Abstract
Timed Up and Go (TUG) test is a widely used clinical paradigm to evaluate balance and mobility. Although TUG includes several complex subcomponents, namely: sit-to-stand, gait, 180° turn and turn-to-sit; the only outcome is the total time to perform the task. We have proposed an instrumented TUG, called iTUG, using portable inertial sensors to improve TUG in several ways: automatic detection and separation of subcomponents, detailed analysis of each one of them and a higher sensitivity than TUG. Twelve subjects in early stages of Parkinson's Disease (PD) and twelve age matched control subjects were enrolled. Stop-watch measurements did not show a significant difference between the two groups. The iTUG, however, showed a significant difference in cadence between early PD and control subjects (111.1±6.2 vs. 120.4±7.6 step/min, p < 0.006) as well as in angular velocity of arm-swing (123±32.0 vs. 174.0 ± 50.4 °/sec, p < 0.005), turning duration (2.18 ± 0.43 vs. 1.79 ± 0.27 seconds, p < 0.023) and time to perform turn-to-sits (2.96 ± 0.68 vs. 2.40 ± 0.33 seconds, p < 0.023). By repeating the tests for a second time, the test-retest reliability of iTUG was also evaluated. Among the subcomponents of iTUG, gait, turning and turn-to-sit were the most reliable and sit-to-stand was the least reliable.
Keywords: Gait, balance, mobility, objective assessment, wearable sensors
I. Introduction
Timed Up and Go test (TUG) is a widely used clinical test to evaluate balance and mobility [1]-[3]. The TUG test uses a stop-watch to time how long it takes a subject to stand up from a chair, walk 3 meters, turn 180 degrees, walk back to the chair and sit down. The TUG test has been shown to predict risk of falls in the elderly [4], [5], reflect balance deficits [6], [7] and correlate with severity of moderate-to-severe Parkinson's disease (PD) [8]-[11]. The clinical utility of the TUG is probably due to sequencing several mobility skills, such as turning and sit to stand transitions that require balance control, as well as straight-ahead gait [12]-[14].
Despite its widespread use in the clinic and clinical studies, TUG is limited. The main limitations are 1) it focuses only on time and ignores any other deficiency of movements and 2) it measures the total time to perform a series of complex activities without separating the performance of the subject in each part. In cross-sectional studies where a new medication, exercise program or other therapies affects some aspects of gait or balance, the effects might go unnoticed if the total time of TUG is not significantly changed. In some neurological diseases with slow rate of progress, TUG might not be sensitive enough to pick up the evolution of the disease unless the study is extended over several years. To address some of these problems, [13] used a stop-watch to measure the duration of each of the four subcomponents of the TUG, namely sit-to-stand, gait, turning and turn-to-sit. However, using a stop-watch to find exact time of a rapid sequence of activities is difficult and somewhat subjective. In a recent study researchers used inertial sensors to measure the duration of each subcomponent of the TUG test automatically [15]. However, the main limitation of TUG, i.e. focusing only on time was not addressed by either of these methods. A main goal of our study was to address both issues by providing detailed, objective evaluation of TUG's subcomponents.
The subcomponents of TUG are complex activities on their own. Lately, there has been progress in developing objective methods to characterize and assess these complex activities in clinical environments using wearable, inertial sensors [16]. Specifically, gyroscopes and accelerometers on the shanks and sternum have been used to record and analyze gait [17]-[20] , sit-to-stand and stand-to-sit transitions [21], [22]. We apply these methods as a foundation to analyze subcomponents of the TUG test.
An important step needed to build a complete system to analyze all subcomponents of the TUG test is to analyze 180° turns. A difficulty in turning analysis is defining the onset and offset of turns [23], [24]. Higashi and colleagues used angular velocity in the yaw-axis and a fixed threshold to define onset and offset of turning [15]. While [24] did not clearly define turning duration, it is assumed a similar approach was used. In this study, we use a new concept to define turn duration based on mathematical modeling. We hypothesize that it is possible to automatically analyze all subcomponents of the TUG test using inertial sensors.
Besides providing detailed assessment of subjects' performance, a useful movement analysis system should be reliable. The TUG, itself, is a reliable test [25] and a recent study has shown good test-retest reliability for the duration of its subcomponents [26]. The present study evaluates the test-retest reliability of using inertial sensors to automatically quantify measures within each subcomponent of the TUG.
A useful clinical assessment tool also needs to be sensitive to pathology. While it is known that patients with moderate to advanced stages of Parkinson's disease (PD) show impaired motor performance compared to control subjects [9], [27], it is difficult to identify mobility deficits in very early stages of their disease [28]. To demonstrate the improved sensitivity of instrumented TUG (iTUG), we compared the performance of TUG and iTUG of a group of unmedicated PD subjects early in the disease who had minimal gait impairment with age-matched healthy control subjects.
The present manuscript focuses on describing the proposed analysis algorithm for automatic analysis of iTUG. We believe the scope of applications of iTUG go beyond assessment of mobility in patients with PD; thus only the most important clinical results are presented. A separate manuscript presents our clinical findings related to early PD in more detail [28]. A draft version of the turning analysis method was previously presented in an IEEE conference [29].
II. Method
A. Subjects
Twelve patients with idiopathic Parkinson's disease and 12 control subjects participated in this study. The two groups were age matched (60.4 ± 8.5 vs. 60.2 ± 8.2 years old). There were seven males in the PD group and three males in the control group. Healthy control subjects were either spouses of the PD subjects or recruited from the community. PD subjects were in early-to-moderate stage of disease (H & Y score between 1 and 2.5, UPDRS motor score 20.0 ± 9.4) and have never taken anti-Parkinsonian medications. Subjects were excluded if they had any neurological disorders other than PD, orthopedic disorders or other impairments that could potentially interfere with gait, or if they used orthotic devices or had artificial joints. All participants provided informed consent approved by the Oregon Health & Science University Institutional Review Board.
