DTR mouse harbors the CD11b-DTR transgene where the diphtheria toxin receptor is regulated by the CD11b promoter/enhancer. (B) By injecting minute amounts of DT into these mice only cells expressing the DTR are susceptible to its lethal effect. (C) Graph showing the effect of a single injection of DT on peripheral blood mononuclear cell populations (PBMCs). Note the selective ablation of CD11b+, Ly6G-, NK1.1 cells which are monocytes. Neutrophils are also not affected (not shown). (D) Schematic of late ablation of monocytes and Mϕs in the nephrotoxic nephritis (NTN) model of crescentic glomerulonephritis and Collagen-III stained lower power images of kidney d20 of NTN. Note the deposition of interstitial collagen is attenuated by Mϕ ablation as is tubular atrophy (E) Schematic of late ablation of monoctes and Mϕs in the chronic obstructive injury model and images of Sirius red stained sections of kidneys after 10d of injury. Note Mϕ ablation attenuated fibrosis in this model of chronic kidney disease also.
Using this model we have been able to target monocytes and Mϕs specifically in models of kidney disease, at different time-points. In a model of crescentic GN induced by immune-complex formation at the basement membrane of the glomerulus (Nephrotoxic nephritis [NTN]), Mϕs promote disease progression (Figure 5D). One manifestation of this progression was interstitial fibrosis, another was tubular atrophy. In a second model of immune complex deposition GN, which is analogous to membranoproliferative GN seen in human diseases including cryoglobulinemia or SLE, Mϕ ablation also ameliorated disease. In both of these models the data suggest that while monocytes and Mϕs normally promote safe, non-phlogisitc clearance of ICs in the glomerulus, the normal safety mechanisms in the innate immune system are overwhelmed allowing monocyte/Mϕ activation and consequent local tissue injury 68,73. Although within the heterogeneous mix of inflammatory Mϕs some may still be performing reparative functions, the net consequence of widespread of Mϕ ablation in these models of glomerular disease is amelioration of tissue injury.
In order to explore the role of Mϕs in fibrosis progression further in ‘non-immunological’ inflammatory disease of the kidney we used a simple model of mechanical injury caused by obstruction of the ureter of the kidney, that results in inflammation and fibrosis 52,60, reminiscent of chronic kidney disease (Figure 5E). We discovered that Mϕs also promote fibrosis in response to mechanical injury, suggesting a generalized role for Mϕs in fibrosis progression but also indicating that much of the interstitial disease seen in immunological kidney disease may be in response to secondary cellular injury rather than glomerular ICs. This finding has been recapitulated by others by preventing recruitment of monocytes from the circulation 100,101.
Next we studied Mϕ function in the bilateral ischemia reperfusion injury model (IRI), which shares similarities with human acute tubular necrosis. Surprisingly, Mϕ recruitment coincides with repair not injury (Figure 1). This surprising finding led us to speculate that Mϕs have the capacity to repair the kidney in the absence of an injury stimulus. In the CD11b-DTR mouse model, ablation of Mϕs during the repair phase of IRI model indeed prevented normal repair 55. Studies are currently underway to dissect the mechanisms by which in single injury followed by repair and regeneration Mϕs promote repair whereas in repetitive injury or chronic injury they promote cell loss and fibrosis.
Rodent Congenics
Strains of inbred mice and rats have widely differing susceptibility to diseases of the kidney. These differences have been exploited experimentally to identify the genes that govern increased susceptibility. Not surprisingly, many of the genes identified play roles in the innate and adaptive immune response 102,103. The Wistar Kyoto rat strain is extremely susceptible to the model of immunecomplex GN, called NTN. This rat was crossed with Lewis rats that are resistant to developing disease in response to nephrotoxic serum. By backcrossing and testing for disease susceptibility, two novel disease susceptibility genes have been identified and these are both monocyte/ Mϕ genes. One, Fcγ receptor 3 (FcγRIII) is present in an alternate form in susceptible rats that renders its Mϕs unable to efficiently phagocytose ICs, and renders them more activatable by pathways other than FcγRIII 67. The other disease susceptibility gene is JunD, a transcription factor in the AP-1 family that regulates cellular activation of Mϕs74. These studies both serve to highlight the central role of Mϕ non-phlogistic clearance of ICs and intracellular regulation of Mϕ activation as key facets that regulate disease progression. Furthermore, rodent FcγRIII is analogous to human FcγRIIA, a major activating FcγR. This receptor has many polymorphisms that determine susceptibility to the development of SLE and lupus nephritis 104,105.
In congenic studies in mice that develop spontaneous SLE, several disease susceptibility chromosomal loci have been identified. The mouse Sle1 locus is syntenic with the human SLE susceptibility loci containing genes involved in the complement cascade and FcγRs, that are present on monocytes and Mϕs. It also contains genes for B cell survival signals (SLAM), regulatory T cells, complement receptors (CR2), and regulates the development of autoantibody and nephritis 102,106–108. These studies therefore also place monocytes and Mϕs at the centre of the immune response in these models of lupus kidney disease. Collectively these powerful genetic studies place Mϕ regulation of activation and signaling through Mϕ FcγRs at the centre of the immune response in the glomerulus
Mechanisms of macrophage mediated fibrosis
From many studies in vivo in different organ systems, Mϕs have been shown to play a key role in the progression of fibrosis, a major harbinger of organ failure. These cross-organ findings, suggest that the pro-fibrotic role of Mϕs is a stereotyped response to chronic injury or repetitive injury 26,60,99,100,109–115. Multiple mechanisms by which Mϕs cause fibrosis have been proposed (Figure 6), and merit review. In parasitic infections, of liver, recruited inflammatory Mϕs are a major cellular mediators of fibrosis 35,96,116. In this setting Mϕ derived arginase and IL-13 are molecular factors driving fibrosis. It has been postulated that Mϕ arginase may directly promote fibrosis by hydrolyzing arginine to ornithine which can be used to generate the polyamines glutamate and proline which are necessary for collagen synthesis 117. IL13 generated by both TH2 skewed T cells and Mϕs, directly drives myofibrobalsts to generate collagenous matrix. (Figure 6). In lung diseases, production of IL13 and YM1 by Mϕs has been strongly implicated in directly driving myofibroblast activation (Figure 6) 95,118. However, in contrast to lung and skin, in the injured kidney Mϕs do not generate IL-13, Fizz1 or YM1, and arginase is not significantly regulated in response to injury 60. These mechanisms are probably not important in the pathology of renal fibrosis.
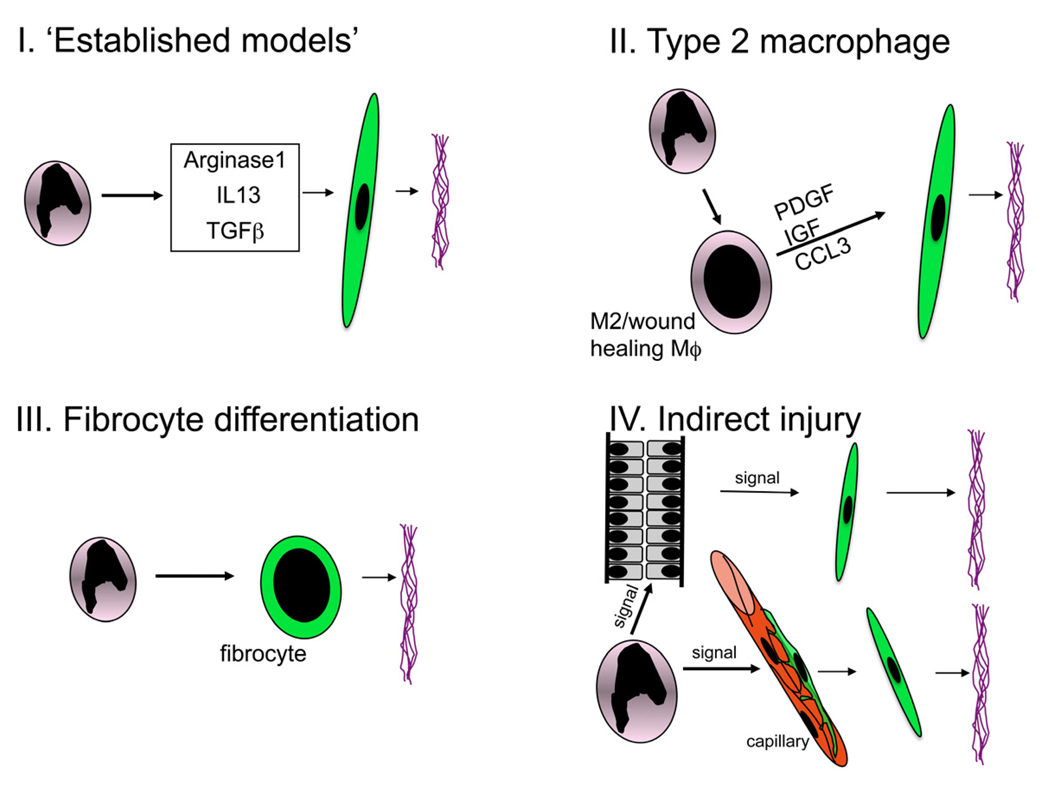
Figure 6. Schematics of proposed models of macrophage mediated fibrosis.
(I) Arginase, TGFβ and IL13 have been shown in pathogen triggered liver fibrosis, lung fibrosis and skin diseases and kidney diseases (TGFβ only) to be significant Mϕ factors in fibrogenesis (II) Activated Mϕs differentiate into Type 2 (M2 or wound healing) Mϕs liberate cytokines that can drive pericyte or myofibroblast activation and consequent deposition of fibrillar collagens I and III, (III) A subpopulation of monocytes differentiates directly into a scar forming cell called fibrocyte (IV) Activated Mϕs injure endothelial cells which sequentially trigger pericyte migration and differentiation into myofibroblasts, or they injure epithelial cells which sequentially liberate factors that promote pericyte migration from capillaries and differentiation into myofibroblasts.
The cytokine TGFβ has been implicated in Mϕ-driven fibrosis in many organ systems by local release and local activation119,120. In the kidney it is clear that Mϕs are but one of many sources of TGFβ and epithelial cells, rather than Mϕs, are the main source of activation of TGFβ from its latent form. The major activating factor is αvβ6 integrin mediated cleavage of the inactive molecule at the epithelial basolateral membrane 121,122. Although TGFβ can activate and trigger proliferation of myofibroblasts, it also promotes cell cycle arrest and cell death in epithelial cells which is also deleterious to the kidney and it remains therefore an attractive target for therapeutics. It is but one factor, however released by Mϕs that can play a role in fibrogenesis (Figure 6). Therefore although Mϕ derived TGFβ may play a role in fibrogenesis it is not likely to be the major Mϕ effector cytokine.
A third mechanism by which Mϕs promote fibrosis is by differentiation into a cell called fibrocyte (myeloid cell that generates fibrotic matrix directly). Despite reports providing evidence for the presence of fibrocytes in models of kidney disease 123–125, our recent exhaustive studies of these cells indicates that in the mouse, at least, they are very rare and do not contribute to fibrogenesis (Figure 6) 52,55,126. More likely is that Mϕs signal directly or indirectly to myofibroblasts and their precursors via cellular (paracrine) cross talk.
We have recently defined Mϕ heterogeneity in vivo in mouse models of chronic kidney disease. Three populations of kidney Mϕs can be identified (Ly6Chigh, Ly6Cint, Ly6Clow). All three derive from a single population of circulating inflammatory Ly6Chigh monocytes (Figure 4) 52,55,126. Ly6Chigh Mϕs in the kidney are activated, produce pro-inflammatory cytokines including IL1β and chemokines including Mip1α, Mip2 and are similar to M1 activated Mϕs defined previously. In stark contrast, Ly6Clow Mϕs, which derive from Ly6Chigh Mϕs generate low levels of IL1β and Mip2, but instead produce cytokines Ccl17, Ccl22, Pdgf, Igf1, all factors that have been associated with fibrogenesis and define Ly6Clow Mϕs as M2 or wound healing. Myofibroblasts have receptors for Pdgf, Igf1 and receptors for the type 2 chemokines. While it is possible that one Mϕ derived factor is responsible for the pro-fibrotic biology of Mϕs, far more likely is that many cytokines converge on myofibroblasts or pericytes in driving fibrosis. Nevertheless, the Ly6Clow Mϕs, by virtue of their M2 skewed transcriptional profile, fulfill several of the criteria for paracrine signaling, and are therefore a target for therapy (Figure 4). Despite clear evidence for wound healing Mϕs in kidney fibrosis, it remains possible that either Ly6Chigh Mϕs producing M1 type cytokines or Ly6Clow Mϕs producing M2 type cytokines act indirectly to drive myofibroblast activation and differentiation. We have recently discovered that myofibroblasts derive from pericytes, a newly described cell type in the kidney 126,127. Pericytes are perivascular cells of capillaries, derived from metanephric mesenchyme during development, and are necessary for angiogenesis and vascular stability through two-way signaling between pericytes and endothelial cells. It is therefore possible that Mϕ-signaling to endothelial cells, directs pericyte migration, and differentiation into myofibroblasts. In that context, Ly6Chigh Mϕs generate high levels of Ang2 which may signal deleteriously to endothelial cells (Figure 6).