B. Measurement protocol
Subjects performed timed up and go test three times. They all used an armless chair and were instructed to not to use their arms to stand up. Although in traditional TUG an armchair is used, we used an armless chair to make the task slightly more challenging. Several other studies explored using armless chairs in the past [6], [15], [25], [30]. Using armless chairs could reduce the variability between subjects by eliminating the choice to use or not to use the armrests to arise. In traditional TUG walkway is only 3 meters. Extended Timed Get Up and Go (ETGUG) used a 10-meters walkway to include more gait cycles during the test [30]. However a larger room is required to accommodate a longer pathway. We decided to make a compromise and used a 7-meter walkway so that we could have more gait cycles than TUG but use less space than ETGUG. The beginning and end of the walkway were clearly marked with 2½ cm wide tape on the floor. These markings were shown to the subjects before the test. The end line was 3 meters away from the wall. Subjects were instructed to sit straight on the chair with their hands on their thighs and their backs touching the back of the chair. After they were given the go signal by the tester, they arose from the chair, walked at their normal speed, turned around right after passing the tape at the end of the pathway, returned back to the chair, turned around and sat down. The tester timed their performance with a stop-watch. All sessions were recorded on video to verify performance.
To assess test-retest reliability of the measures, nine subjects in each group repeated the protocol a second time. After finishing the three iTUG tests, sensors were removed from their body. After one hour, the sensors were replaced, and the protocol was repeated. We assumed that the subjects' performance remained the same within this time period. The same examiner used the same device and same protocol to test the subject for the second time.
In summary, subjects had 3 TUG (3 meters) and 3 iTUG (7 meters) trials. Subjects that also participated in the test-retest reliability had an additional 3 trials of iTUG.
C. Systems and devices
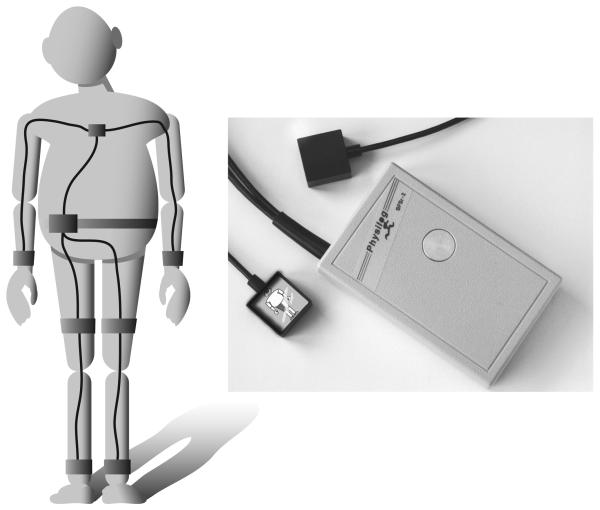
Subjects wore a small data-logger in a waist-worn pack, Physilog (BioAGM, CH), with seven inertial sensors attached on the forearms, shanks, thighs and sternum as shown in Fig. 1 [17]. To limit the number of recorded channels, a different combination of accelerometers and gyroscopes was used for each body site: a sensor with a 3D accelerometer (±5 g) and a 2D gyroscope (±400 °/s, yaw and pitch axes) was fixed on sternum [22] with double stick tape. A 2D gyroscope (±1200 °/s, yaw and pitch axes) was attached on each forearm and a single axis gyroscope (±600 °/s, pitch axes) was placed on each thigh and shank [31] with Velcro straps. Sampling rate was 200 Hz. The data were recorded on a 128 MB SD flash card.
Fig 1.
Sensors were attached on the upper and lower limbs using elastic bands. On the trunk, sensors were attached using a double sided adhesive tape.
D. Components of the iTUG
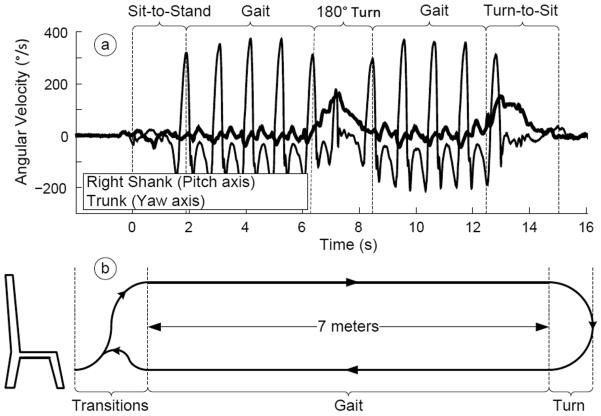
The iTUG was divided into 4 major components: sit-to-stand, stead-state gait, turning and turn-to-sit (see Fig. 2 ).
Fig 2.
Components of the iTUG and representative recorded raw signals from angular velocity of a shank and sternum
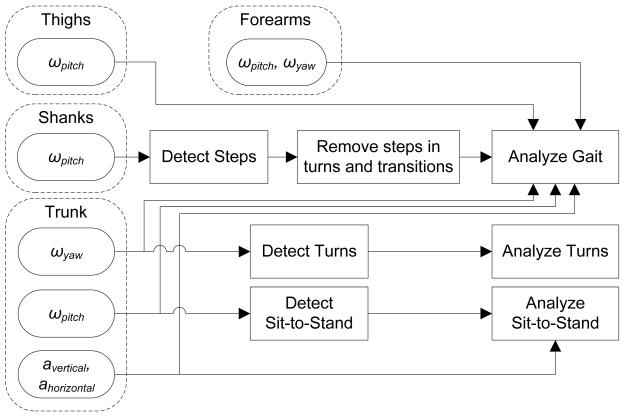
Each subcomponent of the iTUG was detected with a unique subset of sensors: sit-to-stand and turn-to-sit transitions were detected and analyzed using the trunk sensor with a method published elsewhere [22]. Steps during gait and turns were detected using the two sensors on the shanks [31]. Turns were detected using the trunk sensor with a new method described in the next section. Fig. 3 shows how signals from each sensor were used to detect and analyze different components of the iTUG.