Monocytes and Macrophages as new targets for therapy
A) Targeting macrophage activation of phenotype
We have recently been able to test, serendipitously, whether Mϕ-ativation in the injured kidney is necessary for fibrosis progression. Understanding of the mechanisms by which Mϕs become activated in sterile inflammation remains incomplete. Although foreign proteins, lipoproteins and nucleic acids, (pathogen associated molecular patterns or PAMPs) readily bind to Mϕ cell surface pattern recognition receptors (PRRs), and activate cells, these foreign proteins are not present in sterile injured tissues and therefore cannot be a mechanism of activation. Increasing evidence suggests that in addition to activating IFNγ released from NK cell, soluble factors and debris released from injured cells and injured tissues, known as danger associated molecular patterns (DAMPS) also bind to PRRs including TLRs and other receptors such as RAGE (Receptor for advanced glycation endproducts) (Figure 2, 3) 83,85. Selective blockade of myeloid cell activation triggered by sterile injury while permitting activation triggered by foreign pathogen epitopes is a highly attractive approach to the treatment of chronic inflammation since it will not pose a risk of increased infection susceptibility, always a concern when targeting leukocyte activation.
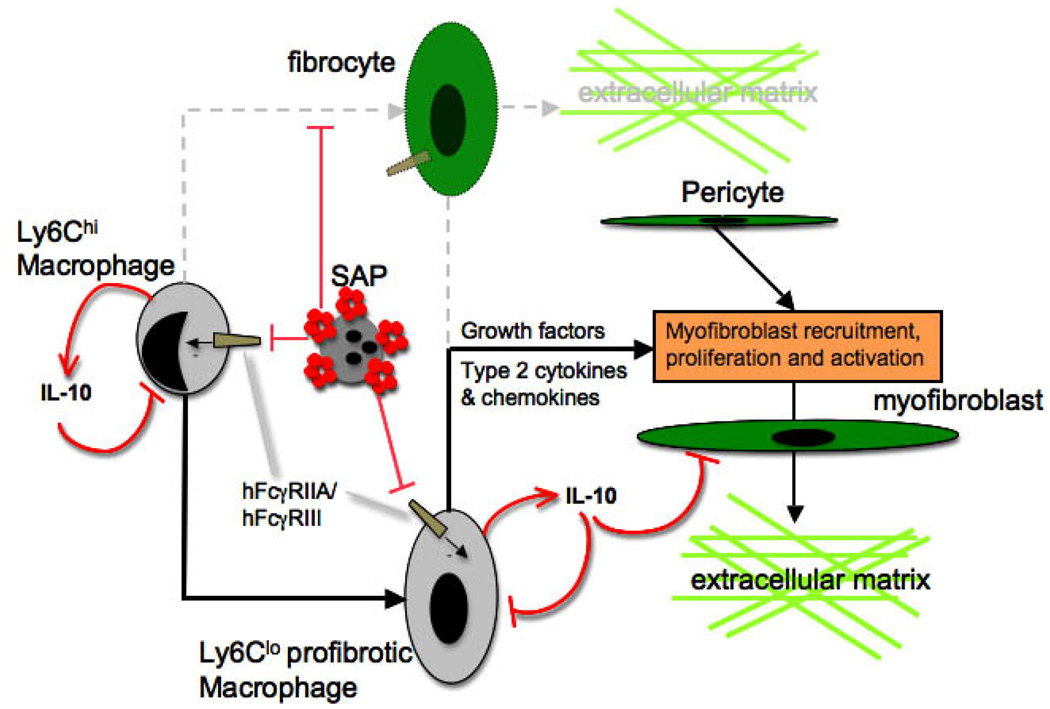
Serum Amyloid P (SAP) or pentraxin-2 (PTX2), with structural similarities to C-reactive protein (CRP) is a circulating pentameric protein of the innate immune system and is strongly anti-fibrotic in diseases of the kidney 52. Unexpectedly, its antifibrotic effect is not mediated through binding to myofibroblasts, rather it binds to Mϕ FcγRs. PTX-2 deposits in injured tissues by opsonizing dead cells and debris in injured tissues. Once it has opsonized targets, it undergoes a conformational change converting to a high affinity ligand for the activating FcγRs, hFcγRIIA and hFcγRIII (mFcγRIII and mFcγRIV). Cross-linking of FcγRs by PTX-2 does not activate Mϕs (unlike crosslinking of FcγRs by immunoglobulin) 52. Conversely it inhibits activation mediated by other activating stimuli (Figure 7). Part of the mechanism by which SAP inhibits Mϕ activation is through local release of IL10. IL10 is an anti-inflammatory cytokine that is well recognized for inhibiting inflammation and has direct antifibrotic effects on myofibroblasts. IL10 is very short-lived and therefore difficult to administer systemically. In addition systemic administration may stimulate B-cell proliferation and may thereofre paradoxically stimulate adaptive immune responses. However our studies show that by endocytosing and phagocytosing PTX-2-opsonized debris, Mϕs release IL10 locally in the injured kidney resulting in less activated Mϕs (both M1 and M2 subtypes) that are unable to drive fibrosis. Furthermore, IL10 generation by inflammatory Mϕs defines them as regulatory or immunsuppressive Mϕs. It maybe therefore that PTX-2 not only inhibits Mϕ activation but causes differentiation toward the regulatory Mϕ phenotype (Figure 3) 52,88. Importantly, PTX-2 does not inhibit activation triggered by the bacterial cell wall lipoprotein LPS, implicating PTX-2 as a novel and safe endogenous inhibitor of sterile inflammation and fibrosis. Recombinant (PTX-2), is currently in Phase I trials as an anti-fibrotic therapy, and it clearly may have broad therapeutic indications. Although still in its infancy new PRRs and new DAMPs that active the innate immune system are being identified and may become new targets for therapy 85. Novel inhibitors of Mϕ activation including inhibitors of the MAP kinase and Jun kinase activating cell-signaling pathway are currently in development.
Figure 7. Mechanism by which Pentraxin-2/Serum Amyloid P inhibits macrophage directed fibrogenesis in the kidney.
PTX-2 (red pentamers) opsonization of apoptotic cells, debris and oxidized matrix, triggers a conformational change that renders PTX-2 a high affinity ligand for activating immunoglobulin Fcγ receptors hFcγRIIA and III. Ligation of activating receptors on inflammatory kidney macrophages triggers differentiation of inflammatory Mϕs into regulatory Mϕs which generate IL10. This inhibits both Ly6Chi and Ly6Clo Mϕ activation and also directly inhibits collagen synthesis by myofibroblasts. In other organ systems PTX-2 has been reported to trigger differentiation of fibrocytes but these are not detected in kidney disease.
B) Targeting monocytes
Rodent studies using liposomal clodronate or the CD11b-DTR ablation system indicate that ablation is effective in limiting tissue injury and fibrosis in chronic injury models in rodents. Selective cellular ablation is widely accepted in humans, in the form of monoclonal or polyclonal antibodies that target T cells or B cells (Anti-thymocyte globulin (ATG), anti-CD3 antibodies (OKT3), anti-CD20 antibodies (rituxumab), anti-CD52 (Campath) antibodies). All of these therapies are highly efficacious but come with considerable infection risk (except anti-CD20 antibodies). ATG likely also ablates monocytes in addition to T cells. In addition, a wide range of other therapies, including Mycophenolic acid, Cyclosporine A, and Cyclophosphamide, function either to ablate or profoundly inhibit proliferation of lymphocytes. It is quite likely therefore that monocyte specific ablative therapies would be successful in humans, but there may be unacceptable side effects, particularly if they are to considered in the treatment of chronic diseases. Nevertheless, these therapies could find a role in the management of acute inflammatory diseases of the kidney including acute interstitial nephritis and rapidly progressive GN. The monocyte and Mϕ receptor for CSF or M-CSF is Csf1R or C-FMS. This tyrosine kinase dependent receptor drives monocyte proliferation in tissues and may be an alternative target for therapy. Several tyrosine kinase inhibitors that are selective for the Csf1R have been developed and are in early trial phases 128.
C) Targeting macrophage recruitment
Alternative strategies to Mϕ ablation have been investigated and tested with varying degrees of success. Chemokines and their receptors are important factors in monocyte recruitment. One problem encountered with targeting monocyte recruitment has been the redundancy of chemokines and their receptors, relegating single chemokine receptor blockade due to its limited capacity to prevent monocyte entry into the injured organ including the kidney 129. Moreover chemokine receptors such as CCR2 have broader roles in release of monocytes from bone marrow which may pose additive risks of infection. In addition, the finding that two or more subpopulations of monocytes exist in the circulation, one with high CCR2 receptor, another with high CX3CR1 (fractalkine) receptor renders targeting strategies more complicated since there may be redundancy of function of these populations 130. New small molecules that provide broader blockade of chemokine receptors, including compounds such as BMS-A (Bristol-Myers Squibb) which block both CCR2 and CCR5 in humans and rodents, or combination inhibitors that block individual receptors including CCR1, CCR2 and CCR5 hold promise as new therapies in fibrosing inflammatory diseases, not only in the kidney but in other organs systems including liver 131–135. Since the innate cellular immune response to pathogens is important in health, and since almost all rodent studies are performed in sterile facilities, safety in addition to efficacy studies will need to be completed before these compounds can be used in human diseases. In this context the CCR1 antagonist CP-481,715 (Pfizer) is currently in phase I trials for Rheumatoid Arthritis 136,137.
D) Targeting macrophage differentiation
Several studies support the model in which Mϕs differentiate into a wound remodeling or fibrotic macrophage 56,84,88,138,139. The mechanisms by which this differentiation occurs remain incompletely understood. Several candidate factors driving such differentiation have been described including PTX-2 described earlier. Local release of adenosine, which binds to adenosine receptors has been identified as another candidate. Adenosine has been ascribed as ‘anti-inflammatory’ since it can result in appearance of wound healing Mϕs with angiogenic properties and can prevent pro-inflammatory cytokine production 81. However the role of adenosine in appearance of pro-fibrotic Mϕs remains to be explored. Mechanisms to selectively target Adenosine receptors or target the extracellular enzymes such as CD73 that generate extracellular adenosine should be tested in models of sterile inflammation with fibrosis to determine efficacy 140.
E) Other potential targets
The list of Mϕ paracrine effector molecules is extensive, but the precise role of these factors individually or in concert has been inadequately tested. The cytokines PDGF, IGF1, and Angiopoietin2, PGE2 are all liberated by M2 or wound healing Mϕs in vivo and may play deleterious roles. Whether single molecule blockade will be effective remains to be established. However, promising studies that target the receptor for PDGF, PDGFRβ using tyrosine kinase inhibitors are currently underway in human transplant nephropathy to determine whether selective blockade of this paracrine pathway will impact on human fibrosis progression.
Mechanisms of macrophage mediated cellular loss
In additional to a fibrogenic role of Mϕs in chronic or repetitive kidney injuries, Mϕ not only promote fibrosis but also promote loss of epithelial cells and microvasculature 26,59,141. Mϕ-directed loss of epithelial cells can be detected by the presence of increased cellular apoptosis, but apoptotic cell death occurs while epithelial cells engaged in cell cycle. In chronic disease states Mϕs drive both the cell cycle and apoptotic cell death 26,59. The mechanisms by which this occurs have not been completely elucidated., but it is likely that growth factors drive cell cycle entry and progression and other factors promote cell death at cell cycle checkpoints. Possible roles for iNOS, and Tnfα have been explored but no consistent cytokine signals have been clearly identified 142, and M1 Mϕ type functions are implicated in this process. Regardless of the specific cellular crosstalk, two important facets of macrophage biology are highlighted. Firstly, macrophage activation is required for epithelial cell death and secondly, a common theme emerges by which macrophages provoke cells into cell cycle and target their untimely death at DNA cell cycle checkpoints. This manifestation of macrophage function has been recapitulated in several organ systems, and seems to be a generalized function of activated macrophages. It is possible that macrophages function in this context to test cell health. By triggering epithelial (and other) cells into cell cycle they are testing cell integrity. If a cell pauses at a DNA checkpoint it is likely due to stress, inadequate energy or resources, or excessive damage to its DNA. It is widely acknowledged that cells pausing at DNA checkpoints are more susceptible to apoptotic cell death, so the macrophage functions as a policeman of interstitial cells checking for health and driving rapid death and clearance if the stress testing does not go well. Clearly although this kind of function might appear desirable, in chronic inflammation with persistent activation of macrophages, excessive cell loss can ensue leading to tubule atrophy and peritubular capillary rarefaction. New studies are required to understand the molecular mechanisms underlying these observations.