Fig 3.
diagram showing how inertial sensors were used for the iTUG analysis algorithms.
To analyze steady-state gait, after detecting sit-to-stand and stand-to-sit transitions [32] and turns (explained in the next section), steps within turns and transitions were removed. The remaining steps, which were taken only during straight walking, were analyzed using the signals from the 4 sensors attached to the lower-limbs [31]. We also analyzed arm swing during gait using the signals from sensors attached on the forearms.
E. Analysis of turning
An interesting problem in analyzing turns in the iTUG is identifying the onset and offset of the turns. The iTUG includes two 180° turns: one after walking 7 meters and the other during final turn-to-sit transition. Unlike initial and terminal contacts in gait analysis, onset and offset of turns are not events marked by sudden, distinct movements of the body or impacts with the floor, but are rather a slow transition from one form of activity (straight walk) to another (turn). [15] defined onset and offset of turns by finding the time trunk angular velocity crossed a fixed threshold level. However, gait and transitions produce noticeable signals in yaw angular velocity (see Fig. 3.a). As a result threshold-based methods are sensitive to noise and will probably have low reliability, especially with slow gait and turning speeds. In our approach, we avoided fixed thresholds and used a mathematical model to find these transitions from straight to curved walking and back to straight walking (or sitting at the end of TUG) using an optimization method.
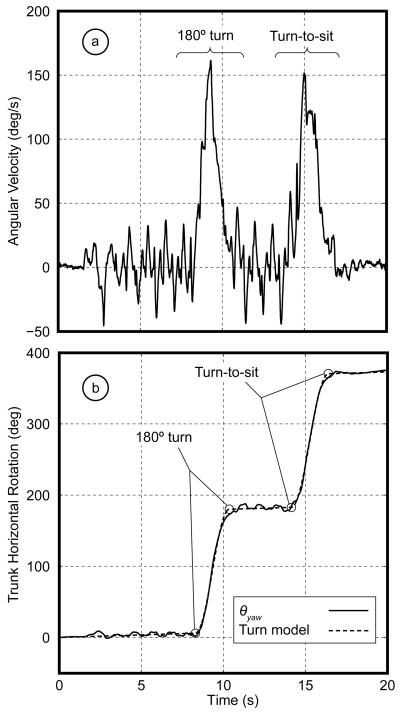
To detect 180° turns, we analyzed the signal from the yaw gyroscope on the sternum (ωy(t), Fig. 4.a). This signal showed large variations associated with shoulder girdle rotation during walking. As seen in the raw signal, although it is relatively easy to identify when turns occurred in this signal, due to marked peaks in yaw velocity amplitude, it is difficult to see exactly where turns begin and end.
Fig 4.
a) Raw signal of the yaw gyroscope on the trunk during iTUG. The first peak corresponds to 180° turn and the second peak corresponds to the turn-to-sit transition b) Relative angle of the trunk in the horizontal plane and fitted mathematical model.
After integrating ωy(t) signal, the relative trunk angle in the horizontal plane (θy(t)) was obtained. Since the initial angle of the trunk at the beginning of recording is unknown, a value of zero was assumed. Thus, θy(t) showed how much the trunk was turned to the left or right relative to the beginning of the test. As seen in Fig. 4.b, walking components appear in θy(t) as nearly flat lines with some variations due to yaw rotation oscillations of trunk. Turning components appear as positive or negative ramps, depending on the direction of turns. Since the sensor attached on the trunk could have a small inclination relative to the horizontal plane due to anatomy of this part of the body, differences in θy(t) before and after turns could be smaller than 180°. A continuous mathematical model was used to describe θy(t) during iTUG:
| (1) |
where E(t) is a continuous piecewise function changing smoothly between two levels:
| (2) |
In M(t) drift and offset of the gyroscopes were assumed to be constant during the short period of iTUG and were described as constant slope C.
In this model 〈a1, b1, c1〉 described the first turn and 〈a2, b2, c2〉 described the second turn. The 〈ai, bi, ci〉 coefficients used to estimate the amplitude of the turns (usually near, but less than, 180° due to sensors inclination), the time and duration of turns, respectively. In this model, period of the turns would be defined as .
A subspace trust-region based, non-linear, least squares optimization method [33] was use to fit M(t) on θy(t). Coefficient of determination (R2) was used to evaluate the quality of model fit to data.
F. iTUG metrics
The following outcomes were calculated for each subcomponent of iTUG. Sit-to-stand metrics [22], [32]: A) Average and peak angular velocity of trunk in the sagittal plane (in degrees per second). B) Duration (in seconds) and range of motion of the trunk in the sagittal plane (in degrees).
Gait metrics [31]: A) Temporal measures including Cadence (number of steps per minute), Stance (as percentage of gait cycle time), Double support (as percentage of gait cycle time), Limp (i.e. difference between initial and terminal double support as a percentage of gait cycle time). B) Performance of the lower limbs including Range of Motion (ROM) of shanks, thighs as well as flexion-extension of knee (all in degrees), stride-length and stride-velocity (normalized by stature) and peak angular velocity of shanks (in degrees per second). C) Performance of the upper limbs including arm-swing in Pitch and Yaw axes (in degrees), peak angular velocity (by combining Pitch and Yaw axes, reported in degrees per second) and left/right asymmetry (difference of the left and right divided by the fastest, reported as percentage). D) Performance of the trunk including ROM in horizontal and sagittal planes as well as peak angular velocity in horizontal and sagittal planes.
Turning metrics: A) Duration of turns (in seconds). B) Peak angular velocity of the trunk in horizontal plane (in degrees per seconds). C) Number of steps, average step time (from heel-strike to heel-strike, in seconds), time for the longest step (in seconds), last step time right before turn (in seconds) and number steps of double steps, i.e. successive steps with the same foot.
Turn-to-sit metrics: A) Turning metrics. B) Range of motion of trunk in the sagittal plane (in degrees).
G. Data analysis
All analysis algorithms, as well as statistical evaluation of outcomes were performed in MATLAB.
Mean value of the straight walking gait metrics across all three trials were reported. The median of metrics of the transitions and turns in the three trials were reported to eliminate outliers.
To compare differences between the mild PD and control groups, Wilcoxon's non-parametric test (rank-sum) was used. Since the tests for the metrics where pre-planned, the p-values were not adjusted for multiple-comparisons. To evaluate test-retest reliability of the iTUG, Intra-Class Correlation (ICC) was used [34]. Since the same subjects and same device was used for reliability, an ICC(1,1) was used. ρ and 95% confidence intervals were reported.
III. Results
A. Sensitivity
The total time to perform either 3 meter TUG or 7 meter iTUG was not significantly different between the early PD and control groups (10.8 ± 0.5 vs. 9.9 ± 0.3, p > 0.18 for TUG and 15.4 ± 0.6 vs. 14.3 ± 0.5 seconds, p > 0.18 for iTUG). After combining three repetitions of iTUG and removing gait cycles overlapping with turns and transitions, there was no significant difference in number of gait cycles between groups: the PD group had 15.6 ± 3.5 gait cycles and the control group had 15.5 ± 3.9 gait cycles > 0.95). None of the common spatio-temporal gait measures, except Cadence, were significantly different between the two groups (see Table 1).
TABLE I.
Metrics in iTUG grouped by type of activity. Those highlighted in boldface were significantly different (i.e. p < 0.05) between early PD and control subjects.
| PD | Control | rank-sum | ||||
|---|---|---|---|---|---|---|
| Activity | Metric | Mean | S.D. | Mean | S.D. | p-value |
| Gait | ||||||
| Temporal | Cadence (steps/min) | 111.1 | 6.2 | 120.4 | 7.6 | 0.0061 |
| Stance (%) | 58.9 | 1.6 | 59.4 | 2.4 | 0.2366 | |
| Double Support (%) | 17.8 | 3.1 | 18.8 | 4.9 | 0.2366 | |
| Limp (%) | 2.3 | 1.1 | 2.1 | 1.2 | 0.6236 | |
|
| ||||||
| Lower Limbs | RoM-Shank (°) | 69.6 | 7.3 | 69.6 | 7.0 | 0.8852 |
| RoM-Thigh (°) | 43.7 | 5.7 | 43.4 | 3.8 | 0.6650 | |
| RoM-Knee (°) | 54.6 | 6.7 | 56.0 | 7.3 | 0.5444 | |
| Stride-Length (%h) | 75.6 | 7.6 | 74.8 | 5.8 | 0.7508 | |
| Stride-Velocity (%h/s) | 70.3 | 9.7 | 75.0 | 7.2 | 0.1939 | |
| Peak Swing Velocity (°/s) | 333.5 | 47.1 | 359.8 | 43.6 | 0.2602 | |
|
| ||||||
| Upper Limbs | Arm-Swing Pitch (°) | 17.6 | 4.3 | 24.0 | 10.6 | 0.0783 |
| Arm-Swing Yaw (°) | 28.9 | 9.8 | 39.4 | 13.1 | 0.0464 | |
| Peak Arm Swing Velocity (°/s) | 123.2 | 32.0 | 174.0 | 50.4 | 0.0051 | |
| Arm Swing Speed Asym (%) | 36.3 | 17.9 | 23.1 | 12.4 | 0.0531 | |
|
| ||||||
| Trunk | Peak Trunk Horiz. Velocity (°/s) | 34.7 | 9.0 | 44.2 | 10.1 | 0.0226 |
| Peak Trunk Sagit. Velocity (°/s) | 30.7 | 6.9 | 38.4 | 9.2 | 0.0783 | |
| RoM-Trunk Horiz (°) | 7.7 | 2.4 | 8.8 | 1.4 | 0.1260 | |
| RoM-Turnk Sagit (°) | 4.4 | 0.7 | 4.7 | 0.7 | 0.4705 | |
| Turning | ||||||
| Trunk | Peak Angular Velocity (°/s) | 162.3 | 30.85 | 172.44 | 30.13 | 0.7950 |
| Duration (s) | 2.18 | 0.43 | 1.79 | 0.27 | 0.0226 | |
|
| ||||||
| Lower Limbs | Steps | 4.08 | 1.00 | 3.50 | 0.52 | 0.1422 |
| Average Step Time (s) | 0.57 | 0.07 | 0.56 | 0.07 | 0.7508 | |
| Max Step Time (s) | 0.71 | 0.15 | 0.69 | 0.12 | 0.7507 | |
| Last Step Time Before Turn (s) | 0.56 | 0.04 | 0.52 | 0.04 | 0.0302 | |
| Number of Double Steps | 0.33 | 0.49 | 0.00 | 0.00 | 0.0357 | |
| Sit-to-Stand | ||||||
| Trunk | Peak Angular Velocity (°/s) | 89.13 | 17.20 | 92.69 | 15.77 | 0.8399 |
| Average Angular Velocity (°/s) | 29.43 | 3.22 | 30.81 | 3.85 | 0.3123 | |
| Duration (s) | 2.18 | 0.25 | 2.10 | 0.26 | 0.4178 | |
| RoM-Trunk (°) | 37.11 | 3.11 | 37.49 | 6.22 | 0.8399 | |
| Turn-to-Sit | ||||||
| Trunk | Duration (s) | 2.96 | 0.68 | 2.40 | 0.33 | 0.0226 |
| RoM-Trunk (°) | 29.22 | 3.80 | 30.20 | 6.01 | 0.9770 | |
|
| ||||||
| Lower Limbs | Steps | 3.00 | 0.85 | 3.17 | 1.34 | 1.0000 |
| Average Step Time (s) | 0.72 | 0.17 | 0.55 | 0.16 | 0.0304 | |
| Max Step Time (s) | 1.08 | 0.41 | 0.77 | 0.34 | 0.0734 | |
| Last Step Time Before Turn (s) | 0.56 | 0.04 | 0.52 | 0.04 | 0.0375 | |
| Number of Double Steps | 0.00 | 0.00 | 0.08 | 0.29 | 0.3593 | |
The algorithms automatically detected all transitions and all turns. The residuals of fitting the mathematical model of turning (M(t)) on raw data were small (average R2 = 0.9989, min 0.9973 , max 0.9997).