Macrophages in repair and regeneration
A common theme throughout this article is that the natural state of being for a Mϕ is non-phlogistic clearance of debris, and unwanted things from the body, and liberation of safe helpful cytokines that promote well-being. Chronic, repetitive or severe injury states overcome the inbuilt mechanisms that prevent activation and the Mϕ becomes chronically activated leading to deleterious consequences. There is evidence from multiple organ settings that following single injury Mϕs provide reparative functions 55,143,144. At the current time, the factors that dictate how Mϕs become predominantly reparative vs. deleterious remain obscure. One candidate pathway that is activated by reparative Mϕs in the kidney is the Wnt signaling pathway, a cell-cell signaling pathway with profound importance in kidney development. Doubtless multiple mechanisms by which Mϕs promote repair in the kidney will be uncovered. Nevertheless, by understanding how Mϕs repair and regenerate tissue we may be able to artificially impose a repair program on chronically injured tissues to drive healthy repair processes and disable the deleterious processes that lead to chronic disease. Further in targeting ‘bad’ Mϕs as a novel therapeutic option, it will be important to understand when inflammatory Mϕs function positively rather than negatively.
Therapeutic options in the treatment of chronic kidney diseases and immune mediated kidney diseases
To summarize, Mϕs are an innate immune cell that is widespread in diseases of the kidney. Increasing evidence that targeting Mϕs and their functions will lead to improved outcomes in many kidney diseases has emerged. The final common pathway of chronic kidney disease that leads to organ failure, death or renal replacement therapy, appears increasingly to be driven, at least in part, by chronically activated Mϕs, and similar deleterious roles for Mϕs in immunologically mediated diseases have been identified. New therapeutic targets are emerging and undergoing investigation in human trials as potential novel therapies in a range of kidney diseases.
Acknowledgements
Funding: The laboratory is funded by National Institutes of Health DK73299, DK84077 and DK87389, grants from Genzyme renal innovation program, Gottschalk Award from American Society of Nephrology, and Grants from Promedior Inc, Baxter Inc, Regulus Pharmaceuticals Inc.
Thanks to Mark Lupher Jr (Promedior), Richard A. Lang (Cincinnati Children’s Hospital, OH, Jeremy Hughes (University of Edinburgh, UK), Charles Alpers (University of Washington, WA_ for valuable discussions and collaboration, Ana P. Castano (HMS) and Helmut Rennke (HMS) for help with images
Abbreviations
- GN
glomerulonephritis
- Mϕ
macrophage
- PMN
neutrophil
- ANCA
anti-neutrophil cytoplasmic antibody
- GBM
glomerular basement membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Furth R. Macrophage activity and clinical immunology. Origin and kinetics of mononuclear phagocytes. Ann N Y Acad Sci. 1976;278:161–175. doi: 10.1111/j.1749-6632.1976.tb47027.x. [DOI] [PubMed] [Google Scholar]
- 3.Magil AB, Wadsworth LD. Monocyte involvement in glomerular crescents: a histochemical and ultrastructural study. Lab Invest. 1982;47(2):160–166. [PubMed] [Google Scholar]
- 4.Germain MJ, Anderson RW, Keane WF. Renal disease in cryoglobulinemia type II: response to therapy. A case report and review of the literature. Am J Nephrol. 1982;2(4):221–226. doi: 10.1159/000166650. [DOI] [PubMed] [Google Scholar]
- 5.Ferrario F, Castiglione A, Colasanti G, et al. The detection of monocytes in human glomerulonephritis. Kidney Int. 1985;28(3):513–519. doi: 10.1038/ki.1985.158. [DOI] [PubMed] [Google Scholar]
- 6.Cole EH, Cardella CJ, Schulman J, Levy GA. Monocyte procoagulant activity and plasminogen activator. Role in human renal allograft rejection. Transplantation. 1985;40(4):363–371. doi: 10.1097/00007890-198510000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Sanfilippo F, Kolbeck PC, Vaughn WK, Bollinger RR. Renal allograft cell infiltrates associated with irreversible rejection. Transplantation. 1985;40(6):679–685. doi: 10.1097/00007890-198512000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Nolasco FE, Cameron JS, Hartley B, et al. Intraglomerular T cells and monocytes in nephritis: study with monoclonal antibodies. Kidney Int. 1987;31(5):1160–1166. doi: 10.1038/ki.1987.123. [DOI] [PubMed] [Google Scholar]
- 9.Bolton WK, Innes DJ, Jr, Sturgill BC, Kaiser DL. T-cells and macrophages in rapidly progressive glomerulonephritis: clinicopathologic correlations. Kidney Int. 1987;32(6):869–876. doi: 10.1038/ki.1987.288. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulos E, Seron D, Hartley RB, Nolasco F, Cameron JS. The role of interstitial infiltrates in IgA nephropathy: a study with monoclonal antibodies. Nephrol Dial Transplant. 1989;4(3):187–195. doi: 10.1093/oxfordjournals.ndt.a091854. [DOI] [PubMed] [Google Scholar]
- 11.van Goor H, van der Horst ML, Fidler V, Grond J. Glomerular macrophage modulation affects mesangial expansion in the rat after renal ablation. Lab Invest. 1992;66(5):564–571. [PubMed] [Google Scholar]
- 12.Roccatello D, Isidoro C, Mazzucco G, et al. Role of monocytes in cryoglobulinemia-associated nephritis. Kidney Int. 1993;43(5):1150–1155. doi: 10.1038/ki.1993.161. [DOI] [PubMed] [Google Scholar]
- 13.Van Goor H, Ding G, Kees-Folts D, et al. Macrophages and renal disease. Lab Invest. 1994;71(4):456–464. [PubMed] [Google Scholar]
- 14.Young BA, Burdmann EA, Johnson RJ, et al. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 1995;48(2):439–448. doi: 10.1038/ki.1995.312. [DOI] [PubMed] [Google Scholar]
- 15.Rovin BH, Doe N, Tan LC. Monocyte chemoattractant protein-1 levels in patients with glomerular disease. Am J Kidney Dis. 1996;27(5):640–646. doi: 10.1016/s0272-6386(96)90097-9. [DOI] [PubMed] [Google Scholar]
- 16.Hill GS, Delahousse M, Nochy D, Mandet C, Bariety J. Proteinuria and tubulointerstitial lesions in lupus nephritis. Kidney Int. 2001;60(5):1893–1903. doi: 10.1046/j.1523-1755.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 17.Becker GJ, Hancock WW, Stow JL, et al. Involvement of the macrophage in experimental chronic immune complex glomerulonephritis. Nephron. 1982;32(3):227–233. doi: 10.1159/000182850. [DOI] [PubMed] [Google Scholar]
- 18.Kramer AA, Postler G, Salhab KF, et al. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55(6):2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 19.Lan HY, Mitsuhashi H, Ng YY, et al. Macrophage apoptosis in rat crescentic glomerulonephritis. Am J Pathol. 1997;151(2):531–538. [PMC free article] [PubMed] [Google Scholar]
- 20.Rabb H, Mendiola CC, Dietz J, et al. Role of CD11a and CD11b in ischemic acute renal failure in rats. Am J Physiol. 1994;267(6 Pt 2):F1052–F1058. doi: 10.1152/ajprenal.1994.267.6.F1052. [DOI] [PubMed] [Google Scholar]