Table I shows list of the outcome metrics. Despite the similarity of the total time to perform iTUG and the similar number of gait cycles between the two groups, several measures related to some components of iTUG, namely gait, turns and turn-to-sit, showed significant differences between groups. The only component of iTUG that was not significantly different between the two groups was the sit-to-stand.
B. Reliability
Temporal measures of gait, with the exception of limp, showed an excellent reliability (ρ > 0.90). Spatial measures (stride-length and stride-velocity) showed somewhat lower, but yet good, reliability (ρ > 0.75). Reliability of the estimated duration of turn and turn-to-sit transitions was also very high. Sit-to-stand measures, however, did not show good test-retest reliability. Table II summarized the test-retest reliability results.
TABLE II.
Test-retest reliability of the iTUG metrics.
| Test1 | Test2 | ICC | 95% CI bounds | |||||
|---|---|---|---|---|---|---|---|---|
| Activity | Metric | Mean | S.D. | Mean | S.D. | ρ | lower | Upper |
| Gait | ||||||||
| Temporal | Cadence(steps/min) | 116.38 | 8.85 | 114.61 | 9.17 | 0.94 | 0.84 | 0.98 |
| Stance (%) | 58.89 | 2.10 | 59.29 | 2.14 | 0.92 | 0.79 | 0.97 | |
| Double Support (%) | 17.78 | 4.19 | 18.58 | 4.27 | 0.92 | 0.79 | 0.97 | |
| Limp (%) | 2.02 | 0.91 | 2.13 | 1.10 | 0.25 | −0.23 | 0.64 | |
|
| ||||||||
| Lower Limbs | RoM-Shank (°) | 2.39 | 8.78 | 71.72 | 7.39 | 0.64 | 0.26 | 0.85 |
| RoM-Thigh (°) | 45.23 | 4.92 | 44.47 | 5.12 | 0.63 | 0.25 | 0.85 | |
| RoM-Knee (°) | 55.79 | 8.99 | 54.20 | 7.41 | 0.55 | 0.12 | 0.81 | |
| Stride-Length (%h) | 79.37 | 8.25 | 78.53 | 7.05 | 0.67 | 0.30 | 0.86 | |
| Stride-Velocity (%h/s) | 76.98 | 9.52 | 75.26 | 9.82 | 0.78 | 0.51 | 0.92 | |
| Peak Swing Velocity (°/s) | 364.24 | 56.25 | 355.89 | 54.26 | 0.78 | 0.50 | 0.91 | |
|
| ||||||||
| Arm Swing | RoM-Arm Pitch (°) | 21.83 | 11.02 | 21.31 | 9.36 | 0.68 | 0.32 | 0.87 |
| RoM Arm Yaw (°) | 31.92 | 12.30 | 33.47 | 13.68 | 0.84 | 0.62 | 0.94 | |
| Peak Velocity (°/s) | 147.41 | 49.07 | 146.98 | 53.99 | 0.90 | 0.75 | 0.96 | |
| Speed Asym (%) | 34.24 | 17.38 | 34.03 | 17.82 | 0.94 | 0.85 | 0.98 | |
|
| ||||||||
| Trunk | Horiz. Velocity (°/s) | 40.06 | 11.97 | 39.67 | 12.13 | 0.95 | 0.86 | 0.98 |
| Sagit. Velocity (°/s) | 37.65 | 11.83 | 34.65 | 10.97 | 0.83 | 0.61 | 0.94 | |
| RoM-Trunk Horiz. (°) | 7.97 | 2.26 | 8.26 | 2.42 | 0.78 | 0.50 | 0.91 | |
| RoM-Turnk Sagit. (°) | 4.72 | 1.26 | 4.46 | 0.73 | 0.52 | 0.08 | 0.79 | |
| Turning | ||||||||
| Trunk | Peak Angular Velocity (°/s) | 164.88 | 33.27 | 159.61 | 31.79 | 0.86 | 0.67 | 0.95 |
| Duration (s) | 2.01 | 0.52 | 1.99 | 0.41 | 0.89 | 0.74 | 0.96 | |
|
| ||||||||
| Lower Limbs | # of Steps | 4.00 | 0.94 | 3.82 | 0.88 | 0.75 | 0.45 | 0.90 |
| Average Step Time (s) | 0.58 | 0.09 | 0.59 | 0.07 | 0.61 | 0.21 | 0.84 | |
| Max Step Time (s) | 0.73 | 0.22 | 0.71 | 0.13 | 0.50 | 0.05 | 0.78 | |
| Step Before Turn (s) | 0.54 | 0.04 | 0.54 | 0.05 | 0.85 | 0.64 | 0.94 | |
| # of Double Steps | 0.18 | 0.39 | 0.18 | 0.39 | 0.22 | −0.27 | 0.62 | |
| Sit-to-Stand | ||||||||
| Trunk | Peak Angular Velocity (°/s) | 92.07 | 24.32 | 91.71 | 18.59 | 0.43 | −0.04 | 0.75 |
| Average Angular Velocity (°/s) | 29.65 | 4.54 | 30.15 | 3.87 | 0.22 | −0.27 | 0.62 | |
| Duration (s) | 2.20 | 0.26 | 2.12 | 0.26 | 0.04 | −0.42 | 0.50 | |
| RoM-Trunk (°) | 36.73 | 4.64 | 36.98 | 5.02 | 0.22 | −0.27 | 0.62 | |
| Turn-to-Sit | ||||||||
| Trunk | Duration (s) | 2.59 | 0.53 | 2.77 | 0.67 | 0.84 | 0.61 | 0.94 |
| RoM-Trunk (°) | 32.35 | 8.38 | 30.75 | 5.96 | 0.77 | 0.47 | 0.91 | |
|
| ||||||||
| Lower Limbs | # of Steps | 2.82 | 0.53 | 3.00 | 0.94 | 0.23 | −0.26 | 0.63 |
| Average Step Time (s) | 0.62 | 0.14 | 0.67 | 0.16 | 0.52 | 0.08 | 0.79 | |
| Max Step Time (s) | 0.78 | 0.33 | 0.97 | 0.39 | 0.38 | −0.10 | 0.72 | |
| Last Step Time Before Turn (s) | 0.53 | 0.04 | 0.54 | 0.05 | 0.78 | 0.50 | 0.91 | |
| # of Double Steps | 0.06 | 0.24 | 0.00 | 0.00 | 0.00 | −0.46 | 0.46 | |
IV. Discussion
The iTUG is a technological evolution of the TUG, a familiar and well-established test of mobility. By instrumenting the TUG and designing automatic analysis algorithms we could provide objective and comprehensive assessment that went beyond temporal measures. The idea of looking at performance of subjects in each component of TUG separately was previously explored by [30] with Extended Timed Get Up and Go (ETGUG). They used a 10-meter walkway and recorded the time of each of 4 components of the tests. To our knowledge, only one other group used inertial sensors to quantify subcomponents of the TUG [15]. However, besides durations and amplitude of accelerations during gait, they did not report any other objective outcomes related to components of the TUG. The iTUG, on the other hand, provides a detailed assessment of each subcomponent of the test. For example results in Table I show that range and amplitude of arm-swing were sensitive to early PD while TUG total time was not. This is a new finding that could not be measured by TUG, ETGUG or accelerometery methods focusing on temporal measures.