- 21.Morris L, Graham CF, Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991;112(2):517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161(3):475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hume DA, Gordon S. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J Exp Med. 1983;157(5):1704–1709. doi: 10.1084/jem.157.5.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76(7):1015–1022. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 25.Lange-Sperandio B, Fulda S, Vandewalle A, Chevalier RL. Macrophages induce apoptosis in proximal tubule cells. Pediatr Nephrol. 2003;18(4):335–341. doi: 10.1007/s00467-003-1116-2. [DOI] [PubMed] [Google Scholar]
- 26.Duffield JS, Tipping PG, Kipari T, et al. Conditional Ablation of Macrophages Halts Progression of Crescentic Glomerulonephritis. Am J Pathol. 2005;167(5):1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holdsworth SR, Tipping PG, Hooke DH, Atkins RC. Role of the macrophage in immunologically induced glomerulonephritis. Contrib Nephrol. 1985;45:105–114. doi: 10.1159/000410453. [DOI] [PubMed] [Google Scholar]
- 28.Masaki T, Chow F, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Heterogeneity of antigen expression explains controversy over glomerular macrophage accumulation in mouse glomerulonephritis. Nephrol Dial Transplant. 2003;18(1):178–181. doi: 10.1093/ndt/18.1.178. [DOI] [PubMed] [Google Scholar]
- 29.D'Souza MJ, Oettinger CW, Shah A, et al. Macrophage depletion by albumin microencapsulated clodronate: attenuation of cytokine release in macrophage-dependent glomerulonephritis. Drug Dev Ind Pharm. 1999;25(5):591–596. doi: 10.1081/ddc-100102213. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30(6):766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Hume DA, Underhill DM, Sweet MJ, et al. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2(1):11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 35.Wilson MS, Mentink-Kane MM, Pesce JT, et al. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85(2):148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbert DR, Holscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20(5):623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 37.Lan HY, Nikolic-Paterson DJ, Mu W, Atkins RC. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int. 1995;48(3):753–760. doi: 10.1038/ki.1995.347. [DOI] [PubMed] [Google Scholar]
- 38.Paul LC, Grothman GT, Benediktsson H, Davidoff A, Rozing J. Macrophage subpopulations in normal and transplanted heart and kidney tissues in the rat. Transplantation. 1992;53(1):157–162. doi: 10.1097/00007890-199201000-00032. [DOI] [PubMed] [Google Scholar]
- 39.Kinoue K, Hattori M, Horita S, Kawaguchi H, Ito K. Crescent formation in children with Henoch-Schonlein purpura nephritis: a pathological and immunohistochemical study. Nippon Jinzo Gakkai Shi. 1996;38(8):364–371. [PubMed] [Google Scholar]
- 40.Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005;68(4):1866–1874. doi: 10.1111/j.1523-1755.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 41.Pilmore HL, Painter DM, Bishop GA, McCaughan GW, Eris JM. Early up-regulation of macrophages and myofibroblasts: a new marker for development of chronic renal allograft rejection. Transplantation. 2000;69(12):2658–2662. doi: 10.1097/00007890-200006270-00028. [DOI] [PubMed] [Google Scholar]
- 42.Burkhard K, Hofmann GO, Bosnecker A, et al. Early infiltration of renal allografts with 27E10-positive macrophages and graft outcome. Transpl Int. 1994;7 Suppl 1:S577–S579. doi: 10.1111/j.1432-2277.1994.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto K, Wada T, Furuichi K, et al. CD68 and MCP-1/CCR2 expression of initial biopsies reflect the outcomes of membranous nephropathy. Nephron Clin Pract. 2004;98(1):c25–c34. doi: 10.1159/000079924. [DOI] [PubMed] [Google Scholar]
- 44.Lewis A, Steadman R, Manley P, et al. Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol Histopathol. 2008;23(6):731–739. doi: 10.14670/HH-23.731. [DOI] [PubMed] [Google Scholar]
- 45.Kikuchi Y, Kobayashi S, Hemmi N, et al. Galectin-3-positive cell infiltration in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19(3):602–607. doi: 10.1093/ndt/gfg603. [DOI] [PubMed] [Google Scholar]
- 46.Ikezumi Y, Suzuki T, Karasawa T, et al. Use of mizoribine as a rescue drug for steroid-resistant pediatric IgA nephropathy. Pediatr Nephrol. 2008;23(4):645–650. doi: 10.1007/s00467-007-0664-2. [DOI] [PubMed] [Google Scholar]
- 47.Alexopoulos E, Gionanlis L, Papayianni E, et al. Predictors of outcome in idiopathic rapidly progressive glomerulonephritis (IRPGN) BMC Nephrol. 2006;7:16. doi: 10.1186/1471-2369-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill GS, Delahousse M, Nochy D, et al. A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney Int. 2000;58(3):1160–1173. doi: 10.1046/j.1523-1755.2000.00272.x. [DOI] [PubMed] [Google Scholar]
- 49.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104(1):27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 50.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 51.Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-·, PGE2 and PAF. Journal of Clinical Investigation. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castano AP, Lin SL, Surowy T, et al. Serum Amyloid P Inhibits Fibrosis Through FcγR-Dependent Monocyte-Macrophage Regulation in Vivo. Science Tran Med. 2009;1(5) doi: 10.1126/scitranslmed.3000111. 5ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61(4):375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 54.Savill J, Gregory C, Haslett C. Cell biology. Eat me or die. Science. 2003;302(5650):1516–1517. doi: 10.1126/science.1092533. [DOI] [PubMed] [Google Scholar]
- 55.Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical in kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0912228107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macedo L, Pinhal-Enfield G, Alshits V, et al. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171(6):1774–1788. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cooper L, Johnson C, Burslem F, Martin P. Wound healing and inflammation genes revealed by array analysis of 'macrophageless' PU.1 null mice. Genome Biol. 2005;6(1):R5. doi: 10.1186/gb-2004-6-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang XR, Tipping PG, Apostolopoulos J, et al. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol. 1997;109(1):134–142. doi: 10.1046/j.1365-2249.1997.4091307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duffield JS, Erwig LP, Wei X, et al. Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol. 2000;164(4):2110–2119. doi: 10.4049/jimmunol.164.4.2110. [DOI] [PubMed] [Google Scholar]
- 60.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183(10):6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 61.Reynolds J, Tam FW, Chandraker A, et al. CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Invest. 2000;105(5):643–651. doi: 10.1172/JCI6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279(5353):1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 63.Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int. 1997;51(1):94–103. doi: 10.1038/ki.1997.12. [DOI] [PubMed] [Google Scholar]
- 64.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189(10):1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynolds J, Moss J, Duda MA, et al. The evolution of crescentic nephritis and alveolar haemorrhage following induction of autoimmunity to glomerular basement membrane in an experimental model of Goodpasture's disease. J Pathol. 2003;200(1):118–129. doi: 10.1002/path.1336. [DOI] [PubMed] [Google Scholar]
- 66.Kalluri R, Danoff TM, Okada H, Neilson EG. Susceptibility to anti-glomerular basement membrane disease and Goodpasture syndrome is linked to MHC class II genes and the emergence of T cell-mediated immunity in mice. J Clin Invest. 1997;100(9):2263–2275. doi: 10.1172/JCI119764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aitman TJ, Dong R, Vyse TJ, et al. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature. 2006;439(7078):851–855. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- 68.Guo S, Muhlfeld AS, Wietecha TA, et al. Deletion of activating Fcgamma receptors does not confer protection in murine cryoglobulinemia-associated membranoproliferative glomerulonephritis. Am J Pathol. 2009;175(1):107–118. doi: 10.2353/ajpath.2009.081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez W, Mold C, Kataranovski M, et al. C-reactive protein-mediated suppression of nephrotoxic nephritis: role of macrophages, complement, and Fcgamma receptors. J Immunol. 2007;178(1):530–538. doi: 10.4049/jimmunol.178.1.530. [DOI] [PubMed] [Google Scholar]
- 70.Cunningham MA, Kitching AR, Tipping PG, Holdsworth SR. Fibrin independent proinflammatory effects of tissue factor in experimental crescentic glomerulonephritis. Kidney Int. 2004;66(2):647–654. doi: 10.1111/j.1523-1755.2004.00785.x. [DOI] [PubMed] [Google Scholar]
- 71.Kitching AR, Kong YZ, Huang XR, et al. Plasminogen activator inhibitor-1 is a significant determinant of renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14(6):1487–1495. doi: 10.1097/01.asn.0000065550.13931.00. [DOI] [PubMed] [Google Scholar]
- 72.Holdsworth SR, Tipping PG. Macrophage-induced glomerular fibrin deposition in experimental glomerulonephritis in the rabbit. J Clin Invest. 1985;76(4):1367–1374. doi: 10.1172/JCI112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo S, Wietecha TA, Hudkins K, et al. Macrophages are essential contributors to kidney injury in murine cryoglobiulinemia-associated membranoproliferative glomerulonephritis. J Am Soc Nephrol. 2008;19 doi: 10.1038/ki.2011.249. (Abstract in press.) [DOI] [PubMed] [Google Scholar]
- 74.Behmoaras J, Bhangal G, Smith J, et al. Jund is a determinant of macrophage activation and is associated with glomerulonephritis susceptibility. Nat Genet. 2008;40(5):553–559. doi: 10.1038/ng.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fanciulli M, Norsworthy PJ, Petretto E, et al. FCGR3B copy number variation is associated with susceptibility to systemic, but not organ-specific, autoimmunity. Nat Genet. 2007;39(6):721–723. doi: 10.1038/ng2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith J, Lai PC, Behmoaras J, et al. Genes expressed by both mesangial cells and bone marrow-derived cells underlie genetic susceptibility to crescentic glomerulonephritis in the rat. J Am Soc Nephrol. 2007;18(6):1816–1823. doi: 10.1681/ASN.2006070733. [DOI] [PubMed] [Google Scholar]
- 77.Kanamaru Y, Arcos-Fajardo M, Moura IC, et al. Fc alpha receptor I activation induces leukocyte recruitment and promotes aggravation of glomerulonephritis through the FcR gamma adaptor. Eur J Immunol. 2007;37(4):1116–1128. doi: 10.1002/eji.200636826. [DOI] [PubMed] [Google Scholar]
- 78.Bao L, Osawe I, Puri T, et al. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol. 2005;35(8):2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- 79.Brandt J, Pippin J, Schulze M, et al. Role of the complement membrane attack complex (C5b-9) in mediating experimental mesangioproliferative glomerulonephritis. Kidney Int. 1996;49(2):335–343. doi: 10.1038/ki.1996.50. [DOI] [PubMed] [Google Scholar]
- 80.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110(7):955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nemeth ZH, Lutz CS, Csoka B, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175(12):8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okamura DM, Pennathur S, Pasichnyk K, et al. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol. 2009;20(3):495–505. doi: 10.1681/ASN.2008010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24(4):155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 84.Csoka B, Nemeth ZH, Virag L, et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110(7):2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park JS, Gamboni-Robertson F, He Q, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 86.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10(2):137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 88.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duffield JS, Lupher ML. PRM-151 (recombinant human serum amyloid P/Pentraxin-2) for the treatment of fibrosis Drug News Perspect. 2010 doi: 10.1358/dnp.2010.23.5.1444206. in press. [DOI] [PubMed] [Google Scholar]
- 90.Hume DA, Underhill DM, Sweet MJ, et al. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geissmann F, Auffray C, Palframan R, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86(5):398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 92.Strobl H, Scheinecker C, Riedl E, et al. Identification of CD68+lin- peripheral blood cells with dendritic precursor characteristics. J Immunol. 1998;161(2):740–748. [PubMed] [Google Scholar]
- 93.Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17(9):424–428. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 94.Skold M, Behar SM. Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J Immunol. 2008;181(9):6349–6360. doi: 10.4049/jimmunol.181.9.6349. [DOI] [PubMed] [Google Scholar]
- 95.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18(1):60–65. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 96.Pesce JT, Ramalingam TR, Mentink-Kane MM, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4):e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Auffray C, Fogg DK, Narni-Mancinelli E, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206(3):595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87(1):373–377. [PubMed] [Google Scholar]
- 99.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lange-Sperandio B, Cachat F, Thornhill BA, Chevalier RL. Selectins mediate macrophage infiltration in obstructive nephropathy in newborn mice. Kidney Int. 2002;61(2):516–524. doi: 10.1046/j.1523-1755.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- 101.Lange-Sperandio B, Schimpgen K, Rodenbeck B, et al. Distinct roles of Mac-1 and its counter-receptors in neonatal obstructive nephropathy. Kidney Int. 2006;69(1):81–88. doi: 10.1038/sj.ki.5000017. [DOI] [PubMed] [Google Scholar]