A major contribution of this study was to introduce a new mathematical model to quantify turning during gait. Our novel, mathematical model to detect turning is reliable and fits the data very well. It is based on modeling the whole dataset, so unlike previous methods, it is not sensitive to local noise and artifacts near the moment of onset and offset of turns. The model is fit over the angle of trunk rotation in the yaw axis rather than over the angular velocity. Thus, it can be used even in extreme cases of very slow or rapid turns; e.g patients with PD turn more slowly than control subjects. This is an advantage over yaw angular velocity threshold methods [15], [24] that could have the drawback of population-dependence (the threshold is hard to generalize and tends to be influenced by noise and movement artifacts).
It is noteworthy that although we focused on 180° turns in this study, the mathematical model can be used to analyze turning by 90° or any other value. In fact, due to alignment of the sensor on the body turns were not exactly 180° thus as part of the fitting process, the model measures the turning angle. We hypothesize that this approach can be used to analyze turns under various other conditions, including unplanned, free turns during continuous measurement of spontaneous activity.
Gait was the most reliable subcomponent of the test. Cadence was its most reliable metric (ρ = 0.94). Stride-length and stride-velocity showed moderate to good reliability (ρ = 0.67 and 0.78 respectively). These findings are comparable to a recent study [35] though it reported a slightly lower ICC for cadence and slightly better ICC for stride-velocity. The reason that spatial measures were less reliable than temporal might be due to the sensitivity of these measures to the alignment of the sensors on the body [31]. There is a potential to improve the reliability of these measures by improving the gait analysis method with 3D, instead of 1-D, gyroscopes. Limp, i.e. difference between initial and terminal double-support, was the least reliable gait measure (ρ = 0.25) probably due to its very short duration (20 ms) compared to the sampling period of the system (5 ms). These results suggest that gait analysis within iTUG is feasible and reliable.
Duration of turns was the most reliable turning metric. The reason might be due to the turning model that uses the whole signal, including gait prior to and after the turn. Also, the model is not particularly sensitive to local noise near onset and offset of turns. The least reliable measure related to turning was the number of double steps. The relatively mild PD subjects rarely took double steps and did not need to use double steps consistently in all trials. Healthy subjects never took double steps. Reliability of the turn-to-sit metrics were comparable to the turning metrics.
The sit-to-stand component was the least reliable part of the test. This finding is similar to the findings of a previous study looking at duration of the TUG test components [26]. The poor reliability of the sit-to-stand metrics might be due to the large degrees of freedom available to subjects who can use a variety of strategies to perform this activity [36], [37], despite our efforts to limit this freedom by giving subjects explicit instructions on how to perform the test and using the same chair during all trials. Another reason for poor reliability might be due to our analysis method that relies on detection of peak angular velocity, though we have used appropriate filters to minimize the effect of noise in peak-identification [22].
Significant differences between the early PD and age-matched control groups could be observed in three, out of four, components of the iTUG, although the total time to perform the iTUG test was not sensitive enough to separate the groups' performance. Subjects with early PD had especially slow turning, arm swing, cadence, and trunk rotation during straight walk. They performed normally in sit-to-stand. A more detailed clinical discussion of the differences found between the two groups, as well as the relationship between severity of PD and the outcomes measures of the iTUG is published in a separate article [28].