- 102.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci U S A. 2001;98(4):1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rahman ZS, Niu H, Perry D, et al. Expression of the autoimmune Fcgr2b NZW allele fails to be upregulated in germinal center B cells and is associated with increased IgG production. Genes Immun. 2007;8(7):604–612. doi: 10.1038/sj.gene.6364423. [DOI] [PubMed] [Google Scholar]
- 104.Hellquist A, Jarvinen TM, Koskenmies S, et al. Evidence for genetic association and interaction between the TYK2 and IRF5 genes in systemic lupus erythematosus. J Rheumatol. 2009;36(8):1631–1638. doi: 10.3899/jrheum.081160. [DOI] [PubMed] [Google Scholar]
- 105.Kyogoku C, Tsuchiya N, Matsuta K, Tokunaga K. Studies on the association of Fc gamma receptor IIA, IIB, IIIA and IIIB polymorphisms with rheumatoid arthritis in the Japanese: evidence for a genetic interaction between HLA-DRB1 and FCGR3A. Genes Immun. 2002;3(8):488–493. doi: 10.1038/sj.gene.6363921. [DOI] [PubMed] [Google Scholar]
- 106.Kumar KR, Li L, Yan M, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312(5780):1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 107.Giles BM, Tchepeleva SN, Kachinski JJ, et al. Augmentation of NZB autoimmune phenotypes by the Sle1c murine lupus susceptibility interval. J Immunol. 2007;178(7):4667–4675. doi: 10.4049/jimmunol.178.7.4667. [DOI] [PubMed] [Google Scholar]
- 108.Namjou B, Kilpatrick J, Harley JB. Genetics of clinical expression in SLE. Autoimmunity. 2007;40(8):602–612. doi: 10.1080/08916930701510962. [DOI] [PubMed] [Google Scholar]
- 109.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172(2):288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haudek SB, Trial J, Xia Y, et al. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci U S A. 2008;105(29):10179–10184. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frangogiannis NG, Dewald O, Xia Y, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115(5):584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 112.Pulichino AM, Wang IM, Caron A, et al. Identification of transforming growth factor beta1-driven genetic programs of acute lung fibrosis. Am J Respir Cell Mol Biol. 2008;39(3):324–336. doi: 10.1165/rcmb.2007-0186OC. [DOI] [PubMed] [Google Scholar]
- 113.Stoneman V, Braganza D, Figg N, et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100(6):884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martin P, D'Souza D, Martin J, et al. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13(13):1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 115.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reiman RM, Thompson RW, Feng CG, et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74(3):1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thompson RW, Pesce JT, Ramalingam T, et al. Cationic amino acid transporter-2 regulates immunity by modulating arginase activity. PLoS Pathog. 2008;4(3):e1000023. doi: 10.1371/journal.ppat.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moore BB, Kolodsick JE, Thannickal VJ, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kang HR, Cho SJ, Lee CG, Homer RJ, Elias JA. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem. 2007;282(10):7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- 120.Vicencio AG, Lee CG, Cho SJ, et al. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol. 2004;31(6):650–656. doi: 10.1165/rcmb.2004-0092OC. [DOI] [PubMed] [Google Scholar]
- 121.Horan GS, Wood S, Ona V, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177(1):56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 122.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 123.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171(10):5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79(6):1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 126.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173(6):1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma FY, Liu J, Kitching AR, Manthey CL, Nikolic-Paterson DJ. Targeting renal macrophage accumulation via c-fms kinase reduces tubular apoptosis but fails to modify progressive fibrosis in the obstructed rat kidney. Am J Physiol Renal Physiol. 2009;296(1):F177–F185. doi: 10.1152/ajprenal.90498.2008. [DOI] [PubMed] [Google Scholar]
- 129.Tesch GH, Schwarting A, Kinoshita K, et al. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103(1):73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 131.Anders HJ, Vielhauer V, Frink M, et al. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest. 2002;109(2):251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao Q, Pang J, McIntyre K, et al. A CCR2/CCR5-dual antagonist, BMS-A, offers a potential novel oral therapy for the treatment of autoimmune disease. Journal of Immunology. 2009;182 92.96. [Google Scholar]
- 133.Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117(4):902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eis V, Luckow B, Vielhauer V, et al. Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15(2):337–347. doi: 10.1097/01.asn.0000111246.87175.32. [DOI] [PubMed] [Google Scholar]
- 135.Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clucas AT, Shah A, Zhang YD, Chow VF, Gladue RP. Phase I evaluation of the safety, pharmacokinetics and pharmacodynamics of CP-481,715. Clin Pharmacokinet. 2007;46(9):757–766. doi: 10.2165/00003088-200746090-00003. [DOI] [PubMed] [Google Scholar]
- 137.Gladue RP, Tylaska LA, Brissette WH, et al. CP-481,715, a potent and selective CCR1 antagonist with potential therapeutic implications for inflammatory diseases. J Biol Chem. 2003;278(42):40473–40480. doi: 10.1074/jbc.M306875200. [DOI] [PubMed] [Google Scholar]
- 138.Lin S-L, Castano AP, Nowlin BT, Lupher ML, Duffield JS. Bone Marrow Ly6Chigh Monocytes Are Selectively Recruited to Injured Kidney and Differentiate into Functionally Distinct Populations. Journal of Immunology. 2009;183 doi: 10.4049/jimmunol.0901473. in press. [DOI] [PubMed] [Google Scholar]
- 139.Ramanathan M, Pinhal-Enfield G, Hao I, Leibovich SJ. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol Biol Cell. 2007;18(1):14–23. doi: 10.1091/mbc.E06-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grenz A, Zhang H, Eckle T, et al. Protective role of ecto-5'-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18(3):833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 141.Lenda DM, Kikawada E, Stanley ER, Kelley VR. Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol. 2003;170(6):3254–3262. doi: 10.4049/jimmunol.170.6.3254. [DOI] [PubMed] [Google Scholar]
- 142.Cattell V, Cook HT, Ebrahim H, et al. Anti-GBM glomerulonephritis in mice lacking nitric oxide synthase type 2. Kidney Int. 1998;53(4):932–936. doi: 10.1111/j.1523-1755.1998.00892.x. [DOI] [PubMed] [Google Scholar]
- 143.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]