The potential applications of iTUG is not be limited to testing subjects with PD. The iTUG provides a large number of quantitative outcomes for gait and postural transitions that are relevant for testing anyone with balance or gait deficits. A different subset of measures in the iTUG might be sensitive to different neurological or musculoskeletal constraints. The traditional TUG has been shown to predict falls in the elderly [4]. Our new, instrumented version, the iTUG, may be even more sensitive to earlier stages of mobility disability. A better understanding of the subcomponents affected in mobility disability will also help focus rehabilitation to remediate these constraints [38].
Some important components of the test that have not been fully explored in this study are gait initiation and termination, in particular the sit-to-walk movement. Further work is required to evaluate feasibility of using inertial sensor to analyze these components.
In conclusion, the iTUG is an objective, fast, reliable and sensitive test of mobility. We recommend using the iTUG to replace the TUG for clinical trials and clinical practice.
Acknowledgement
We thank Caroline Paquette and Triana Nagel-Nelson for assistance with data collection. We also thank Pascal Morel and Jean Gramiger for designing and development of the Physilog system. Dr. Horak was a consultant for the Kinetics Foundation. This potential conflict of interest has been reviewed and managed by OHSU. The technology described herein is patent pending and available for licensing from OHSU.
This work was supported by the Kinetics Foundation and the National Institutes of Health (grants AG006457 and DC01849).
Contributor Information
Arash Salarian, Laboratory of Movement Analysis and Measurement (LMAM), Ecole Polytechnique Fédéral de Lausanne (EPFL), Switzerland. He is now with the Department of Neurology, Oregon Health & Science University, Portland, OR, USA.
Fay B Horak, Department of Neurology, Oregon Health & Science University, Portland, OR, USA..
Cris Zampieri, Department of Neurology, Oregon Health & Science University, Portland, OR, USA. She is now with the Rehabilitation Medicine Department, National Institutes of Health, Bethesda, MD, USA..
Kamiar Aminian, Laboratory of Movement Analysis and Measurement (LMAM), Ecole Polytechnique Fédéral de Lausanne (EPFL), Switzerland..
References
- 1.Berg KO, Maki BE, Williams KI. Clinical and laboratory measures of postural balance in an elderly population. Archives of Physical Medicine and Rehabilitation. 1992;73:1073–1080. [PubMed] [Google Scholar]
- 2.Thompson M, Medley A. Performance of community dwelling elderly on the timed up and go test. Physical and Occupational Therapy in Geriatrics. 1995;13:17–29. [Google Scholar]
- 3.Lin M, Hwang H, Hu M, Wu H, Wang Y, Huang F. Psychometric comparisons of the timed up and go, oneleg stand, functional reach, and tinetti balance measures in community-dwelling older people. Journal of the American Geriatrics Society. 2004;52:1343–1348. doi: 10.1111/j.1532-5415.2004.52366.x. [DOI] [PubMed] [Google Scholar]
- 4.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up and go test. Physical Therapy. 2000;80(no. 9):896–903. [PubMed] [Google Scholar]
- 5.Whitney JC, Lord SR, Close JCT. Streamlining assessment and intervention in a falls clinic using the timed up and go test and physiological profile assessments. Age and Ageing. 2005;34(no. 6):567–571. doi: 10.1093/ageing/afi178. [DOI] [PubMed] [Google Scholar]
- 6.Mathias S, Nayak USL, Isaacs B. Balance in elderly patients: The ‘get-up and go’ test. Archives of Physical Medicine and Rehabilitation. 1986;67(no. 6):387–389. [PubMed] [Google Scholar]
- 7.Whitney SL, Poole JL, Cass SP. A review of balance instruments for older adults. American Journal of Occupational Therapy. 1998;52(no. 8):666–671. doi: 10.5014/ajot.52.8.666. [DOI] [PubMed] [Google Scholar]
- 8.Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with parkinson disease. Physical Therapy. 2005;85(no. 2):134–41. [PubMed] [Google Scholar]
- 9.Martínez-Martín P, Urra DG, Quijano TS, Gómez B, Utrero EG, Piñeiro P, Andrés MT. A new clinical tool for gait evaluation in parkinson's disease. Clinical Neuropharmacology. 1997;20(no. 3):183–194. doi: 10.1097/00002826-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the timed ”up & go” test in people with parkinson disease. Physical Therapy. 2001;81(no. 2):810–819. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 11.Dibble LE, Lange M. Predicting falls in individuals with parkinson disease: A reconsideration of clinical balance measures. Journal of Neurologic Physical Therapy. 2006;30(no. 2):60–67. doi: 10.1097/01.npt.0000282569.70920.dc. [DOI] [PubMed] [Google Scholar]
- 12.Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Disturbance of sequential movements in patients with parkinson's disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 13.Rogers MA, Phillips JG, Bradshaw JL, Iansek R, Jones D. Provision of external cues and movement sequencing in parkinson's disease. Motor Control. 1998;2(no. 2):125–32. doi: 10.1123/mcj.2.2.125. [DOI] [PubMed] [Google Scholar]
- 14.Bloem BR, Valkenburg VV, Slabbekoorn M, van Dijk JG. The multiple tasks test. strategies in parkinson's disease. Experimental Brain Research. 2001;137(no. 3-4):478–86. doi: 10.1007/s002210000672. [DOI] [PubMed] [Google Scholar]
- 15.Higashi Y, Yamakoshi K, Fujimoto T, Sekine M, Tamura T. Quantitative evaluation of movement using the timed up-and-go test. IEEE Engineering in Medicine and Biology Magazine. 2008;27(no. 4):38–46. [Google Scholar]
- 16.Zijlstra W, Aminian K. Mobility assessment in older people: new possibilities and challenges. European Journal of Ageing. 2007;4(no. 1):3–12. doi: 10.1007/s10433-007-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aminian K, Najafi B, Bula C, Leyvraz PF, Robert P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. Journal of Biomechanics. 2002;35(no. 5):689–699. doi: 10.1016/s0021-9290(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Allum J, Carpenter M. A speedy solution for balance and gait analysis: angular velocity measured at the centre of body mass. Current Opinion in Neurology. 2005;18(no. 1):15. doi: 10.1097/00019052-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Brandes M, Zijlstra W, Heikens S, van Lummel R, Rosenbaum D. Accelerometry based assessment of gait parameters in children. Gait & Posture. 2006;24(no. 4):482–486. doi: 10.1016/j.gaitpost.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Moore ST, MacDougall HG, Gracies J-M, Cohen HS, Ondo WG. Long-term monitoring of gait in parkinson's disease. Gait & Posture. 2007;26(no. 2):200–207. doi: 10.1016/j.gaitpost.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Najafi B, Aminian K, Paraschiv-Ionescu A, Loew F, Bula C, Robert P. Ambulatory system for human motion analysis using a kinematic sensor: monitoring of daily physical activity in the elderly. IEEE Transactions on Biomedical Engineering. 2003;50(no. 6):711–723. doi: 10.1109/TBME.2003.812189. [DOI] [PubMed] [Google Scholar]
- 22.Salarian A, Russmann H, Vingerhoets FJG, Burkhard PR, Aminian K. Ambulatory monitoring of the physical activities in patients with parkinson's disease. IEEE Transactions on Biomedical Engineering. 2007;54(no. 12):2296–2299. doi: 10.1109/tbme.2007.896591. [DOI] [PubMed] [Google Scholar]
- 23.Stack E, Jupp K, Ashburn A. Developing methods to evaluate how people with parkinson's disease turn 180°: an activity frequently associated with falls. Disability and Rehabilitation. 2004;26(no. 8):478–484. doi: 10.1080/09638280410001663085. [DOI] [PubMed] [Google Scholar]
- 24.Visser JE, Voermans NC, Nijhuis LBO, van der Eijk M, Nijk R, Munneke M, Bloem BR. Quantification of trunk rotations during turning and walking in parkinson's disease. Clinical Neurophysiology. 2007;118(no. 7):1602–1606. doi: 10.1016/j.clinph.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Podsiadlo D, Richardson S. The timed ‘up and go’: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(no. 2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 26.Botolfsen P, Helbostad JL, Moe-nilssen R, Wall JC. Reliability and concurrent validity of the expanded timed up-and-go test in older people with impaired mobility. Physiotherapy Research International. 2008;13(no. 2):94–106. doi: 10.1002/pri.394. [DOI] [PubMed] [Google Scholar]
- 27.Campbell CM, Rowse JL, Ciol MA, Shumway-Cook A. The effect of cognitive demand on timed up and go performance in older adults with and without parkinson disease. Neurology report. 2003;27(no. 1):2–7. [Google Scholar]
- 28.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. An instrumented timed up and go test characterizes gait and postural transitions in untreated parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81(no. 2):171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salarian A, Zampieri C, Horak FB, Carlson-Kuhta P, Nutt JG, Aminian K. Analyzing 180° turns using an inertial system reveals early signs of progression of parkinson's disease. Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE. 2009 Sep.:224–227. doi: 10.1109/IEMBS.2009.5333970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wall JC, Bell C, Campbell S, Davis J. The timed get-up-and-go test revisited: measurement of the component tasks. Journal of Rehabilitation Research and Development. 2000;37(no. 1):109–13. [PubMed] [Google Scholar]
- 31.Salarian A, Russmann H, Vingerhoets FJG, Dehollain C, Blanc Y, Burkhard PR, Aminian K. Gait assessment in parkinson's disease: Toward an ambulatory system for long-term monitoring. IEEE Transactions on Biomedical Engineering. 2004;51(no. 8):1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 32.Najafi B, Aminian K, Loew F, Blanc Y, Robert PA. Measurement of stand-sit and sit-stand transitions using a miniature gyroscope and its application in fall risk evaluation in the elderly. IEEE Transactions on Biomedical Engineering. 2002;49(no. 8):843–851. doi: 10.1109/TBME.2002.800763. [DOI] [PubMed] [Google Scholar]
- 33.Coleman TF, Li Y. An interior trust region approach for nonlinear minimization subject to bounds. SIAM Journal on Optimization. 1996;6(no. 2):418–445. [Google Scholar]
- 34.McGraw KO, Wong SP. Forming inferences about some interclass correlation coefficients. Psychological Methods. 1996;1(no. 1):30–46. [Google Scholar]
- 35.Najafi B, Helbostad JL, Moe-Nilssen R, Zijlstra W, Aminian K. Does walking strategy in older people change as a function of walking distance? Gait & Posture. 2009;29(no. 2):261–266. doi: 10.1016/j.gaitpost.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Janssen WGM, Bussmann HBJ, Stam HJ. Determinants of the sit-to-stand movement: A review. Physical Therapy. 2002;82(no. 9):866–879. [PubMed] [Google Scholar]
- 37.Millington PJ, Myklebust BM, Shambes GM. Biomechanical analysis of the sit-to-stand motion in elderly persons. Archives of Physical Medicine and Rehabilitation. 1992;73(no. 7):609–617. [PubMed] [Google Scholar]
- 38.King LA, Horak FB. Delaying mobility disability in parkinson's disease with a sensorimotor agility exercise program. Physical Therapy. 2009;89(no. 4):384–393. doi: 10.2522/ptj.20080214. [DOI] [PMC free article] [PubMed] [Google Scholar]